Aquatic Mercury Pollution from Artisanal and Small-Scale Gold Mining in Sub-Saharan Africa: Status, Impacts, and Interventions
Abstract
:1. Introduction
2. Methodological Approach
3. Mercury Pollution in Sub-Saharan Africa: Trends and Impacts
3.1. Mercury Pollution to Air, Land, and Aquatic Ecosystems: MIA Synthesis
3.1.1. Eastern Africa
3.1.2. Southern Africa
3.1.3. West Africa
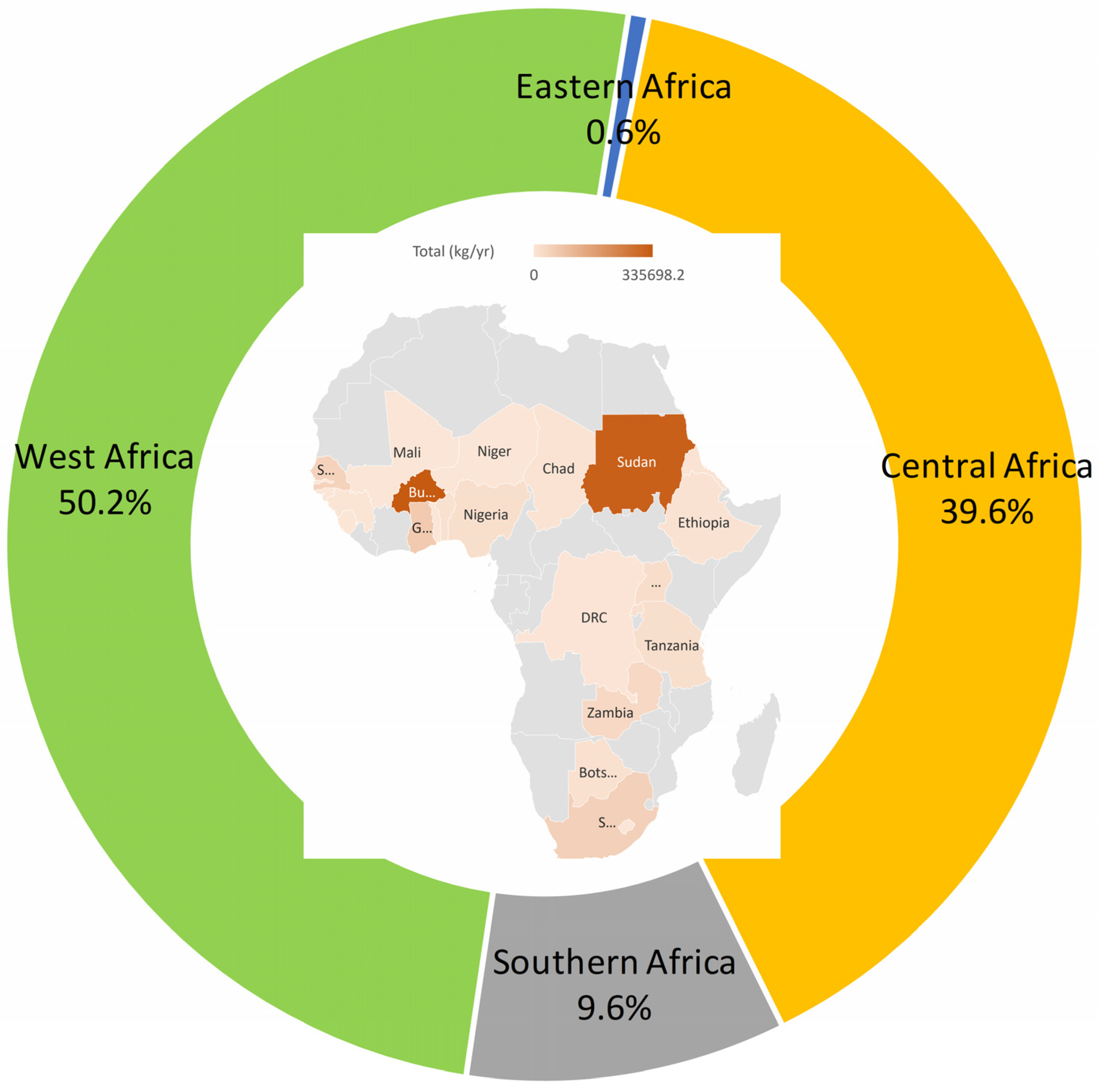
3.2. Regional Patterns of Aquatic Hg Pollution from ASGM
3.2.1. Eastern Africa
Uganda
Tanzania
Kenya
Sudan
3.2.2. Southern Africa
South Africa
Zimbabwe
3.2.3. Central Africa
3.2.4. West Africa
Ghana
Senegal
Nigeria
| Region and Country | Aquatic Matrix | Hg Category and Value | References |
|---|---|---|---|
| 1. Eastern | |||
| Uganda | Okame River | Hg: 0.019 mg/L (Water) | [55] |
| Nankuke River | Hg: 0.0163 mg/L (Water) | ||
| Nabweo River | Hg: 0.0158 mg/L (Water) | [56,57] | |
| Namukombe stream | THg: 0.11 µg/g (Fish, Oreochromis niloticus); 1.21 mg/L (Water); 0.14 µg/g (Sediment) | ||
| Rwizi River | Hg: 0.01–0.1 ug/g (Sediment); 0.01–0.3 µg/L (Water); 0.04 µg/g (Fish, Barbus altianalis); 0.09 µg/g (Fish, Brycinus sadleri) | [58] | |
| Lake Victoria | THg: 1.7–5.8 ng/L; MeHg: 0.2–1.0 ng/L (Water) | ||
| Tanzania | Lake Victoria Goldfield streams | Hg: 7.0 µg/kg (Fish, Tilapia sp.); ug/g (Sediment) | [58,59,60,61,63] |
| Mugusu mine streams | Hg: 0.35–6.0 mg/kg (Sediment) | ||
| L. Victoria, Nungwe Bay | Hg: 2–35 µg/kg (Fish, Tilapia sp.; Lates niloticus; Protopterus sp.) | ||
| Rwamagasa Gold mine region streams | Hg: 0.01–1.6 mg/kg (Sediment); 0.006–3.5 mg/kg (Fish, Lates sp., Oreochromis spp., Clarias sp.) | ||
| Kenya | Migori Goldbelt, Lake Victoria Basin | Hg: 430 µg/kg–149.9 mg/kg (Sediment) | [64,65,66,67,68] |
| Hg: 0.36 µg/L–0.66 mg/L (Water); 0.26–355 mg/kg (Fish, Rastrineobola argentea) | |||
| Sudan | River Nile State Albenda region streams | Hg: 0.001–0.005 mg/L (Water); 0.017–0.094 mg/kg (Fish: Oreochromis niloticus); 0.085–0.172 mg/kg (Fish: Lates niloticus) | [72,74] |
| 2. Southern | |||
| South Africa | Berg River | THg: 50 ng/g (Sediment); MeHg: 1.3 ng/g (Sediment) | [76,79,80,81,84] |
| Olifants River | MeHg: 68 ng/g (Sediment); THg: 0.1–6.1 µg/g (Fish, Clarias sp.; Hydrocynus sp., Labeo sp., Schilbe sp., Labeobarbus sp., Oreochromis sp.); 0.001–0.078 mg/g (Sediment) | ||
| Ga-Selati River | MeHg: 0.036 µg/L (Water) | ||
| Randfontein, West Johannesburg stream | Hg: 10 µg/g (Sediment); 2 ng/L (Surface water); 223 ng/L (Ground water) | ||
| Zimbabwe | Farvic Gold mine area streams, Bulawayo. Chimanimani, East Zimbabwe | Hg: 6–154 mg/kg (Sediment); 0.06–0.4 mg/L (Water) Hg: 0.1–0.3 mg/L (Water) | [86,87,88] |
| Mazowe and Chivaro Reservoirs | THg: Above 0.5 µg/g (Fish) | ||
| 3. Central | |||
| Cameroon | Togo Gold District, Kianke, and Tchangue Basins | Hg: 0.106 mg/kg | [93] |
| Kadey River, East Cameroon | Hg: 0.02 mg/L (Water) | [96] | |
| Lom River, Adamawa | Hg: 0.01–1.83 mg/L (Water) | [97] | |
| Lom Basin | Hg: 0.007–0.008 mg/L (Water) | [98] | |
| Lom River, Gankombol | Hg: 0.004–0.005 mg/L (Water) | [99,100] | |
| Democratic Republic of Congo | Fizi Basin River systems, south Kivu | Hg: 7.8–41.3 ng/L (Water); 17.1–89.8 mg/kg (Sediment) | [94,95,96] |
| 4. West | |||
| Ghana | Kejetia Gorogo and Bolgatanga ASGM area streams, Upper East Ghana | Hg: 0.05–0.248 µg/g (Sediment); 0.024–0.22 µg/g (Fish; species unidentified) | [101] |
| Pra River Basin | Hg: 0.01–2.92 mg/kg (Sediment) | [36] | |
| THg: 0.019–0.265 mg/kg (Sediment) | |||
| MeHg: 0.001 mg/kg (Sediment) | [37] | ||
| Bonsa River | Hg: 0.18 µg/L–0.061 mg/L (Water); 1.13–1.21 mg/kg (Sediment) | [102,103] | |
| River Apepe and Ankora | MeHg: 0.24–14.82 ng/g (Sediment) | [104] | |
| Ankobrah and Pra Rivers | Hg: 0.006–0.0093 mg/L (Water); 0.04–0.6 mg/kg (Fish, Oreochromis niloticus; Clarias angularis) | [40] | |
| Senegal | Gambia River | THg: 0.03–0.5 mg/kg (Fish); 0.5–1.05 mg/kg (Shellfish); 4.2 ng/g–9.9 mg/kg (Sediment); 22 ng/L (Water) | [106,107] |
| Nigeria | Manyera River, Niger State | Hg: 0.014–0.025 mg/L (Water); 0.021 mg/kg (Sediment); 0.008 mg/kg (Fish—Heterotis niloticus) | [108] |
| Northwest Anka ASGM Region | Hg: 2.12 mg/g (Sediment) | [109] | |
| Igade mining area, Niger State | Hg: 0.01–0.012 mg/L (Water) | [110] |
3.3. Regional Summary and Intercontinental Hg Pollution from ASGM
| Sub-Region | Country | Environmental Compartment | References | ||
|---|---|---|---|---|---|
| Water | Sediment/Tailings | Biota | |||
| Eastern | Uganda | 5.8 ng/L–1.21 mg/L | 0.14–0.4 µg/g | Fish: 0.11 µg/g | [53,54,55] |
| Tanzania | 629 ng/g–6 mg/kg | Fish: 2 µg/kg–3.5 mg/kg Shrimp: 8µg/L Snail: 68 µg/L | [51,59,60,61] | ||
| Kenya | 0.36 ug/L–0.66 mg/L | 430 ug/kg–1920 mg/kg (tailings) 0.001–24.6 mg/kg (sediment) | Fish: 0.26–355 mg/kg | [62,63,65,66] | |
| Sudan | 0.001–0.005 mg/L | 0.001–0.005 mg/kg (sediment) | Fish: 0.017–0.172 mg/kg | [70,72,118] | |
| Southern | South Africa | 0.036 µg/L | 50 ng/g–1.3 mg/g | Fish: 0.001–6.1 ng/g | [50,74,77,78,82] |
| Zimbabwe | 0.06–154 mg/kg | Fish: 17–32% with Hg > 0.2 µg/g | [84,85,86] | ||
| Central | DRC | 7.8–41.3 ng/L | 13.6–88.8 mg/kg | [92] | |
| Cameroon | 7.8 ng/L–0.83 mg/L | 2–25 mg/kg | [91,93,94,97,98] | ||
| West | Ghana | 0.18 µg/L–0.0093 mg/L | 0.005 µg/g–2.92 mg/kg | Fish: 0.024 µg/g–0.6 mg/kg | [36,37,40,100,102,119] |
| Senegal | 22 ng/L | 4.2 ng/g–9.9 mg/kg | [105] | ||
| Nigeria | 0.01–0.05 mg/L | 2.12 ug/g–0.021 mg/kg | Fish: 0.008 mg/kg | [106,107,108] | |
| Asia | Indonesia | 0.7–9.9 µg/L | [34] | ||
| Myanmar | 0.002–0.008 µg/L | [109] | |||
| Philippines | 0.009–80 µg/L | [110] | |||
| Thailand | 0.7–6 µg/L | [120] | |||
| Cambodia | 64 ng/g | Fish: 90 ng/g–50 µg/g | [111] | ||
| Latin America | Ecuador | Sediment: 2.7 µg/g–0.02 mg/kg Tailings: 89–1535 µg/g | [112,121] | ||
| Brazil | 100 ng/g–1207 mg/kg | Fish: 100 ng/g–1242 µg/kg | [113,122,123] | ||
| Venezuela | 4.60 µg/L | 0.12–1.92 mg/kg | [114] | ||
| Brazil | Fish (Carnivorous 0.4 µg/g; Omnivore-Pimelodus ornatus, 1.8 µg/g; Herbivore-Mylesinus paraschombourgkii, 1 µg/g; M. ternetzi, 0.85 ug/g Hg) | [115] | |||
| Brazil, Colombia, Peru | Fish (Carnivore, Hoplias malabaricus, 0.21–282 µg/g; Herbivore-Pterygoplichthys pardalis, 0.09–0.28 µg/g THg) | [116] | |||
| Peru | Lake sediment: 64–86 ng/g River sediment: 20–53 ng/g | Fish(“chambira”, 1215 ng/g: “palomata”, 80 ng/g; “Huasaco”, 500 ng/g) | [117] | ||
4. Socioeconomic, Environmental, and Human Health Impacts of ASGM in SSA
5. Considerations for Interventions and Remediation
5.1. Mitigation and Remediation Strategies
5.2. Minamata Convention Parties’ Commitment in SSA
5.3. Towards Mercury-Free ASGM Technologies: Selected Case Studies
