Abstract
The signal crayfish Pacifastacus leniusculus is one of the most widespread non-indigenous crayfish in Europe and is of interest to aquaculture in many countries. Once they escape into the wild, they disperse and become a potential source of food exploited for consumption by local inhabitants. The ingestion of plastics by the invasive signal crayfish in the Wieprza River, a tributary to the Baltic Sea, was determined by the FTIR identification of plastic compounds found in stomachs. The occurrence of plastic debris in the stomachs of P. leniusculus is reported for the first time. Plastic particles with the size range between 70 and 450 µm were observed only among crayfish inhabiting the lower, urbanized part of the river (7.3% of specimens). The presence of PTFE (polytetrafluoroethylene), cellophane, PP (polypropylene), PE (polyethylene), PMMA (polymethyl methacrylate), and nylon was confirmed with the highest frequency of PTFE in the stomachs of crayfish. Fibres were the predominant type of microplastics in crayfish stomachs. The results indicated the size-selective uptake and ingestion of plastics depending on the traits of the species and environment.
1. Introduction
Pollution by plastic debris, widely recognized as a growing global problem [1,2,3] is caused by the production of synthetic polymers. Over recent years, the production of plastics has increased, while in Europe, fossil-based plastics production has decreased, and recycled plastics production has increased [4]. There are different types of plastics. Some types of plastic ingredients, applied in a wide variety of cosmetics and personal care products (PCCP), occur as particulate microplastics < 5 mm [5]. Larger plastics, under environmental conditions, may fragment to microplastics [6].
Plastics are widely used by humans, and, as waste, they become a pollutant in the environment. Plastics may pose risks for aquatic ecosystems [7]. Plastics are water-insoluble, have low degradation rates [8], may be ingested by various organisms ranging from zooplankton to fish and mammals, and accumulate in the intestines through the aquatic food web [9]. Moreover, plastic can adsorb organic contaminants from the surrounding media, which can be transferred to organisms upon ingestion [10].
Most studies on plastic debris are limited to the marine environment [7,10,11]. However, rivers are significant sources of plastic pollution for coastal and offshore areas [12,13].
The bioaccumulation of plastics was previously confirmed in many aquatic species, e.g., Gammarus setosus (Dementieva, 1931) [14], Carcinus maenas (Linnaeus, 1758) [15], Palaemonetes pugio (Holthuis, 1949) [16], Amphibalanus amphitrite (Darwin, 1854) [17], and Mytilus edulis Linnaeus, 1758 [18]. However, to our knowledge, their presence in the signal crayfish Pacifastacus leniusculus (Dana, 1852) has not been confirmed.
The signal crayfish is one of the most widespread non-indigenous crayfish in Europe [19]. They are large, euryhaline, attractive for consumption and for this reason were introduced for aquaculture into many countries [20,21]. In the 1990s, this species was introduced into the Wieprza River drainage area [22,23]. Once they escaped into the wild, they became exploited by local inhabitants.
The aim of this study was to determine the level of contamination by plastics in the invasive signal crayfish from the Baltic coastal tributary that is considered as a potential local food source. The specific objectives were:
- determination of plastic contamination in different river sections,
- assess microplastic amount and type,
- verification if there are any differences between sexes, sizes and condition of organisms resulting from distinct traits of individuals.
2. Materials and Methods
2.1. Material Collection and Preparation
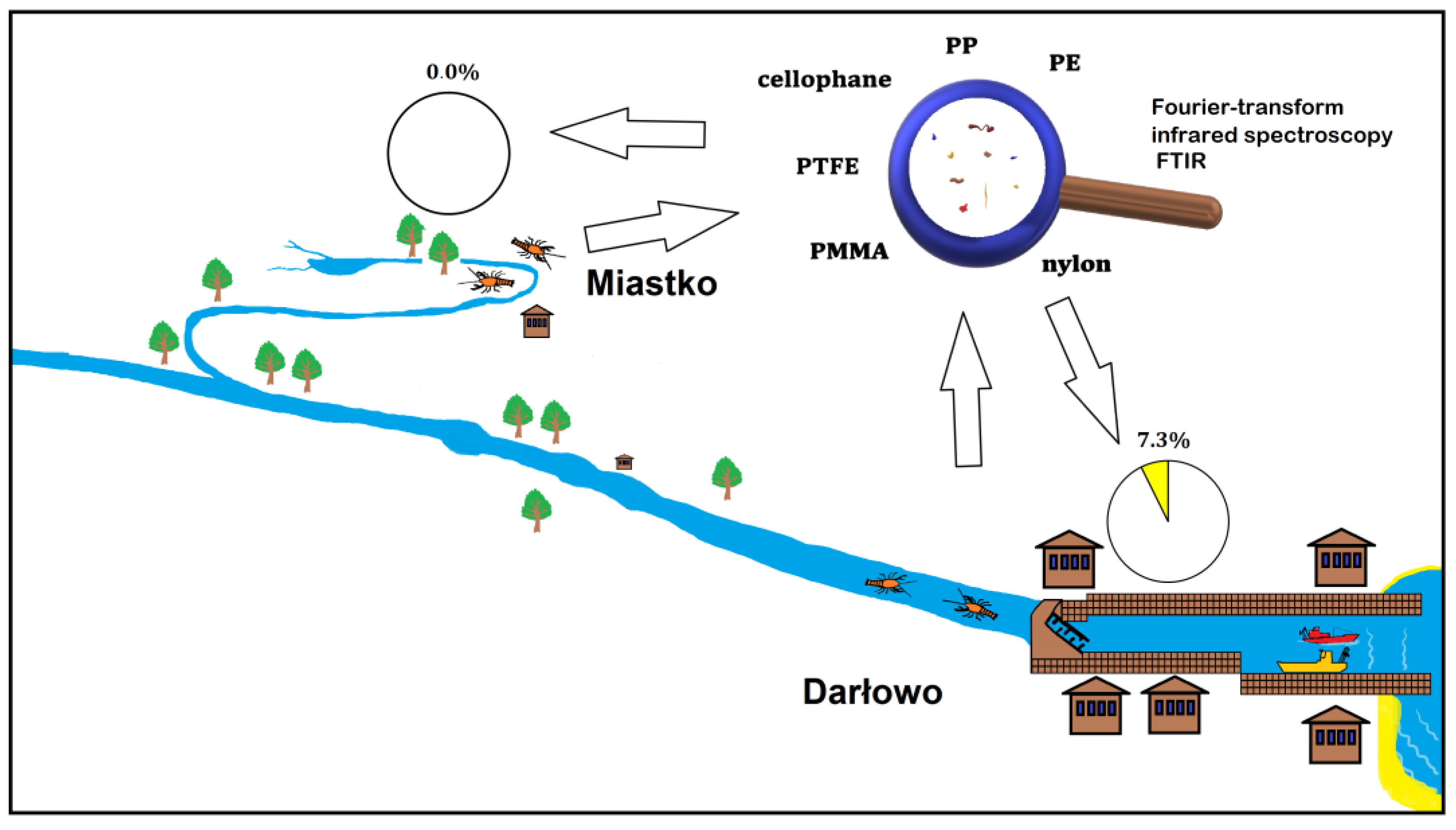
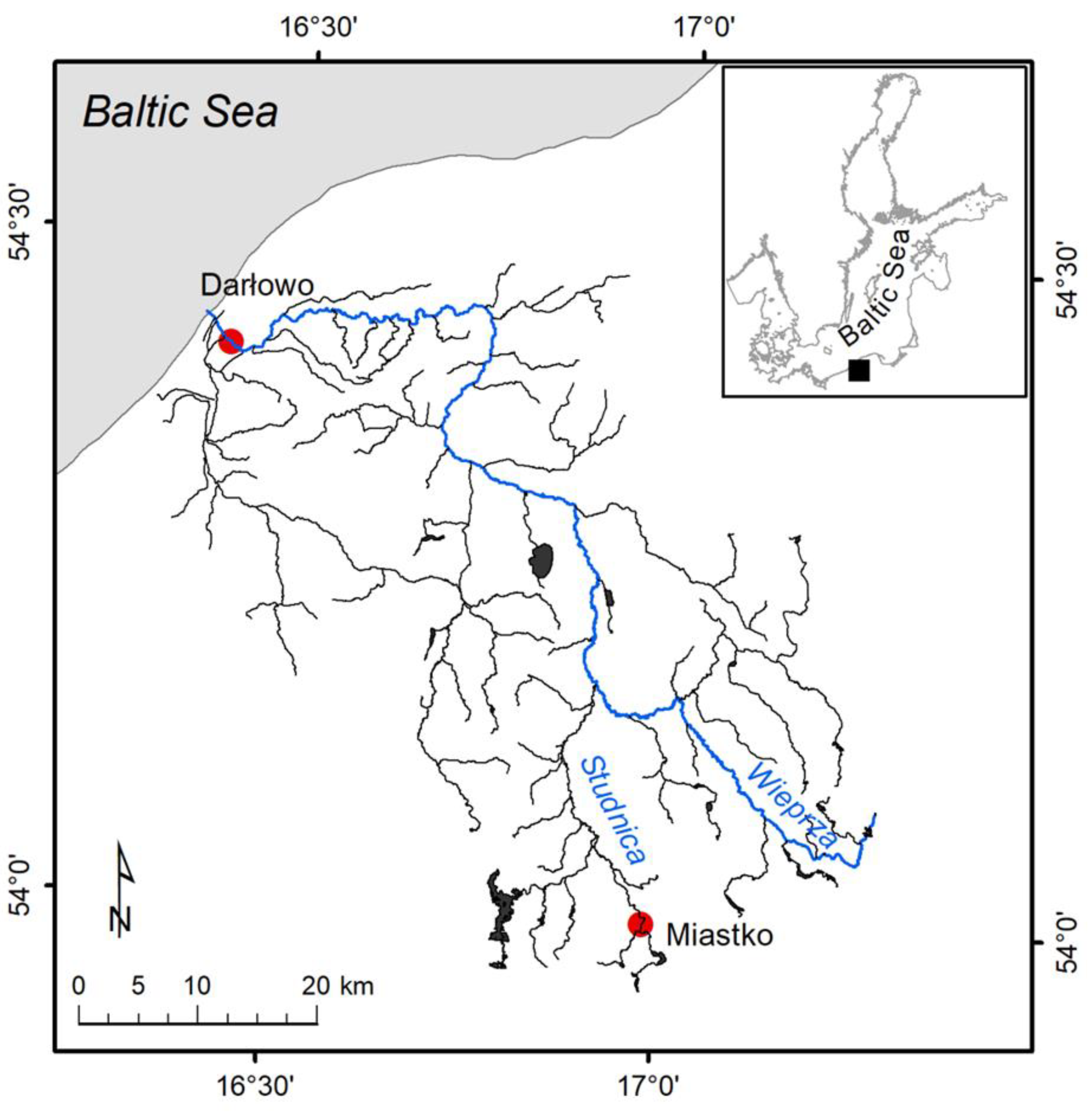
Specimens of P. leniusculus were collected from the Wieprza River and its tributary in the summer months between 2014 and 2018 (Figure 1 and Figure 2). Crayfish were caught at two sites: in the upstream section of the River Studnica above Miastko (n = 25) and the downstream section of the Wieprza River which is close to Baltic Sea in Darłowo (Poland) (n = 205). Crustaceans were caught using the “Pirate” crayfish traps at both sites and by the hand collecting method to increase the sample size from Miastko [24]. The distance between traps was between 5 and 20 m within the studied areas. After the capture, animals were immediately frozen (−20 °C). In the laboratory, thawed crayfish were sexed and measured for total length (LT) with digital callipers. The wet mass (MW) was determined using a Mettler Toledo XS 205 scale (Greifensee, Switzerland). The LT and MW were expressed as a mean with a standard deviation (mean ± SD).
