Abstract
The significant interest in the islands in the Russian Arctic has been in terms of available oil reserves, which determine the direction of economic development and associated environmental risks for this sector of the Arctic in the near future. Kotelny Island is the largest island of the New Siberian Islands Archipelago included in the protected zone of the Lena Delta Nature Reserve, which is located at 76° N, washed from the west by the Laptev Sea, washed from the east by the East Siberian Sea in a permafrost zone, and characterized by harsh climatic conditions defined by the northeast winds that prevail in vegetative season. January sees temperatures ranging from −32 to −35 °C, and July from +6 to +8 °C, which causes a short growing season. Samples were taken between August 3 and 8, 2018 in 12 freshwater bodies where 210 taxa were revealed. Aquatic communities were dominated by zygnematophycean and diatom algae, grouped in the basins of two rivers and associated with the position on the island’s landscape, which suggests the influence of cold north-east winds, leading to the avoidance of habitats in open and high places, which was revealed by statistical methods and also confirms the high individuality of taxa composition. Bioindication methods showed that water bodies are slightly alkaline, with low ion concentrations, with the presence of sulfides in low-lying habitats, and average saturation with organic matter. The mesotrophic status of the studied water bodies was evaluated through an assessment and the type of nutrition in the communities of algae and cyanobacteria indicates they formed there as true autotrophs, which corresponds to the status of a protected area and can serve as a reference level for monitoring anthropogenic impact.
1. Introduction
Arctic freshwater flora, especially in protected areas, have long attracted the attention of researchers since the organisms that comprise them survive in extreme conditions. In addition, the state of aquatic ecosystems, as a criterion of disturbance or anthropogenic impact, is an important indicator of the stability of ecosystems at the border of survival, when environmental factors put the existence of an ecosystem on the brink of existence. Of particular interest is the study of the patterns of distribution and formation of biodiversity in the Arctic regions. The protected areas of the Arctic are delineated by the common border of the Arctic Biodiversity Monitoring Zone of the Biodiversity Working Group of the Arctic Council (CAFF), which is in the interests of the international community [1], and within which three zones are distinguished, High Arctic, Low Arctic, and Subarctic. In addition, it is important to study the Arctic biodiversity, as it can be an indicator of the impact of climatic changes [2].
Kotelny Island is part of the New Siberian Islands archipelago on the Arctic shelf of Eastern Siberia, which still remains poorly studied algologically. The first information about algae in the reservoirs of Kotelny Island, which is part of the archipelago, is provided by E.K. Kosinskaya (1956) [3]. The work provides a list that includes 141 taxa of algae of various taxonomic groups, except for Cryptophyceae, Bacillariophyceae, and Dinophyceae, which the authors intentionally did not identify. Since there is no geographical reference of sampling points in the work, the species list given by the authors can only be attributed, in general, to Kotelny Island. This makes it impossible to use these data for full-fledged environmental analysis using bioindicators and statistical mapping tasks. Preliminary results of the limnological studies of 11 water bodies on the Faddeevsky Peninsula of Kotelny Island are presented in a brief report by L. A. Ushnitskaya et al. [4]. The authors report the discovery of 148 species of diatoms from 22 genera. According to researchers, the greatest diversity is found in the genera Navicula (23 species), Eunotia (21), Cymbella (20), and Pinnularia (17). The authors explain the absence of representatives from the class Centrophyceae by the shallowness of the studied reservoirs, in which there is no appropriate biotope for the development of phytoplankton. The publication does not contain a species list. The work of S.I. Genkal and V.A. Gabyshev (2020) [5] provides a species list of diatoms containing 70 taxa. The study is based on the study of plankton samples from six water bodies on Kotelny Island using electron microscopy. The species composition of silica-scaled chrysophytes, Rotosphaerida, Thaumatomonadida, and centrohelid heliozoans in the water bodies of Kotelny Island was studied in the work of A.Y. Bessudova (2023) [6], and as a result, information was obtained on 25 taxa from the genera Chrysosphaerella, Paraphysomonas, Spiniferomonas, Mallomonas, Synura, Acanthocystis, Choanocystis, Raineriophrys, Raphidocystis, Pterocystis, Thaumatomastix, and Rabdiophrys [6]. Thus, the available work on the microflora of the island’s water bodies is limited to a few botanical reports.
Attention to studying the diversity and conditions influencing the formation of Arctic aquatic communities has a significant history [7,8]. There are still no works devoted to bioindication, the search for the most important environmental factors that determine the biodiversity of aquatic organisms in the conditions of the High Arctic island. It should be noted that part of the Russian Arctic remains non-impact and is part of the protective zone of the Lena Delta Wildlife Reserve. The significant interest in this territory in terms of available oil reserves determines the direction of economic development and associated environmental risks for this sector of the Arctic in the near future. These main reasons determine the relevance of studying the aquatic ecosystems of the New Siberian Islands. Inaccessibility and harsh climatic conditions make it very difficult to collect samples, and restrictions on the weight of accompanying equipment often do not allow for measuring environmental parameters in natural conditions. As a consequence of these difficulties, rare expeditions have collected specimens of organisms from some of the island’s accessible water bodies, but habitat data are lacking. This gap can be filled using bioindicator analysis.
The objective of this research was to determine the taxonomic composition of algae and cyanobacteria in 12 small, diverse aquatic objects of Kotelny Island, identify indicator taxa and analyze their spatial distribution, conduct an ecological-geographical analysis, and determine environmental factors affecting the diversity of photosynthetic microorganisms in the studied Arctic habitats.
2. Materials and Methods
2.1. Site Description
Kotelny Island is a part of the Anzhu Islands group of the New Siberian Islands archipelago (Figure 1 and Figure 2). This is the largest island of the archipelago, with an area of 23.2 thousand km2. A large peninsula, Faddeevsky, is separated from Kotelny Island by Gedenstrom Bay. The island is located at 76° N between the Laptev Sea and the East Siberian Sea. The study area is located in a continuous permafrost zone, the thickness of which reaches 500 m [9] and is marked by severe climatic conditions. The mean air temperature in January falls between −32 and −35 °C, and in July it reaches up to +6–8 °C. The duration of the ice-free period in the reservoirs of the archipelago reaches 30–45 days [10], which causes a short growing season. The island is situated in the Arctic tundra.
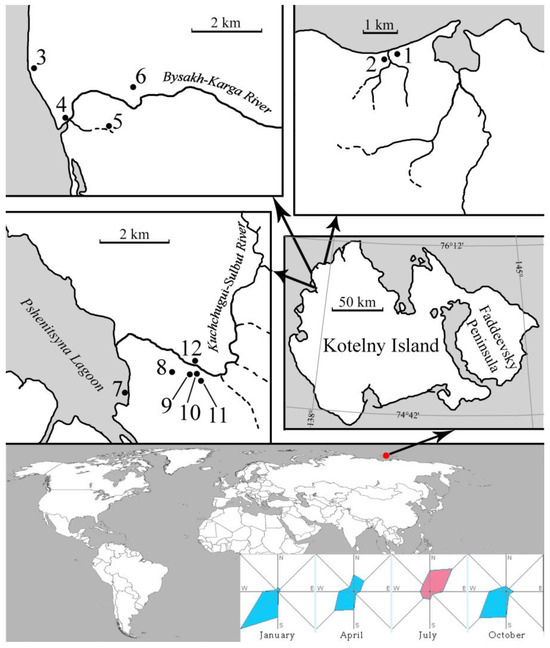
Figure 1.
Sampling points in the studied area of Kotelny Island along with wind rose seasonality, numbers according to Table 1.

Figure 2.
Natural landscape of investigated water bodies of Kotelny Island. Arctic tundra with a pool (station 3) (a) and stream (station 2) (b).
2.2. Sampling and Laboratory Study
Sampling was carried out by the executive director of the Russian Geographical Society (RGS) for the Republic of Sakha (Yakutia) D.I. Solovyov during the period from 3 to 8 August 2018, as part of a complex expedition of the Russian Geographical Society. Plankton samples were collected from 12 different types of water bodies in the northwest of the island, including the Kuchchugui-Sulbut and Bysakh-Karga Rivers, a puddle within the Pshenitsyna Lagoon tidal zone, streams, and small tundra reservoirs (Figure 1 and Figure 2, Table 1). An Apstein plankton net (SEFAR NITEX fabric, mesh size 15 µm) was used for sampling. The samples were fixed by adding 4% neutral formaldehyde. All samples were transported for further treatment at the Institute for Biological Problems of Cryolithozone, Yakutsk.

Table 1.
Description of study sites with their geographical coordinates in Kotelny Island, New Siberian Islands Archipelago, Yakutia.
The diatom shells were cleaned in 30% hydrogen peroxide with a 6 h heat treatment in a thermostat at 85 °C [11] and examined in permanent slides under an Olympus BH-2 (Olympus; Tokyo, Japan) light microscope and JSM-6510 LV (JEOL Ltd.; Tokyo, Japan) scanning electron microscope.
Data on the taxa composition of the Bacillariophyceae, Mediophyceae, and, partly, Chrysophyceae of the studied water bodies were partly published by us earlier [6,8] and their list was borrowed from our works. Handbooks and articles were used for the diatom taxa identification [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. The identification of representatives of other classes was carried out with an Olympus BH-2 light microscope using handbooks [35,36,37,38,39,40,41,42,43]. Algaebase.org (accessed on 15 January 2024) was used to adopt the modern taxa names [44]. Since ‘Algae’ is a large polyphyletic grouping, in this work this term is taken in its customary sense as diverse group of photosynthetic, eukaryotic organisms [45,46].
The species-specific ecological preferences of revealed taxa were used to perform bioindicator analysis [47,48,49].