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Driscoll, C.T.; Mason, R.P.; Chan, H.M.; Jacob, D.J.; Pirrone, N. Mercury as a Global Pollutant: Sources, Pathways, and Effects. Environ. Sci. Technol. 2013, 47, 4967–4983. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of Heavy Metals on the Environment and Human Health: Novel Therapeutic Insights to Counter the Toxicity. J. King Saud Univ. Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Teng, H.; Altaf, A.R. Journal of Hazardous Materials Advances Elemental Mercury (Hg 0) Emission, Hazards, and Control: A Brief Review. J. Hazard. Mater. Adv. 2022, 5, 100049. [Google Scholar] [CrossRef]
- Park, J.; Zheng, W. Human Exposure and Health Effects of Inorganic and Elemental Mercury. J. Prev. Med. Public Health 2012, 45, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Streets, D.G.; Horowitz, H.M.; Jacob, D.J.; Lu, Z.; Levin, L.; Ter Schure, A.F.H.; Sunderland, E.M. Total Mercury Released to the Environment by Human Activities. Environ. Sci. Technol. 2017, 51, 5969–5977. [Google Scholar] [CrossRef] [PubMed]
- Marrugo-Negrete, J.; Verbel, J.O.; Ceballos, E.L.; Benitez, L.N. Total Mercury and Methylmercury Concentrations in Fish from the Mojana Region of Colombia. Environ. Geochem. Health 2008, 30, 21–30. [Google Scholar] [CrossRef]
- Harika, V.K.; Penki, T.R.; Loukya, B.; Samanta, A.; Xu, G.; Sun, C.; Grinberg, I.; Deepak, F.L.; Amine, K.; Aurbach, D.; et al. Sustainable Existence of Solid Mercury Nanoparticles at Room Temperature and Their Applications. Chem. Sci. 2021, 12, 3226–3238. [Google Scholar] [CrossRef]
- Sawicka, B.; Umachandran, K.; Fawzy, M.; Mahmoud, A.E.D. 27—Impacts of Inorganic/Organic Pollutants on Agroecosystems and Eco-Friendly Solutions. In Egbuna the Military and Health; Mtewa, A.G., Egbuna, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 523–552. ISBN 978-0-12-821556-2. [Google Scholar]
- Zhang, W.; Zhang, X.; Tian, Y.; Zhu, Y.; Tong, Y.; Li, Y.; Wang, X. Risk Assessment of Total Mercury and Methylmercury in Aquatic Products from Offshore Farms in China. J. Hazard. Mater. 2018, 354, 198–205. [Google Scholar] [CrossRef]
- Fleck, J.A.; Marvin-DiPasquale, M.; Eagles-Smith, C.A.; Ackerman, J.T.; Lutz, M.A.; Tate, M.; Alpers, C.N.; Hall, B.D.; Krabbenhoft, D.P.; Eckley, C.S. Mercury and Methylmercury in Aquatic Sediment across Western North America. Sci. Total Environ. 2016, 568, 727–738. [Google Scholar] [CrossRef]
- Kalisińska, E.; Łanocha-Arendarczyk, N.; Kosik-Bogacka, D.I. Mercury, Hg. In Mammals and Birds as Bioindicators of Trace Element Contaminations in Terrestrial Environments; Kalisińska, E., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 593–653. ISBN 978-3-030-00121-6. [Google Scholar]
- de Almeida Rodrigues, P.; Ferrari, R.G.; dos Santos, L.N.; Conte Junior, C.A. Mercury in Aquatic Fauna Contamination: A Systematic Review on Its Dynamics and Potential Health Risks. J. Environ. Sci. 2019, 84, 205–218. [Google Scholar] [CrossRef]
- Celo, V.; Lean, D.R.S.; Scott, S.L. Abiotic Methylation of Mercury in the Aquatic Environment. Sci. Total Environ. 2006, 368, 126–137. [Google Scholar] [CrossRef] [PubMed]
- La Colla, N.S.; Botté, S.E.; Marcovecchio, J.E. Mercury Cycling and Bioaccumulation in a Changing Coastal System: From Water to Aquatic Organisms. Mar. Pollut. Bull. 2019, 140, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Regnell, O.; Watras, C.J. Microbial Mercury Methylation in Aquatic Environments—A Critical Review of Published Field and Laboratory Studies Published Field and Laboratory Studies. Environ. Sci. Technol. 2018, 53, 4–19. [Google Scholar] [CrossRef] [PubMed]
- UNEP. Global Mercury Assessment; UNEP: Nairobi, Kenya, 2018. [Google Scholar]
- Amoatey, P.; Baawain, M.S. Effects of Pollution on Freshwater Aquatic Organisms. Water Environ. Res. 2019, 91, 1272–1287. [Google Scholar] [CrossRef] [PubMed]
- Amundsen, P.; Henriksson, M.; Poste, A.; Prati, S.; Power, M. Ecological Drivers of Mercury Bioaccumulation in Fish of a Subarctic Watercourse. Environ. Toxicol. Chem. 2023, 42, 873–887. [Google Scholar] [CrossRef] [PubMed]
- Cuellar-Valencia, O.M.; Murillo-García, O.E.; Rodriguez-Salazar, G.A.; Bolívar-García, W. Bioaccumulation of Mercury in Direct-Developing Frogs: The Aftermath of Illegal Gold Mining in a National Park. Herpetol. J. 2023, 33, 6–13. [Google Scholar] [CrossRef]
- Kraus, J.M.; Wanty, R.B.; Schmidt, T.S.; Walters, D.M.; Wolf, R.E. Variation in Metal Concentrations across a Large Contamination Gradient Is Reflected in Stream but Not Linked Riparian Food Webs. Sci. Total Environ. 2021, 769, 144714. [Google Scholar] [CrossRef]
- Lino, A.S.; Kasper, D.; Guida, Y.S.; Thomaz, J.R.; Malm, O. Total and Methyl Mercury Distribution in Water, Sediment, Plankton and Fish along the Tapajós River Basin in the Brazilian Amazon. Chemosphere 2019, 235, 690–700. [Google Scholar] [CrossRef]
- Niane, B.; Moritz, R.; Guédron, S.; Ngom, P.M.; Pfeifer, H.R.; Mall, I.; Poté, J. Effect of Recent Artisanal Small-Scale Gold Mining on the Contamination of Surface River Sediment: Case of Gambia River, Kedougou Region, Southeastern Senegal. J. Geochem. Explor. 2014, 144, 517–527. [Google Scholar] [CrossRef]
- Niane, B.; Devarajan, N.; Poté, J.; Moritz, R. Quantification and Characterization of Mercury Resistant Bacteria in Sediments Contaminated by Artisanal Small-Scale Gold Mining Activities, Kedougou Region, Senegal. J. Geochem. Explor. 2019, 205, 106353. [Google Scholar] [CrossRef]
- Streets, D.G.; Horowitz, H.M.; Lu, Z.; Levin, L.; Thackray, C.P.; Sunderland, E.M. Five Hundred Years of Anthropogenic Mercury: Spatial and Temporal Release Profiles. Environ. Res. Lett. 2019, 14, 084004. [Google Scholar] [CrossRef]
- Streets, D.G.; Horowitz, H.M.; Lu, Z.; Levin, L.; Thackray, C.P.; Sunderland, E.M. Global and Regional Trends in Mercury Emissions and Concentrations, 2010–2015. Atmos. Environ. 2019, 201, 417–427. [Google Scholar] [CrossRef]
- Seccatore, J.; Veiga, M.; Origliasso, C.; Marin, T.; De Tomi, G. An Estimation of the Artisanal Small-Scale Production of Gold in the World. Sci. Total Environ. 2014, 496, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Hilson, G. Farming, Small-Scale Mining and Rural Livelihoods in Sub-Saharan Africa: A Critical Overview. Extr. Ind. Soc. 2016, 3, 547–563. [Google Scholar] [CrossRef]
- Al-Sulaiti, M.M.; Soubra, L.; Al-Ghouti, M.A. The Causes and Effects of Mercury and Methylmercury Contamination in the Marine Environment: A Review. Curr. Pollut. Rep. 2022, 8, 249–272. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Q.; Xu, Q.; Xue, J.; Zhang, C.; Wang, D. Mercury Bioaccumulation in Fish in an Artificial Lake Used to Carry out Cage Culture. J. Environ. Sci. 2019, 78, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Calao-Ramos, C.; Bravo, A.G.; Paternina-Uribe, R.; Marrugo-Negrete, J.; Díez, S. Occupational Human Exposure to Mercury in Artisanal Small-Scale Gold Mining Communities of Colombia. Environ. Int. 2021, 146, 106216. [Google Scholar] [CrossRef] [PubMed]
- Taux, K.; Kraus, T.; Kaifie, A. Mercury Exposure and Its Health Effects in Workers in the Artisanal and Small-Scale Gold Mining (ASGM) Sector—A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 81. [Google Scholar] [CrossRef]
