Research Progress on Novel Electrochemical Descaling Technology for Enhanced Hardness Ion Removal
Abstract
:1. Introduction
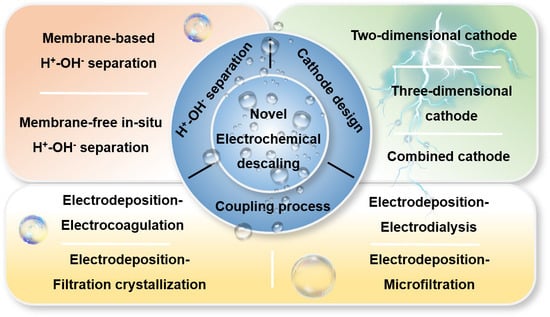
2. H+-OH− Separation
2.1. Membrane-Based H+-OH− Separation
2.1.1. Ion Exchange Membrane (IEMs)
2.1.2. Polytetrafluoroethylene (PTFE) Membrane
2.1.3. Confined Crystallization Membrane (CCM)
2.2. In Situ Membrane-Free H+-OH− Separation
2.2.1. Extraction of Boundary Layer Solution
2.2.2. Bubbles and Water Flow
3. Cathode Design
3.1. Two-Dimensional Cathode
3.2. Three-Dimensional Cathode
3.3. Combined Cathode
4. Coupling Process
4.1. Electrodeposition–Electrocoagulation
4.2. Electrodeposition–Filtration Crystallization
4.3. Electrodeposition–Microfiltration
4.4. Electrodeposition–Electrodialysis
5. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, J.-Q.; Graham, N.; André, C.; Kelsall, G.H.; Brandon, N. Laboratory study of electro-coagulation–flotation for water treatment. Water Res. 2002, 36, 4064–4078. [Google Scholar] [CrossRef]
- Lee, J.-B.; Park, K.-K.; Eum, H.-M.; Lee, C.-W. Desalination of a thermal power plant wastewater by membrane capacitive deionization. Desalination 2006, 196, 125–134. [Google Scholar] [CrossRef]
- Liao, Z.; Gu, Z.; Schulz, M.C.; Davis, J.R.; Baygents, J.C.; Farrell, J. Treatment of cooling tower blowdown water containing silica, calcium and magnesium by electrocoagulation. Water Sci. Technol. 2009, 60, 2345–2352. [Google Scholar] [CrossRef]
- Liu, Z.; Li, N.; Yan, M.; Guo, R.; Liu, Z. The research progress of water treatment technology on recirculated cooling water. IOP Conf. Ser. Earth Environ. Sci. 2020, 508, 012041. [Google Scholar] [CrossRef]
- Kirilina, A.V.; Suslov, S.Y.; Kozlovskii, V.V.; Larin, A.B. Water Chemistry Development for a Thermal Power Plant Circulating Cooling System Using the VTIAMIN EKO-1 Chemical Agent. Therm. Eng. 2019, 66, 750–759. [Google Scholar] [CrossRef]
- Wang, J.; Qu, D.; Tie, M.; Ren, H.; Peng, X.; Luan, Z. Effect of coagulation pretreatment on membrane distillation process for desalination of recirculating cooling water. Sep. Purif. Technol. 2008, 64, 108–115. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, C.; He, D.; Zhang, F.; Wei, Y.; Yang, Z.; Yan, Y. Research on Gravity Energy Saving Reconstruction Technology of Circulating Cooling Water in Mechanical Ventilation Cooling Tower of a Steel Plant. Energies 2023, 16, 6274. [Google Scholar] [CrossRef]
- Chatterjee, A.; Huang, H.; Davis, K.R.; Layton, A. A Multigraph Modeling Approach to Enable Ecological Network Analysis of Cyber Physical Power Networks. In Proceedings of the 2021 IEEE International Conference on Communications, Control, and Computing Technologies for Smart Grids (SmartGridComm), Aachen, Germany, 25–28 October 2021; pp. 239–244. [Google Scholar]
- Xu, J.; Zhao, J.; Jia, Y. Experimental study on the scale inhibition effect of the alternating electromagnetic field on CaCO3 fouling on the heat exchanger surface in different circulating cooling water conditions. Int. J. Therm. Sci. 2023, 192, 108388. [Google Scholar] [CrossRef]
- Tijing, L.D.; Yu, M.-H.; Kim, C.-H.; Amarjargal, A.; Lee, Y.C.; Lee, D.-H.; Kim, D.-W.; Kim, C.S. Mitigation of scaling in heat exchangers by physical water treatment using zinc and tourmaline. Appl. Therm. Eng. 2011, 31, 2025–2031. [Google Scholar] [CrossRef]