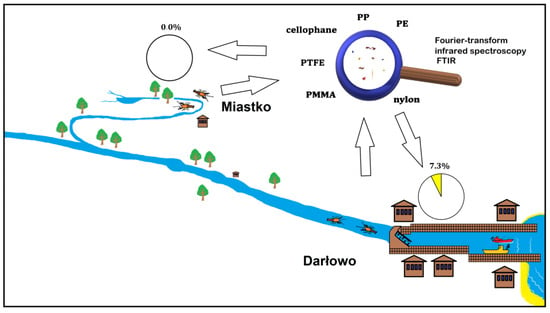
Figure 1.
Graphic representation of the study on the signal crayfish Pacifastacus leniusculus in the Wieprza River system (Poland).
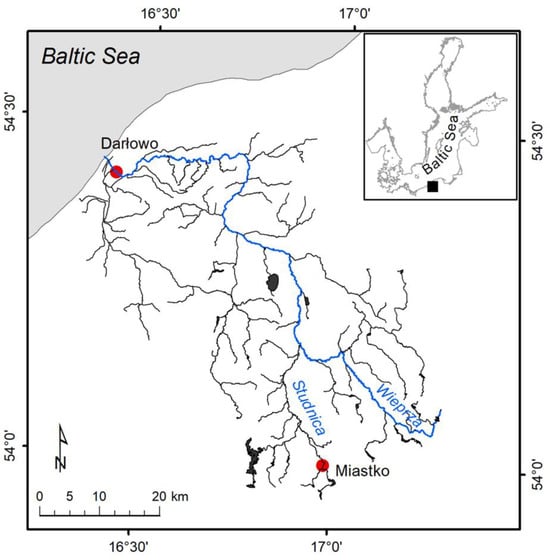
Figure 2.
Sampling sites of the signal crayfish Pacifastacus leniusculus individuals collected in the Wieprza River system (Poland).
2.2. Characteristics of the Sampling Area
The upper, semi-natural area close to Miastko differs from the urbanized, downstream area in Darłowo. It is located on the stretch of the tributary that flows out from the Lake Studzieniczno, passes through the forest, enters into a fish farm, then flows downstream with meadows on both riverbanks into an urban area of Miastko. The potential source of plastic is limited by a low number of people walking across a small bridge near the crayfish collecting site. The sampling site in Darłowo is situated in the urban area and is under more anthropogenic stress. Anthropogenic pressure increases there during summer months due to increased tourism in the coastal area. The river passing through the town enters the marine harbour. Traps were placed approximately 3 km from the river mouth and close to the upstream side of a weir.
2.3. Identification of Plastics Items
The stomachs of crayfish were extracted and analysed in the laboratory. The fragments of tissues containing microplastics were isolated using visual inspection on clean glass Petri dishes under an optical stereoscopic microscope NIKON SMZ800 (Tokyo, Japan) at 6.3× magnification to determine the plastic content. The microplastic particles were removed from isolated samples using tweezers and were not further cleaned. The isolated samples were studied in detail using μFTIR reflectance spectroscopy with the use of a Thermo Scientific™ Nicolet™ Continuμm™ Infrared Microscope (Madison, WI, USA) equipped with an MCT-A detector and a 15× objective lens. Each isolated sample was deposited on a clean glass slide covered with thin layer of silver and flattened with the use of a roller tool cleaned with alcohol after each sample to avoid contamination. The spectrum of the glass slide as a background was automatically subtracted from the spectrum of each sample. The spectra of organic clothing were not collected as a background, because of the difficulty in establishing its average composition within the same sample. Therefore, the identification of the samples was based on the recognition of the characteristic vibration of chemical bonds present in particular polymer.
FTIR spectra were recorded as changes in absorption as a function of wave number ranging from 600 cm−1 to 4000 cm−1. The following conditions were used: measurement recording accuracy—4 cm−1, optical velocity—0.4747 cm−1, aperture—100, minimal scan number—32, data spacing—0.482. The microscope was supplied with the camera which provided the images of the sample’s structure. The spectral analysis of obtained spectra was controlled by the OMNIC version 9.8.372 software package equipped with tools allowing the identification of the size, shape, and colour of found microplastics. For samples with irregular shapes, the longest dimension was determined. The microplastics were identified by the presence of characteristic vibrations of characteristic chemical bonds as well as visual comparison with the reference spectra in accordance with the literature [25,26,27]. The obtained spectra were additionally compared with the spectra of the commercially available infrared library database implemented in OMNIC: HR Nicolet Sampler Library.
2.4. Quality Assurance
To minimize the risk of plastic contamination from researchers’ clothes, clean cotton lab coats were used for all laboratory work.
Sample processing was performed in clean facilities. Samples were covered with aluminium foil to prevent any contamination of samples from atmospheric microplastic particles. Equipment, such as scalpels, tweezers, and dissection boards, were inspected for plastics under a stereomicroscope prior to use and were rinsed with distilled water until all contamination was removed. There is no standardized methodology for blanks in the scientific literature, so our approach is described. Clean glass Petri dishes in the work area during sample processing and analysis were used as laboratory blanks, which were analysed to account for any contamination of plastics, and no microplastics were detected.
2.5. Statistical Analyses
The statistical analyses were performed using STATISTICA 12.0 Software (Statsoft, Krakow, Poland). The difference between crayfish that were contaminated and uncontaminated by plastic debris in relation to crayfish sex, size, and mass was analysed using regression analysis at p = 0.05.
Moreover, the relationships between the ingested plastic length, type, and the total length of the crayfish were calculated according to the following equation:
where:
- LP—plastic length in μm,
- LT—crayfish total length in mm,
- a—intercept, b—slope.
The length–mass relationships are used to establish the physiological condition of the individuals. This condition of the signal crayfish individuals was calculated based on the length–mass relationship according to the equation below:
where:
- MW—wet mass in mg,
- LT—crayfish total length in mm,
- a—intercept, b—slope.
The coefficients of variation R2 for both above-mentioned relationships were determined at p = 0.05. Microsoft Excel 10.0 was used to calculate the relationships.
Differences between slopes (b) and intercepts (a) of length–mass regressions of specimens contaminated and uncontaminated by plastics were tested using analysis of covariance (ANCOVA) at p = 0.05. Before the analysis, both y and x data were log transformed to linearize the relationship (ln y = b × ln x + ln a) to improve the normality and homoscedasticity of the data.