- Mallongi, A.; Parkpian, P.; Pataranawat, P.; Chinwetkitvanich, S. Mercury Distribution and Its Potential Environmental and Health Risks in Aquatic Habitat at Artisanal Buladu Gold Mine in Gorontalo Province, Indonesia. Pak. J. Nutr. 2015, 14, 1010–1025. [Google Scholar] [CrossRef]
- Meutia, A.A.; Bachriadi, D.; Gafur, N.A. Environment Degradation, Health Threats, and Legality at the Artisanal Small-Scale Gold Mining Sites in Indonesia. Int. J. Environ. Res. Public Health 2023, 20, 6774. [Google Scholar] [CrossRef]
- Barkdull, N.M.; Carling, G.T.; Rey, K.; Yudiantoro, D.F. Comparison of Mercury Contamination in Four Indonesian Watersheds Affected by Artisanal and Small-Scale Gold Mining of Varying Scale. Water. Air. Soil Pollut. 2019, 230, 214. [Google Scholar] [CrossRef]
- Hilson, G. The Environmental Impact of Small-Scale Gold Mining in Ghana. Geogr. J. 2002, 168, 57. [Google Scholar] [CrossRef]
- Donkor, A.K.; Bonzongo, J.-C.J.; Nartey, V.K.; Adotey, D.K. Heavy Metals in Sediments of the Gold Mining Impacted Pra River Basin, Ghana, West Africa. Soil Sediment Contam. Int. J. 2005, 14, 479–503. [Google Scholar] [CrossRef]
- Donkor, A.K.; Bonzongo, J.C.; Nartey, V.K.; Adotey, D.K. Mercury in Different Environmental Compartments of the Pra River Basin, Ghana. Sci. Total Environ. 2006, 368, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Telmer, K.H.; Veiga, M.M. World Emissions of Mercury from Artisanal and Small—Scale Gold Mining. In Mercury Fate and Transport in the Global Environment: Emissions, Measurements and Models; Mason, R., Pirrone, N., Eds.; Springer US: Boston, MA, USA, 2009; pp. 131–172. [Google Scholar] [CrossRef]
- Yoshimura, A.; Suemasu, K.; Veiga, M.M. Estimation of Mercury Losses and Gold Production by Artisanal and Small-Scale Gold Mining (ASGM). J. Sustain. Metall. 2021, 7, 1045–1059. [Google Scholar] [CrossRef]
- Kortei, N.K.; Heymann, M.E.; Essuman, E.K.; Kpodo, F.M.; Akonor, P.T.; Lokpo, S.Y.; Boadi, N.O.; Ayim-Akonor, M.; Tettey, C. Health Risk Assessment and Levels of Toxic Metals in Fishes (Oreochromis noliticus and Clarias anguillaris) from Ankobrah and Pra Basins: Impact of Illegal Mining Activities on Food Safety. Toxicol. Rep. 2020, 7, 360–369. [Google Scholar] [CrossRef]
- Basta, P.C.; de Vasconcellos, A.C.S.; Hallwass, G.; Yokota, D.; Pinto, D.d.O.d.R.; de Aguiar, D.S.; de Souza, C.C.; Oliveira-da-Costa, M. Risk Assessment of Mercury-Contaminated Fish Consumption in the Brazilian Amazon: An Ecological Study. Toxics 2023, 11, 800. [Google Scholar] [CrossRef]
- Barbieri, F.L.; Gardon, J. Hair Mercury Levels in Amazonian Populations: Spatial Distribution and Trends. Int. J. Health Geogr. 2009, 8, 71. [Google Scholar] [CrossRef]
- UNEP. Minamata Convention on Mercury; UNEP: Nairobi, Kenya, 2013. [Google Scholar]
- Anan, T.; Toda, E. Analysis of National Priorities from Minamata Initial Assessments Minamata Convention on Mercury. In Proceedings of the 14th International Conference on Mercury as a Global Pollutant (ICMGP 2019), Krakow, Poland, 8–13 September 2019. [Google Scholar]
- van Straaten, P. Mercury Contamination Associated with Small-Scale Gold Mining in Tanzania and Zimbabwe. Sci. Total Environ. 2000, 259, 105–113. [Google Scholar] [CrossRef]
- Nuwagira, U.; Mubiru, D.; Yasin, I.; Nasasira, P. Impact of Artisanal Gold Mining on Wetland Health in Buhweju District, Southwestern Uganda. East Afr. J. Environ. Nat. Resour. 2023, 6, 297–310. [Google Scholar] [CrossRef]
- Fayiga, A.O.; Ipinmoroti, M.O.; Chirenje, T. Environmental Pollution in Africa. Environ. Dev. Sustain. 2018, 20, 41–73. [Google Scholar] [CrossRef]
- Abdoul, K.A.M.; Alassane, Y.A.K.; Alphonse, S.A.; Emmanuel, A.; Olivier, D.; Daouda, M.; Dominique, C.K.S. Mercury Pollution in Africa: A Review. J. Environ. Chem. Ecotoxicol. 2022, 14, 32–49. [Google Scholar] [CrossRef]
- Yabe, J.; Ishizuka, M.; Umemura, T. Current Levels of Heavy Metal Pollution in Africa. J. Vet. Med. Sci. 2010, 72, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Walters, C.R.; Somerset, V.S.; Leaner, J.J.; Nel, J.M. A Review of Mercury Pollution in South Africa: Current Status. J. Environ. Sci. Health Part A 2011, 46, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.M.; Dixon, D.G.; Hecky, R.E. A Review of Mercury in Lake Victoria, East Africa: Implications for Human and Ecosystem Health. J. Toxicol. Environ. Health Part B 2003, 6, 325–356. [Google Scholar] [CrossRef] [PubMed]
- Issah Musah-Surugu, J.; Ahenkan, A.; Bawole, J.N.; Yeboah-Assiamah, E. Rural Poverty and Artisanal Mining in Sub-Saharan Africa. Int. J. Rural Manag. 2017, 13, 162–181. [Google Scholar] [CrossRef]
- Barasa, B.; Kakembo, V.; Karl, T. Characterization of Artisanal Gold Mining Activities in the Tropics and Their Impact on Sediment Loading and Stream Flow in the Okame River Catchment, Eastern Uganda. Environ. Earth Sci. 2016, 75, 1076. [Google Scholar] [CrossRef]
- Omara, T.; Karungi, S.; Kalukusu, R.; Nakabuye, B.V.; Kagoya, S.; Musau, B. Mercuric Pollution of Surface Water, Superficial Sediments, Nile tilapia (Oreochromis nilotica Linnaeus 1758 [Cichlidae]) and Yams (Dioscorea alata) in Auriferous Areas of Namukombe Stream, Syanyonja, Busia, Uganda. PeerJ 2019, 7, e7919. [Google Scholar] [CrossRef]
- Basooma, A.; Teunen, L.; Semwanga, N.; Bervoets, L. Trace Metal Concentrations in the Abiotic and Biotic Components of River Rwizi Ecosystem in Western Uganda, and the Risks to Human Health. Heliyon 2021, 7, e08327. [Google Scholar] [CrossRef]
- Campbell, L.M.; Hecky, R.E.; Muggide, R.; Dixon, D.G.; Ramlal, P.S. Variation and Distribution of Total Mercury in Water, Sediment and Soil from Northern Lake Victoria, East Africa. Biogeochemistry 2003, 65, 195–211. [Google Scholar] [CrossRef]
- Ikingura, J.R.; Akagi, H. Monitoring of Fish and Human Exposure to Mercury Due to Gold Mining in the Lake Victoria Goldfields, Tanzania. Sci. Total Environ. 1996, 191, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Ikingura, J.R.; Mutakyahwa, M.K.D.; Kahatano, J.M.J. Mercury and Mining in Africa with Special Reference to Tanzania. Water Air Soil Pollut. 1997, 97, 223–232. [Google Scholar] [CrossRef]
- Ikingura, J.R.; Akagi, H.; Mujumba, J.; Messo, C. Environmental Assessment of Mercury Dispersion, Transformation and Bioavailability in the Lake Victoria Goldfields, Tanzania. J. Environ. Manag. 2006, 81, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Chibunda, R.T. Comparative Sensitivity of Caridina nilotica, Haplochromis nubilus, Bulinus africanus and Bulinus forskalii from Lake Victoria, Tanzania to Mercury Chloride. Chem. Ecol. 2008, 24, 207–212. [Google Scholar] [CrossRef]
- Taylor, H.; Appleton, J.D.; Lister, R.; Smith, B.; Chitamweba, D.; Mkumbo, O.; Machiwa, J.F.; Tesha, A.L.; Beinhoff, C. Environmental Assessment of Mercury Contamination from the Rwamagasa Artisanal Gold Mining Centre, Geita District, Tanzania. Sci. Total Environ. 2005, 343, 111–133. [Google Scholar] [CrossRef]
- Ogola, J.S.; Mitullah, W.V.; Omulo, M.A. Impact of Gold mining on the Environment and Human Health: A Case Study in the Migori Gold Belt, Kenya. Environ. Geochem. Health 2002, 24, 141–157. [Google Scholar] [CrossRef]
