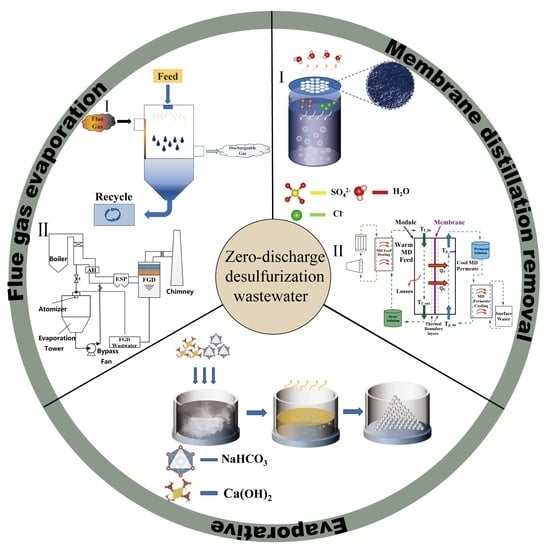
Current Status of Zero Liquid Discharge Technology for Desulfurization Wastewater
Abstract
1. Introduction
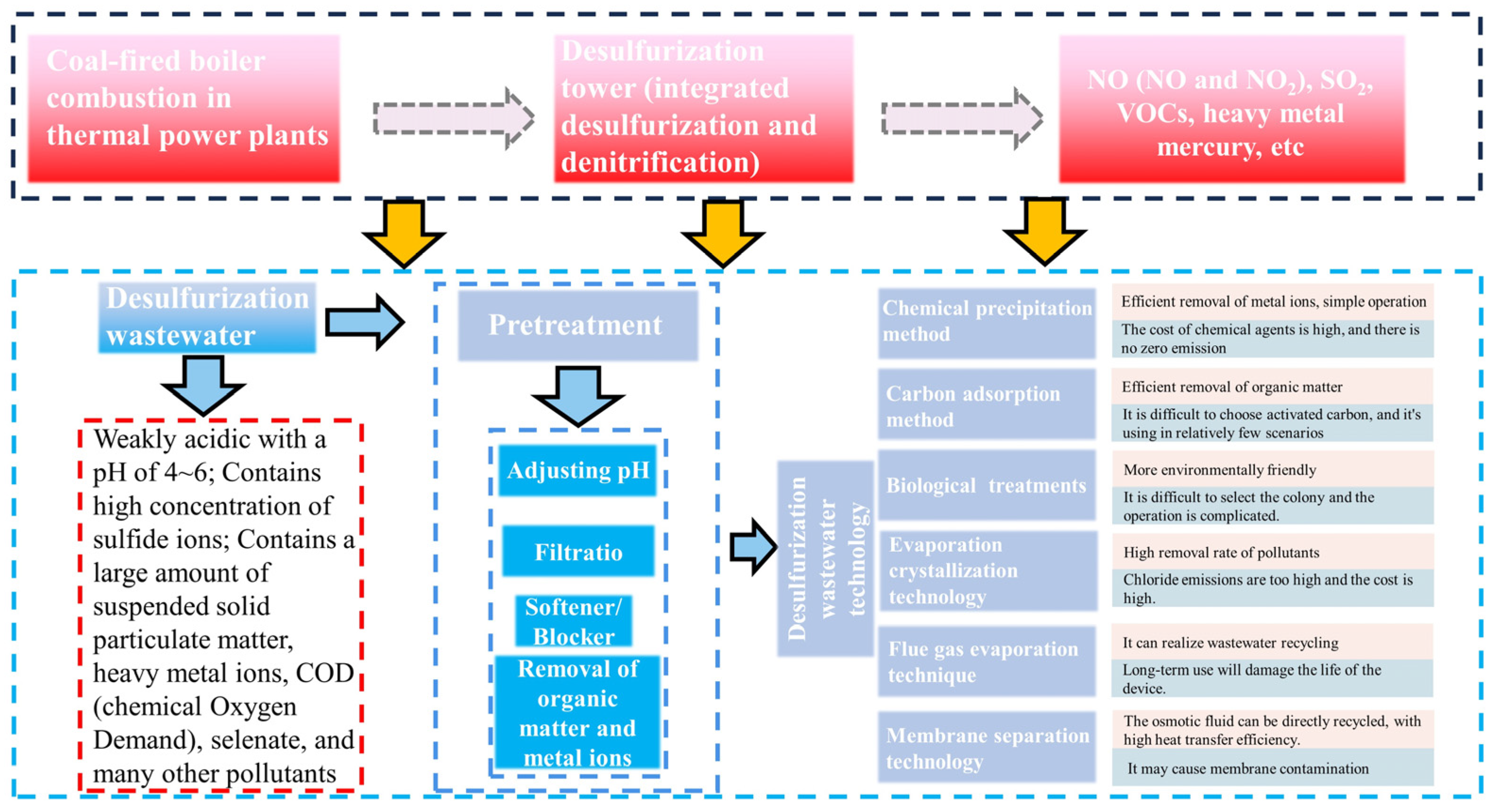
2. Conventional Treatment Technologies for Desulfurization Wastewater
2.1. Chemical Precipitation
2.2. Activated Carbon Adsorption
2.3. Biological Treatment
3. Zero Liquid Discharge Technologies for Desulfurization Wastewater
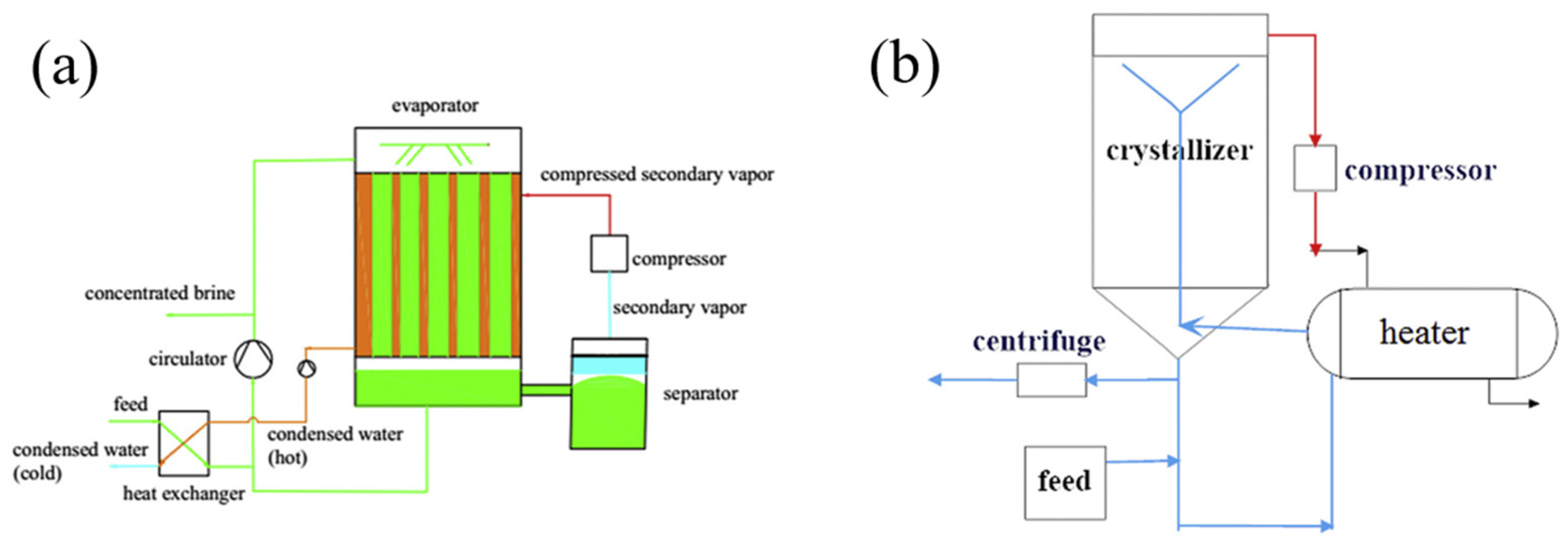
3.1. Evaporation Crystallization Technology
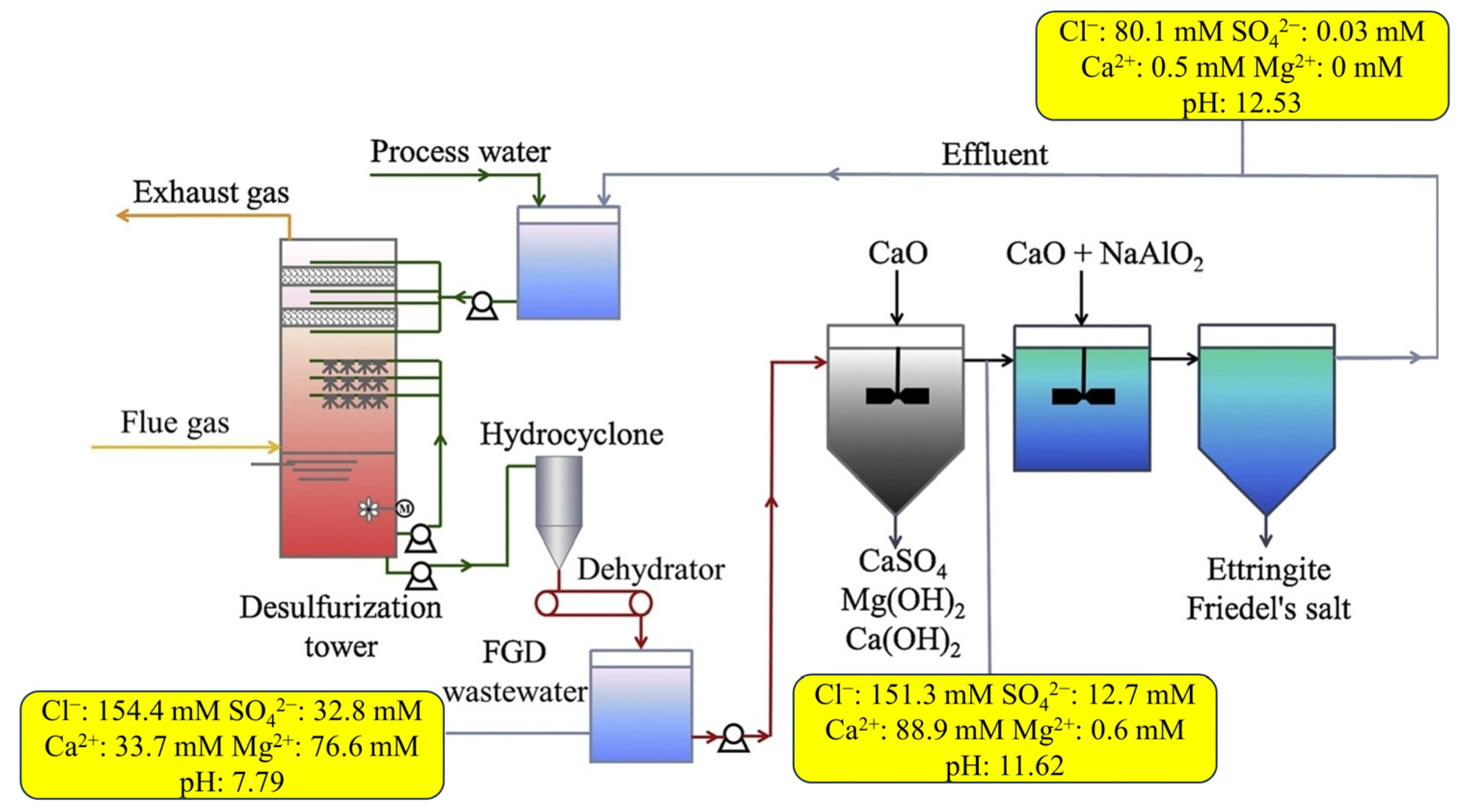
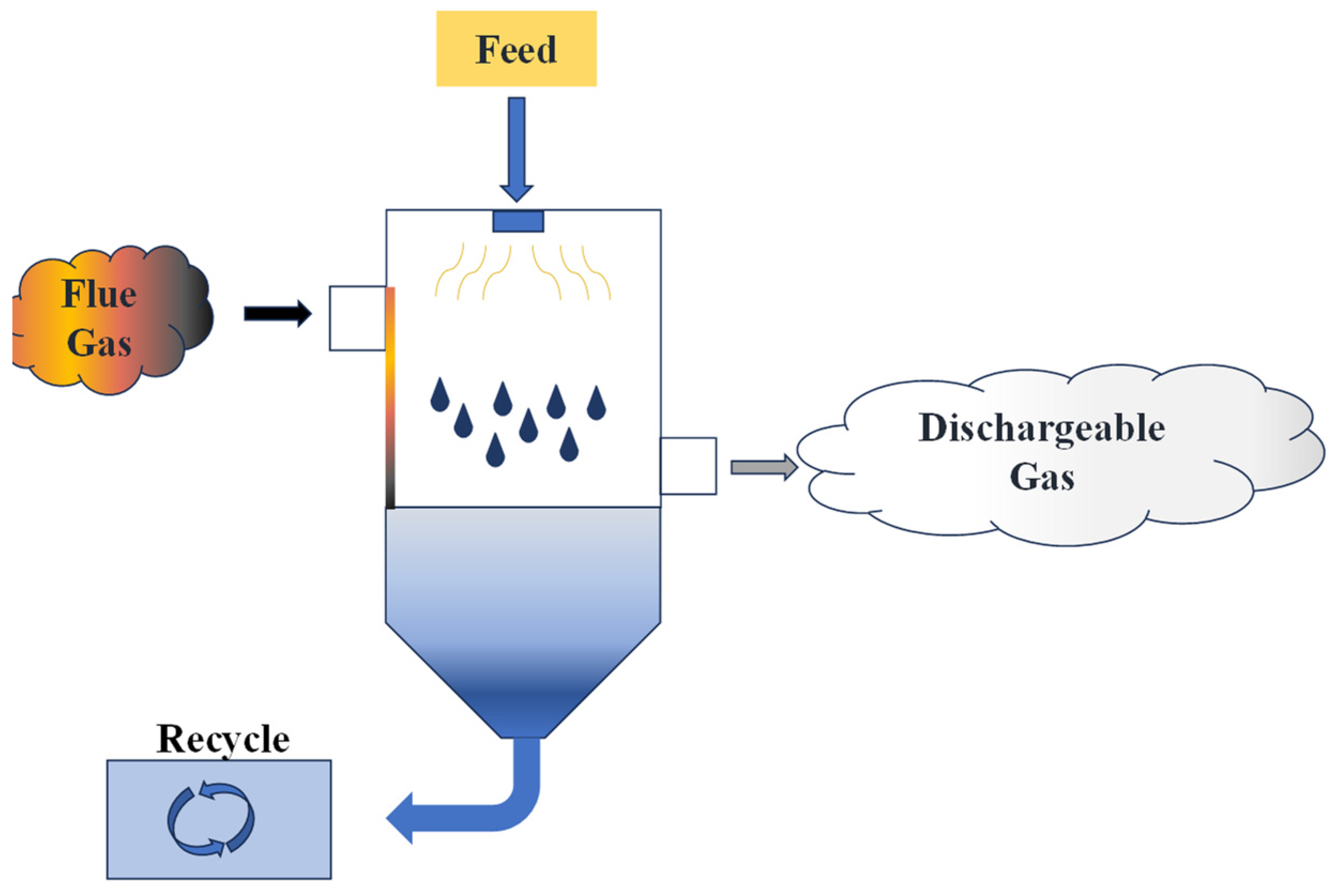
3.2. Flue Gas Evaporation Technology
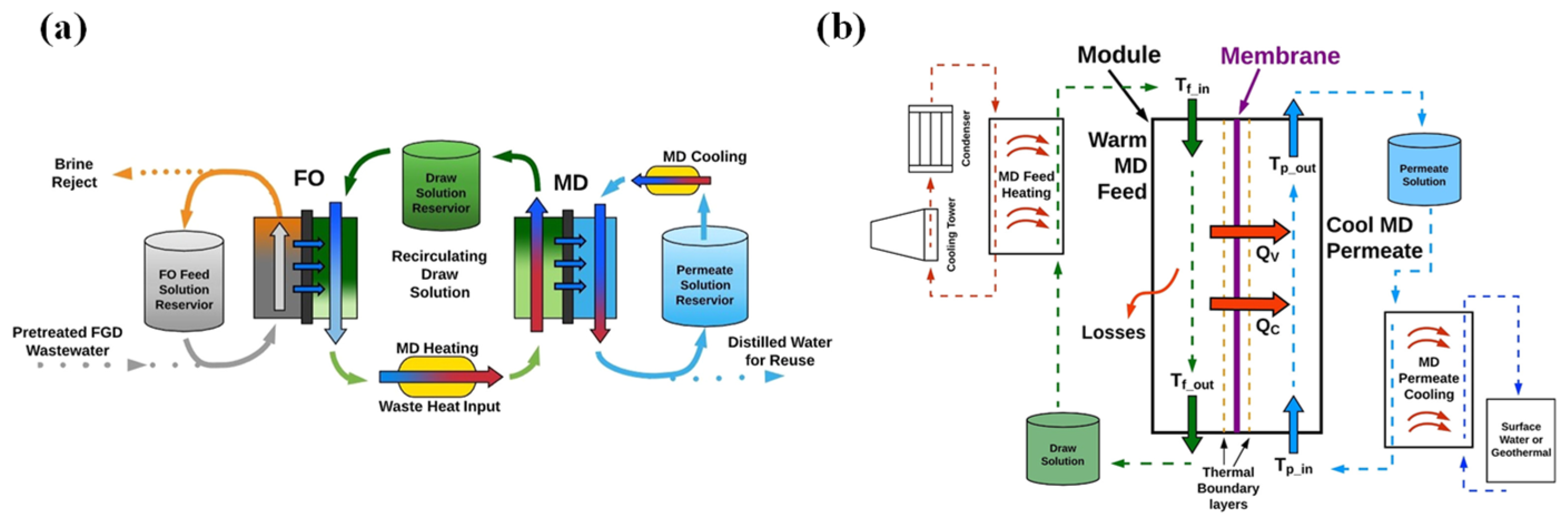
3.3. Membrane Distillation Removal Technology
4. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Sung, Y. Advances in Reduction Technologies of Gas Emissions (CO2, NOx, and SO2) in Combustion-Related Applications. Energies 2023, 16, 3469. [Google Scholar] [CrossRef]
- Mazziotti Di Celso, G.; Karatza, D.; Lancia, A.; Musmarra, D.; Prisciandaro, M.J.C.E.T. Limestone-Gypsum Flue Gas Desulfuration Process: Modeling of Catalyzed Bisulfite Oxidation. Chem. Eng. Trans. 2013, 32, 781–786. [Google Scholar]