3. Results
3.1. Crayfish Characteristics
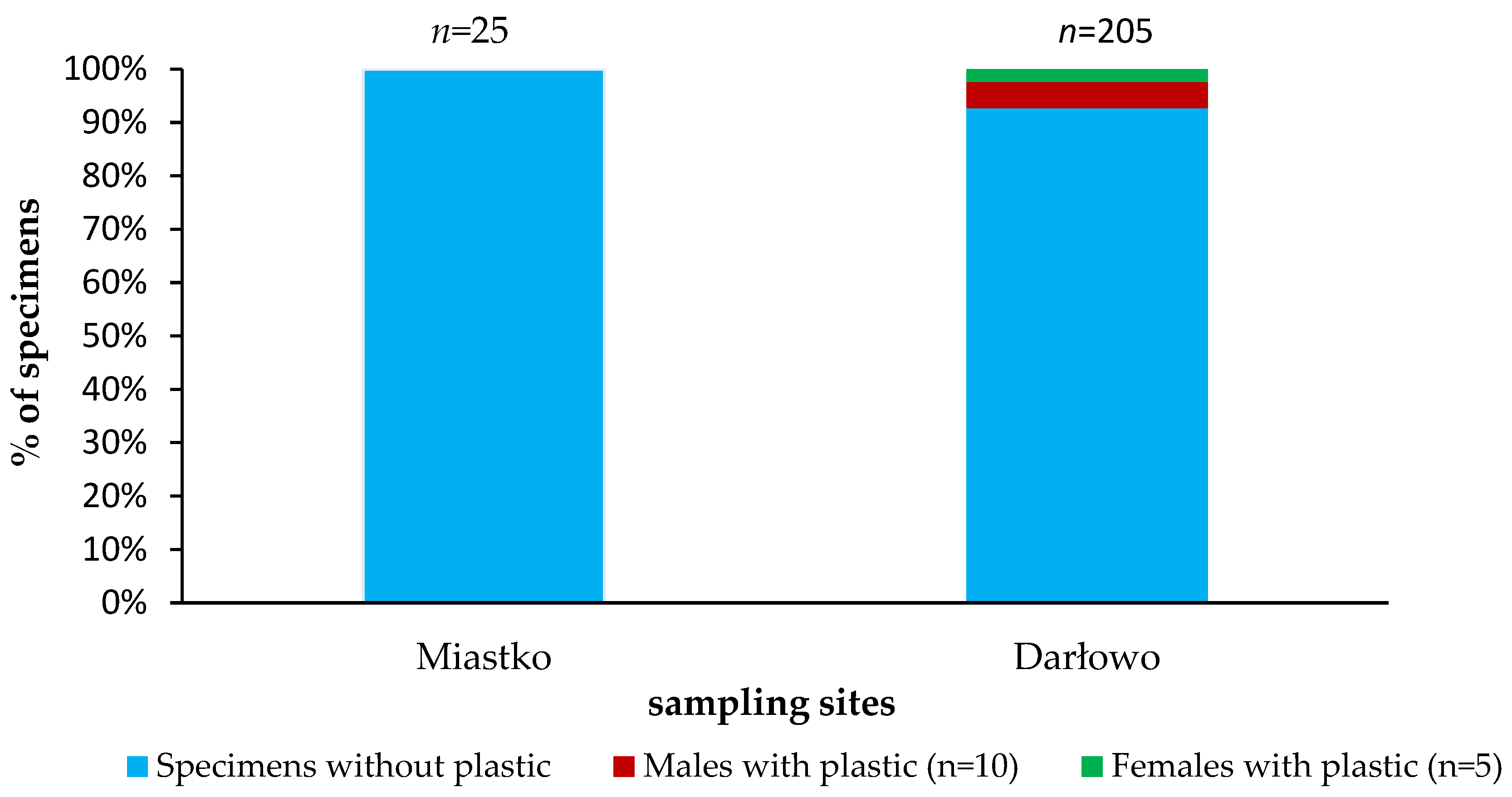
In total, 230 specimens of the signal crayfish in the size range from 45.7 to 133.23 mm and wet mass range from 2.87 to 69.19 g were analysed. No plastic debris was found in stomachs of crayfish caught in Miastko. In the lower river section—Darłowo—plastics were detected in the stomachs of crayfish of both sexes (Figure 3, Table S1 in Supplementary Materials).
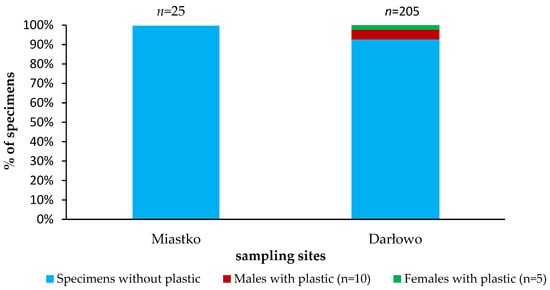
Figure 3.
Plastic debris in the stomachs of the signal crayfish Pacifastacus leniusculus collected in the Wieprza River system.
Males had higher plastic content in their stomach than females (67% of contaminated crayfish were identified as males). Crayfish with plastic debris from Darłowo were characterized by a total length (mean ± SD) of 91.42 ± 12.54 mm and a wet mass of 23.49 ± 9.43 g in comparison to all others collected in Darłowo (99 ± 12.51 mm and 31.29 ± 12.97 g, respectively). From the regression analysis, it was found that the presence of plastics in crayfish varies significantly with the length, mass, and sex of crayfish with correlation coefficients of R2 = 0.15, 0.02, and 0.02. The corresponding obtained F-value is 4.95 with a significance level of p = 0.05.
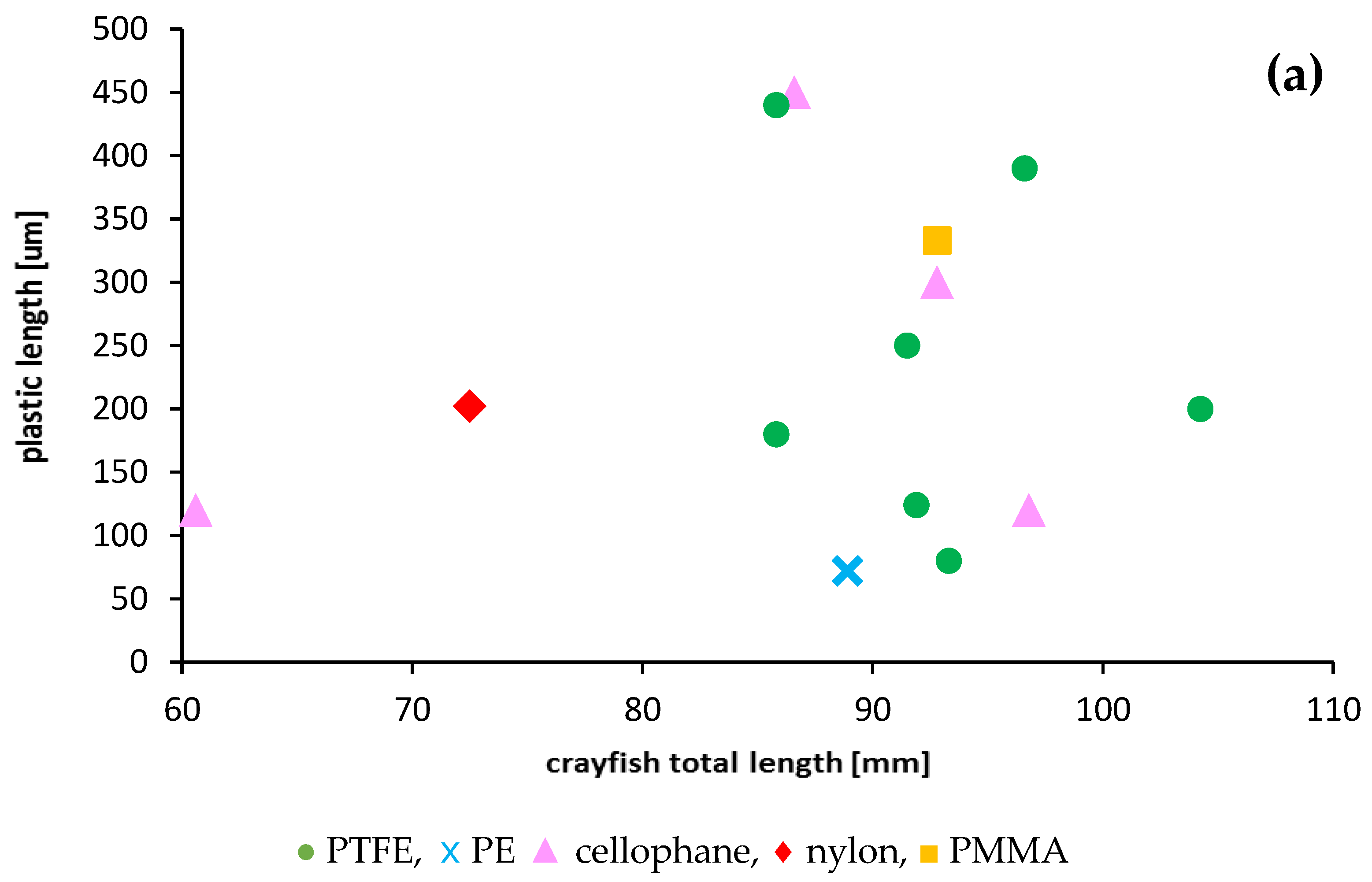
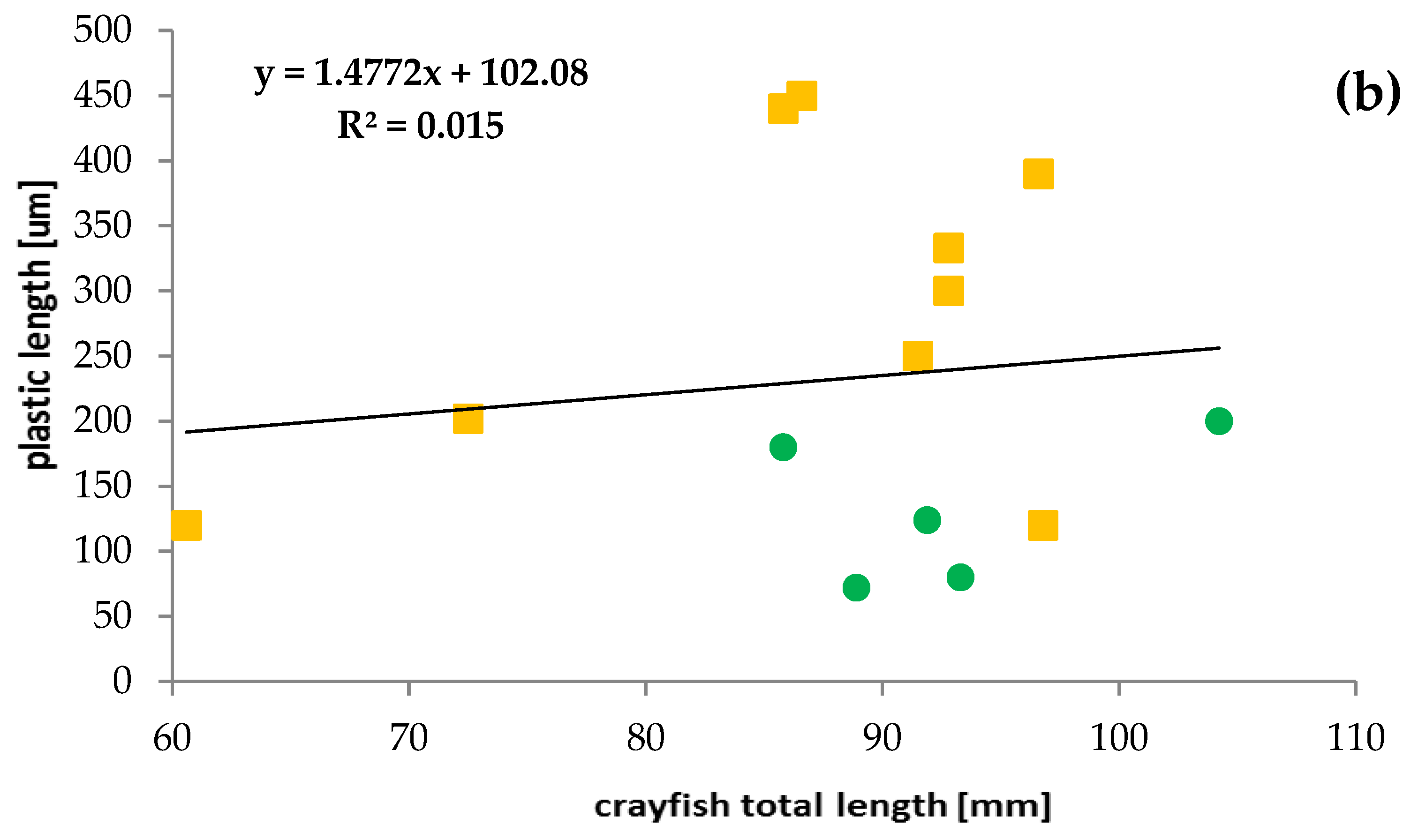
Most crayfish (12) had a single plastic particle in their stomachs. Two particles in stomachs were found only in three specimens. The presence of plastics was mainly limited to crayfish in the length range from 80 to 100 mm (67% of specimens), which contained mainly PTFE and a higher number of plastic particles—up to two per individual (Figure 4). The relationship between crayfish size and plastic particle size consumed was poor and not significant (R2 = 0.015) (Figure 4). A stronger relationship was found between microplastic fibres and crayfish sizes (R2 = 0.1628) but was also not significant.