- AzadiAghdam, M.; Park, M.; Lopez-Prieto, I.J.; Achilli, A.; Snyder, S.A.; Farrell, J. Pretreatment for water reuse using fluidized bed crystallization. J. Water Process Eng. 2020, 35, 101226. [Google Scholar] [CrossRef]
- Choi, D.-J.; You, S.-J.; Kim, J.-G. Development of an environmentally safe corrosion, scale, and microorganism inhibitor for open recirculating cooling systems. Mater. Sci. Eng. A 2002, 335, 228–235. [Google Scholar] [CrossRef]
- Rahmani, K.; Jadidian, R.; Haghtalab, S. Evaluation of inhibitors and biocides on the corrosion, scaling and biofouling control of carbon steel and copper–nickel alloys in a power plant cooling water system. Desalination 2016, 393, 174–185. [Google Scholar] [CrossRef]
- Seo, S.-J.; Jeon, H.; Lee, J.K.; Kim, G.-Y.; Park, D.; Nojima, H.; Lee, J.; Moon, S.-H. Investigation on removal of hardness ions by capacitive deionization (CDI) for water softening applications. Water Res. 2010, 44, 2267–2275. [Google Scholar] [CrossRef]
- Zhang, Z.; Jia, Y.; Zhao, J. Effect of Magnesium Ion Concentration on the Scale Inhibition of Heat Exchanger in Circulating Cooling Water under Alternating Electric Field. Appl. Sci. 2020, 10, 5491. [Google Scholar] [CrossRef]
- Chaussemier, M.; Pourmohtasham, E.; Gelus, D.; Pécoul, N.; Perrot, H.; Lédion, J.; Cheap-Charpentier, H.; Horner, O. State of art of natural inhibitors of calcium carbonate scaling. A review article. Desalination 2015, 356, 47–55. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, C.; Zhu, L.; Gao, Q.; Wu, L.; Zhang, Q.; Zhao, R. Effect of alternating electromagnetic field and ultrasonic on CaCO3 scale inhibitive performance of EDTMPS. J. Taiwan Inst. Chem. Eng. 2019, 99, 104–112. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, J.-D.; Jia, Y.; Li, T.; Yang, S.-B.; Zhang, Z.-H. Effect of fouling resistance in heat exchanger and the crystal form of CaCO3 in hard circulating cooling water with electrostatic field and alternating current electric field. Water Sci. Technol. 2021, 84, 1608–1622. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xu, Y.; Xie, M.; Huang, M.; Chen, G. Characterization of scalants and strategies for scaling mitigation in membrane distillation of alkaline concentrated circulating cooling water. Desalination 2022, 527, 115534. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, H.; Chen, E.; Li, M.; Liu, S. Physicochemical Characteristics and the Scale Inhibition Effect of Air Nanobubbles (A-NBs) in a Circulating Cooling Water System. Langmuir 2023, 39, 1629–1639. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zheng, F.; Lu, S.; Hao, L.; Li, B.; Mao, Z.; Long, Y.; Yao, C.; Wu, H.; Zheng, X.; et al. Effect of electrochemical pretreatment on the control of scaling and fouling caused by circulating cooling water on heat exchanger and side-stream reverse osmosis membrane. J. Water Process Eng. 2021, 43, 102261. [Google Scholar] [CrossRef]
- Bogart, S.J.; Woodman, S.; Steinkey, D.; Meays, C.; Pyle, G.G. Rapid changes in water hardness and alkalinity: Calcite formation is lethal to Daphnia magna. Sci. Total Environ. 2016, 559, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Vera, M.L.; Palacios, C.J.O.; Díaz Nieto, C.H.; Palacios, N.A.; Di Carlantonio, N.; Luna, F.G.; Torres, W.R.; Flexer, V. A strategy to avoid solid formation within the reactor during magnesium and calcium electrolytic removal from lithium-rich brines. J. Solid State Electrochem. 2022, 26, 1981–1994. [Google Scholar] [CrossRef]