- Odumo, O.B.; Mustapha, A.O.; Patel, J.P.; Angeyo, H.K. Multielemental Analysis of Migori (Southwest, Kenya) Artisanal Gold Mine Ores and Sediments by EDX-ray Fluorescence Technique: Implications of Occupational Exposure and Environmental Impact. Bull. Environ. Contam. Toxicol. 2011, 86, 484–489. [Google Scholar] [CrossRef]
- Ngure, V.; Davies, T.; Kinuthia, G.; Sitati, N.; Shisia, S.; Oyoo-Okoth, E. Concentration Levels of Potentially Harmful Elements from Gold Mining in Lake Victoria Region, Kenya: Environmental and Health Implications. J. Geochem. Explor. 2014, 144, 511–516. [Google Scholar] [CrossRef]
- Odumo, B.O.; Carbonell, G.; Angeyo, H.K.; Patel, J.P.; Torrijos, M.; Rodríguez Martín, J.A. Impact of Gold Mining Associated with Mercury Contamination in Soil, Biota Sediments and Tailings in Kenya. Environ. Sci. Pollut. Res. 2014, 21, 12426–12435. [Google Scholar] [CrossRef]
- Tampushi, L.L.; Onyari, J.M.; Muthama, N.J. Environmental Distribution and Risk of Exposure of Heavy Metal Pollutants from Lolgorian Artisanal Gold Mining in Kenya. Bull. Environ. Contam. Toxicol. 2022, 109, 310–316. [Google Scholar] [CrossRef]
- Ondayo, M.A.; Watts, M.J.; Hamilton, E.M.; Mitchell, C.; Mankelow, J.; Osano, O. Artisanal Gold Mining in Kakamega and Vihiga Counties, Kenya: Potential Human Exposure and Health Risk. Environ. Geochem. Health 2023, 45, 6543–6565. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, M.S. How Mercury Is Poisoning a Nation and Gross Mismanagement Is Aggravating the Problem; Sudan’s Gold Curse Briefing Paper No. 1; Sudan Transparency Tracker: Khartoum, Sudan, 2022. [Google Scholar]
- Yan, J.; Li, R.; Ali, M.U.; Wang, C.; Wang, B.; Jin, X.; Shao, M.; Li, P.; Zhang, L.; Feng, X. Mercury Migration to Surface Water from Remediated Mine Waste and Impacts of Rainfall in a Karst Area—Evidence from Hg Isotopes. Water Res. 2023, 230, 119592. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Purwanto, P.; Sunoko, H. Consequences of Mercury Used by Artisanal and Small-Scale Gold Mining Processes—A Case of River Nile State Sudan. J. Ecol. Eng. 2019, 20, 106–115. [Google Scholar] [CrossRef]
- Ali, M.; Elhagwa, A.; Elfaki, J. An Investigation of Mercury Distribution in the Soils around Gold Mining Area at Dar-Mali Locality, River Nile State, Sudan. Eurasian J. Soil Sci. 2018, 7, 365–372. [Google Scholar] [CrossRef]
- Mubarak, E.A.T.; Ali, A.A. Determination of Some Heavy Metals Content in the Body of Two Popular Fish Species O. niloticus and L. niloticus, in Lake Nubia, Wadi Halfa, Sudan. J. Aquac. Mar. Biol. 2020, 9, 170–175. [Google Scholar] [CrossRef]
- Oosthuizen, M.A.; John, J.; Somerset, V. Mercury Exposure in a Low-Income Community in South Africa. S. Afr. Med. J. 2010, 100, 366–371. [Google Scholar] [CrossRef]
- van Rooyen, D.; Erasmus, J.H.H.; Gerber, R.; Nachev, M.; Sures, B.; Wepener, V.; Smit, N.J.J. Bioaccumulation and Trophic Transfer of Total Mercury through the Aquatic Food Webs of an African Sub-Tropical Wetland System. Sci. Total Environ. 2023, 889, 164210. [Google Scholar] [CrossRef]
- Dabrowski, J.M.; Ashton, P.J.; Murray, K.; Leaner, J.J.; Mason, R.P. Anthropogenic Mercury Emissions in South Africa: Coal Combustion in Power Plants. Atmos. Environ. 2008, 42, 6620–6626. [Google Scholar] [CrossRef]
- Pacyna, E.G.; Pacyna, J.M.; Steenhuisen, F.; Wilson, S. Global Anthropogenic Mercury Emission Inventory for 2000. Atmos. Environ. 2006, 40, 4048–4063. [Google Scholar] [CrossRef]
- Kading, T.J.; Mason, R.P.; Leaner, J.J. Mercury Contamination History of an Estuarine Floodplain Reconstructed from a 210Pb-Dated Sediment Core (Berg River, South Africa). Mar. Pollut. Bull. 2009, 59, 116–122. [Google Scholar] [CrossRef]
- Govaerts, A.; Verhaert, V.; Covaci, A.; Jaspers, V.L.B.; Berg, O.K.; Addo-Bediako, A.; Jooste, A.; Bervoets, L. Distribution and Bioaccumulation of POPs and Mercury in the Ga-Selati River (South Africa) and the Rivers Gudbrandsdalslågen and Rena (Norway). Environ. Int. 2018, 121, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Lusilao-Makiese, J.G.; Cukrowska, E.M.; Tessier, E.; Amouroux, D.; Weiersbye, I. The Impact of Post Gold Mining on Mercury Pollution in the West Rand Region, Gauteng, South Africa. J. Geochem. Explor. 2013, 134, 111–119. [Google Scholar] [CrossRef]
- Lusilao-Makiese, J.G.; Tessier, E.; Amouroux, D.; Tutu, H.; Chimuka, L.; Weiersbye, I.; Cukrowska, E.M. Seasonal Distribution and Speciation of Mercury in a Gold Mining Area, North-West Province, South Africa. Toxicol. Environ. Chem. 2014, 96, 387–402. [Google Scholar] [CrossRef]
- Lusilao-Makiese, J.G.; Tessier, E.; Amouroux, D.; Tutu, H.; Chimuka, L.; Weiersbye, I.; Cukrowska, E.M. Mercury Speciation and Dispersion from an Active Gold Mine at the West Wits Area, South Africa. Environ. Monit. Assess. 2016, 188, 47. [Google Scholar] [CrossRef] [PubMed]
- Verhaert, V.; Teuchies, J.; Vlok, W.; Wepener, V.; Addo-Bediako, A.; Jooste, A.; Blust, R.; Bervoets, L. Bioaccumulation and Trophic Transfer of Total Mercury in the Subtropical Olifants River Basin, South Africa. Chemosphere 2019, 216, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, S.J.; Veiga, M.M. Building Capacity in Small-Scale Mining Communities: Health, Ecosystem Sustainability, and the Global Mercury Project. Ecohealth 2005, 2, 361–369. [Google Scholar] [CrossRef]
- Green, C.S.; Lewis, P.J.; Wozniak, J.R.; Drevnick, P.E.; Thies, M.L. A Comparison of Factors Affecting the Small-Scale Distribution of Mercury from Artisanal Small-Scale Gold Mining in a Zimbabwean Stream System. Sci. Total Environ. 2019, 647, 400–410. [Google Scholar] [CrossRef]
- Dalu, T.; Dube, T.; Dondofema, F.; Cuthbert, R.N. Illegal Mining Impacts on Freshwater Potamonautid Crab in a Subtropical Austral Highland Biosphere Reserve. Sci. Total Environ. 2023, 896, 165251. [Google Scholar] [CrossRef]
- Makaure, J.; Dube, T.; Stewart, D.; Razavi, N.R. Mercury Exposure in Two Fish Trophic Guilds from Protected and ASGM-Impacted Reservoirs in Zimbabwe and Possible Risks to Human Health. Arch. Environ. Contam. Toxicol. 2023, 84, 199–213. [Google Scholar] [CrossRef]
- UNEP. Environmental Assessment of Mercury Pollution in Two Artisanal Gold Mining Sites in Eastern Democratic Republic of the Congo; UNEP: Nairobi, Kenya, 2016. [Google Scholar]
- Shandro, J.A.; Veiga, M.M.; Chouinard, R. Reducing Mercury Pollution from Artisanal Gold Mining in Munhena, Mozambique. J. Clean. Prod. 2009, 17, 525–532. [Google Scholar] [CrossRef]
- Esdaile, L.J.; Chalker, J.M. The Mercury Problem in Artisanal and Small-Scale Gold Mining. Chem. A Eur. J. 2018, 24, 6905–6916. [Google Scholar] [CrossRef] [PubMed]
- Bashir, I.; Lone, F.A.; Bhat, R.A.; Mir, S.A.; Dar, Z.A.; Dar, S.A. Concerns and Threats of Contamination on Aquatic Ecosystems. In Bioremediation and Biotechnology; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–26. ISBN 9783030356910. [Google Scholar]