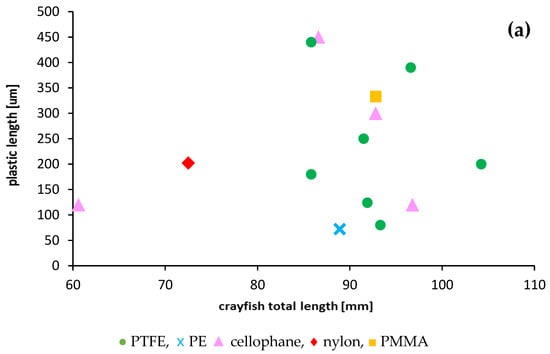
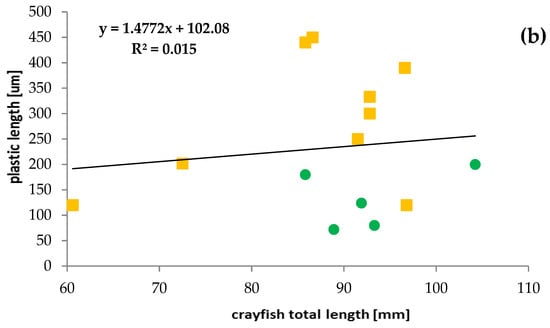
Figure 4.
Relationships between the size of ingested microplastics confirmed by FTIR and the total length of the signal crayfish Pacifastacus leniusculus collected in the Wieprza River in Darłowo: (a) information on the type of plastics, (b) information on the plastic length.
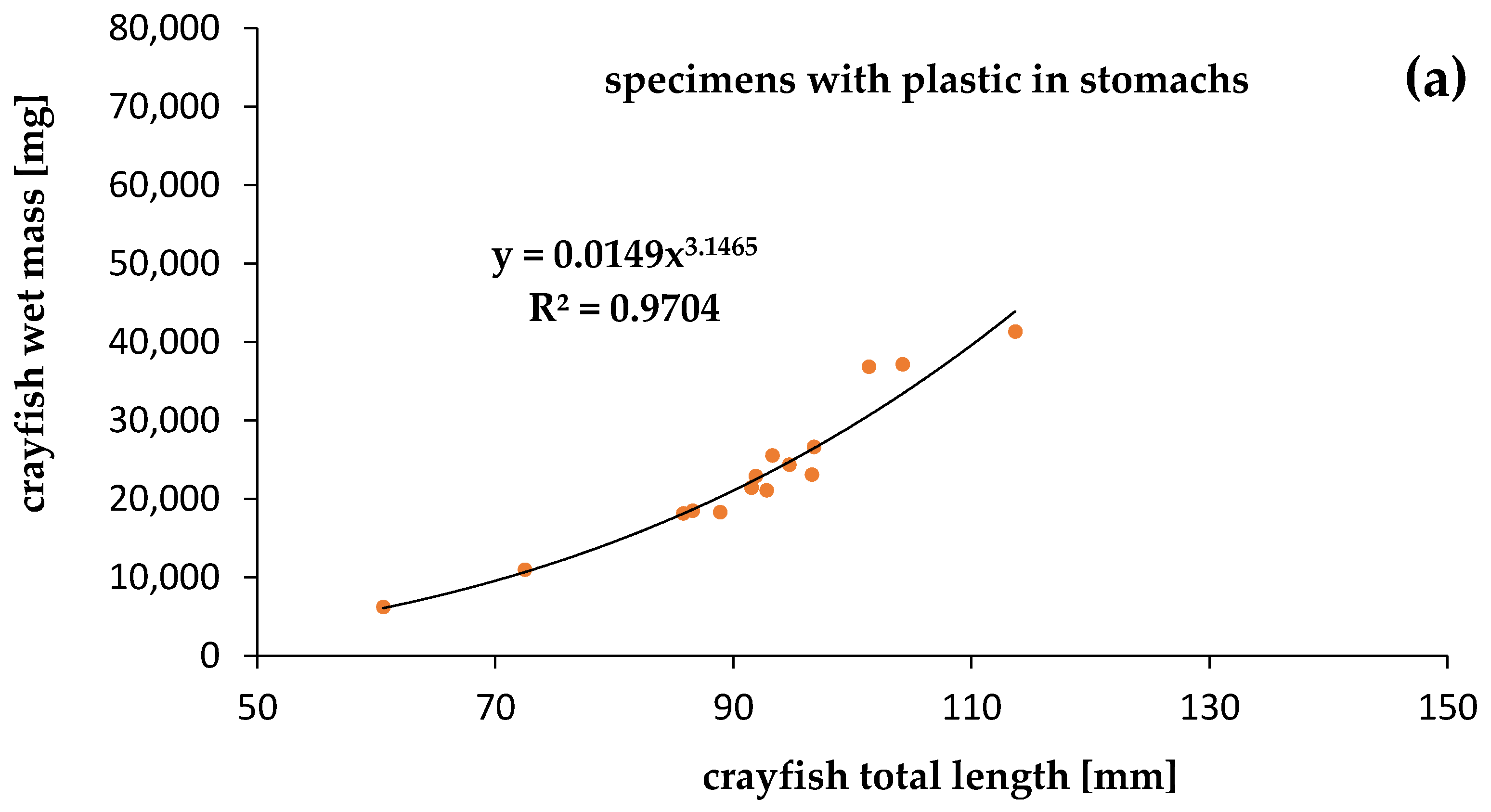
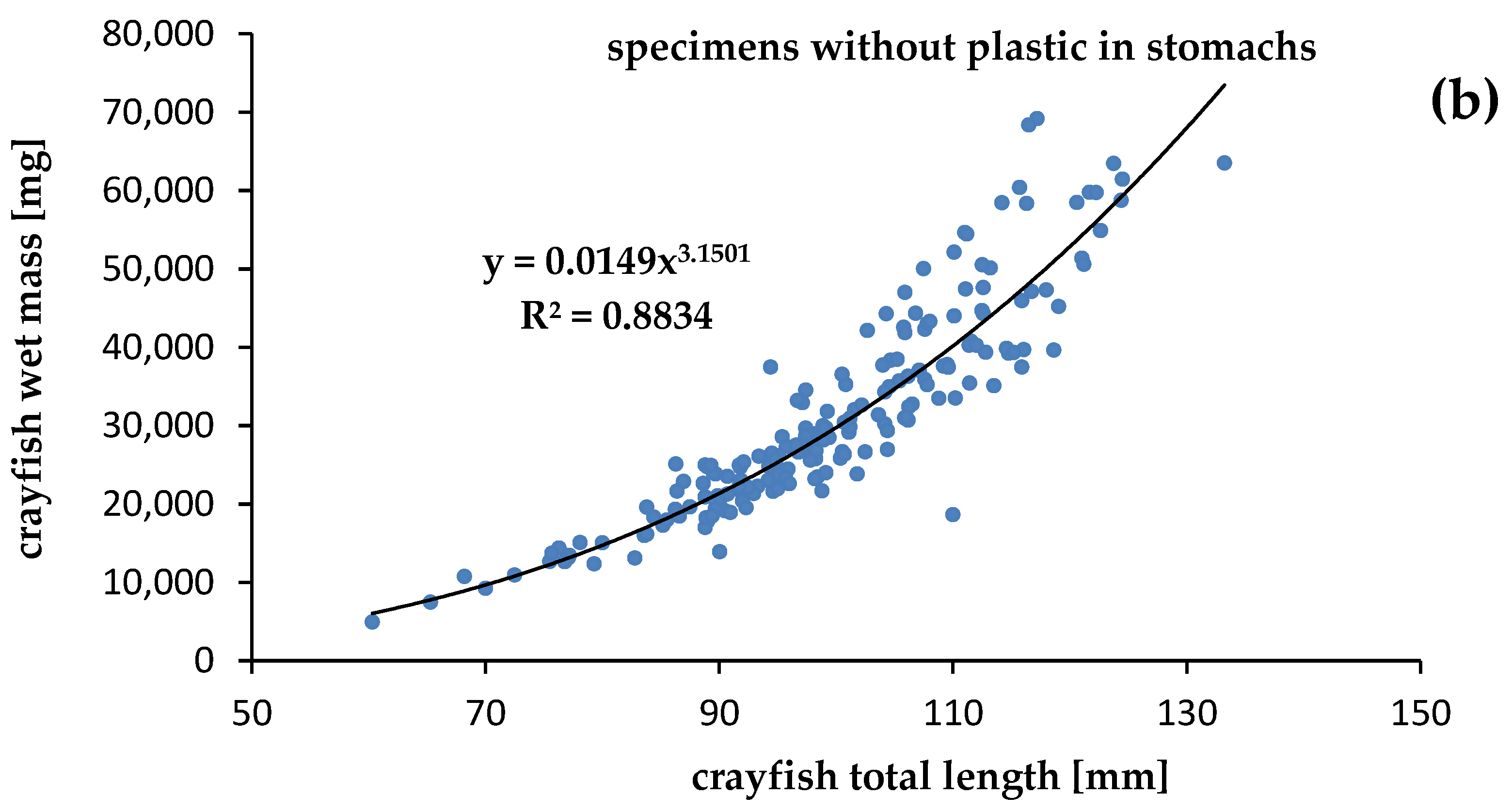
The mass of the specimens was significantly correlated with the length of both crayfish contaminated by plastics (R2 = 0.9704) and uncontaminated (R2 = 0.8834), at p = 0.05. Although we expected a lower condition of specimens contaminated by plastics, the relationships between the mass and length of specimens that were contaminated and uncontaminated by plastics showed that the calculated slope “b” was high and similar. The physiological condition of the crayfish collected in Darłowo was almost the same for specimens with (b = 3.1465) (Figure 5) and without (b = 3.1501) plastic debris in the stomachs (Figure 5). Statistical differences between slopes (b) and intercepts (a) of specimens contaminated and uncontaminated by plastics were not significant (ANCOVA, p > 0.05).
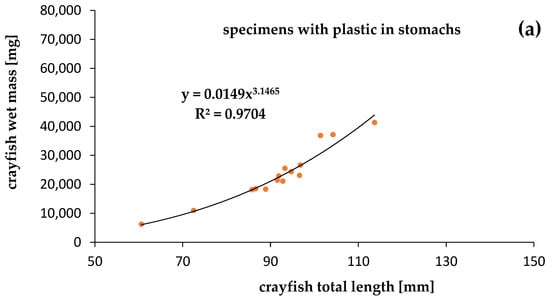
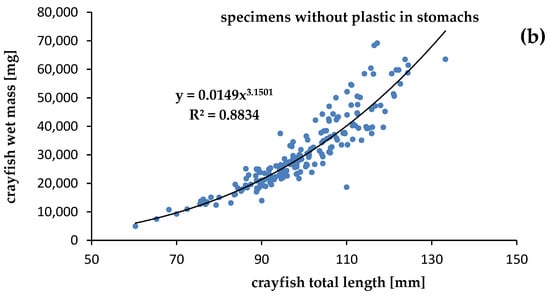
Figure 5.
Relationships between total length and wet mass of the signal crayfish Pacifastacus leniusculus (a) with plastics and (b) without plastics in stomachs collected in the Wieprza River in Darłowo.
3.2. Plastic Debris Characteristics
All plastic debris found in the crayfish stomachs (Figure S1 in Supplementary Materials) were different types, sizes, shapes, and colours (Table 1). Detailed polymer analysis indicated that plastics consisted of round particles with mainly irregular fragments (opaque), fibres (transparent, blue, and red) or thin pieces (blue or colourless). The dimensions of microplastics ranged from 70 to 450 µm. The presence of PTFE (polytetrafluoroethylene), cellophane, PP (polypropylene), PE (polyethylene), PMMA (polymethyl methacrylate), and nylon was identified by FTIR spectroscopy.