- Gongye, F.; Zhou, J.; Peng, J.; Zhang, H.; Peng, S.; Li, S.; Deng, H. Study on the Removal of Oxide Scale Formed on 300 M Steel Special-Shaped Hot Forging Surfaces during Heating at Elevated Temperature by a High-Pressure Water Descaling Process. Materials 2023, 16, 1745. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xu, Z.; Guo, Y.; Guo, S.; Xu, X.; Gao, X.; Wang, L.; Yan, W. Research and application progress of electrochemical water quality stabilization technology for recirculating cooling water in China: A short review. J. Water Process Eng. 2020, 37, 101433. [Google Scholar] [CrossRef]
- Al-Hamzah, A.A.; Fellows, C.M. A comparative study of novel scale inhibitors with commercial scale inhibitors used in seawater desalination. Desalination 2015, 359, 22–25. [Google Scholar] [CrossRef]
- Wang, Y.; Niu, X.; Xing, X.; Wang, S.; Jing, X. Curing behaviour and properties of a novel benzoxazine resin via catalysis of 2-phenyl-1,3,2-benzodioxaborole. React. Funct. Polym. 2017, 117, 60–69. [Google Scholar] [CrossRef]
- Lin, Y.-P.; Singer, P.C. Inhibition of calcite crystal growth by polyphosphates. Water Res. 2005, 39, 4835–4843. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, L.; Sun, W.; Yang, Z.; Wang, S.; Zhou, Y.; He, S.; Wang, Y.; Liu, G. Corrosion-Induced Performance Degradation of Phosphorus-Containing Scale Inhibitors at Carbon Steel–Water Interface. Ind. Eng. Chem. Res. 2018, 57, 5183–5189. [Google Scholar] [CrossRef]
- Ansari, S.Z.; Pandit, A.B. Optimising hydrodynamic conditions for inhibiting scale deposition on metal surfaces in the presence of aspartic acid. Indian Chem. Eng. 2022, 64, 337–347. [Google Scholar] [CrossRef]
- Wang, Y.; Kuntke, P.; Saakes, M.; van der Weijden, R.D.; Buisman, C.J.N.; Lei, Y. Electrochemically mediated precipitation of phosphate minerals for phosphorus removal and recovery: Progress and perspective. Water Res. 2022, 209, 117891. [Google Scholar] [CrossRef]
- Lei, Y.; Saakes, M.; van der Weijden, R.D.; Buisman, C.J.N. Electrochemically mediated calcium phosphate precipitation from phosphonates: Implications on phosphorus recovery from non-orthophosphate. Water Res. 2020, 169, 115206. [Google Scholar] [CrossRef]
- Wang, Q.; Lenhart, J.J.; Walker, H.W. Recovery of metal cations from lime softening sludge using Donnan dialysis. J. Membr. Sci. 2010, 360, 469–475. [Google Scholar] [CrossRef]
- Kausley, S.B.; Desai, K.S.; Patil, R.A.; Malhotra, C.P.; Pandit, A.B. Comparative study of lime softening, soda ash process, and electrocoagulation for the removal of hardness from groundwater. Proc. Indian Natl. Sci. Acad. 2022, 88, 379–391. [Google Scholar] [CrossRef]
- Zhao, K.; Wu, J.; Li, X.; Li, Z.; Chen, Y. Advances of Ultrasonic Scaling Removal Technology and Heat Transfer Enhancement Technology. ChemBioEng Rev. 2021, 8, 134–144. [Google Scholar] [CrossRef]
- Karabelas, A.J.; Mitrouli, S.T.; Kostoglou, M. Scaling in reverse osmosis desalination plants: A perspective focusing on development of comprehensive simulation tools. Desalination 2020, 474, 114193. [Google Scholar] [CrossRef]