- Warych, J.; Szymanowski, M. Model of the Wet Limestone Flue Gas Desulfurization Process for Cost Optimization. Ind. Eng. Chem. Res. 2001, 40, 2597–2605. [Google Scholar] [CrossRef]
- Peng, W.; Wagner, F.; Ramana, M.V.; Zhai, H.; Small, M.J.; Dalin, C.; Zhang, X.; Mauzerall, D.L. Managing China’s coal power plants to address multiple environmental objectives. Nat. Sustain. 2018, 1, 693–701. [Google Scholar] [CrossRef]
- Han, X.; Chen, N.; Yan, J.; Liu, J.; Liu, M.; Karellas, S. Thermodynamic analysis and life cycle assessment of supercritical pulverized coal-fired power plant integrated with No.0 feedwater pre-heater under partial loads. J. Clean. Prod. 2019, 233, 1106–1122. [Google Scholar] [CrossRef]
- Liu, C.; Ma, L.; Xu, Y.; Wang, F.; Tan, Y.; Huang, L.; Ma, S. Experimental and theoretical study of a new CDI device for the treatment of desulfurization wastewater. Environ. Sci. Pollut. Res. 2022, 29, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhan, L.; Chen, H.; Yang, L. Concentration of desulfurization wastewater using low-temperature flue gas and associated corrosion risk. Environ. Sci. Pollut. Res. 2023, 30, 18395–18407. [Google Scholar] [CrossRef]
- Zheng, L.; Jiao, Y.; Zhong, H.; Zhang, C.; Wang, J.; Wei, Y. Insight into the magnetic lime coagulation-membrane distillation process for desulfurization wastewater treatment: From pollutant removal feature to membrane fouling. J. Hazard. Mater. 2020, 391, 122202. [Google Scholar] [CrossRef] [PubMed]
- Córdoba, P. Partitioning and speciation of selenium in wet limestone flue gas desulphurisation systems: A review. Fuel 2017, 202, 184–195. [Google Scholar] [CrossRef]
- Kayan, G.Ö.; Kayan, A. Composite of Natural Polymers and Their Adsorbent Properties on the Dyes and Heavy Metal Ions. J. Polym. Environ. 2021, 29, 3477–3496. [Google Scholar] [CrossRef]
- Kayan, A. Inorganic-organic hybrid materials and their adsorbent properties. Adv. Compos. Hybrid Mater. 2019, 2, 34–45. [Google Scholar] [CrossRef]