- Mandeng, E.P.B.; Bidjeck, L.M.B.; Bessa, A.Z.E.; Ntomb, Y.D.; Wadjou, J.W.; Doumo, E.P.E.; Dieudonné, L.B. Contamination and Risk Assessment of Heavy Metals, and Uranium of Sediments in Two Watersheds in Abiete-Toko Gold District, Southern Cameroon. Heliyon 2019, 5, e02591. [Google Scholar] [CrossRef] [PubMed]
- Pascal, N.M.; Kiteba, S.; Dieudonne, M.; Jean-Noel, M. Physicochemical Characterization of the Waters of Nine Rivers Draining the FIZI Gold Panning Areas in South Kivu: Environmental Impact Study. Int. J. Eng. Appl. Sci. 2020, 7, 29. [Google Scholar] [CrossRef]
- Pascal, N.M.; Dieudonné, M.E.; Jean-Noël, M.K. Evaluation of the Level of Mercury Pollution in the Sediments of the Rivers Draining the Gold Panning Sites in the Territory of Fizi, Eastern Democratic Republic of Congo. J. Geosci. Environ. Prot. 2020, 08, 97–111. [Google Scholar] [CrossRef]
- Ngueyep, M.L.L.; Kingni, K.S.; Ngounouno, N.M.; Ndi, A.A. The Impact of Gold Mining Exploitation on the Physicochemical Quality of Water: Case of Batouri (Cameroon). Int. J. Energy Water Resour. 2021, 5, 159–173. [Google Scholar] [CrossRef]
- Ngounouno, M.A.; Ngueyep, L.L.M.; Kingni, S.T.; Nforsoh, S.N.; Ngounouno, I. Evaluation of the Impact of Gold Mining Activities on the Waters and Sediments of Lom River, Wakaso, Cameroon and the Restorative Effect of Moringa Oleifera Seeds. Appl. Water Sci. 2021, 11, 113. [Google Scholar] [CrossRef]
- Veiga, M.M.; Angeloci-Santos, G.; Meech, J.A. Review of Barriers to Reduce Mercury Use in Artisanal Gold Mining. Extr. Ind. Soc. 2014, 1, 351–361. [Google Scholar] [CrossRef]
- Bella Atangana, M.; Ndam Ngoupayou, J.; Deliege, J.-F. Hydrogeochemistry and Mercury Contamination of Surface Water in the Lom Gold Basin (East Cameroon): Water Quality Index, Multivariate Statistical Analysis and Spatial Interpolation. Water 2023, 15, 2502. [Google Scholar] [CrossRef]
- Ayiwouo, M.N.; Yamgouot, F.N.; Ngueyep Mambou, L.L.; Kingni, S.T.; Ngounouno, I. Impact of Gold Mining on the Water Quality of the Lom River, Gankombol, Cameroon. Heliyon 2022, 8, e12452. [Google Scholar] [CrossRef]
- Rajaee, M.; Long, R.; Renne, E.; Basu, N. Mercury Exposure Assessment and Spatial Distribution in A Ghanaian Small-Scale Gold Mining Community. Int. J. Environ. Res. Public Health 2015, 12, 10755–10782. [Google Scholar] [CrossRef]
- Affum, A.O.; Dede, S.O.; Nyarko, B.J.B.; Acquaah, S.O.; Kwaansa-Ansah, E.E.; Darko, G.; Dickson, A.; Affum, E.A.; Fianko, J.R. Influence of Small-Scale Gold Mining and Toxic Element Concentrations in Bonsa River, Ghana: A Potential Risk to Water Quality and Public Health. Environ. Earth Sci. 2016, 75, 178. [Google Scholar] [CrossRef]
- Obiri-Yeboah, A.; Nyantakyi, E.K.; Mohammed, A.R.; Yeboah, S.I.I.K.; Domfeh, M.K.; Abokyi, E. Assessing Potential Health Effect of Lead and Mercury and the Impact of Illegal Mining Activities in the Bonsa River, Tarkwa Nsuaem, Ghana. Sci. Afr. 2021, 13, e00876. [Google Scholar] [CrossRef]
- Tulasi, D.; Fajon, V.; Kotnik, J.; Shlyapnikov, Y.; Adotey, D.K.; Serfor-Armah, Y.; Horvat, M. Mercury Methylation in Cyanide Influenced River Sediments: A Comparative Study in Southwestern Ghana. Environ. Monit. Assess. 2021, 193, 180. [Google Scholar] [CrossRef] [PubMed]
- Persaud, A.W.; Telmer, K.H.; Costa, M.; Moore, M.L. Artisanal and Small-Scale Gold Mining in Senegal: Livelihoods, Customary Authority, and Formalization. Soc. Nat. Resour. 2017, 30, 980–993. [Google Scholar] [CrossRef]
- Niane, B.; Guédron, S.; Feder, F.; Legros, S.; Ngom, P.M.; Moritz, R. Impact of Recent Artisanal Small-Scale Gold Mining in Senegal: Mercury and Methylmercury Contamination of Terrestrial and Aquatic Ecosystems. Sci. Total Environ. 2019, 669, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Gerson, J.R.; Driscoll, C.T.; Hsu-Kim, H.; Bernhardt, E.S. Senegalese Artisanal Gold Mining Leads to Elevated Total Mercury and Methylmercury Concentrations in Soils, Sediments, and Rivers. Elementa 2018, 6, 11. [Google Scholar] [CrossRef]
- Idowu, O.S.; Muruf, A.K.; Osaguona, P.; Ajayi, J. Mercury Contamination in Artisanal Gold Mining Area of Manyera River, Niger State Nigeria. E3 J. Environ. Res. Manag. 2013, 4, 326–0333. [Google Scholar]
- Adewumi, A.J.; Laniyan, T.A. Contamination, Sources and Risk Assessments of Metals in Media from Anka Artisanal Gold Mining Area, Northwest Nigeria. Sci. Total Environ. 2020, 718, 137235. [Google Scholar] [CrossRef]
- Sani, A.H.; Amanabo, M.; Achimugu, M.D. Assessment of Heavy Metal Pollution of Drinking Water Sources and Staple Food Cultivars around Artisanal Mining Site in Igade-Mashegu, Niger State, Nigeria. World J. Biol. Pharm. Health Sci. 2023, 14, 306–319. [Google Scholar] [CrossRef]
- Kawakami, T. Diffusin of Mercury from Artisanal Small-Scale Gold Mining (ASGM) Sites in Myanmar. Int. J. GEOMATE 2019, 17, 228–235. [Google Scholar] [CrossRef]
- Akagi, H.; Castillo, E.S.; Cortes-Maramba, N.; Francisco-Rivera, A.T.; Timbang, T.D. Health Assessment for Mercury Exposure among Schoolchildren Residing near a Gold Processing and Refining Plant in Apokon, Tagum, Davao Del Norte, Philippines. Sci. Total Environ. 2000, 259, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.P.; Irvine, K.N.; Sampson, M.; Guo, J.; Parr, T. Mercury Contamination along the Mekong River, Cambodia. Asian J. Water Environ. Pollut. 2008, 6, 1–9. [Google Scholar] [CrossRef]
- Abrahan, M.; Diana, J.F.; Jürgen, M. Levels of MN, ZN, PB and HG in Sediments of the Zamora River, Ecuador. Rev. Int. Contam. Ambient. 2018, 34, 245–249. [Google Scholar]
- Bastos, W.R.; Dórea, J.G.; Bernardi, J.V.; Lauthartte, L.C.; Mussy, M.H.; Lacerda, L.D.; Malm, O. Mercury in fish of the Madeira river (temporal and spatial assessment), Brazilian Amazon. Environ. Res. 2015, 140, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Sanchez, A.; Contreras, F.; Adams, M.; Santos, F. Mercury Contamination of Surface Water and Fish in a Gold Mining Region (Cuyuní River Basin, Venezuela). Int. J. Environ. Pollut. 2008, 33, 260–274. [Google Scholar] [CrossRef]
- Hacon, S.d.S.; Oliveira-da-Costa, M.; Gama, C.d.S.; Ferreira, R.; Basta, P.C.; Schramm, A.; Yokota, D. Mercury Exposure through Fish Consumption in Traditional Communities in the Brazilian Northern Amazon. Int. J. Environ. Res. Public Health 2020, 17, 5269. [Google Scholar] [CrossRef]
- da Silva, S.F.; Oliveira, D.C.; Pereira, J.P.G.; Castro, S.P.; Costa, B.N.S.; de Oliveira Lima, M. Seasonal Variation of Mercury in Commercial Fishes of the Amazon Triple Frontier, Western Amazon Basin. Ecol. Indic. 2019, 106, 105549. [Google Scholar] [CrossRef]
- Moreno-Brush, M.; Rydberg, J.; Gamboa, N.; Storch, I.; Biester, H. Is Mercury from Small-Scale Gold Mining Prevalent in the Southeastern Peruvian Amazon? Environ. Pollut. 2016, 218, 150–159. [Google Scholar] [CrossRef]
- Ali, M.; Hery, S.; Putri, S.T. Mercury Toxicity Potential from Artisanal and Small—Scale Gold Mines in Lebong Regency, Bengkulu Province. E3S Web Conf. 2018, 73, 06002. [Google Scholar] [CrossRef]