- Cinperi, N.C.; Ozturk, E.; Yigit, N.O.; Kitis, M. Treatment of woolen textile wastewater using membrane bioreactor, nanofiltration and reverse osmosis for reuse in production processes. J. Clean. Prod. 2019, 223, 837–848. [Google Scholar] [CrossRef]
- Altmann, T.; Das, R. Process improvement of sea water reverse osmosis (SWRO) and subsequent decarbonization. Desalination 2021, 499, 114791. [Google Scholar] [CrossRef]
- Wang, X.-X.; Wu, Y.-H.; Zhang, T.-Y.; Xu, X.-Q.; Dao, G.-H.; Hu, H.-Y. Simultaneous nitrogen, phosphorous, and hardness removal from reverse osmosis concentrate by microalgae cultivation. Water Res. 2016, 94, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hong, S. A novel single-pass reverse osmosis configuration for high-purity water production and low energy consumption in seawater desalination. Desalination 2018, 429, 142–154. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, Z.; Guo, S.; Chen, S.; Xu, H.; Xu, X.; Gao, X.; Yan, W. Selection of anode materials and optimization of operating parameters for electrochemical water descaling. Sep. Purif. Technol. 2021, 261, 118304. [Google Scholar] [CrossRef]
- Ba, X.; Chen, J.; Wang, X.; Xu, H.; Sun, J.; Qi, Y.; Li, Y.; Wang, J.; Jiang, B. An integrated electrolysis-microfiltration-ion exchange closed-loop system for effective water softening without chemicals input and spent regenerant discharge. Desalination 2023, 553, 116481. [Google Scholar] [CrossRef]
- Sanjuán, I.; Benavente, D.; García-García, V.; Expósito, E.; Montiel, V. Electrochemical softening of concentrates from an electrodialysis brackish water desalination plant: Efficiency enhancement using a three-dimensional cathode. Sep. Purif. Technol. 2019, 208, 217–226. [Google Scholar] [CrossRef]
- Clauwaert, P.; De Paepe, J.; Jiang, F.; Alonso-Fariñas, B.; Vaiopoulou, E.; Verliefde, A.; Rabaey, K. Electrochemical tap water softening: A zero chemical input approach. Water Res. 2020, 169, 115263. [Google Scholar] [CrossRef] [PubMed]
- Hasson, D.; Sidorenko, G.; Semiat, R. Calcium carbonate hardness removal by a novel electrochemical seeds system. Desalination 2010, 263, 285–289. [Google Scholar] [CrossRef]
- Tlili, M.M.; Benamor, M.; Gabrielli, C.; Perrot, H.; Tribollet, B. Influence of the interfacial pH on electrochemical CaCO3 precipitation. J. Electrochem. Soc. 2003, 150, C765–C771. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, J.; Chen, J.; Liu, S.; Xu, H.; Yang, Q.; Xu, F.; Guo, Y.; Jiang, B. Robust electrolysis system divided by bipolar electrode and non-conductive membrane for energy-efficient calcium hardness removal. Chemosphere 2023, 331, 138797. [Google Scholar] [CrossRef]
- Sanjuán, I.; Benavente, D.; Expósito, E.; Montiel, V. Electrochemical water softening: Influence of water composition on the precipitation behaviour. Sep. Purif. Technol. 2019, 211, 857–865. [Google Scholar] [CrossRef]
- Zeppenfeld, K. Electrochemical water softening: The role of alkalinity. Water Solut. 2019, 4, 28–31. [Google Scholar]