Table 1.
The sizes, shapes, and colours of polymers found in the stomachs of the signal crayfish Pacifastacus leniusculus collected in Darłowo.
Polytetrafluoroethylene (PTFE) polymer was isolated as black rugged clods from six crayfish. It was the most frequent polymer in the analysed stomachs. The FTIR spectrum shows the presence of two very intense bands of stretching vibrations of the CF2 group at about 1220 and 1157 cm−1.
Cellophane was isolated from five specimens. It was characterized with the following bands: a broad peak at 3324 cm−1 representing OH stretching vibrations, 2913 and 2850 cm−1 representing CH stretching, a peak at about 1363 cm−1 attributed to OH bending, 1310 cm−1 representing CH2 wagging, and a band at about 1153 cm−1 characteristic of C–O antisymmetric bridge stretching and C–O–C pyranose ring skeletal vibrations. Moreover, in the spectrum of cellophane, a strong band at about 1013 cm−1 is present, which can be assigned as C–O–C stretching, and a small band at 889 cm−1 that corresponds to the glycosidic C–H deformation with ring vibration contribution and O–H bending.
Polypropylene (PP) was isolated from three crayfish as fragments of blue foil or thin red filaments and the most characteristic vibrations for PP are 2952, 2915, and 2837 cm−1, typical of symmetric and asymmetric C–H stretching, as well as 1455 and 1377 cm−1, which represent CH2 and CH3 bending vibrations, respectively.
Polyethylene (PE) was found in two crayfish and had the forms of colourless, blue, and red film pieces. The characteristic absorbance bands for PE are located at 2901 and 2843 cm−1, typical of asymmetric and symmetric vibrations, as well as 1460 and 713 cm−1, typical of bending and rocking CH2 deformations.