- Rajaee, M.; Obiri, S.; Green, A.; Long, R.; Cobbina, S.J.; Nartey, V.; Buck, D.; Antwi, E.; Basu, N. Integrated Assessment of Artisanal and Small-Scale Gold Mining in Ghana—Part 2: Natural Sciences Review. Int. J. Environ. Res. Public Health 2015, 12, 8971–9011. [Google Scholar] [CrossRef]
- Pataranawat, P.; Parkpian, P.; Polprasert, C.; Delaune, R.D.; Jugsujinda, A. Mercury Emission and Distribution: Potential Environmental Risks at a Small-Scale Gold Mining Operation, Phichit Province, Thailand. J. Environ. Sci. Health—Part A Toxic/Hazard. Subst. Environ. Eng. 2007, 42, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Ramírez Requelme, M.E.; Ramos, J.F.F.; Angélica, R.S.; Brabo, E.S. Assessment of Hg-Contamination in Soils and Stream Sediments in the Mineral District of Nambija, Ecuadorian Amazon (Example of an Impacted Area Affected by Artisanal Gold Mining). Appl. Geochem. 2003, 18, 371–381. [Google Scholar] [CrossRef]
- da Penha Rhodes, V.; de Lena, J.C.; Santolin, C.V.A.; da Silva Pinto, T.; Mendes, L.A.; Windmöller, C.C. Speciation and Quantification of Hg in Sediments Contaminated by Artisanal Gold Mining in the Gualaxo Do Norte River, Minas Gerais, SE, Brazil. Environ. Monit. Assess. 2018, 190, 49. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Ferreira, C.M.; Egler, S.G.; Yallouz, A.V.; Ignácio, Á.R.A. Semiquantitative Determination of Total Mercury in Pygocentrus Nattereri Kner, 1858 and Sediment at the Plateau of Upper Paraguai River, Brazil. Chemosphere 2017, 174, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Hilson, G. “Creating” Rural Informality: The Case of Artisanal Gold Mining in Sub-Saharan Africa. SAIS Rev. Int. Aff. 2013, 33, 51–64. [Google Scholar] [CrossRef]
- Hilson, G. Small-Scale Mining, Poverty and Economic Development in Sub-Saharan Africa: An Overview. Resour. Policy 2009, 34, 1–5. [Google Scholar] [CrossRef]
- Hilson, G. Artisanal Mining, Small Holder Farming and Livelihood Diversificatio in Rural Sub-Saharan Africa: An Introduction. J. Int. Dev. 2011, 23, 1031–1041. [Google Scholar] [CrossRef]
- Schwartz, F.W. A Review of the Scope of Artisanal and Small-Scale Mining Worldwide, Poverty, and the Associated Health Impacts. GeoHealth 2021, 5, e2020GH000325. [Google Scholar] [CrossRef]
- Mkodzongi, G. Artisanal and Small-Scale Gold Mining and Rural Transformation in Post-Land Reform Zimbabwe: A Broad Overview. J. Rural Stud. 2023, 100, 103027. [Google Scholar] [CrossRef]
- Hilson, G.; Goumandakoye, H.; Diallo, P. Land Use Policy Formalizing Artisanal Mining ‘Spaces’ in Rural Sub-Saharan Africa: The Case of Niger. Land Use Policy 2019, 80, 259–268. [Google Scholar] [CrossRef]
- Mkodzongi, G. The Extractive Industries and Society The Rise of ‘Mashurugwi’ Machete Gangs and Violent Conflicts in Zimbabwe’s Artisanal and Small-Scale Gold Mining Sector. Extr. Ind. Soc. 2020, 7, 1480–1489. [Google Scholar] [CrossRef]
- WHO. Environmental and Occupational Health Hazards Associated with Artisanal and Small-Scale Gold Mining; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Steckling, N.; Tobollik, M.; Plass, D.; Hornberg, C.; Ericson, B.; Fuller, R.; Bose-O’Reilly, S. Global Burden of Disease of Mercury Used in Artisanal Small-Scale Gold Mining. Ann. Glob. Health 2017, 83, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Shoko, D.S.M. Small-Scale Mining and Alluvial Gold Panning within the Zambezi Basin: An Ecological Time Bomb and Tinderbox for Future Conflicts among Riparian States. Bloom. Businessweek 2012, 10, 12–14. [Google Scholar]
- Hilson, G. Artisanal and Small-Scale Mining and Agriculture Exploring Their Links in Rural Sub-Saharan Africa; International Institute for Environment and Development, JSTOR: London, UK, 2016. [Google Scholar]
- Ncube-Phiri, S.; Ncube, A.; Mucherera, B.; Ncube, M. Artisanal Small-Scale Mining: Potential Ecological Disaster in Mzingwane District, Zimbabwe. Jàmbá J. Disaster Risk Stud. 2015, 7, 158. [Google Scholar] [CrossRef] [PubMed]
- Mhangara, P.; Tsoeleng, L.T.; Mapurisa, W. Monitoring the Development of Artisanal Mines in South Africa. J. S. Afr. Inst. Min. Metall. 2020, 120, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Girard, V.; Molina-Millán, T.; Vic, G. Artisanal Mining in Africa; NOVAFRICA Working Paper; NOVAFRICA: Carcavelos, Portugal, 2022. [Google Scholar]
- Fisher, J.A.; Schneider, L.; Fostier, A.-H.; Guerrero, S.; Guimarães, J.R.D.; Labuschagne, C.; Leaner, J.J.; Martin, L.G.; Mason, R.P.; Somerset, V.; et al. A Synthesis of Mercury Research in the Southern Hemisphere, Part 2: Anthropogenic Perturbations. Ambio 2023, 52, 918–937. [Google Scholar] [CrossRef] [PubMed]
- Hsu-Kim, H.; Eckley, C.S.; Achá, D.; Feng, X.; Gilmour, C.C.; Jonsson, S.; Mitchell, C.P.J. Challenges and Opportunities for Managing Aquatic Mercury Pollution in Altered Landscapes. Ambio 2018, 47, 141–169. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; Bradley, T.; Moore, J.; Kuykindall, T.; Minella, L. Acute and Chronic Toxicity of Nano-Scale TiO2 Particles to Freshwater Fish, Cladocerans, and Green Algae, and Effects of Organic and Inorganic Substrate on TiO2 Toxicity. Nanotoxicology 2009, 3, 91–97. [Google Scholar] [CrossRef]
- Bodaly, R.A.; Jansen, W.A.; Majewski, A.R.; Fudge, R.J.P.; Strange, N.E.; Derksen, A.J.; Green, D.J. Post-impoundment Time Course of Increased Mercury Concentrations in Fish in Hydroelectric Reservoirs of Northern Manitoba, Canada. Arch. Environ. Contam. Toxicol. 2007, 53, 379–389. [Google Scholar] [CrossRef]
- Baptista-Salazar, C.; Quadra, G.R.; Sobek, A.; Jonsson, S. Insights into the Factors Influencing Mercury Concentrations in Tropical Reservoir Sediments. Environ. Sci. Process. Impacts 2021, 23, 1542–1553. [Google Scholar] [CrossRef]
- Selin, H. Global Environmental Law and Treaty-Making on Hazardous Substances: The Minamata Convention and Mercury Abatement. Glob. Environ. Polit. 2014, 14, 1–19. [Google Scholar] [CrossRef]
- Keane, S.; Bernaudat, L.; Davis, K.J.; Stylo, M.; Mutemeri, N.; Singo, P.; Twala, P.; Mutemeri, I.; Nakafeero, A.; Etui, I.D. Mercury and Artisanal and Small-Scale Gold Mining: Review of Global Use Estimates and Considerations for Promoting Mercury-Free Alternatives. Ambio 2023, 52, 833–852. [Google Scholar] [CrossRef] [PubMed]
- Poloko, N. Physical Separation Methods, Part 1: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2019, 641, 012023. [Google Scholar] [CrossRef]
- AMDC. Artisanal and Small–Scale Mining in Africa: Selected Countries Policy Profile Review on ASM; Africa Mining Development Centre/African Union: Addis Ababa, Ethiopia, 2017. [Google Scholar]
- Drace, K.; Kiefer, A.M.; Veiga, M.M.; Williams, M.K.; Ascari, B.; Knapper, K.A.; Logan, K.M.; Breslin, V.M.; Skidmore, A.; Bolt, D.A.; et al. Mercury-Free, Small-Scale Artisanal Gold Mining in Mozambique: Utilization of Magnets to Isolate Gold at Clean Tech Mine. J. Clean. Prod. 2012, 32, 88–95. [Google Scholar] [CrossRef]
- NEMA. The National Action Plan for Artisanal and Small–Scale Gold Mining in Uganda; National Environmental Management Authority NEMA: Kampala, Uganda, 2019.