- Wang, Z.; Lin, W.; Wang, W.; Wang, Z.; Li, J.; Xu, J.; Yu, J. Research on performance optimization and mechanism of electrochemical water softening applied by pulse power supply. Water Sci. Technol. 2021, 84, 2432–2445. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Liu, H. The j–pH diagram of interfacial reactions involving H+ and OH−. J. Energy Chem. 2020, 50, 339–343. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, J.; Rao, Y.; Yang, L.; Jiang, B.; Yan, W.; Xu, X.; Xu, H. Application of titanium suboxide electrode in electrochemical polarity reversal descaling-filtration crystallization coupling system. Desalination 2023, 568, 117028. [Google Scholar] [CrossRef]
- Jin, H.; Yu, Y.; Chen, X. Membrane-based electrochemical precipitation for water softening. J. Membr. Sci. 2020, 597, 117639. [Google Scholar] [CrossRef]
- Zaslavschi, I.; Shemer, H.; Hasson, D.; Semiat, R. Electrochemical CaCO3 scale removal with a bipolar membrane system. J. Membr. Sci. 2013, 445, 88–95. [Google Scholar] [CrossRef]
- Liu, Y.; Niu, Q.; Zhu, J.; Li, Y.; Sun, H.; Jiang, B. Efficient and green water softening by integrating electrochemically accelerated precipitation and microfiltration with membrane cleaning by periodically anodic polarization. Chem. Eng. J. 2022, 449, 137832. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Wang, L. Facilitating removal efficiency of electrochemical descaling system using confined crystallization membranes. J. Water Process Eng. 2023, 56, 104338. [Google Scholar] [CrossRef]
- Ba, X.; Chen, J.; Wang, X.; Feng, F.; Shi, X.; Qi, Y.; Wang, J.; Jiang, B. Anode Boundary Layer Extraction Strategy for H+–OH– Separation in Undivided Electrolytic Cell: Modeling, Electrochemical Analysis, and Water Softening Application. ACS ES&T Eng. 2023, 3, 2183–2193. [Google Scholar]
- Kang, W.; Li, L.; Yan, L.; Mao, W.; Wang, X.; Yu, H.; Ma, C. Spatial and temporal regulation of homogeneous nucleation and crystal growth for high-flux electrochemical water softening. Water Res. 2023, 232, 119694. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Gu, Y.; Kang, W.; Yu, H. Facilitated OH¯ diffusion via bubble motion and water flow in a novel electrochemical reactor for enhancing homogeneous nucleation of CaCO3. Water Res. 2023, 242, 120195. [Google Scholar] [CrossRef] [PubMed]
- Comstock, S.E.H.; Boyer, T.H. Combined magnetic ion exchange and cation exchange for removal of DOC and hardness. Chem. Eng. J. 2014, 241, 366–375. [Google Scholar] [CrossRef]
- Feng, S.; Zhong, Z.; Wang, Y.; Xing, W.; Drioli, E. Progress and perspectives in PTFE membrane: Preparation, modification, and applications. J. Membr. Sci. 2018, 549, 332–349. [Google Scholar] [CrossRef]
- Zhang, C.; Tang, J.; Zhao, G.; Tang, Y.; Li, J.; Li, F.; Zhuang, H.; Chen, J.; Lin, H.; Zhang, Y. Investigation on an electrochemical pilot equipment for water softening with an automatic descaling system: Parameter optimization and energy consumption analysis. J. Clean. Prod. 2020, 276, 123178. [Google Scholar] [CrossRef]
- Huang, L.; Li, D.; Liu, J.; Yang, L.; Dai, C.; Ren, N.; Feng, Y. CFD simulation of mass transfer in electrochemical reactor with mesh cathode for higher phenol degradation. Chemosphere 2021, 262, 127626. [Google Scholar] [CrossRef]