Polymethyl methacrylate (PMMA) was found in a single crayfish and identified with vibrations in the 2960–2840 cm−1 range, typical of a stretching region of CH, CH2, and CH3. Pure PMMA can be described by the 1736 cm−1 band assigned to free C=O stretching, although in the spectrum of the analysed sample, this band is shifted to a lower wavenumber (1707 cm−1). C–O stretching frequency is one of the intense bands that appear in the fingerprint region, usually within 1000–1400 cm−1. The bands present in the 1260–1000 region can be assigned to C–O stretching modes, whereas the vibrations of C=O in the plane and out of plane bending are assigned to a medium-strong band in the IR spectrum at about 720 cm−1.
Nylon as a blue small filament was identified in a single crayfish with the presence of stretching vibrations of NH groups at 3290 cm−1 and bending NH vibrations at 1535 cm−1, as well as the stretching carbonyl C=O group at 1638 cm−1 and the CH vibrations at 2914 and 2847 cm−1.
4. Discussion
Recently, the problem of the land-based transport of plastics to river systems has been described [28]. Microplastics in macroinvertebrates can indicate plastic pollution within a catchment [29], and our study has, for the first time, shown the occurrence of plastic debris in the stomachs of signal crayfish, although previous observations reported the plastics in different aquatic organisms, e.g., described in refs. [11,30] and summarized by de Sá et al. in [31].
In our study, plastic debris was confirmed in 7.3% of analysed specimens in the lower, urbanized part of the Wieprza River, which has been under severe anthropogenic pressure for a long period. Such an impact is not only observed in Darłowo but also in many areas under anthropogenic pressure worldwide [32]. Our study found a lack of plastic debris in the upper, semi-natural section of the Wieprza River tributary which flows from the Lake Studzieniczno to Miastko (Figure 2). However, 2 out of 25 specimens collected there should have contained plastics, so the difference in sampling size of 25 vs. 205 is not crucial for our conclusion. The smaller number of crayfish collected upstream in comparison to the downstream river section was related to lower numbers of signal crayfish in this part of the Wieprza River system. This was confirmed in a previous study [24]. However, microplastic accumulation in the sediment is modified by fluvial processes, which result in lower levels in the upper river course, as was observed in the Inde River [33].
Plastic debris seems to be rather rare in crayfish stomachs and was identified in only 7.3% among specimens caught in Darłowo. Similarly low plastic content (9%) was observed in Chinese mitten crab Eriocheir sinensis H. Milne Edwards, 1853 in the Vistula Lagoon (Poland) [34]. These results differ from another study where plastics were found in approximately 50% of analysed macroinvertebrate samples from UK rivers [30], which might be explained by larger historic plastic production in western European countries compared to Poland (central states) [35,36]. The occurrence of plastics in crayfish stomachs, based on our results, does not appear to be a good indicator for measuring the contamination of the river environment. Present results indicating relatively low plastic debris content in crayfish stomachs may be explained by animal behaviour and, to some extent, by the local environmental conditions. Specimens of the signal crayfish are characterized by their activity in the river manifested through burrowing behaviour and movement [37,38,39]. They may spend a long time in burrows or may migrate in both upstream and downstream directions, crossing sediments contaminated by plastics. In the case of crayfish that reached the trapping site in Darłowo, most move downstream. Therefore, they might spend more time in the stretches of the river with a relatively lower level of contamination by plastic compared with the urbanized river mouth. The ingestion of plastics by the signal crayfish (determined as the occurrence of plastics in stomachs) differs from the sessile species such as mussels, which experience permanent exposure to plastic pollution and accumulate significant quantities of microplastics, depending on the pollution loading where they live [40].
Visual classification with the use of a microscope allowed us to identify “plastic debris” in 52 individuals (22.6% of all analysed crayfish). But, to avoid misidentification and the underestimation of microplastics it was necessary to use the more accurate method, such as Fourier transform infrared spectroscopy (FTIR). FTIR analysis confirmed plastics in 15 individuals (28.8% of visually identified presence of plastics). This step was critical since often about 70% of particles that visually resemble microplastics are not confirmed as plastics by FTIR spectroscopy [41]. Additionally, based on FTIR analysis, the determination of the type of polymer was possible by the identification of six different types of polymers in the stomachs of P. leniusculus from the Wieprza River system, namely PTFE (polytetrafluoroethylene), cellophane, PP (polypropylene), PE (polyethylene), PMMA (polymethyl methacrylate), and nylon. According to Duis and Coors [42], the most frequent polymers in aquatic environments are PE, PP, and PS. However, in our study, PTFE was the most common. This is a high-performance fluoropolymer, commonly used in the kitchen (pans, baking trays, etc.), and has wide applications in chemical, electronic, construction and car industries. As a highly inert and nontoxic polymer, it also finds use in medical applications for cardiovascular grafts, heart patches, etc. [43]. It is also the most chemically resistant polymer with very high stability, which is not subject to degradation in the environment [44].
One of the most important factors affecting microplastics distribution in aquatic environments is the density of the materials. The relatively high frequency of PTFE in the crayfish stomachs may be explained to some extent by different accumulation among various types of plastics in the riverine sediment [45]. Due to the hydrodynamic properties of the plastic particles, such as density, particle size, shape, surface roughness, and the hydrodynamic transport conditions in the river, PTFE is the most frequent type of plastic deposited between 0 and 30 cm depth of sediment, that is, the habitat zone of the signal crayfish [46]. Other polymers, such as PE and PP, transfer to deeper layers of fluvial sediments [45].
Fibres were the predominant type of microplastics in crayfish stomachs, and also the most common form of plastics described in previous studies [47,48,49]. The size of plastic debris ingested depends on the animal size [50]. However, during the digestion process, the fragmentation of plastics occurs in crustaceans such as Norway lobster Nephrops norvegicus (Linnaeus, 1758) [51] and results in the occurrence of tinier plastic fragments in the environment. Such particles might be available for smaller organisms at lower trophic levels. The size of plastic particles is a crucial factor in determining their uptake, digestion, and toxicity. Smaller particles (<10 µm) may have a higher negative impact with increased potential to interact at the molecular level than larger ones (summarized in [52]).
The ingestion of plastics might be harmful by blocking the crustaceans’ stomachs, causing a reduction in their feeding [53]. The blockage and injury of the digestive tract and reduction in feeding caused by ingested plastic and the absorption of polychlorinated biphenyls in fish has been recorded over the past decades and considered by ref. [54] after refs. [55,56]. The chemical decomposition of some polymers may also lead to the formation of toxic compounds, e.g., the oxidative degradation of PTFE in atmospheric conditions results in the evolution of gaseous carbonyl fluoride COF2, which is highly toxic and hydrolyses further to hydrofluoric acid, HF, and carbon dioxide, CO2 [57]. The condition of collected crayfish in this study with plastic debris in stomachs was as good as specimens without plastics. This is not surprising, because microplastics cause no effects on the survival and growth of many invertebrates, but they still may affect the functioning of only sensitive aquatic species [58]. Moreover, recent studies [59] showed that the negative impacts of microplastics may not be readily visible at the ecosystem scale.
The size (70–450 µm) and abundance of plastic particles (1–2 per individual) were very low considering the signal crayfish stomach size (including cardiac and pyloric parts of the stomach) is about 20% of total body length [60]. Moreover, the presence of plastics, limited mainly to crayfish specimens in the length range between 80 and 100 mm, with predomination in males, may be connected with food preferences and mechanisms related to the crayfish size, sex (considering diet specialization), and moulting. Such crayfish feed mainly on small food [61], so microplastic particles are more frequently swallowed by this group of specimens. The lower percentage of females with plastics in their stomachs than males may result from sexual dimorphism and different behaviour. Females are characterized by smaller carapace size and claws, different mandible shape, and lower feeding [61,62], which may result in a lower probability of plastic consumption. The smallest crayfish shed their carapaces more frequently than larger specimens. During the process of moulting, they may potentially eliminate plastic debris. Crustaceans lose their integument (the foregut and hindgut) during ecdysis, which is moulted along with the exoskeleton [53]. This process explains the lack of plastics in the smallest crayfish. Low plastic content in crayfish stomachs is also connected with higher water temperature during summer months when the process of moulting is frequent [63]. Moreover, the largest crayfish do not have plastics in their stomachs, because of different feeding preferences—they prefer larger food size [61]. Small particles (even detritus) may be also consumed by large crayfish [46]; similarly, plastic might be accidentally eaten by crayfish during normal feeding. It is possible that crayfish mistake microplastics for prey.
The only eaten parts of the crayfish are the abdomen and chelae that consist of muscle tissue, and the remains (cephalothorax) with microplastics in the stomachs and intestines are thrown away. The plastic debris may potentially transfer toxic substances [64], but without data on their concentration in the muscle tissue of the crayfish, their impact on human health remains under speculation. However, potential contamination of plastic particles in muscle tissue by monomers which occur in the circulatory system of these animals should be studied in the future.