- Babut, M.; Sekyi, R.; Rambaud, A.; Potin-Gautier, M.; Tellier, S.; Bannerman, W.; Beinhoff, C. Improving the Environment Management of Small-Scale Gold Mining in Ghana: A Case Study of Dumasi. J. Clean. Prod. 2003, 11, 215–221. [Google Scholar] [CrossRef]
- Styles, M.T.; Amankwah, R.K.; Al-Hassan, S.; Nartey, R.S. The Identification and Testing of a Method for Mercury-Free Gold Processing for Artisanal and Small-Scale Gold Miners in Ghana. Int. J. Environ. Pollut. 2010, 41, 289. [Google Scholar] [CrossRef]
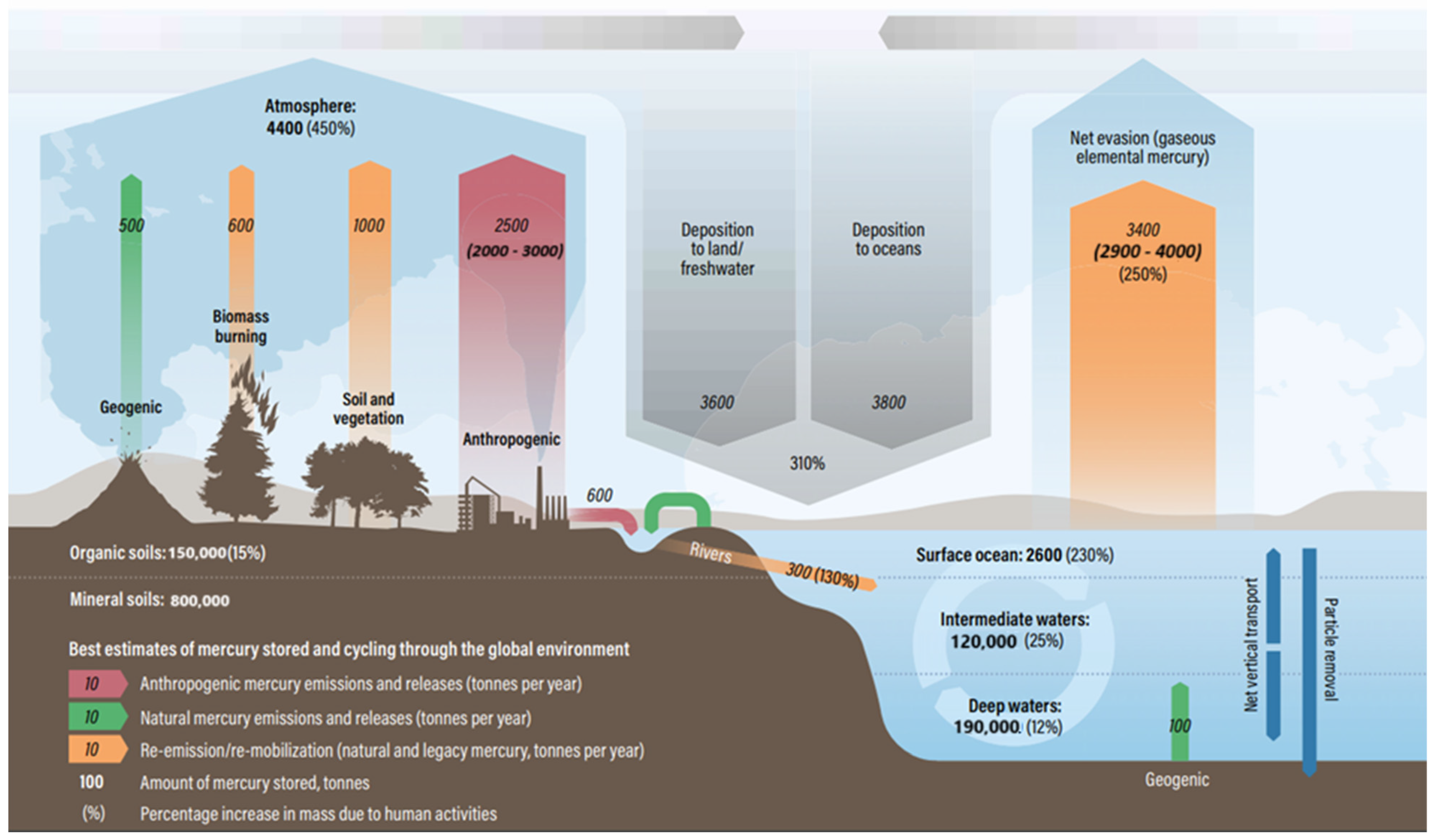


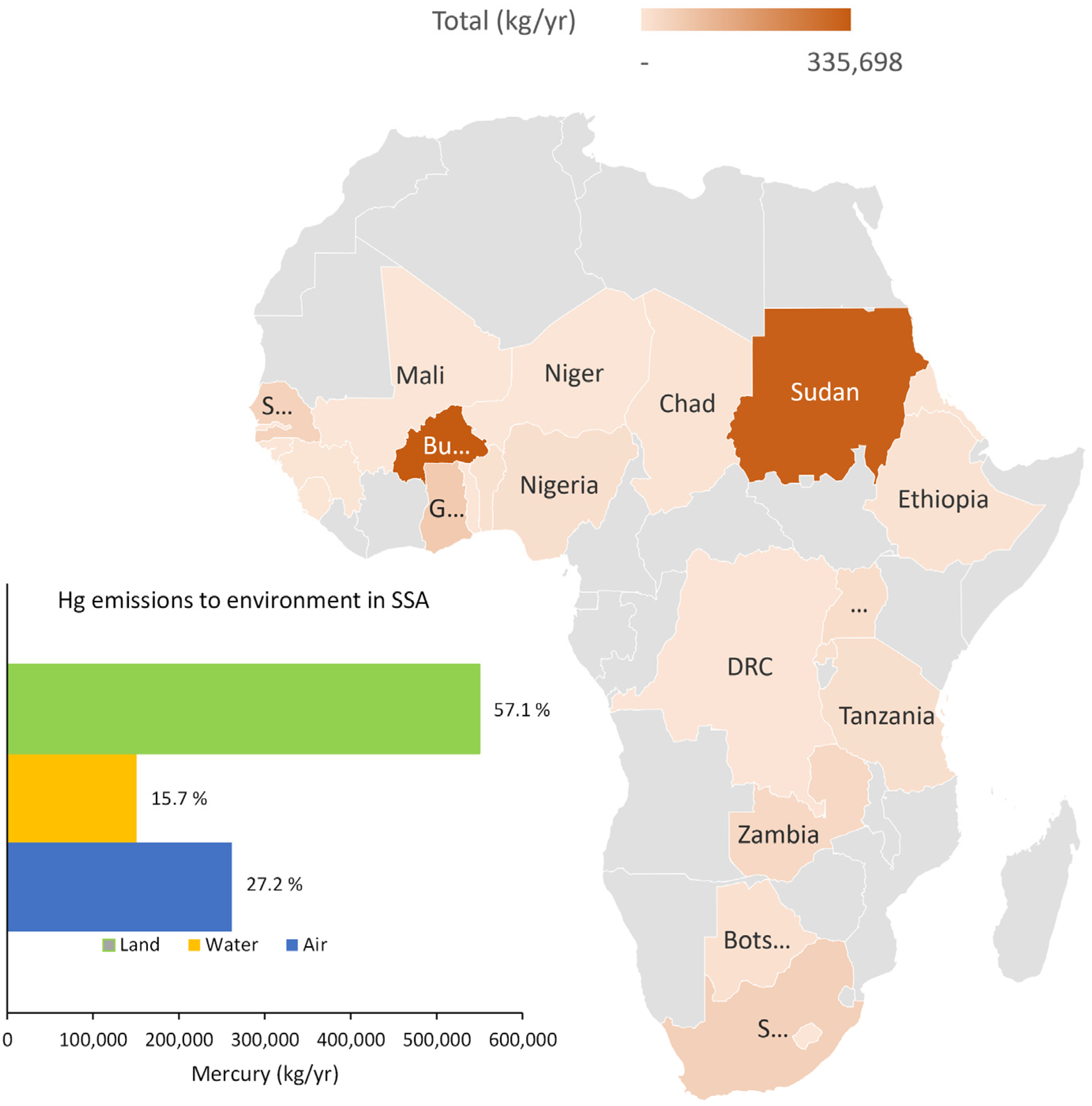
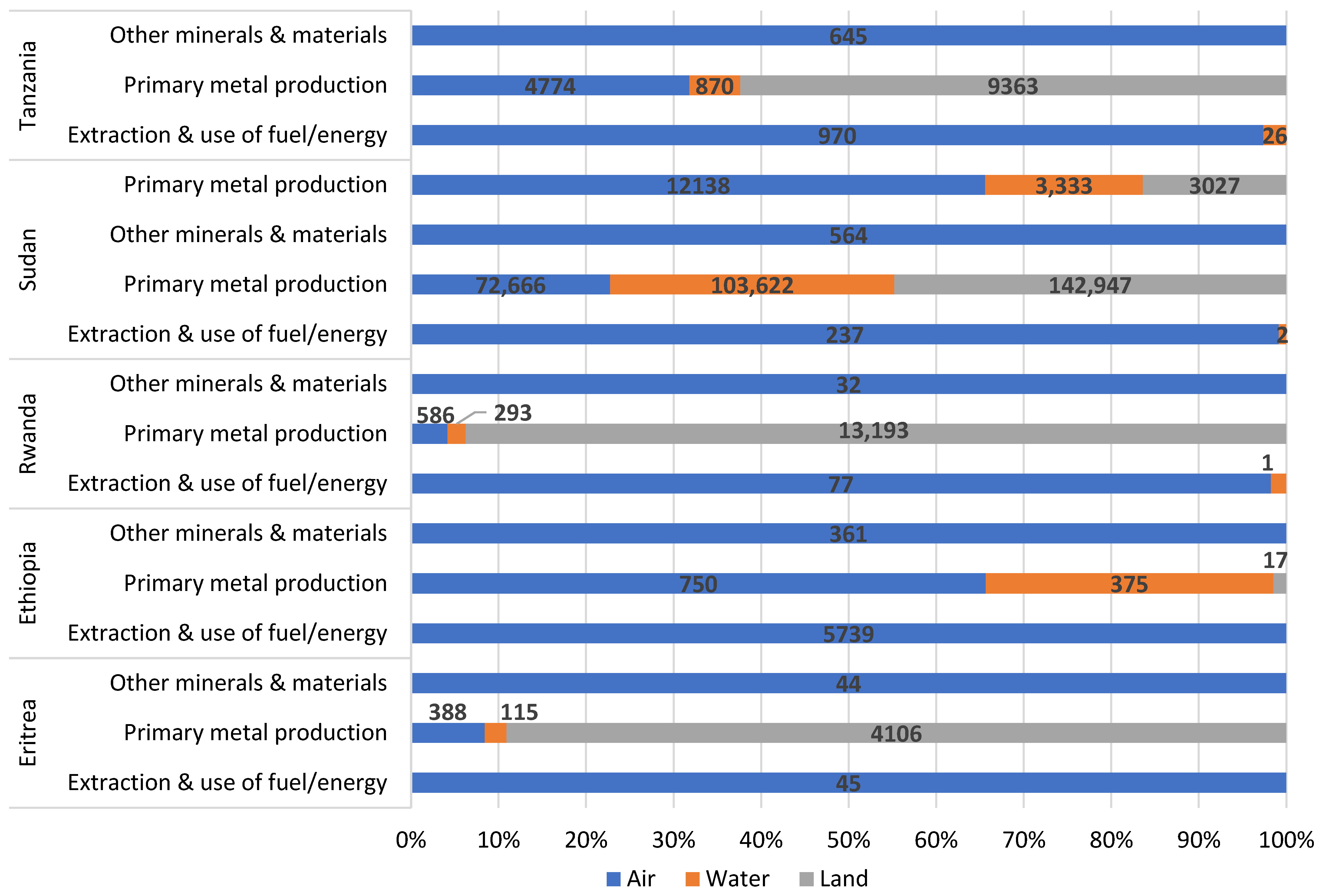

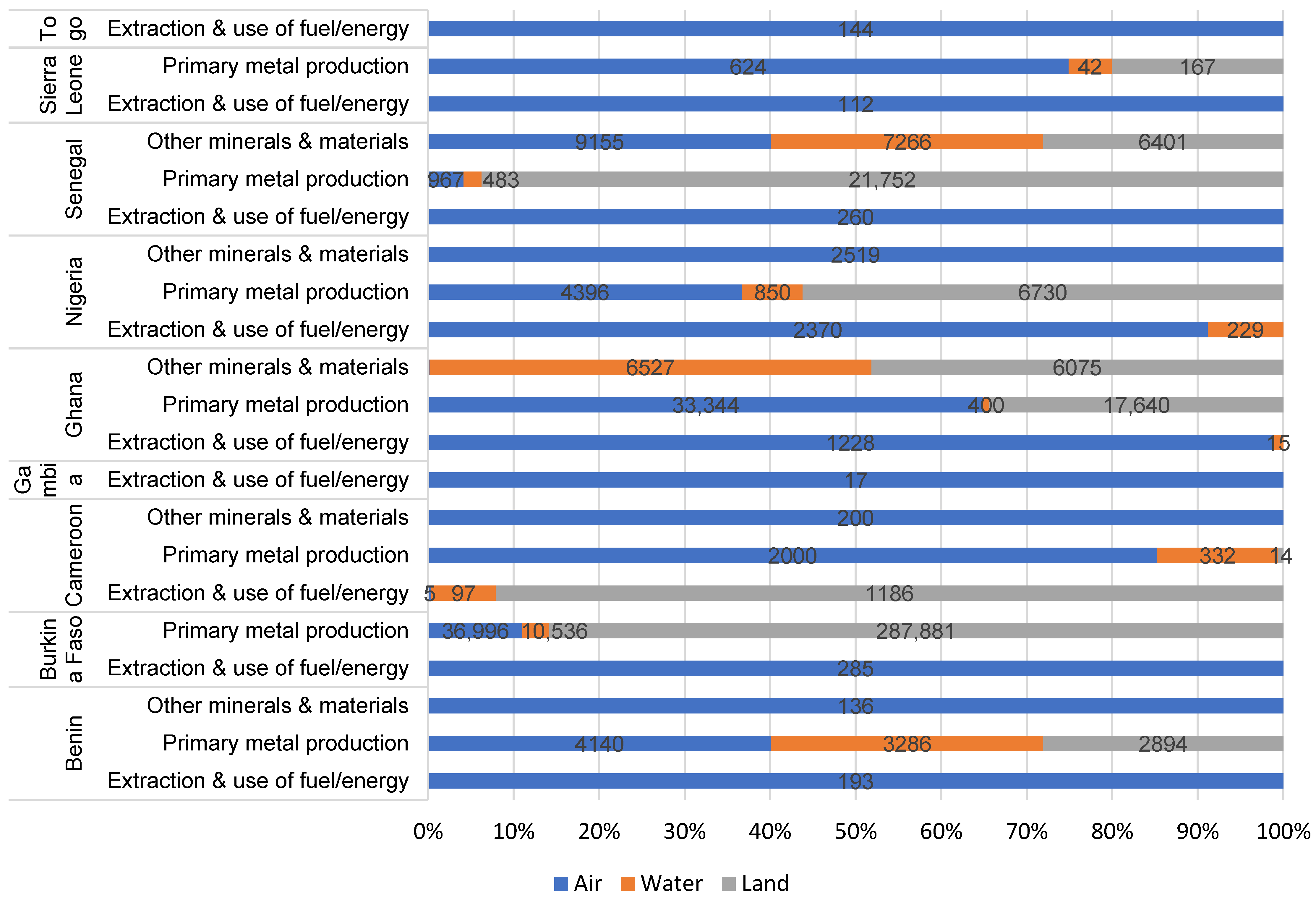

| No. | Action | Year | Party | % of SSA |
|---|---|---|---|---|
| 1 | Signature | 2013–2014 | Benin, Burkina Faso, CAR, Côte d’Ivoire, Djibouti, Gambia, Kenya, Malawi, Mali, Niger, Nigeria, South Africa, Togo, Uganda, Tanzania, Zambia, Ethiopia, Mozambique, Angola, Burundi, Cameroon, Chad, Congo, Gabon, Ghana, Guinea, Guinea-Bissau, Liberia, Senegal, Sierra Leone, Sudan, Zimbabwe | 76 |
| 2 | Accession | 2014–2017 | Botswana, Equatorial Guinea, Eritrea, Eswatini, Lesotho, Namibia, Rwanda | 15 |
| 3 | Ratification | 2014–2015 | Djibouti, Chad | 4 |