- Lin, W.; Wang, Z.; Wang, W.; Chen, Q.; Xu, J.; Yu, J. Comparative analysis the performance of electrochemical water softening between high frequency electric fields and direct current electric fields based on orthogonal experimental methods. Water Sci. Technol. 2021, 83, 1677–1690. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Alshawabkeh, A.N.; Hojabri, S.; Sun, M.; Xu, G.; Li, J. A Robust Flow-Through Platform for Organic Contaminant Removal. Cell Rep. Phys. Sci. 2021, 2, 100296. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Li, K.; Zhao, Z.; Li, Y.; Yang, Q.; Jiang, B. A pH self-regulated three-dimensional electro-Fenton system with a bifunctional Fe-Cu-C particle electrode: High degradation performance, wide working pH and good anti-scaling ability. Sep. Purif. Technol. 2022, 298, 121672. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, M.; Chen, Y.; Zou, Y.; Gong, Y. Study on electrolytic bubble size and its distribution by means of laser diffraction method. Acta Chim. Sinica 1992, 50, 967–972. [Google Scholar]
- Emmel, B.U.; Gawel, K.M.; Bhuiyan, M.H.; Torsæter, M.; Edvardsen, L. Electrochemically Enhanced Deposition of Scale from Chosen Formation Waters from the Norwegian Continental Shelf. Energies 2022, 15, 542. [Google Scholar] [CrossRef]
- Gabrielli, C.; Maurin, G.; Francy-Chausson, H.; Thery, P.; Tran, T.T.M.; Tlili, M. Electrochemical water softening: Principle and application. Desalination 2006, 201, 150–163. [Google Scholar] [CrossRef]
- Gao, J.; Shi, N.; Guo, X.; Li, Y.; Bi, X.; Qi, Y.; Guan, J.; Jiang, B. Electrochemically Selective Ammonia Extraction from Nitrate by Coupling Electron- and Phase-Transfer Reactions at a Three-Phase Interface. Environ. Sci. Technol. 2021, 55, 10684–10694. [Google Scholar] [CrossRef]
- Hasson, D.; Lumelsky, V.; Greenberg, G.; Pinhas, Y.; Semiat, R. Development of the electrochemical scale removal technique for desalination applications. Desalination 2008, 230, 329–342. [Google Scholar] [CrossRef]
- Luan, J.; Wang, L.; Sun, W.; Li, X.; Zhu, T.; Zhou, Y.; Deng, H.; Chen, S.; He, S.; Liu, G. Multi-meshes coupled cathodes enhanced performance of electrochemical water softening system. Sep. Purif. Technol. 2019, 217, 128–136. [Google Scholar] [CrossRef]
- Yu, Y.; Jin, H.; Quan, X.; Hong, B.; Chen, X. Continuous Multistage Electrochemical Precipitation Reactor for Water Softening. Ind. Eng. Chem. Res. 2019, 58, 461–468. [Google Scholar] [CrossRef]
- Marchesiello, M.; Thivel, P.X. Electrochemical antiscaling treatment using a fluidized bed. Sep. Purif. Technol. 2018, 194, 480–487. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, L.; He, Q.; Wu, D. Reduced cathodic scale and enhanced electrochemical precipitation of Ca2+ and Mg2+ by a novel fenced cathode structure: Formation of strong alkaline microenvironment and favorable crystallization. Water Res. 2022, 209, 117893. [Google Scholar] [CrossRef]
- Muddemann, T.; Haupt, D.; Engelke, M.; Sievers, M.; Fischer, A.; Kiefer, C.; Filip, K.; Zielinski, O.; Hickmann, T.; Kunz, U. Combination of magnetically actuated flexible graphite–polymer composite cathode and boron-doped diamond anode for electrochemical water softening or wastewater treatment. Electrochim. Acta 2020, 354, 136729. [Google Scholar] [CrossRef]
- Yu, Y.; Jin, H.; Meng, P.; Guan, Y.; Shao, S.; Chen, X. Electrochemical water softening using air-scoured washing for scale detachment. Sep. Purif. Technol. 2018, 191, 216–224. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Sun, W.; Yan, Z.; He, Y.; Shu, X.; Liu, G. Study on electrochemical water softening mechanism of high-efficient multi-layer mesh coupled cathode. Sep. Purif. Technol. 2020, 247, 117001. [Google Scholar] [CrossRef]