Statistica 12.0 software was used for statistical mapping. The network correlation analysis was done in JASP (significant only) on the botnet package in R Statistica software of [50]. The BioDiversity Pro 2.0 software was used for the Pearson similarity calculation [51].
3. Results
3.1. Taxonomic Content
Altogether 210 taxa of algae and cyanobacteria were identified in 12 studied aquatic habitats of Kotelny Island (Table A1 and Table A2 in Appendix A). Figure 3 shows that taxa richness in the communities was varied between 17 in the Kuchchugui-Sulbut River and 56 in the mouth of the Bysakh-Karga River, excluding a small pool (St. 8), where only one taxon, Nostoc, was found. It can be seen that diatom taxa prevailed in the studied water bodies, along with desmids. Ulvophyceae species were absent from the freshwater habitats studied.
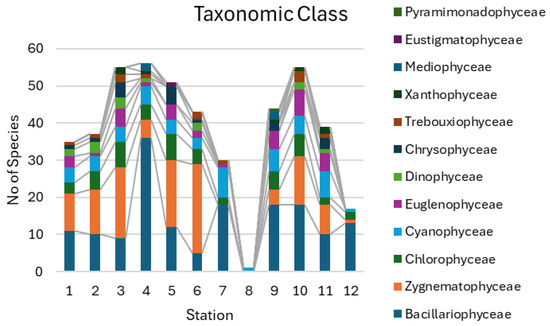
Figure 3.
Distribution of taxonomic components at the class level for the sampling points of Kotelny Island.
Two major bursts of diversity were confined to the Bysakh-Karga River and Kuchchugui-Sulbut River basins (Figure 3) and were related mostly to diatoms, but in the Bysakh-Karga River a sufficient role was also played by desmids.
The analysis of the structure of dominant taxa at the genus level (Table 2), which includes 144 of the 210 identified taxa, however, shows that desmidian algae predominated in the studied communities, and diatoms were represented by a significantly smaller number of taxa. The standard deviation for the sum of dominated genera (14.62) cut off only two genera, Cosmarium and Staurastrum, both from desmids.

Table 2.
Richest genera in the communities of studied aquatic habitats of Kotelny Island in August 2018.
A comparison of the taxa represented in each sampling point by Pearson coefficients based on Table A1 in Appendix A shows a similarity of communities in stations 1–6 in the Bysakh-Karga River basin, a small river in the north, and in two small pools (stations 10, 11) of the Kuchchugui-Sulbut River on the level of 70% (Figure 4). Only communities of small pools at stations 7, 8, and in the Kuchchugui-Sulbut River station 12 were noticeably different, with a low taxa richness.
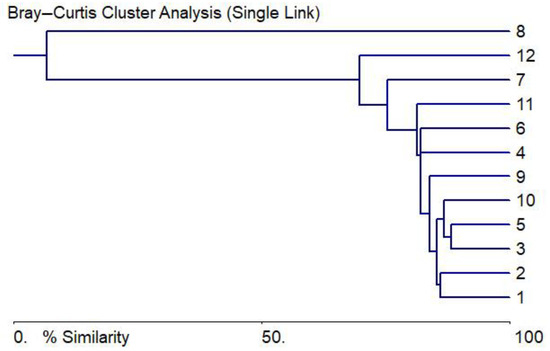
Figure 4.
Tree of similarity for taxonomical and ecological data on the studied water bodies of Kotelny Island based on Table A1 and Table A2 in Appendix A.
The geographical distribution of the revealed taxa was considered as four categories where cosmopolitan taxa was the major category present, and Holarctic taxa with a few Boreal taxa were partly presented (Table A1 in Appendix A, Table 3). Attention can especially be given to the category of Arcto-alpine taxa, which was a noticeable part of communities in station 4, station 5 on the west, and mostly in stations 7, 10, and 12 in the Kuchchugui-Sulbut River in the south-west habitats. This allows us to assume that habitat conditions have a crucial impact in the shaping of the studied Arctic communities and thus draws attention to the ecological preferences of the captured taxa.

Table 3.
Spreading of taxa number in groups by geographic distribution in the aquatic communities of Kotelny Island, New Siberian Islands Archipelago, Yakutia.
3.2. Bioindicators
The ecological characteristics of the identified taxa for nine environmental variables are presented in Table A2 in Appendix A, and summarized for the 12 sampling stations in Table 4. Both planktonic and benthic inhabitants predominate, which is due to the shallowness of the studied water bodies, as both true plankters and drifting phytobenthic taxa enter the net. Indicators of the temperature range preferences suggested that there were mostly temperate taxa but eurythermal taxa also presented and there were even a few warm-water inhabitants. Oxygen saturation indicators were mostly in the group of middle oxygenated waters. Only one taxon indicator of sulfide-enriched waters was found in station 11, where diatom taxa domination was found along with Euglenophyceae and cyanobacteria, without any desmids. Indicators of water pH and salinity as a whole were related to the groups of neutral and low saline waters. Indicators of organic pollution, according to the Watanabe system that uses diatoms, only show low to middle organic pollution in the studied water bodies. Indicators of the nutrition type (autotrophy–heterotrophy) were mostly autotrophs, which indicates an oligo-mesotrophic to mesotrophic environment. Organic pollution indicators based on the species-specific index of saprobity in the Sládeček system indicated water quality was mostly in Classes 2 and 3.

Table 4.
Distribution of taxa indicator numbers in the aquatic communities of Kotelny Island, New Siberian Islands Archipelago, Yakutia.
A comparison of the data in Table A1 in Appendix A and Table 4 was performed for the purpose of characterizing the taxonomical and ecological “face” of each of the studied communities using the JASP program. The results are presented in Figure 5. Figure 5 shows two different cores, the first of which included communities from the north unnamed river habitats and also communities from the Bysakh-Karga River basin, excluding the station 4 data. The second core was formed by communities from the Kuchchugui-Sulbut River basin, which also included station 4. This demonstrates the division of the taxonomic composition and ecological preferences of taxa at the level of geographic location.
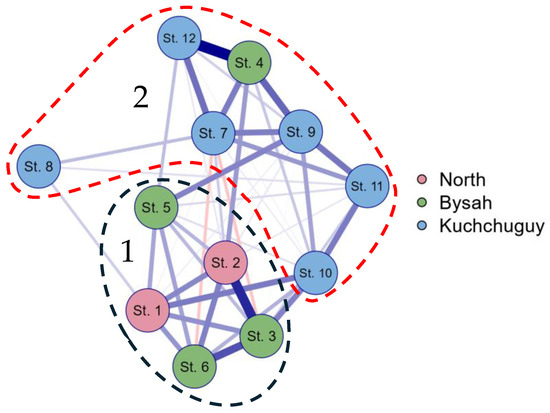
Figure 5.
Correlation graph of JASP for taxonomical and ecological data of the water bodies studied on Kotelny Island based on Table A1 in Appendix A and Table 4. 1, 2—community cores.
A detailed analysis of the distribution of the ecological preferences of taxa in each studied habitat (excluding station 8 because there was only one taxon) by category of bioindicators is presented in Figure 6, Figure 7 and Figure 8. The data were divided into corresponding river basins in histograms. Habitat preferences in each station were as for the all-indicator content distribution but benthic taxa played a sufficient part in stations 1 and 12 (Figure 6a). Geographic distribution categories show a cosmopolitan prevalence but stations 7 and 12 included up to 20% of Arcto-alpine taxa (Figure 6b).
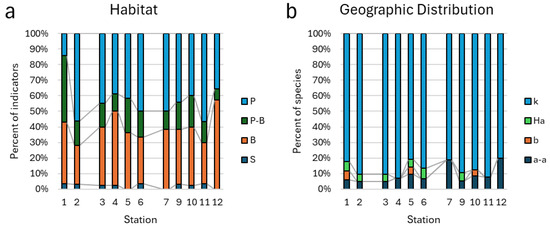
Figure 6.
Distribution of habitat indicators (a) and geographic distribution (b) of taxa in aquatic communities of Kotelny Island. Abbreviations in the legend: habitat (P—planktonic, P-B—plankto-benthic, B—benthic, S—soil) and geographic distribution (a-a—Arcto-alpine, b—Boreal, Ha—Holarctic, k—cosmopolitan).
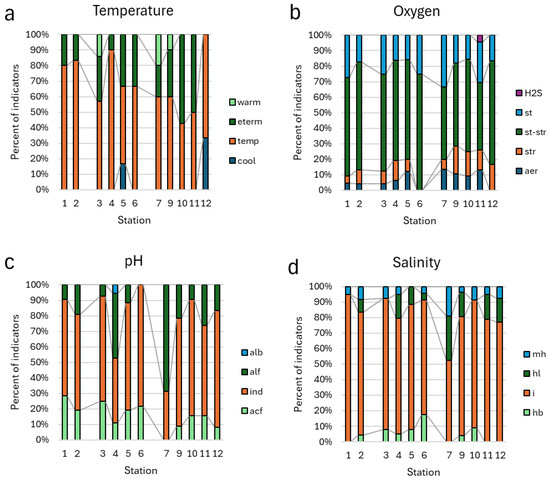
Figure 7.
Distribution of taxa indicators of temperature (a), oxygen (b), pH (c), and salinity (d) in aquatic communities of Kotelny Island.
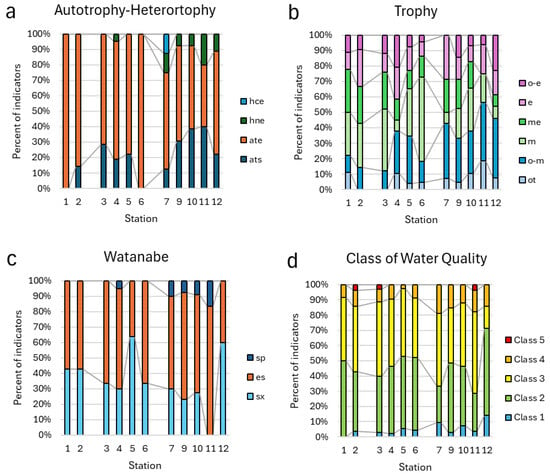
Figure 8.