- Yan, J.; Yuan, W.; Liu, J.; Ye, W.; Lin, J.; Xie, J.; Huang, X.; Gao, S.; Xie, J.; Liu, S.; et al. An integrated process of chemical precipitation and sulfate reduction for treatment of flue gas desulphurization wastewater from coal-fired power plant. J. Clean. Prod. 2019, 228, 63–72. [Google Scholar] [CrossRef]
- Pakzadeh, B.; Wos, J.; Renew, J. Flue Gas Desulfurization Wastewater Treatment for Coal-Fired Power Industry. In Proceedings of the ASME 2014 Power Conference, Baltimore, MD, USA, 29–31 July 2014. [Google Scholar]
- Shuangchen, M.; Jin, C.; Gongda, C.; Weijing, Y.; Sijie, Z. Research on desulfurization wastewater evaporation: Present and future perspectives. Renew. Sustain. Energy Rev. 2016, 58, 1143–1151. [Google Scholar] [CrossRef]
- Enoch, G.D.; Spiering, W.; Tigchelaar, P.; Niet, J.D.; Lefers, J.B. Treatment of Waste Water from Wet Lime (Stone) Flue Gas Desulfurization Plants with Aid of Crossflow Microfiltration. Sep. Sci. Technol. 1990, 25, 1587–1605. [Google Scholar] [CrossRef]