- Jiang, B.; Ren, X.; Liu, Q.; Yue, X.; Yang, Q.; Liu, Y.; Xu, H.; Zhou, J. Electrochemical water softening technology: From fundamental research to practical application. Water Res. 2024, 250, 121077. [Google Scholar] [CrossRef] [PubMed]
- Mahasti, N.N.N.; Shih, Y.-J.; Vu, X.-T.; Huang, Y.H. Removal of calcium hardness from solution by fluidized-bed homogeneous crystallization (FBHC) process. J. Taiwan Inst. Chem. Eng. 2017, 78, 378–385. [Google Scholar] [CrossRef]
- Tiangco, K.A.A.; de Luna, M.D.G.; Vilando, A.C.; Lu, M.-C. Removal and recovery of calcium from aqueous solutions by fluidized-bed homogeneous crystallization. Process Saf. Environ. Prot. 2019, 128, 307–315. [Google Scholar] [CrossRef]
- Jiang, B.; Ren, X.; Liu, Q.; Yue, X.; Yang, Q.; Liu, Y.; Xu, H.; Zhou, J. A critical review on the electrochemical water softening technology from fundamental research to practical application. Water Res. 2023, 250, 121077. [Google Scholar] [CrossRef]
- Zhi, S.; Zhang, K. Hardness removal by a novel electrochemical method. Desalination 2016, 381, 8–14. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, M.; Li, Q.; Chen, X.; Chen, D.; Jin, H. Subtle introduction of membrane polarization-catalyzed H2O dissociation actuates highly efficient electrocoagulation for hardness ion removal. Water Res. 2023, 242, 120240. [Google Scholar] [CrossRef] [PubMed]
- Yifei, G.; Zhicheng, X.; Siyuan, G.; Jianyi, L.; Hao, X.; Xing, X.; Xian, G.; Wei, Y. Practical optimization of scale removal in circulating cooling water: Electrochemical descaling-filtration crystallization coupled system. Sep. Purif. Technol. 2022, 284, 120268. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, D.; Liu, H.; Zhang, X.; Chen, X.; Jin, H. Membrane deposition electrodialysis for cooling water treatment: Ion step removal and stable membrane regeneration. Chem. Eng. J. 2023, 451, 138908. [Google Scholar] [CrossRef]
- Medina-Collana, J.T.; Reyna-Mendoza, G.E.; Montaño-Pisfil, J.A.; Rosales-Huamani, J.A.; Franco-Gonzales, E.J.; Córdova García, X. Evaluation of the Performance of the Electrocoagulation Process for the Removal of Water Hardness. Sustainability 2023, 15, 590. [Google Scholar] [CrossRef]
- Choi, J.; Dorji, P.; Shon, H.K.; Hong, S. Applications of capacitive deionization: Desalination, softening, selective removal, and energy efficiency. Desalination 2019, 449, 118–130. [Google Scholar] [CrossRef]
- Karabacakoğlu, B.; Tezakıl, F.; Güvenç, A. Removal of hardness by electrodialysis using homogeneous and heterogeneous ion exchange membranes. Desalination Water Treat. 2015, 54, 8–14. [Google Scholar] [CrossRef]
- Panagopoulos, A. Energetic, economic and environmental assessment of zero liquid discharge (ZLD) brackish water and seawater desalination systems. Energy Convers. Manag. 2021, 235, 113957. [Google Scholar] [CrossRef]




| Separation Method | Water Hardness | pH | Hardness Removal Rate | Hardness Precipitation | Energy Consumption | References | ||
|---|---|---|---|---|---|---|---|---|
| Ion exchange membrane (IEMs) | Cation exchange membrane (CEMs) | 188 mg/L Ca2+ | 9.8 | 75~86% | - | 7.0~10.1 kWh/kg CaCO3 | [44] | |