Distribution of taxa indicators of autotrophy–heterotrophy (a), trophic state (b), organic pollution according to categories of Watanabe (c), and the class of water quality based on species-specific Index Saprobity S (d) in aquatic communities of Kotelny Island.
Indicators of the temperature preferences were mostly in the categories of temperate and cool water in basins of the north and west, whereas the eurythermic and warm water categories were about 50% and more in communities of the south basin habitats (Figure 7a). Oxygen-enriched categories were sufficient (up to 30%) in the south basin habitat (Figure 7b). Acidophilic indicators were mostly in north and north-west habitats, whereas alkaliphilic taxa were up to 30% in the south basin (Figure 7c). The same distribution was found for the content of the salinity categories of halophiles and mesohalobes, which were more in the south basin habitats (Figure 7d).
At the same time, as indicators of autotrophic categories contain 95–100% of taxa in basins of the north and north-west, the indicators in the south basin represent more complicated content with a significant portion of mixotrophs (Figure 8a). Trophic state indicators represent a diverse distribution pattern (Figure 8b) with a greater role played by oligotrophic taxa in the south basin habitats, whereas mesotrophic taxa prevailed in the north and north-west communities. The decreasing role of clear water saproxenic diatom taxa from north to south habitats can be seen in Figure 8c with the increasing percentage of saprophiles. The organic pollution indicators for Class 4 water quality were slightly more in percentage in the habitats of the southern basin (Figure 8d).
3.3. Statistical Mapping
Therefore, the spatial distribution differences for the different groups of taxonomic content and indicators can be seen. For the purpose of clarifying the role of habitat distribution over the Kotelny Island landscape, a series of statistical maps was constructed (Figure 9 and Figure 10). A map of the total taxa number distribution shows that taxa-rich habitats were concentrated in the north-west of the studied area (Figure 9a). This prevalence is supported mostly by Chlorophyceae taxa (Figure 9b) rather than desmids (Figure 9c) or diatoms (Figure 9d). Figure 9e,f show the controversial distribution of Holarctic taxa concentrated in the coastal area of north-west and Arcto-alpine taxa, which preferred the habitats in the valley of the Kuchchugui-Sulbut River in the south.
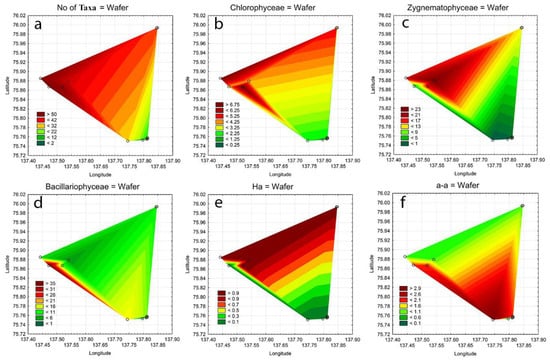
Figure 9.
Statistical maps of the distribution of the total taxa number (a), Chlorophyceae taxa (b), Zygnematophyceae taxa (c), Bacillariophyceae taxa (d), Holarctic taxa (e), and Arcto-alpine taxa (f) in aquatic communities of Kotelny Island.
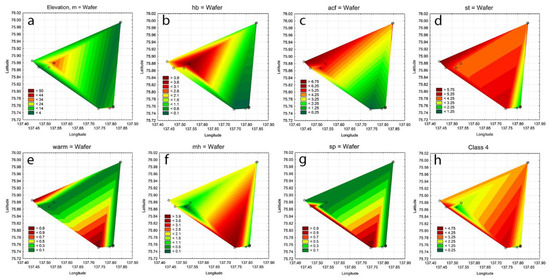
Figure 10.
Statistical maps of altitude (a), halophobe taxa indicators (b), acidophile taxa indicators (c), standing water taxa indicators (d), warm-water taxa indicators (e), mesohalobe taxa indicators (f), saprophyle taxa indicators (g), and Class 4 water quality taxa indicators in aquatic communities of Kotelny Island (h).
The spatial distribution of indicator groups was also mapped in order to identify the habitat preferences of the identified taxa in the landscape of the studied part of Kotelny Island, thereby identifying environmental properties that regulate the formation of communities of algae and cyanobacteria. Figure 10a shows the altitude of the studied habitat distribution as one of the major and stable environment variables. The study area is divided by one hill, 53 m high, which is significant on the surface of the tundra. Communities with a predominance of halophobes (Figure 10b) were concentrated around the top of the hill, while most acidophiles were found in the coastal northern and northwestern parts of the landscape (Figure 10c). Figure 10d reflects a fairly uniform distribution of indicator taxa in moderately oxygenated waters. However, warm-water, mesohalobes, saprophiles, and indicators of Class 4 organically saturated waters (Figure 10c–h) were found towards the southern part of the study area, revealing the Kuchchugui-Sulbut River valley as an environment-forming direction.
4. Discussion
Data on the algal flora of the archipelago were replenished as a result of comprehensive botanical studies of the Arctic tundra of Yakutia, carried out during airborne expeditions of the YSC SB AS USSR in the late 1970s [57]. Information about diatoms from sediments of reservoirs of the Arctic Anzhu Archipelago is presented in the work devoted to the paleogeographic reconstruction of the region based on the analysis of lagoon sediments of Zhokhov island, part of the De Long group of the New Siberian Islands [58]. The authors identified 66 species of diatoms, 17 of them are listed in the text of the work, and a complete list of species is not provided. The work of O.V. Palagushkina et al. [59] is dedicated to studying the taxonomic and ecological composition of communities of fossil and modern diatoms on Bolshoy Lyakhovsky Island, part of the Lyakhovsky Islands group of the New Siberian Islands archipelago. Climatically, the islands of Bolshoy Lyakhovsky and Kotelny are very similar [60]. The authors examined samples of water and surface bottom sediments from 15 reservoirs of polygonal tundra, as well as permafrost sediments from the profile located on a coastal cliff near the D. Laptev Strait.
Only in this work, among the islands of the immediate environment, are data on environmental indicators provided, so it was decided to compare the taxa composition of both islands and identify similarities and differences that make it possible to characterize the properties of water in the water bodies of Kotelny Island. The work provides a list of 159 species of diatoms and identifies the main environmental factors that impact the distribution of diatoms in the studied area. The major ecological factors that specify the distribution of diatoms in the studied data set are mean air temperature in July, pH, conductivity, water depth, and the concentrations of Si4+ and Al3+. Bolshoy Lyakhovsky Island is the closest neighbor of the studied Kotelny island, so it the taxa composition of diatoms from both islands was compared. This research identified 79 diatom taxa from 12 reservoirs, while the list of recent diatoms from Palagushkina’s article contains 84 taxa from 15 reservoirs [59], so it is comparable in number. The samples in this study were homogeneously collected planktonic samples, and on Bolshoi Lyakhovsky Island they studied not only plankton samples, but also benthos and bottom sediments. The areas where sampling points were located on both islands were comparable. It can be noted that only 22 taxa were common to the diatom leaves of both islands.
Because Palagushkina’s list includes both modern and fossil species, comparisons of the diatoms found on both islands revealed only 15 species found in modern communities, representing only 18% of our list. This indicates the high individuality of the floras of the Arctic islands. The same high individuality was noted when studying a group of reservoirs in the Tiksi region, in the territory adjacent to the protective zone of the Lena Delta Wildlife Reserve [61].
It should be noted that in the fossil state there were diverse representatives of the planktonic species Stephanodiscus and Aulacoseira, living in reservoirs with a large mass of water, while in the reservoirs of both islands at present these indicators are poor or not found at all, as on Kotelny Island, the focus of this study. All of this leads to the assumption that, currently, water bodies are becoming shallower and, therefore, more susceptible to fluctuations in environmental parameters. This work using indicator species allows us to describe the studied water bodies as slightly saturated with salts and dissolved organic matter, with a neutral pH.
For the diatom communities of Bolshoi Lyakhovsky Island, the factors of pH and the electrical conductivity of water, as well as the depth of the reservoir, are noted as ecologically important, determining the taxonomic composition of water bodies of the Arctic archipelago. The variability of the data measured there is not given; however, the figures show that the pH values vary in the range of 7.8–8.5, which is typical for slightly alkaline waters. In addition, the electrical conductivity was between 180 and 250 mSm cm−1, which rules out the possibility that the waters under study contain a lot of ions.
Since the composition of the indicator taxa identified in relation to these two environmental parameters is very similar, it can be assumed that the water bodies of Kotelny Island also had water within the specified conductivity and pH parameters.
It should be noted once again that diatoms are well-studied indicators of the environment, but it was determined that the entire available taxa composition of algae and cyanobacteria can enrich conclusions about the properties of the environment, since non-diatoms made up more than 60% of the list and the parameters for bioindication also included trophic and other characteristics of the studied communities.
Thus, the use of groups of indicators of trophic status in the water bodies of Kotelny Island showed that mesotrophic taxa predominate, in contrast to the oligotrophic assessments of waters on Bolshoi Lyakhovsky Island. Assessments of the presence of dissolved organic matter using Watanabe’s methods and Sladechek’s saprobity indices showed that the waters of Kotelny Island are weakly or moderately saturated, while no such assessments were made on Bolshoi Lyakhovsky Island.