- Huang, Y.H.; Peddi, P.K.; Tang, C.; Zeng, H.; Teng, X. Hybrid zero-valent iron process for removing heavy metals and nitrate from flue-gas-desulfurization wastewater. Sep. Purif. Technol. 2013, 118, 690–698. [Google Scholar] [CrossRef]
- Xin, Y.; Zhou, Z.; Ming, Q.; Sun, D.; Han, J.; Ye, X.; Dai, S.; Jiang, L.-M.; Zhao, X.; An, Y. A two-stage desalination process for zero liquid discharge of flue gas desulfurization wastewater by chloride precipitation. J. Hazard. Mater. 2020, 397, 122744. [Google Scholar] [CrossRef] [PubMed]
- Bandosz, T.J. Chapter 5 Desulfurization on activated carbons. In Interface Science and Technology; Bandosz, T.J., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 7, pp. 231–292. [Google Scholar]
- Hsu, C.-J.; Chen, Y.-H.; Hsi, H.-C. Adsorption of aqueous Hg2+ and inhibition of Hg0 re-emission from actual seawater flue gas desulfurization wastewater by using sulfurized activated carbon and NaClO. Sci. Total Environ. 2020, 711, 135172. [Google Scholar] [CrossRef]
- Blázquez, E.; Baeza, J.A.; Gabriel, D.; Guisasola, A. Treatment of real flue gas desulfurization wastewater in an autotrophic biocathode in view of elemental sulfur recovery: Microbial communities involved. Sci. Total Environ. 2019, 657, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Barrington, D.J.; Ho, G. Towards zero liquid discharge: The use of water auditing to identify water conservation measures. J. Clean. Prod. 2014, 66, 571–576. [Google Scholar] [CrossRef]
- EPA US. Steam Electric Power Generating Point Source Category: Final Detailed Study Report; Environmental Protection Agency: Washington, DC, USA, 2009. [Google Scholar]
- Ruozheng, L.; Chong, Z.; Wanqiang, Y.; Wenming, M.; Zhilong, J.; Can, W.; Xuan, C.; Haibin, J. Experimental Study of Flue Gas Desulfurization Wastewater Zero Discharge from Coal-Fired Power Plant, Proceedings of the 2016 International Forum on Energy, Environment and Sustainable Development, 2016/05, 2016; Atlantis Press: Dordrecht, The Netherlands, 2016; pp. 1000–1005. Available online: https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=P1005J8A.txt (accessed on 7 July 2023).