5. Conclusions
Plastic debris was found in the stomach of the invasive signal crayfish from the Wieprza River, a Baltic tributary. Plastic contamination depended on anthropogenic impact on the river and distance from the river mouth. Plastic debris had a size range between 70 and 450 µm. Six types of polymers, namely PTFE (polytetrafluoroethylene), cellophane, PP (polypropylene), PE (polyethylene), PMMA (polymethyl methacrylate), and nylon, were identified using the Fourier transform infrared spectroscopy (FTIR) technique. PTFE was the most common polymer, but according to the literature, it is rarely detected in the environment and organisms. Transparent, blue, and red fibres dominated over other types of plastic debris. Microplastics did not affect crayfish growth rate in our study. The lower percentage of females with plastics may result from sexual dimorphism and different behaviour. Future study should concentrate on signal crayfish contamination by other chemicals, e.g., persistent organic pollutants (POPs), and the potential risk to humans [65].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w16060903/s1, Figure S1: Images and micro-Fourier transform infrared spectroscopy peaks of different microplastic polymers found in stomachs of the Pacifastacus leniusculus caught in the Wieprza River in Darłowo between 2014 and 2018. The blue frames on the pictures of analysed samples show the scanned region. Table S1: The sex, mean total length, and mean wet mass of the signal crayfish Pacifastacus leniusculus collected from Miastko and Darłowo.
Author Contributions
Conceptualization, A.D.-K., M.E.S. and A.P.; methodology, A.D.-K., M.E.S. and A.P.; software, A.D.-K.; validation, A.D.-K.; formal analysis, A.D.-K., M.E.S. and A.P.; investigation, A.D.-K., M.E.S. and A.P.; resources, A.D.-K., M.E.S. and A.P.; writing—original draft preparation, A.D.-K., M.E.S. and A.P.; writing—review and editing, A.D.-K., M.E.S. and A.P.; visualization, A.D.-K., M.E.S. and A.P.; supervision, A.D.-K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available within the article.
Acknowledgments
We thank Patrick Armitage for improving the English of the final version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in marine environment: A review. Mar. Pollut. Bull. 2011, 61, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Burns, E.E.; Boxall, B.A. Microplastics in the Aquatic Environment: Evidence for or Against Adverse Impacts and Major Knowledge Gaps. Environ. Toxicol. Chem. 2018, 37, 2776–2896. [Google Scholar] [CrossRef] [PubMed]
- Besseling, E.; Redondo-Hasselerharm, P.; Foekema, E.M.; Koelmans, A.A. Quantifying ecological risks of aquatic micro- and nanoplastic. Crit. Rev. Environ. Sci. Technol. 2019, 49, 32–80. [Google Scholar] [CrossRef]
- Plastics Europe. Plastics Europe Launches Plastics—The Fast Facts 2023. Available online: https://plasticseurope.org/media/plastics-europe-launches-the-plastics-the-fast-facts-2023/ (accessed on 8 February 2024).
- UNEP. Plastic in Cosmetics. Are We Polluting the Environment through Our Personal Care? 2015, p. 38, ISBN 978-92-807-3466-9. Available online: https://www.unep.org/resources/report/plastic-cosmetics-are-we-polluting-environment-through-our-personal-care (accessed on 30 January 2024).
- Wagner, M.; Scherer, C.; Alvarez-Muñoz, D.; Brennhol, N.; Bourrain, X.; Buchinger, S.; Fries, E.; Grosbois, C.; Klasmeier, J.; Marti, T.; et al. Microplastic in freshwater ecosystems: What we know and what we need to know. Environ. Sci. Eur. 2014, 26, 12. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, A.A. Modeling the role of microplastics in bioaccumulation of organic chemicals to marine aquatic organisms: A critical review. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer International Publishing: London, UK; Cham, Switzerland, 2015; pp. 309–324. [Google Scholar] [CrossRef]
- Wright, S.; Thompson, R.; Galloway, T. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef]
- Dekiff, J.H.; Klasmeier, J.; Fries, E. Occurrence and spatial distribution of microplastics in sediments from Norderney. Environ. Pollut. 2014, 186, 248–256. [Google Scholar] [CrossRef]
- Dris, R.; Imhof, H.; Sanchez, W.; Gasperi, J.; Galgani, F.; Tassin, B.; Laforsch, C. Beyond the ocean: Contamination of freshwater ecosystems with (micro-) plastic particles. Environ. Chem. 2015, 12, 539–550. [Google Scholar] [CrossRef]
- Eerkes-Medrano, D.; Thompson, R.C.; Aldridge, D.C. Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritization of research needs. Water Res. 2015, 75, 63–82. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–81605. [Google Scholar] [CrossRef]
- Gasperi, J.; Dris, R.; Bonin, T.; Rocher, V.; Tassin, B. Assessment of floating plastic debris in surface water along the Seine River. Environ. Pollut. 2014, 195C, 163–166. [Google Scholar] [CrossRef]
- Iannilli, V.; Pasquali, V.; Setini, A.; Corami, F. First evidence of microplastics ingestion in benthic amphipods from Svalbard. Environ. Res. 2019, 179, 198811. [Google Scholar] [CrossRef]
- Watts, A.J.R.; Urbina, M.A.; Goodhead, R.; Moger, J.; Lewis, C.; Galloway, T.S. Effect of microplastic on the gills of the shore Crab Carcinus maenas. Environ. Sci. Technol. 2016, 50, 5364–5369. [Google Scholar] [CrossRef]
- Gray, A.D.; Weinstein, J.E. Size- and shape-dependent effects of microplastic particles on adult daggerblade grass shrimp (Palaemonetes pugio). Environ. Toxicol. Chem. 2017, 36, 3074–3080. [Google Scholar] [CrossRef]
- Gambardella, C.; Morgana, S.; Ferrando, S.; Bramini, M.; Piazza, V.; Costa, E.; Garaventa, F.; Faimali, M. Effects of polystyrene microbeads in marine planktonic crustaceans. Ecotoxicol. Environ. Saf. 2017, 145, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberghe, L.V.; Claessens, M.; Vandegehuchte, M.N. Microplastics are taken up by mussels (Mytilus edulis) and lugworms (Arenicola marina) living in natural habitats. Environ. Pollut. 2015, 199, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Holdich, D.M.; Reynolds, J.D.; Souty-Grosset, C.; Sibley, P.J. A review of the ever increasing threat to European crayfish from non-indigenous crayfish species. Knowledge Manag. Aquat. Ecosyst. 2009, 394–395, 11. [Google Scholar] [CrossRef]
- Holdich, D.M. A review of astaculture—Freshwater crayfish farming. Aquat Liv. Resour. 1993, 6, 307–317. [Google Scholar] [CrossRef]
- Ackefors, H.E.G. Freshwater crayfish farming technology in the 1990s: A European and global perspective. Fish Fish. 2000, 1, 337–359. [Google Scholar] [CrossRef]
- Śmietana, P. Pacifastacus leniusculus (Dana, 1852). In Alien Species in the Fauna of Poland; Głowaciński, Z., Okarma, H., Pawłowski, J., Solarz, W., Eds.; Institute of Nature Conservation, Polish Academy of Sciences: Cracow, Poland, 2011; pp. 201–205. [Google Scholar]
- Mastyński, J.; Andrzejewski, W. Chów i Hodowla Raków (Crayfish Breeding and Culture); Wyd. AR: Poznan, Poland, 2005; p. 168. (In Polish) [Google Scholar]
- Dobrzycka-Krahel, A.; Skóra, M.E.; Raczyński, M.; Szaniawska, A. The signal crayfish Pacifastacus leniusculus—Distribution and invasions in the southern Baltic coastal river. Pol. J. Ecol. 2017, 65, 445–452. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 5th ed.; Wiley: Hoboken, NY, USA, 1997. [Google Scholar]
- Jung, M.R.; Horgen, F.D.; Orski, S.V.; Rodriguez, C.V.; Beers, K.L.; Balazs, G.H.; Jones, T.T.; Work, T.M.; Brignac, K.C.; Royer, S.J.; et al. Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Mar. Pollut. Bull. 2017, 127, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, Z.; Ogonowski, M.; Nybom, C.E.K.; Adolfsson-Erici, M.; Barth, A.E.; Gorokhova, E. Microplastic-mediated transport of PCBs? A depuration study with Daphnia magna. PLoS ONE 2019, 14, e0205378. [Google Scholar] [CrossRef]
- Campanale, C.; Stock, F.; Massarelli, C.; Kochleus, C.; Bagnuolo, G.; Reifferscheid, G.; Uricchio, V.F. Microplastics and their possible sources: The example of Ofanto river in southeast Italy. Environ. Pollut. 2020, 258, 113284. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.I. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Windsor, F.; Tilley, R.M.; Tyler, C.M.; Ormerod, S.J. Microplastic ingestion by riverine macroinvertebrates. Sci. Total Environ. 2019, 646, 68–74. [Google Scholar] [CrossRef] [PubMed]
- De Sá, L.C.; Oliveira, M.; Ribeiro, F.; Rocha, T.L.; Futter, M.N. Studies of the effects of microplastics on aquatic organisms: What do we know and where should we focus our efforts in the future? Sci. Total Environ. 2018, 645, 1029–1039. [Google Scholar] [CrossRef]
- Burak, S.; Dog, E.; Gaziog’lu, C. Impact of urbanization and tourism on coastal environment. Ocean Coast. Manag. 2004, 9–10, 515–527. [Google Scholar] [CrossRef]
- Lechthaler, S.; Esser, V.; Schüttrumpf, H.; Stauch, G. Why analysing microplastics in floodplains matters: Application in a sedimentary context. Environ. Sci. Process Impacts 2021, 23, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Wójcik-Fudalewska, D.; Normant-Saremba, M.; Anastacio, P. Occurrence of plastic debris in the stomach of the invasive crab. Mar. Pollut. Bull. 2016, 113, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Plastics the Compelling Facts About Plastics. An Analysis of Plastics Production, Demand and Recovery for 2006 in Europe. 2008, pp. 1–24. Available online: https://www.plasticseurope.org/application/files/2815/1689/9283/2006compelling_fact_PubJan2008.pdf (accessed on 30 January 2024).