Moreover, we were able to track the dynamics of the taxonomical composition and groups of indicator species in the area. Thus, it turned out that the value of taxa richness is grouped in two different surface areas associated with the drainage basins of the Kuchchugui-Sulbut and Bysakh-Karga Rivers and that the presence of zygnematophycean taxa plays a significant role in the communities. A reflection of the difference in environmental conditions for the aquatic communities of these river basins was also found in indicators of temperature, oxygen, pH, salinity, the type of nutrition, trophic state, and organic pollution; for example, in the basin of the more southern Kuchchugui-Sulbut River there were more indicators of warm waters, oxygen, low alkaline water, oligo-mesotrophy, moderate (saprophiles), or slightly increased organic pollution (Classes 3 and 4).
We tried to track the influence of spatial factors on the separation of communities of these two river basins using statistical mapping, which provided an important basis for describing the influences in the nearby protected zone of the Lena Delta Wildlife Reserve, which has the same climatic parameters. The surface map showed that there was a hill between the three surveyed sites. In fact, this hill is part of a small ridge up to 150 m high and running in the direction from northwest to southeast, thus separating the Kuchchugui-Sulbut River basin from the northeast winds that prevail in August on Kotelny Island (Figure 1).
It turned out that the patterns of distribution of both the algal and cyanobacterial taxonomic composition in the communities of Kotelny Island and indicator species show great similarity, which gives us the opportunity to connect some properties of the communities and environment with the position of water bodies on the landscape and, thus, with the influence of the cold north-east winds in August. As may be observed, the total number of taxa in habitats increases to the north and northwest, and concurrently, the number of taxa of zygnemoid and green algae with a Holarctic areal increase. On the other hand, further south the role of diatoms and arctic alpines increases. At the same time, there are more cold-loving taxa and halophobes at altitude. Where the altitudes are lower, there are more warm-water indicators, saprophiles, and indicators of Class 4 water quality. Indicator distribution maps also show that planktonic taxa and acidophiles are more associated with the northern coast. It can thus be concluded that the geographical position of the reservoir on the surface of the island contributes significantly to development of aquatic communities under the influence of the landscape-regulated influence of northeastern cold winds with a speed of about 3.6 m s−1 from the Arctic Ocean for both islands, Kotelny and Bolshoy Lyakhovsky.
The high similarity of climatic variables on the islands of Kotelny and Bolshoy Lyakhovsky [60] in the absence of anthropogenic impact allows us to assume the similarity of water variables, even though only the taxonomic composition was studied, whereas for the Bolshoy Lyakhovsky Island, taxonomic composition, as well as some chemical variables, are known. It was previously established that Arctic cold northeastern winds have a major influence on the development of communities of continental waters of the coastal zone both in the Barents Sea and in the Laptev Sea [7,61].
5. Conclusions
Thus, about the studied habitats of Kotelny Island, it can be said that the 210 taxa of algae and cyanobacteria identified in the 12 studied aquatic habitats are comparable to the previously studied 15 communities of Bolshoi Lyakhovsky Island. The bioindication method used for the first time on Kotelny Island to determine the amplitude of water parameters and the properties of communities, as well as statistical methods for analyzing their distribution over the island’s landscape area, showed that water bodies are slightly alkaline, with low ion concentrations, dominated by zygnematophycean and diatom algae, grouped in the basins of two rivers, and associated with the position on the island’s landscape, which suggests the influence of cold north-east winds, leading to the avoidance of habitats in open and high places.
Statistical methods showed high individuality of taxa composition in the studied habitats. Bioindication methods, in addition, also revealed the presence of sulfides in low-lying habitats and average saturation with organic matter (for diatoms). An assessment was also made of the trophic status of the assessed water bodies as mesotrophic and the type of nutrition in the communities of algae and cyanobacteria indicates they formed there as true autotrophs, which corresponds to the status of a protected area and can serve as a reference level for monitoring anthropogenic impact.
The New Siberian Islands are hard-to-reach regions as remote areas. Therefore, the organization of freshwater habitat research in the region is extremely difficult. However, it is clear that further research is needed. The most important points that should be kept under our close attention are the control of possible anthropogenic transformation and the need to investigate the long-term effects of climate change on the aquatic ecosystems.
Author Contributions
Conceptualization, S.B. and V.G.; methodology, S.B. and V.G.; software, S.B.; validation, S.B. and V.G.; formal analysis, V.G.; investigation, V.G.; resources, V.G.; data curation, S.B. and V.G.; writing—original draft preparation, S.B. and V.G.; writing—review and editing, S.B. and V.G.; visualization, S.B.; supervision, V.G.; project administration, V.G.; funding acquisition, V.G. All authors have read and agreed to the published version of the manuscript.
Funding
The research was carried out within the state assignment of the Ministry of Science and Higher Education of the Russian Federation (theme number FWRS-2021-0023. reg. number AAAA-A21-121012190038-0).
Data Availability Statement
Data published in Appendix A are available on the article’s website.
Acknowledgments
The authors express deep gratitude to the executive director of the Russian Geographical Society for the Republic of Sakha (Yakutia) D.I. Solovyov for the selection of primary algological material, as well as to I.S. Pavlov, a researcher at the Department for the Study of Mammoth Fauna, Academy of Sciences of the Republic of Sakha (Yakutia) for providing the overview photographs of Kotelny Island. The authors are also grateful for the partial support of the Israeli Ministry of Aliyah and Integration.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Algal and cyanobacteria taxa richness in 12 studied water bodies on Kotelny Island, August 2018.
Table A1.
Algal and cyanobacteria taxa richness in 12 studied water bodies on Kotelny Island, August 2018.
| Taxa | St. 1 | St. 2 | St. 3 | St. 4 | St. 5 | St. 6 | St. 7 | St. 8 | St. 9 | St. 10 | St. 11 | St. 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mediophyceae Medlin & Kaczmarska | ||||||||||||
| Lindavia antiqua (W.Smith) Nakov, Guillory, M.L.Julius, E.C.Theriot & A.J.Alverson | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lindavia cf. minuta (Skvortzov) T.Nakov & al. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Pantocsekiella ocellata (Pantocsek) K.T.Kiss & Ács | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Stephanodiscus neoastraea Håkansson & Hickel | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bacillariophyceae Haeckel | ||||||||||||
| Achnanthidium petersenii (Hustedt) C.E.Wetzel, Ector, D.M.Williams & Jüttner 2019 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
| Achnanthidium minutissimum (Kützing) Czarnecki | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Amphora ovalis Kütz. | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| Caloneis westii (W.Smith) Hendey | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Chamaepinnularia soehrensis (Krasske) Lange-Bertalot & Krammer | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Craticula ambigua (Ehrenberg) D.G.Mann | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ctenophora pulchella (Ralfs ex Kützing) D.M.Williams & Round | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cymbella neocistula Krammer | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 |
| Cymbopleura cuspidata (Kützing) Krammer | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Cymbopleura naviculiformis (Auerswald ex Heiberg) Krammer | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Cymbopleura stauroneiformis (Lagerstedt) Krammer | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Diatoma tenuis C.Agardh | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Didymosphenia geminata (Lyngbye) Mart.Schmidt | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 |
| Encyonema fogedii Krammer | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Encyonema lange-bertalotii Krammer | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Encyonema latens (Krasske) D.G.Mann | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Encyonema minutum (Hilse) D.G.Mann | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Encyonema silesiacum (Bleisch) D.G.Mann | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Encyonema ventricosum (C.Agardh) Grunow | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Entomoneis alata (Ehrenberg) Ehrenberg | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Entomoneis paludosa (W.Smith) Reimer | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Eunotia arcus Ehrenberg | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Eunotia bilunaris (Ehrenberg) Schaarschmidt | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eunotia praerupta Ehrenberg | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Fallacia pygmaea (Kützing) Stickle & D.G.Mann | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Fragilaria capucina Desmazières | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Fragilaria radians (Kützing) D.M.Williams & Round | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Fragilaria rumpens (Kützing) G.W.F.Carlson | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Gomphonella olivacea (Hornemann) Rabenhorst | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gomphonema angusticephalum E.Reichardt & Lange-Bertalot | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Gomphonema lagerheimii A.Cleve | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Gomphonema longiceps f. suecicum (Grunow) Hustedt | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gomphonema micropus Kützing | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Gomphonema montanum (Schumann) Grunow | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Hannaea arcus (Ehrenberg) R.M.Patrick | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 |
| Hantzschia amphioxys (Ehrenberg) Grunow | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| Hygropetra balfouriana (Grunow ex Cleve) Krammer & Lange-Bertalot | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Iconella brebissonii (Krammer & Lange-Bertalot) Bukhtiyarova | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mayamaea agrestis (Hustedt) Lange-Bertalot | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Mayamaea permitis (Hustedt) K.Bruder & Medlin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Meridion circulare (Greville) C.Agardh | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 |
| Navicula antonii Lange-Bertalot | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Navicula bottnica Grunow | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Navicula cincta (Ehrenberg) Ralfs | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Navicula cryptocephala Kützing | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Navicula cryptotenelloides Lange-Bertalot | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Navicula digitoconvergens Lange-Bertalot | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Navicula digitoradiata (W.Gregory) Ralfs | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Navicula margalithii Lange-Bertalot | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Navicula radiosa Kützing | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 |
| Navicula rhynchotella Lange-Bertalot | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Navicula streckerae Lange-Bertalot & Witkowski | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Navicula striolata (Grunow) Lange-Bertalot | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Navicula venerablis Hohn & Hellerman | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Navicula viridula (Kützing) Ehrenberg | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Navicula vulpina Kützing | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Nitzschia acicularis (Kützing) W.Smith | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Nitzschia acidoclinata Lange-Bertalot | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Nitzschia alpina Hustedt | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Nitzschia flexoides Geitler | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Nitzschia inconspicua Grunow | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 |