| Anion exchange membrane (AEMs) | 500 mg/L CaCO3 | - | - | 64~85 g/h/m2 | 9~12 kWh/kg CaCO3 | [53] | ||
| Bipolar membrane | 16 mM Ca2+ | - | - | 630 g/h/m2 | 1.2 kWh/kg CaCO3 | [54] | ||
| Polytetrafluoroethylene membrane (PTFE) | 400 mg/L CaCO3 | 11.5 | - | 348.8 g/h/m2 CaCO3 | 1.88 kWh/kg CaCO3 | [55] | ||
| Confined crystallization membrane (CCM) | 500 mg/L CaCO3 | - | - | 60.26 g/h/m2 CaCO3 | 12 kWh/kg CaCO3 | [56] | ||
| Extraction of boundary layer | 500 mg/L CaCO3 | 11.7 | 92% | - | 4.6 kWh/kg CaCO3 | [57] | ||
| Bubbles and water flow | Nylon net | 500 mg/L CaCO3 | 11.5 | 91.5% | - | 12.6 kWh/kg CaCO3 | [58] | |
| Water flow | 400 mg/L CaCO3 | 10.6 | - | 151.2 g/h/m2 CaCO3 | 16.8 kWh/kg CaCO3 | [59] | ||
| Cathode | Water Hardness | Hardness Removal Rate | Hardness Precipitation | Energy Consumption | References | |
|---|---|---|---|---|---|---|
| Two-dimensional cathode | Multi-layer stainless steel | 350 mg/L CaCO3 | - | 29.16 g/h/m2 | 6 kWh/kg CaCO3 | [72] |
| Multiple cathodes | 350 mg/L CaCO3 | - | 71.1 g/h/m2, | 3.17 kWh/kg CaCO3 | [73] | |
| Three-dimensional cathode | Stainless steel wire | 2400 mg/L CaCO3 | 45% | - | - | [43] |
| Stainless steel ball fluidized bed | 64 mg/L Ca2+; 7.5 mg/L Mg2+ | - | - | 0.375 kWh/m3 | [74] | |
| Combined cathode | Stainless steel–carbon felt | 1.8 mmol/L Ca2+; 1.8 mmol/L Mg2+ | Ca2+: 91%; Mg2+: 38.6%, | - | Ca2+: 0.68 kWh/mol; Mg2+: 1.68 kWh/mol | [75] |
| Graphite–polymer composite | 7.845 mmol/L Ca2+ | 90% | - | 45.9 kWh/kg CaCO3 | [76] | |
| Coupling Process | Water Hardness | Hardness Removal Rate | Hardness Precipitation | Energy Consumption | References | |
|---|---|---|---|---|---|---|
| Electrodeposition–electrocoagulation | Electrodeposition–electrocoagulation synergy | 300 mg/L CaCO3 | 65% | - | - | [83] |
| Membrane polarization catalytic electrocoagulation | 350 mg/L CaCO3 | - | 318.9 g/h/m2 | 3.8 kWh/kg CaCO3 | [84] | |
| Electrodeposition– filtration crystallization | Conventional electrodeposition filtration crystallization | 300 mg/L CaCO3 | 15.6~33.4% | - | - | [85] |
| Reverse polarity descaling–filtration crystallization | 300 mg/L CaCO3 | 25.5~27.3% | - | - | [52] | |
| Electrodeposition–microfiltration | Electrochemical accelerated precipitation decaling– microfiltration | 400 mg/L CaCO3 | - | 348.8 g/h/m2 CaCO3 | 1.88 kWh/kg CaCO3 | [55] |
| Electrolysis– microfiltration– ion exchange | 400 mg/L CaCO3 | 90% | - | 1.9 kWh/Kg CaCO3 | [76] | |
| Electrodeposition– Electrodialysis | 6400 mg/L CaCO3 | Ca2+: 57%; Mg2+: 61%, | 5.8–15.9 M/h/m2 | - | [86] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zhou, J.; Chang, Y.; Xu, H. Research Progress on Novel Electrochemical Descaling Technology for Enhanced Hardness Ion Removal. Water 2024, 16, 886. https://doi.org/10.3390/w16060886
Wang L, Zhou J, Chang Y, Xu H. Research Progress on Novel Electrochemical Descaling Technology for Enhanced Hardness Ion Removal. Water. 2024; 16(6):886. https://doi.org/10.3390/w16060886
Chicago/Turabian StyleWang, Liangtian, Jie Zhou, Yuexin Chang, and Hao Xu. 2024. "Research Progress on Novel Electrochemical Descaling Technology for Enhanced Hardness Ion Removal" Water 16, no. 6: 886. https://doi.org/10.3390/w16060886