- Lu, H.; Wang, J.; Wang, T.; Wang, N.; Bao, Y.; Hao, H. Crystallization techniques in wastewater treatment: An overview of applications. Chemosphere 2017, 173, 474–484. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, L.; Yang, Z.; Qiang, T.; Pu, G.; Ran, J. Evaporation and crystallization of a droplet of desulfurization wastewater from a coal-fired power plant. Appl. Therm. Eng. 2017, 119, 52–62. [Google Scholar] [CrossRef]
- Zheng, C.; Zheng, H.; Yang, Z.; Liu, S.; Li, X.; Zhang, Y.; Weng, W.; Gao, X. Experimental study on the evaporation and chlorine migration of desulfurization wastewater in flue gas. Environ. Sci. Pollut. Res. 2019, 26, 4791–4800. [Google Scholar] [CrossRef]
- Liang, Z.; Cheng, X.; Zhang, L.; Yang, Z.; Ran, J.; Ding, L. Study of Main Solutes on Evaporation and Crystallization Processes of the Desulfurization Wastewater Droplet. Energy Fuels 2018, 32, 6119–6129. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Yao, X.; Deng, B.; Kong, H.; Wei, G.; Yang, H. A novel drying tower technology for zero liquid discharge of desulfurization technology. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 1–19. [Google Scholar] [CrossRef]
- Chen, H.; Hou, D.; Zhan, L.; Li, Z.; Zheng, S.; Li, F.; Chen, H.; Wu, H.; Yang, L. A demonstration project for desulfurization wastewater evaporation technology: Field test and performance analysis. J. Water Process Eng. 2023, 53, 103874. [Google Scholar] [CrossRef]
- Fu, J.; Hu, N.; Yang, Z.; Wang, L. Experimental study on zero liquid discharge (ZLD) of FGD wastewater from a coal-fired power plant by flue gas exhausted heat. J. Water Process Eng. 2018, 26, 100–107. [Google Scholar] [CrossRef]
- Ma, S.; Chai, J.; Wu, K.; Xiang, Y.; Jia, S.; Li, Q. Experimental research on bypass evaporation tower technology for zero liquid discharge of desulfurization wastewater. Environ. Technol. 2019, 40, 2715–2725. [Google Scholar] [CrossRef]
- Ma, S.; Chai, J.; Wu, K.; Wan, Z.; Xiang, Y.; Zhang, J.; Fan, Z. Experimental and mechanism research on volatilization characteristics of HCl in desulfurization wastewater evaporation process using high temperature flue gas. J. Ind. Eng. Chem. 2018, 66, 311–317. [Google Scholar] [CrossRef]
- Chen, H.; Zhan, L.; Gu, L.; Feng, Q.; Zhao, N.; Feng, Y.; Wu, H.; Yang, L. Chloride release characteristics of desulfurization wastewater droplet during evaporation process using the single droplet drying method. Fuel 2021, 305, 121551. [Google Scholar] [CrossRef]
- Chen, H.; Zhan, L.; Gu, L.; Feng, Q.; Zhao, N.; Feng, Y.; Wu, H.; Yang, L. Spray drying of desulfurization wastewater: Drying characteristics, product analysis and potential risk assessment. Powder Technol. 2021, 394, 748–756. [Google Scholar] [CrossRef]
- Xu, Y.; Jin, B.; Zhou, Z.; Fang, W. Experimental and numerical investigations of desulfurization wastewater evaporation in a lab-scale flue gas duct: Evaporation and HCl release characteristics. Environ. Technol. 2021, 42, 1411–1427. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, L.; Chen, S.; Bai, L.; Wu, X. Promoting the removal of fine particles and zero discharge of desulfurization wastewater by spray-turbulent agglomeration. Fuel 2020, 270, 117461. [Google Scholar] [CrossRef]
- Shanshan, Z.; Qiaoling, W.; Haihong, J.; Tinghao, L. Research and Application of Zero Discharge Technology for Desulfurization Wastewater of 660MW Coal-Fired Unit. In Proceedings of the 2021 5th International Conference on Green Energy and Applications (ICGEA), Singapore, 6–8 March 2021; pp. 167–171. [Google Scholar]
- Lu, J.; Geng, K.; Zhang, Q.; Yao, J.; Cui, L.; Dong, Y. Effect of charged desulfurization wastewater droplet evaporation on the agglomeration of fine particles. Sep. Purif. Technol. 2022, 283, 120158. [Google Scholar] [CrossRef]
- Wei, H.; Guo, H. New Process for Deep Treatment of Desulfurization Wastewater Successfully Applied for the First Time in Domestic Coal-Fired Power Plants. Available online: http://www.ccoa-news.com/news/202307/07/c173695.html (accessed on 7 July 2023).