| Nitzschia linearis W.Smith | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nitzschia perminuta Grunow | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Nitzschia vermicularis (Kützing) Hantzsch | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Opephora mutabilis Sabbe & Wyverman | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pinnularia bullacostae Krammer & Lange-Bertalot | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Pinnularia lata (Brébisson) W.Smith | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Placoneis amphibola (Cleve) E.J.Cox | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Reimeria sinuata (W.Gregory) Kociolek & Stoermer | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Stauroneis anceps Ehrenberg | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Stauroneis phoenicenteron (Nitzsch) Ehrenberg | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Surirella hibernica (W.Smith) D.Kapustin & O.Kryvosheia | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Tryblionella debilis Arnott ex O’Meara | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Tryblionella hungarica (Grunow) Frenguelli | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Ulnaria ulna (Nitzsch) Compère | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chrysophyceae Pascher | ||||||||||||
| Dinobryon anulatum D.G.Hilliard & B.C.Asmund | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dinobryon sertularia Ehrenberg | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Jaoniella planctonica Skvortsov | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Kephyrion gracile (Hilliard) Starmach | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Kephyrion ovale (Lackey) Huber-Pestalozzi | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Paraphysomonas acuminata Scoble & Cavalier-Smith | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Paraphysomonas uniformis J.M.Scoble & T.Cavalier-Smith | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Synura borealis Škaloud & Škaloudová | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Synura sp. | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eustigmatophyceae D.J.Hibberd & Leedale | ||||||||||||
| Pseudostaurastrum limneticum (Borge) Guiry | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Xanthophyceae P.Allorge ex F.E.Fritsch | ||||||||||||
| Tribonema ambiguum Skuja | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Tribonema elegans Pascher | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tribonema gayanum Pascher | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tribonema viride Pascher | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Tribonema vulgare Pascher | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| Zygnematophyceae Round ex Guiry | ||||||||||||
| Actinotaenium cucurbita (Brébisson ex Ralfs) Teiling | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Closterium lanceolatum Kützing ex Ralfs | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Closterium leibleinii Kützing ex Ralfs | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 |
| Closterium littorale f. minus L.E.Komarenko | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Closterium littorale F.Gay | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Closterium moniliferum Ehrenberg ex Ralfs | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium anceps P.Lundell | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| Cosmarium arctoum Nordstedt | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium bioculatum Brébisson ex Ralfs | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Cosmarium bioculatum var. excavatum Gutwinski | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium botrytis Meneghini ex Ralfs | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 |
| Cosmarium botrytis var. gemmiferum (Brébisson) Nordstedt | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Cosmarium contractum var. ellipsoideum (Elfving) West & G.S.West | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium costatum Nordstedt | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium debaryi var. novae-semliae Wille | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium formosulum Hoff | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Cosmarium granatum Brébisson ex Ralfs | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium holmiense var. integrum P.Lundell | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Cosmarium impressulum Elfving | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium levinotabile var. heterocrenatum (West & G.S.West) Croasdale | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Cosmarium margaritatum (P.Lundell) J.Roy & Bisset | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium pachydermum P.Lundell | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Cosmarium pseudoholmii O.Borge | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Cosmarium punctulatum Brébisson | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium pyonochondrum Nordstedt | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium sexnotatum var. tristriatum (Lütkemuller) Schmidle | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium subarctoum (Lagerheim) Raciborski | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium subcrenatum Hantzsch | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Cosmarium subexcavatum West & G.S.West | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| Cosmarium subprotumidum Nordstedt | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium subspeciosum Nordstedt | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium thwaitesii Ralfs | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium tinctum Ralfs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Cosmarium turpinii Brébisson | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Spirogyra sp. ster. | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Staurastrum avicula var. lunatum (Ralfs) Coesel & Meesters | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Staurastrum basidentatum Borge | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Staurastrum brevispina Brébisson | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Staurastrum dilatatum Ehrenberg ex Ralfs | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Staurastrum furcigerum (Brébisson) W.Archer | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Staurastrum granulosum Ralfs | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Staurastrum muticum Brébisson ex Ralfs | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Staurastrum orbiculare Meneghini ex Ralfs | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Staurastrum polymorphum Brébisson | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Staurastrum punctulatum Brébisson | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 |
| Staurodesmus dejectus var. apiculatus (Brébisson) Croasdale | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Staurodesmus spetsbergensis (Nordstedt) Teiling | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chlorophyceae Wille | ||||||||||||
| Ankistrodesmus falcatus (Corda) Ralfs | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ankistrodesmus fusiformis Corda | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ankistrodesmus spiralis (W.B.Turner) Lemmermann | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Carteria pseudoglobosa Ettl | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Colemanosphaera charkowiensis (Korshikov) H.Nozaki, T.K.Yamada, F.Takahashi, R.Matsuzaki & T.Nakada | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Euastropsis richteri (Schmidle) Lagerheim | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gonium pectorale O.F.Müller | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Monoraphidium contortum (Thuret) Komárková-Legnerová | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Monoraphidium griffithii (Berkeley) Komárková-Legnerová | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Monoraphidium irregulare (G.M.Smith) Komárková-Legnerová | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mychonastes anomalus (Korshikov) Krienitz, C.Bock, Dadheech & Proschold | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mychonastes jurisii (Hindák) Krienitz, C.Bock, Dadheech & Proschold | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Oedogonium sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Pandorina morum (O.F.Müller) Bory | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 |
| Schroederia setigera (Schröder) Lemmermann | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Stigeoclonium sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Tetrabaena socialis (Dujardin) H.Nozaki & M.Itoh | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 |
| Tetradesmus obliquus (Turpin) M.J.Wynne | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Tetraëdron minimum (A.Braun) Hansgirg | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pyramimonadophyceae Moestrup & Daugbjerg | ||||||||||||
| Polyblepharides singularis P.A.Dangeard | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Trebouxiophyceae Friedl | ||||||||||||
| Botryococcus braunii Kützing | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| Closteriopsis acicularis (Chodat) J.H.Belcher & Swale | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Mucidosphaerium pulchellum (H.C.Wood) C.Bock, Proschold & Krienitz | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Neglectella solitaria (Wittrock) Stenclová & Kaštovský | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Willea rectangularis (A.Braun) D.M.John, M.J.Wynne & P.M.Tsarenko | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Cyanophyceae Schaffner | ||||||||||||
| Anabaena inaequalis Bornet & Flahault | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 |
| Anagnostidinema tenue (Anisimova) Strunecky & al. | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chroococcus minutus (Kützing) Nägeli | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Chroococcus turgidus (Kützing) Nägeli | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 |
| Jaaginema subtilissimum (Kützing ex Forti) Anagnostidis & Komárek | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Jaaginema woronichinii (Anisimova) Anagnostidis & Komárek | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Leptolyngbya gracillima (Hansgirg) Anagnostidis & Komárek | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Merismopedia glauca (Ehrenberg) Kützing | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Merismopedia tenuissima Lemmermann | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Merismopedia tranquilla (Ehrenberg) Trevisan | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Microcrocis irregularis (Lagerheim) Geitler | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Microcystis aeruginosa (Kützing) Kützing | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nostoc linckia Bornet ex Bornet & Flahault | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Nostoc microscopicum Carmichael ex Bornet & Flahault | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 |
| Nostoc pruniforme C.Agardh ex Bornet & Flahault | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Oscillatoria tenuis C.Agardh ex Gomont | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Phormidium bohneri Schmidle | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Phormidium breve (Kützing ex Gomont) Anagnostidis & Komárek | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Phormidium grunowianum (Gomont) Anagnostidis & Komárek | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Phormidium tergestinum (Rabenhorst ex Gomont) Anagnostidis & Komárek | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Snowella lacustris (Chodat) Komárek & Hindák | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Spirulina major Kützing ex Gomont | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Trichodesmium lacustre Klebahn | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Woronichinia compacta (Lemmermann) Komárek & Hindák | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Dinophyceae F.E.Fritsch | ||||||||||||
| Peridinium bipes F.Stein | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Peridinium cinctum (O.F.Müller) | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 |
| Peridinium willei Huitfeldt-Kaas | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Euglenophyceae Schoenichen | ||||||||||||
| Euglena oblonga F.Schmitz | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 |
| Euglena texta (Dujardin) Hübner | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Euglena viridis (O.F.Müller) Ehrenberg | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Euglenaformis proxima (P.A.Dangeard) M.S.Bennett & Triemer | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lepocinclis oxyuris (Schmarda) B.Marin & Melkonian | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 |
| Lepocinclis spirogyroides B.Marin & Melkonian | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Monomorphina pyrum (Ehrenberg) Mereschkowsky | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Phacus caudatus Hübner | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Phacus orbicularis Hübner | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trachelomonas dubia Svirenko | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Trachelomonas dybowskii Dreżepolski | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 |
| Trachelomonas hispida (Perty) F.Stein | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 |
| Trachelomonas intermedia P.A.Dangeard | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trachelomonas lacustris Dreżepolski | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Trachelomonas macropunctata (Skvortsov) Deflandre | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trachelomonas planctonica f. oblonga (Drezepolski) T.G.Popova | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Trachelomonas similis A.Stokes | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
Notes: “0“, not found; “1“, present.

Table A2.
Algal and cyanobacteria taxa ecological preferences in 12 studied water bodies on the Kotelny Island, August 2018.
Table A2.