- Chalmin, P. The History of Plastics: From the Capitol to the Tarpeian Rock. Field Actions Science Reports Special Issue 19. Publisher Institut Veolia 2019. pp. 6–11, ISSN 1867-8521. Available online: http://journals.openedition.org/factsreports/5071 (accessed on 30 January 2024).
- Albertson, I.K.; Daniels, M.D. Effects of invasive crayfish on fine sediment accumulation, gravel movement, and macroinvertebrate communities. Freshw. Sci. 2016, 35, 644–653. [Google Scholar] [CrossRef]
- Hudina, A.; Lucič, A.; Žganec, K.; Jankowič, S. Characteristics and movement patterns of a recently established invasive Pacifastacus leniusculus population in the river Mura, Croatia. Knowl. Manag. Aquat. Ecosyst. 2011, 403, 07. [Google Scholar] [CrossRef][Green Version]
- Turley, M.D.; Bilotta, G.S.; Gaspariini, A.; Sera, F.; Mathers, K.L.; Humpheryes, I.; England, J. The effects of non-native signal crayfish (Pacifastacus leniusculus) on fine sediment and sediment-biomonitoring. Sci. Total Environ. 2017, 601–602, 186–193. [Google Scholar] [CrossRef]
- Thushari, G.G.N.; Senevirathna, J.D.M.; Yakupitiyage, A.; Chavanich, S. Effects of microplastics on sessile invertebrates in the eastern coast of Thailand: A approach to coastal conservation. Mar. Pollut. Bull. 2017, 124, 349–355. [Google Scholar] [CrossRef]
- Hidago-Ruz, V.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- Duis, K.; Coors, A. Microplastics in the aquatic and terrestrial environment: Sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 2016, 28, 2. [Google Scholar] [CrossRef]
- Dhanumalayan, E.; Joshi, G.M. Performance properties and applications of polytetrafluoroethylene (PTFE) a review. Adv. Compos. Hybrid Mater. 2018, 1, 247–268. [Google Scholar] [CrossRef]
- Henry, B.J.; Carlin, J.P.; Hammerschmidt, J.A.; Buck, R.C.; Buxton, W.L.; Fiedler, H.; Seed, J.; Hernandez, O. Critical reviews of the application of polymer of low concern and regulatory criteria to fluoropolymers. Integr. Environ. Assess. Manag. 2017, 14, 316–334. [Google Scholar] [CrossRef] [PubMed]
- Frei, S.; Piehl, S.; Gilfedder, B.S.; Löder, M.G.J.; Krutzke, J.; Wilhelm, L.; Laforsch, C. Occurrence of microplastics in the hyporheic zone of rivers. Sci. Rep. 2019, 9, 15256. [Google Scholar] [CrossRef]
- Guan, R.; Wiles, P.R. Feeding ecology of the signal crayfish Pacifastacus leniusculus in a British lowland river. Aquaculture 1998, 169, 177–193. [Google Scholar] [CrossRef]
- Murray, F.; Cowie, P.R. Plastic contamination in the decapod crustacean Nephrops norvegicus (Linnaeus, 1758). Mar. Pollut. Bull. 2011, 62, 1207–1217. [Google Scholar] [CrossRef]
- Tanaka, K.; Takada, H. Microplastic fragments and microbeds in digestive tracts of planktivorous fish from urban coastal waters. Sci. Rep. 2016, 6, 34351. [Google Scholar] [CrossRef] [PubMed]
- Mülayim, A.; Bat, L.; Öztekin, A.; Gunduz, S.K.; Yucedag, E.; Bicak, B. Microplastic Accumulation in Crayfish Astacus leptodactylus (Eschscholtz 1823) and Sediments of Durusu (Terkos) Lake (Turkey). Water Air Soil Pollut. 2022, 233, 449. [Google Scholar] [CrossRef]
- Jȃms, I.B.; Windsor, F.M.; Poudevigne-Durance, T.; Ormerod, S.J.; Durance, I. Estimating the size distribution of plastic ingested by animals. Nat. Commun. 2020, 11, 1594. [Google Scholar] [CrossRef] [PubMed]
- Cau, C.; Avio, C.G.; Dessi, C.; Moccia, D.; Regoli, F.; Cannos, R.; Cristina, M. Benthic crustacean digestion can mudalate the environmental fate of microplastics in the deep sea. Environ. Sci. Technol. 2020, 54, 4886–4892. [Google Scholar] [CrossRef] [PubMed]
- Kögel, T.; Bjorøya, O.; Toto, B.; Bienfait, A.M.; Sanden, M. Micro-and nanoplastic toxicity on aquatic life: Determining factors. Sci. Total Environ. 2020, 709, 136050. [Google Scholar] [CrossRef]
- Welden, N.; Cowie, P. Environment and gut morphology influence microplastic retention in langoustine, Nephrops norvegicus. Environ. Pollut. 2016, 214, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Skóra, M.E.; Sapota, M.R.; Skóra, K.E.; Pawelec, A. Diet of the twaite shad Alosa fallax (Lacépède, 1803) (Clupeidae) in the Gulf of Gdansk, the Baltic Sea. Oceanol. Hydrobiol. Stud. 2012, 41, 24–32. [Google Scholar] [CrossRef]
- Hoss, D.E.; Settle, L.R. Ingestion of plastics by teleost fishes. In Proceedings of the 2nd International Conference on Marine Debris, Honolulu, HI, USA, 2–7 April 1989; Shomura, R.S., Godfrey, M.L., Eds.; NMFS, NOAA-TM-NMFSSWFCS-154. Department of Commerce, NOAA Technical Memorandum: Miami, FL, USA, 1990; pp. 693–709. [Google Scholar]
- Derraik, J.G.B. The pollution of the marine environment by plastic debris: A review. Mar. Pollut. Bull. 2002, 44, 842–852. [Google Scholar] [CrossRef]
- Gilbert, M. Brydson’s Plastics Materials, 8th ed.; Elsevier BH: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Redondo-Hasselerharm, P.E.; Falahudin, D.; Peeters, E.T.H.M.; Koelmans, A.A. Microplastic effect thresholds for freshwater benthic macroinvertebrates. Environ. Sci. Technol. 2018, 52, 2278–2286. [Google Scholar] [CrossRef]
- Marchant, D.J.; Rodriquez, M.A.; Francelle, P.; Jones, J.I.; Kratina, P. Contrasting the effects of microplastic types, concentrations and nutrient enrichment on freshwater communities and ecosystem functioning. Ecotoxicol. Environ. Saf. 2023, 255, 114834. [Google Scholar] [CrossRef]
- Gherardi, F.; Southy-Grosset, C.; Vogt, G.; Dieguez-Uribeondo, J.G.; Crandall, K.A. Infraorder Astacidea Latreille, 1802 p.p.: The freshwater crayfish. In Treatise on Zoology, Anatomy, Taxonomy, Biology—The Crustacea, Decapoda; Schram, F.R., von Vaupel Klein, J.C., Eds.; Brill: Leiden, The Netherlands, 2010; pp. 269–423. [Google Scholar]
- Ermgassen, P.S.E.; Aldridge, D.C. Predation by the invasive American signal crayfish, Pacifastacus leniusculus Dana, on the invasive zebra mussel, Dreissena polymorpha Pallas: The potential for control and facilitation. Hydrobiologia 2011, 658, 303–315. [Google Scholar] [CrossRef]
- Diaz, D.C. Morphological Analyses of Modifications in Signal Crayfish Mandible in Relations to Feeding Habitat. Bachelor’s Thesis, Biology Education Centre, Department of Limnology, Uppsala University, Uppsala, Sweden, 2009. [Google Scholar]
- Kozak, P.; Buric, M.; Kanta, J.; Kouba, A.; Hamr, P.; Policar, T. The effect of water temperature on the number of mouts and growth of juvenile signal crayfish Pacifastacus leniusculus Dana. J. Anim. Sci. 2009, 54, 286–292. [Google Scholar] [CrossRef]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.U.; Barlaz, A.; Jonsson, S.; Björn, A.; Rowland, S.J.; Thompson, R.C.; Galloway, T.S.; Yamashita, R.; et al. Transport and release of chemicals from plastics to the environment and to wildlife. Phil. Trans. R. Soc. 2009, B364, 2027–2045. [Google Scholar] [CrossRef] [PubMed]
- Dobrzycka-Krahel, A.; Skóra, M.E.; Malek, M. Human consumption of invasive species in the circular economy: Determination of persistent organic pollutants in the invasive signal crayfish from the Baltic coastal river and its assessment for consumption. Sustainability, 2024; under review. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).