| 2016–2019 | Benin, Burkina Faso, Côte d’Ivoire, Gambia, Mali, Niger, Nigeria, South Africa, Togo, Uganda, Zambia, the DRC, Ghana, Guinea-Bissau, Senegal, Sierra Leone | 39 | ||
| 2020–2023 | CAR, Kenya, Malawi, Tanzania, Burundi, Cameroon, Zimbabwe | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulenga, M.; Ouma, K.O.; Monde, C.; Syampungani, S. Aquatic Mercury Pollution from Artisanal and Small-Scale Gold Mining in Sub-Saharan Africa: Status, Impacts, and Interventions. Water 2024, 16, 756. https://doi.org/10.3390/w16050756
Mulenga M, Ouma KO, Monde C, Syampungani S. Aquatic Mercury Pollution from Artisanal and Small-Scale Gold Mining in Sub-Saharan Africa: Status, Impacts, and Interventions. Water. 2024; 16(5):756. https://doi.org/10.3390/w16050756
Chicago/Turabian StyleMulenga, Mary, Kennedy O. Ouma, Concillia Monde, and Stephen Syampungani. 2024. "Aquatic Mercury Pollution from Artisanal and Small-Scale Gold Mining in Sub-Saharan Africa: Status, Impacts, and Interventions" Water 16, no. 5: 756. https://doi.org/10.3390/w16050756
APA StyleMulenga, M., Ouma, K. O., Monde, C., & Syampungani, S. (2024). Aquatic Mercury Pollution from Artisanal and Small-Scale Gold Mining in Sub-Saharan Africa: Status, Impacts, and Interventions. Water, 16(5), 756. https://doi.org/10.3390/w16050756