Algal and cyanobacteria taxa ecological preferences in 12 studied water bodies on the Kotelny Island, August 2018.
| Taxa | HAB | T | OXY | pH | pH-Range | SAL | WAT | SAP | Index S | TRO | AUT-HET | GEO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mediophyceae Medlin & Kaczmarska | ||||||||||||
| Lindavia antiqua (W.Smith) Nakov, Guillory, M.L.Julius, E.C.Theriot & A.J.Alverson | P-B | - | - | acf | - | hb | - | o | 1.00 | ot | ats | - |
| Lindavia cf. minuta (Skvortzov) T.Nakov & al. | - | - | - | - | - | - | - | - | - | - | - | - |
| Pantocsekiella ocellata (Pantocsek) K.T.Kiss & Ács | - | - | - | - | - | - | - | - | - | - | - | - |
| Stephanodiscus neoastraea Håkansson & Hickel | P | temp | st-str | alb | 5.5–9 | i | es | o-b | 1.40 | o-m | ate | k |
| Bacillariophyceae Haeckel | ||||||||||||
| Achnanthidium petersenii (Hustedt) C.E.Wetzel, Ector, D.M.Williams & Jüttner 2019 | - | - | - | - | - | - | - | - | - | - | - | - |
| Achnanthidium minutissimum (Kützing) Czarnecki | P-B | eterm | st-str | ind | 4.3–9.2 | i | es | x-b | 0.95 | o-e | ate | k |
| Amphora ovalis Kütz. | B | temp | st-str | alf | 6.2–9.0 | i | sx | o-b | 1.50 | me | ate | k |
| Caloneis westii (W.Smith) Hendey | B | - | - | - | - | hl | - | - | - | - | - | - |
| Chamaepinnularia soehrensis (Krasske) Lange-Bertalot & Krammer | B | - | str | acf | - | hb | - | o | 1.00 | ot | ats | - |
| Craticula ambigua (Ehrenberg) D.G.Mann | B | warm | st | alf | 5.5–6.0 | i | es | b | 2.30 | me | - | k |
| Ctenophora pulchella (Ralfs ex Kützing) D.M.Williams & Round | P-B | - | st-str | alf | - | i | - | b | 2.30 | o-m | ate | - |
| Cymbella neocistula Krammer | B | - | - | ind | - | I | - | o | 1.20 | o-m | - | k |
| Cymbopleura cuspidata (Kützing) Krammer | P-B | temp | - | ind | 6.7 | i | - | o-a | 1.80 | o-m | - | k |
| Cymbopleura naviculiformis (Auerswald ex Heiberg) Krammer | B | - | st-str | ind | - | i | es | o | 1.20 | o-m | ate | b |
| Cymbopleura stauroneiformis (Lagerstedt) Krammer | B | - | - | ind | - | - | - | o | 1.00 | ot | - | - |
| Diatoma tenuis C.Agardh | P-B | - | st-str | ind | - | hl | sx | o | 1.30 | e | ate | k |
| Didymosphenia geminata (Lyngbye) Mart.Schmidt | B | - | st-str | ind | - | i | sx | o-x | 0.70 | ot | - | a-a |
| Encyonema fogedii Krammer | - | - | - | - | - | - | - | - | - | - | - | - |
| Encyonema lange-bertalotii Krammer | - | - | - | - | - | - | - | - | - | - | - | - |
| Encyonema latens (Krasske) D.G.Mann | B | - | - | - | 7.8 | - | sx | o | 1.00 | - | - | - |
| Encyonema minutum (Hilse) D.G.Mann | B | - | st-str | ind | 6.2 | i | sx | o | 1.20 | o-e | ate | k |
| Encyonema silesiacum (Bleisch) D.G.Mann | B | - | st-str | ind | 6.2–7.7 | i | sx | o | 1.20 | o-e | ate | k |
| Encyonema ventricosum (C.Agardh) Grunow | B | - | st-str | ind | 6.2–7.9 | i | sx | o | 1.20 | o-e | ate | k |
| Entomoneis alata (Ehrenberg) Ehrenberg | P-B | - | st | alf | - | mh | - | b | 2.00 | - | - | k |
| Entomoneis paludosa (W.Smith) Reimer | P-B | - | - | alf | - | hl | - | b-a | 2.50 | m | - | k |
| Eunotia arcus Ehrenberg | B | - | st-str | acf | - | i | - | x-o | 0.50 | ot | ats | k |
| Eunotia bilunaris (Ehrenberg) Schaarschmidt | B | temp | st-str | acf | 5.0–6.6 | i | es | o | 1.00 | o-e | ate | k |
| Eunotia praerupta Ehrenberg | P-B | cool | st-str | acf | - | I | sx | x-o | 0.40 | o-m | ats | k |
| Fallacia pygmaea (Kützing) Stickle & D.G.Mann | P-B | - | st-str | alf | 7.55–8.45 | mh | es | a-o | 2.70 | e | hne | - |
| Fragilaria capucina Desmazières | P-B | - | - | ind | 7.7 | i | es | b-o | 1.60 | m | - | k |
| Fragilaria radians (Kützing) D.M.Williams & Round | P-B | - | - | neu | 6.45–6.95 | - | - | x-b | 0.80 | o-m | - | - |
| Fragilaria rumpens (Kützing) G.W.F.Carlson | - | - | - | - | - | - | - | - | - | - | - | - |
| Gomphonella olivacea (Hornemann) Rabenhorst | B | - | st-str | alf | 7.5–8.0 | i | es | o-b | 1.45 | e | ate | k |
| Gomphonema angusticephalum E.Reichardt & Lange-Bertalot | - | - | - | - | - | - | - | - | - | - | - | - |
| Gomphonema lagerheimii A.Cleve | B | - | str | acf | - | hb | - | o | 1.30 | ot | ats | - |
| Gomphonema longiceps f. suecicum (Grunow) Hustedt | - | - | - | - | - | - | - | - | - | - | - | - |
| Gomphonema micropus Kützing | B | - | str | ind | 6.6–7.95 | i | es | o | 1.30 | ot | ate | - |
| Gomphonema montanum (Schumann) Grunow | B | - | str | ind | - | i | es | x-b | 0.85 | m | ats | - |
| Hannaea arcus (Ehrenberg) R.M.Patrick | B | temp | str | alf | 5.5–7.5 | i | es | x | 0.30 | o-m | ats | a-a |
| Hantzschia amphioxys (Ehrenberg) Grunow | B | temp | st-str | ind | - | I | es | o-a | 1.90 | o-e | ate | k |
| Hygropetra balfouriana (Grunow ex Cleve) Krammer & Lange-Bertalot | - | - | - | - | - | - | - | - | - | - | - | - |
| Iconella brebissonii (Krammer & Lange-Bertalot) Bukhtiyarova | B | - | st-str | alf | - | i | - | b-o | 1.70 | - | - | k |
| Mayamaea agrestis (Hustedt) Lange-Bertalot | B | - | - | neu | - | i | es | b-a | 2.40 | - | - | - |
| Mayamaea permitis (Hustedt) K.Bruder & Medlin | B | - | - | alf | - | oh | es | a | 3.20 | e | hne | - |
| Meridion circulare (Greville) C.Agardh | B | - | str | ind | - | i | es | o | 1.10 | o-m | ate | k |
| Navicula antonii Lange-Bertalot | B | - | - | alf | - | oh | - | b-o | 1.60 | - | - | - |
| Navicula bottnica Grunow | B | - | - | - | - | hl | - | - | - | - | - | - |
| Navicula cincta (Ehrenberg) Ralfs | B | warm | st-str | alf | 7–9 | hl | es | x-o | 0.50 | me | ate | - |
| Navicula cryptocephala Kützing | P-B | temp | st-str | ind | - | i | es | b | 2.10 | o-e | ate | k |
| Navicula cryptotenelloides Lange-Bertalot | B | - | - | alf | - | oh | - | b-a | 2.40 | - | - | - |
| Navicula digitoconvergens Lange-Bertalot | - | - | - | - | - | - | - | - | - | - | - | - |
| Navicula digitoradiata (W.Gregory) Ralfs | B | - | - | alf | - | I | es | b | 2.00 | me | - | k |
| Navicula margalithii Lange-Bertalot | B | - | - | alf | - | hl | - | o | 1.00 | - | - | - |
| Navicula radiosa Kützing | B | temp | st-str | ind | 5–9 | i | es | o | 1.30 | me | ate | k |
| Navicula rhynchotella Lange-Bertalot | B | - | - | alf | - | hl | es | b-a | 2.55 | - | - | - |
| Navicula streckerae Lange-Bertalot & Witkowski | - | - | - | - | - | - | - | - | - | - | - | - |
| Navicula striolata (Grunow) Lange-Bertalot | B | - | - | alb | - | i | - | o | 1.00 | - | - | - |
| Navicula venerablis Hohn & Hellerman | - | - | - | - | - | - | - | - | - | - | - | - |
| Navicula viridula (Kützing) Ehrenberg | B | - | st-str | alf | - | hl | es | b | 2.20 | me | ate | k |
| Navicula vulpina Kützing | B | - | str | ind | 7.9 | i | - | b | 2.00 | me | ats | - |
| Nitzschia acicularis (Kützing) W.Smith | P-B | temp | - | alf | 7.85–8.15 | i | es | a-o | 2.70 | e | hce | k |
| Nitzschia acidoclinata Lange-Bertalot | B | - | str | ind | - | hb | es | o-b | 1.40 | o-m | ats | - |
| Nitzschia alpina Hustedt | P-B | - | str | acf | - | I | sx | o | 1.00 | ot | ats | - |
| Nitzschia flexoides Geitler | - | - | - | - | - | - | - | - | - | - | - | - |
| Nitzschia inconspicua Grunow | B | - | st-str | alf | - | I | es | a-o | 2.70 | e | hne | k |
| Nitzschia linearis W.Smith | B | temp | st-str | alf | 7.6 | i | es | b-o | 1.70 | me | ate | k |
| Nitzschia perminuta Grunow | P-B | temp | str | alf | 7.3 | hl | sp | b-o | 1.75 | o-m | ats | - |