- Yin, N.; Liu, F.; Zhong, Z.; Xing, W. Integrated Membrane Process for the Treatment of Desulfurization Wastewater. Ind. Eng. Chem. Res. 2010, 49, 3337–3341. [Google Scholar] [CrossRef]
- Lee, S.; Kim, Y.; Hong, S. Treatment of industrial wastewater produced by desulfurization process in a coal-fired power plant via FO-MD hybrid process. Chemosphere 2018, 210, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Conidi, C.; Macedonio, F.; Ali, A.; Cassano, A.; Criscuoli, A.; Argurio, P.; Drioli, E. Treatment of Flue Gas Desulfurization Wastewater by an Integrated Membrane-Based Process for Approaching Zero Liquid Discharge. Membranes 2018, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Wang, J. Treatment of flue gas desulfurization wastewater with near-zero liquid discharge by nanofiltration-membrane distillation process. Sep. Sci. Technol. 2018, 53, 146–153. [Google Scholar] [CrossRef]
- Azizi Namaghi, H.; Pourafshari Chenar, M.; Haghighi Asl, A.; Esmaeili, M.; Pihlajamäki, A.; Kallioinen, M.; Mänttäri, M. Ultra-desulfurization of sulfur recovery unit wastewater using thin film nanocomposite membrane. Sep. Purif. Technol. 2019, 221, 211–225. [Google Scholar] [CrossRef]
- Anderson, W.V.; Cheng, C.-M.; Butalia, T.S.; Weavers, L.K. Forward Osmosis–Membrane Distillation Process for Zero Liquid Discharge of Flue Gas Desulfurization Wastewater. Energy Fuels 2021, 35, 5130–5140. [Google Scholar] [CrossRef]
- Wei, Y.; Li, C.; Wang, Y.; Zhang, X.; Li, Q.; Xu, T. Regenerating sodium hydroxide from the spent caustic by bipolar membrane electrodialysis (BMED). Sep. Purif. Technol. 2012, 86, 49–54. [Google Scholar] [CrossRef]
- Zhang, X.; Ye, C.; Pi, K.; Huang, J.; Xia, M.; Gerson, A.R. Sustainable treatment of desulfurization wastewater by ion exchange and bipolar membrane electrodialysis hybrid technology. Sep. Purif. Technol. 2019, 211, 330–339. [Google Scholar] [CrossRef]
- Li, B.; Yun, Y.; Liu, G.; Li, C.; Li, X.; Hilal, M.; Yang, W.; Wang, M. Direct contact membrane distillation with softening Pre-treatment for effective reclaiming flue gas desulfurization wastewater. Sep. Purif. Technol. 2021, 277, 119637. [Google Scholar] [CrossRef]
- Cheng, Q.; Wu, Y.; Huang, Y.; Li, F.; Liu, Z.; Nengzi, L.; Bao, L. An integrated process of calcium hydroxide precipitation and air stripping for pretreatment of flue gas desulfurization wastewater towards zero liquid discharge. J. Clean. Prod. 2021, 314, 128077. [Google Scholar] [CrossRef]
- Chen, H.; Wang, M.; Wang, L.; Zhou, M.; Wu, H.; Yang, H. Enhanced separation performance of Hg2+ in desulfurization wastewater using a tannin acid reduced graphene oxide membrane. Sep. Purif. Technol. 2021, 274, 119017. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, F.; Zhao, S.; Li, B.; Li, H.; Ling, Z.; Zhang, G.; Liu, M. Current Status of Zero Liquid Discharge Technology for Desulfurization Wastewater. Water 2024, 16, 900. https://doi.org/10.3390/w16060900
Xu F, Zhao S, Li B, Li H, Ling Z, Zhang G, Liu M. Current Status of Zero Liquid Discharge Technology for Desulfurization Wastewater. Water. 2024; 16(6):900. https://doi.org/10.3390/w16060900
Chicago/Turabian StyleXu, Feng, Sanmei Zhao, Bin Li, Haihua Li, Zhongqian Ling, Guangxue Zhang, and Maosheng Liu. 2024. "Current Status of Zero Liquid Discharge Technology for Desulfurization Wastewater" Water 16, no. 6: 900. https://doi.org/10.3390/w16060900
APA StyleXu, F., Zhao, S., Li, B., Li, H., Ling, Z., Zhang, G., & Liu, M. (2024). Current Status of Zero Liquid Discharge Technology for Desulfurization Wastewater. Water, 16(6), 900. https://doi.org/10.3390/w16060900