| Nitzschia vermicularis (Kützing) Hantzsch | P-B | - | str | alf | - | i | - | b | 2.20 | m | - | k |
| Opephora mutabilis Sabbe & Wyverman | - | - | - | - | - | - | - | - | - | - | - | - |
| Pinnularia bullacostae Krammer & Lange-Bertalot | - | - | - | - | - | - | - | - | - | - | - | - |
| Pinnularia lata (Brébisson) W.Smith | P-B | - | str | acf | - | i | - | o | 1.00 | ot | - | b |
| Placoneis amphibola (Cleve) E.J.Cox | - | - | - | - | - | - | - | - | - | - | - | k |
| Reimeria sinuata (W.Gregory) Kociolek & Stoermer | P-B,aer | - | st | ind | - | i | sx | o | 1.30 | m | ate | - |
| Stauroneis anceps Ehrenberg | P-B | - | st-str | ind | 6.1–6.9 | i | sx | o | 1.30 | o-m | ate | k |
| Stauroneis phoenicenteron (Nitzsch) Ehrenberg | P-B | temp | st-str | ind | 7.3 | i | es | o | 1.30 | me | ate | k |
| Surirella hibernica (W.Smith) D.Kapustin & O.Kryvosheia | - | - | - | - | - | - | - | - | - | - | - | - |
| Tryblionella debilis Arnott ex O’Meara | P-B | - | ae | alf | - | i | es | a-o | 2.60 | - | ate | - |
| Tryblionella hungarica (Grunow) Frenguelli | P-B | - | - | alf | - | mh | sp | a-o | 2.90 | e | ate | k |
| Ulnaria ulna (Nitzsch) Compère | P-B | temp | st-str | ind | 5.0–9.2 | i | es | b | 2.25 | o-e | ate | k |
| Chrysophyceae Pascher | ||||||||||||
| Dinobryon anulatum D.G.Hilliard & B.C.Asmund | P | - | - | - | - | - | - | o | 1.20 | - | - | - |
| Dinobryon sertularia Ehrenberg | P | - | - | - | - | i | - | o | 1.30 | - | - | k |
| Jaoniella planctonica Skvortsov | - | - | - | - | - | - | - | - | - | - | - | - |
| Kephyrion gracile (Hilliard) Starmach | - | - | - | - | - | - | - | - | - | - | - | - |
| Kephyrion ovale (Lackey) Huber-Pestalozzi | B | - | - | - | - | - | - | o-b | 1.50 | - | - | - |
| Paraphysomonas acuminata Scoble & Cavalier-Smith | - | - | - | - | - | - | - | - | - | - | - | - |
| Paraphysomonas uniformis J.M.Scoble & T.Cavalier-Smith | - | - | - | - | - | - | - | - | - | - | - | - |
| Synura borealis Škaloud & Škaloudová | - | - | - | - | - | - | - | - | - | - | - | - |
| Synura sp. | - | - | - | - | - | - | - | - | - | - | - | - |
| Eustigmatophyceae D.J.Hibberd & Leedale | ||||||||||||
| Pseudostaurastrum limneticum (Borge) Guiry | P | - | st-str | - | - | - | - | o-b | 1.50 | - | - | - |
| Xanthophyceae P.Allorge ex F.E.Fritsch | ||||||||||||
| Tribonema ambiguum Skuja | - | - | - | - | - | - | - | - | - | - | - | - |
| Tribonema elegans Pascher | B | - | - | - | - | - | - | x | 1.00 | - | - | - |
| Tribonema gayanum Pascher | - | - | - | - | - | - | - | - | - | - | - | - |
| Tribonema viride Pascher | P-B | - | - | - | - | i | - | o-x | 0.70 | - | - | k |
| Tribonema vulgare Pascher | P-B | - | - | - | - | i | - | o-b | 1.40 | - | - | k |
| Zygnematophyceae Round ex Guiry | ||||||||||||
| Actinotaenium cucurbita (Brébisson ex Ralfs) Teiling | P-B | - | aer | acf | - | - | - | x-b | 0.90 | o | - | - |
| Closterium lanceolatum Kützing ex Ralfs | B | - | st | ind | - | - | - | - | - | e | - | - |
| Closterium leibleinii Kützing ex Ralfs | P-B | - | st-str | ind | - | - | - | a-o | 2.60 | e | - | - |
| Closterium littorale f. minus L.E.Komarenko | - | - | - | - | - | - | - | - | - | - | - | - |
| Closterium littorale F.Gay | P-B | - | - | ind | - | - | - | b-a | 2.40 | e | - | - |
| Closterium moniliferum Ehrenberg ex Ralfs | P-B | - | st-str | ind | - | i | - | b | 2.10 | me | - | k |
| Cosmarium anceps P.Lundell | B,aer | - | aer | acf | - | - | - | - | - | m | - | - |
| Cosmarium arctoum Nordstedt | - | - | - | - | - | - | - | - | - | - | - | - |
| Cosmarium bioculatum Brébisson ex Ralfs | P-B | - | st-str | ind | - | hb | - | x-o | 0.50 | m | - | k |
| Cosmarium bioculatum var. excavatum Gutwinski | - | - | - | - | - | - | - | - | - | - | - | - |
| Cosmarium botrytis Meneghini ex Ralfs | P-B | - | st-str | ind | - | i | - | o-a | 1.90 | m | - | - |
| Cosmarium botrytis var. gemmiferum (Brébisson) Nordstedt | B | - | - | ind | - | - | - | - | - | m | - | - |
| Cosmarium contractum var. ellipsoideum (Elfving) West & G.S.West | - | - | - | - | - | - | - | - | - | - | - | - |
| Cosmarium costatum Nordstedt | - | - | - | acf | - | - | - | - | - | m | - | - |
| Cosmarium debaryi var. novae-semliae Wille | - | - | - | - | - | - | - | - | - | - | - | - |
| Cosmarium formosulum Hoff | P-B | - | - | ind | - | - | - | o-a | 1.80 | me | - | - |
| Cosmarium granatum Brébisson ex Ralfs | B | - | st-str | ind | - | i | - | o | 1.20 | m | - | - |
| Cosmarium holmiense var. integrum P.Lundell | B,aer | - | aer | acf | - | - | - | - | - | m | - | - |
| Cosmarium impressulum Elfving | B | - | - | ind | - | hb | - | b-o | 1.60 | m | - | - |
| Cosmarium levinotabile var. heterocrenatum (West & G.S.West) Croasdale | - | - | - | - | - | - | - | - | - | m | - | - |
| Cosmarium margaritatum (P.Lundell) J.Roy & Bisset | B | - | - | acf | - | - | - | - | - | m | - | - |
| Cosmarium pachydermum P.Lundell | B | - | - | ind | - | i | - | o-x | 0.70 | m | - | - |
| Cosmarium pseudoholmii O.Borge | P | - | - | ind | - | i | - | - | - | m | - | - |
| Cosmarium punctulatum Brébisson | P-B | - | - | ind | - | hb | - | o | 1.30 | m | - | - |
| Cosmarium pyonochondrum Nordstedt | - | - | - | - | - | - | - | - | - | - | - | - |
| Cosmarium sexnotatum var. tristriatum (Lütkemuller) Schmidle | B | - | - | acf | - | - | - | - | - | m | - | - |
| Cosmarium subarctoum (Lagerheim) Raciborski | - | - | - | acf | - | - | - | - | - | m | - | - |
| Cosmarium subcrenatum Hantzsch | B,aer | - | aer | acf | - | - | - | o | 1.10 | m | - | - |
| Cosmarium subexcavatum West & G.S.West | - | - | - | - | - | - | - | - | - | - | - | - |
| Cosmarium subprotumidum Nordstedt | P-B | - | st-str | ind | - | - | - | o-a | 1.90 | me | - | k |
| Cosmarium subspeciosum Nordstedt | B | - | - | acf | - | - | - | - | - | m | - | - |
| Cosmarium thwaitesii Ralfs | - | - | - | - | - | - | - | - | - | - | - | - |
| Cosmarium tinctum Ralfs | B | - | - | acf | - | - | - | - | - | o-m | - | - |
| Cosmarium turpinii Brébisson | P-B | - | - | ind | - | i | - | o-x | 0.70 | me | - | k |
| Spirogyra sp. st. | - | - | - | - | - | - | - | - | - | - | - | - |
| Staurastrum avicula var. lunatum (Ralfs) Coesel & Meesters | P-B | - | - | ind | - | i | - | - | - | me | - | - |
| Staurastrum basidentatum Borge | - | - | - | - | - | - | - | - | - | - | - | - |
| Staurastrum brevispina Brébisson | - | - | - | - | - | - | - | - | - | - | - | - |
| Staurastrum dilatatum Ehrenberg ex Ralfs | P | - | - | - | - | - | - | - | - | - | - | - |
| Staurastrum furcigerum (Brébisson) W.Archer | P-B | - | - | ind | - | i | - | o | 1.20 | m | - | - |
| Staurastrum granulosum Ralfs | - | - | - | ind | - | - | - | - | - | m | - | - |
| Staurastrum muticum Brébisson ex Ralfs | B | - | st | acf | - | i | - | - | - | m | - | - |
| Staurastrum orbiculare Meneghini ex Ralfs | B | - | - | acf | - | - | - | o | 1.30 | m | - | - |
| Staurastrum polymorphum Brébisson | P-B | - | - | ind | - | i | - | - | - | m | - | k |
| Staurastrum punctulatum Brébisson | P-B | - | st-str | ind | - | i | - | o | 1.20 | o-m | - | - |
| Staurodesmus dejectus var. apiculatus (Brébisson) Croasdale | P-B | - | - | ind | - | i | - | - | - | o-m | - | - |
| Staurodesmus spetsbergensis (Nordstedt) Teiling | - | - | - | - | - | - | - | - | - | - | - | - |
| Chlorophyceae Wille | ||||||||||||
| Ankistrodesmus falcatus (Corda) Ralfs | P-B | - | st-str | - | - | hb | - | b | 2.30 | - | - | k |
| Ankistrodesmus fusiformis Corda | P-B | - | st-str | - | - | i | - | b | 2.00 | - | - | k |
| Ankistrodesmus spiralis (W.B.Turner) Lemmermann | P | - | - | - | - | - | - | b | 2.10 | - | - | - |
| Carteria pseudoglobosa Ettl | - | - | - | - | - | - | - | - | - | - | - | - |
| Colemanosphaera charkowiensis (Korshikov) H.Nozaki, T.K.Yamada, F.Takahashi, R.Matsuzaki & T.Nakada | - | - | - | - | - | - | - | - | - | - | - | Ha |
| Euastropsis richteri (Schmidle) Lagerheim | - | - | - | - | - | - | - | - | - | - | - | - |
| Gonium pectorale O.F.Müller | P | - | st | - | - | i | - | a-o | 2.80 | - | - | - |
| Monoraphidium contortum (Thuret) Komárková-Legnerová | P-B | - | st-str | - | - | i | - | b | 2.20 | - | - | k |
| Monoraphidium griffithii (Berkeley) Komárková-Legnerová | P-B | - | st-str | - | - | i | - | b | 2.20 | - | - | k |
| Monoraphidium irregulare (G.M.Smith) Komárková-Legnerová | P-B | - | st-str | - | - | i | - | - | - | - | - | k |
| Mychonastes anomalus (Korshikov) Krienitz, C.Bock, Dadheech & Proschold | P | - | - | - | - | i | - | o-a | 1.80 | - | - | k |
| Mychonastes jurisii (Hindák) Krienitz, C.Bock, Dadheech & Proschold | - | - | - | - | - | - | - | o-a | 1.90 | - | - | - |
| Oedogonium sp. | - | - | - | - | - | - | - | - | - | - | - | - |
| Pandorina morum (O.F.Müller) Bory | P | - | st | - | - | i | - | b | 2.30 | - | - | k |
| Schroederia setigera (Schröder) Lemmermann | P | - | st-str | - | - | i | - | b-o | 1.70 | - | - | k |
| Stigeoclonium sp. | - | - | - | - | - | - | - | - | - | - | - | - |
| Tetrabaena socialis (Dujardin) H.Nozaki & M.Itoh | P | - | st | - | - | - | - | b | 2.30 | - | - | - |
| Tetradesmus obliquus (Turpin) M.J.Wynne | - | - | - | - | - | - | - | - | - | - | - | k |
| Tetraëdron minimum (A.Braun) Hansgirg | P-B | - | st-str | - | - | i | - | b | 2.10 | - | - | - |
| Pyramimonadophyceae Moestrup & Daugbjerg | ||||||||||||
| Polyblepharides singularis P.A.Dangeard | - | - | - | - | - | - | - | - | - | - | - | - |
| Trebouxiophyceae Friedl | ||||||||||||
| Botryococcus braunii Kützing | P-B | - | st | ind | - | i | - | o-b | 1.50 | - | - | k |
| Closteriopsis acicularis (Chodat) J.H.Belcher & Swale | P-B | - | st-str | - | - | i | - | o-a | 1.90 | - | - | k |
| Mucidosphaerium pulchellum (H.C.Wood) C.Bock, Proschold & Krienitz | P-B | - | st-str | ind | - | i | - | b | 2.30 | - | - | k |
| Neglectella solitaria (Wittrock) Stenclová & Kaštovský | - | - | - | - | - | - | - | - | - | - | - | - |
| Willea rectangularis (A.Braun) D.M.John, M.J.Wynne & P.M.Tsarenko | P | - | st-str | ind | - | i | - | b | 2.10 | - | - | k |
| Cyanophyceae Schaffner | ||||||||||||
| Anabaena inaequalis Bornet & Flahault | P-B | - | st | - | - | - | - | b-o | 1.60 | - | - | - |
| Anagnostidinema tenue (Anisimova) Strunecky & al. | - | - | - | - | - | - | - | - | - | - | - | - |
| Chroococcus minutus (Kützing) Nägeli | P-B | - | - | ind | - | i | - | o-a | 1.80 | o-m | - | k |
| Chroococcus turgidus (Kützing) Nägeli | P-B,S | - | aer | alf | - | hl | - | x-b | 0.80 | - | - | k |
| Jaaginema subtilissimum (Kützing ex Forti) Anagnostidis & Komárek | P-B | H2S | st | - | - | - | - | a | 3.20 | o | - | - |
| Jaaginema woronichinii (Anisimova) Anagnostidis & Komárek | B,Ep | - | st | - | - | mh | - | - | - | - | - | - |
| Leptolyngbya gracillima (Hansgirg) Anagnostidis & Komárek | B,S | - | aer | - | - | - | - | - | - | - | - | - |
| Merismopedia glauca (Ehrenberg) Kützing | P-B | - | - | ind | - | i | - | b-o | 1.75 | o-m | - | k |
| Merismopedia tenuissima Lemmermann | P-B | - | - | - | - | hl | - | b-a | 2.40 | e | - | k |
| Merismopedia tranquilla (Ehrenberg) Trevisan | - | - | - | - | - | - | - | - | - | - | - | - |
| Microcrocis irregularis (Lagerheim) Geitler | - | - | - | - | - | - | - | - | - | - | - | - |
| Microcystis aeruginosa (Kützing) Kützing | P | - | - | - | - | hl | - | b | 2.10 | e | - | k |
| Nostoc linckia Bornet ex Bornet & Flahault | P-B, Ep | - | - | - | - | - | - | b-o | 1.60 | - | - | - |
| Nostoc microscopicum Carmichael ex Bornet & Flahault | S | - | - | - | - | i | - | - | - | - | - | k |
| Nostoc pruniforme C.Agardh ex Bornet & Flahault | P-B,S | - | st | - | - | - | - | o-b | 1.50 | - | - | - |
| Oscillatoria tenuis C.Agardh ex Gomont | P-B,S | - | st-str | - | - | hl | - | a-o | 2.60 | me | - | k |
| Phormidium bohneri Schmidle | B,S | - | - | - | - | - | - | - | - | - | - | - |
| Phormidium breve (Kützing ex Gomont) Anagnostidis & Komárek | P-B,S | - | st,aer | - | - | - | - | a | 3.10 | - | - | - |
| Phormidium grunowianum (Gomont) Anagnostidis & Komárek | - | - | - | - | - | - | - | - | - | - | - | - |
| Phormidium tergestinum (Rabenhorst ex Gomont) Anagnostidis & Komárek | P-B,S | - | st-str | - | - | i | - | a-o | 2.60 | e | - | - |
| Snowella lacustris (Chodat) Komárek & Hindák | P | - | - | - | - | i | - | b-o | 1.60 | me | - | k |
| Spirulina major Kützing ex Gomont | P-B,S | warm | st | - | - | hl | - | a | 3.40 | - | - | - |
| Trichodesmium lacustre Klebahn | P | - | st | - | - | - | - | - | - | - | - | - |
| Woronichinia compacta (Lemmermann) Komárek & Hindák | P-B | - | - | - | - | - | - | - | - | o-m | - | a-a |
| Dinophyceae F.E.Fritsch | ||||||||||||
| Peridinium bipes F.Stein | P | - | st-str | - | - | oh | - | o | 1.30 | - | - | - |
| Peridinium cinctum (O.F.Müller) | P-B | - | st-str | - | - | i | - | b-o | 1.60 | - | - | k |
| Peridinium willei Huitfeldt-Kaas | P | - | st | - | - | - | - | o-b | 1.50 | - | - | - |
| Euglenophyceae Schoenichen | ||||||||||||
| Euglena oblonga F.Schmitz | P | eterm | st-str | ind | 6.0–8.0 | - | - | b | 2.10 | - | - | - |
| Euglena texta (Dujardin) Hübner | P | eterm | st-str | ind | - | - | - | b | 2.30 | - | - | - |
| Euglena viridis (O.F.Müller) Ehrenberg | P-B,S | eterm | st-str | ind | 4.0–8.5 | mh | - | i | 4.00 | - | - | k |
| Euglenaformis proxima (P.A.Dangeard) M.S.Bennett & Triemer | P-B | eterm | st-str | ind | 6.8–8.8 | mh | - | p-a | 3.50 | - | - | - |
| Lepocinclis oxyuris (Schmarda) B.Marin & Melkonian | P-B | - | st-str | ind | 7 | mh | - | a-o | 2.60 | - | - | - |
| Lepocinclis spirogyroides B.Marin & Melkonian | P-B | - | st-str | ind | 7.6 | i | - | b-a | 2.40 | - | - | - |
| Monomorphina pyrum (Ehrenberg) Mereschkowsky | P-B | eterm | st-str | ind | 5.5–9.5 | mh | - | b | 2.35 | - | - | - |
| Phacus caudatus Hübner | P-B | eterm | st-str | alf | 7.9 | i | - | b | 2.30 | - | - | - |
| Phacus orbicularis Hübner | P-B | - | st-str | ind | 7.0 | i | - | b-a | 2.45 | - | - | - |
| Trachelomonas dubia Svirenko | P | - | st-str | - | - | - | - | b | 2.00 | - | - | - |
| Trachelomonas dybowskii Dreżepolski | - | - | - | - | - | - | - | b | 2.30 | - | - | - |
| Trachelomonas hispida (Perty) F.Stein | P-B | eterm | st-str | - | - | i | - | b | 2.20 | - | - | k |
| Trachelomonas intermedia P.A.Dangeard | P-B | eterm | - | - | - | i | - | b | 2.20 | - | - | - |
| Trachelomonas lacustris Dreżepolski | - | eterm | - | - | - | hb | - | o-b | 1.50 | - | - | - |
| Trachelomonas macropunctata (Skvortsov) Deflandre | - | - | - | - | - | - | - | b | 2.25 | - | - | - |
| Trachelomonas planctonica f. oblonga (Drezepolski) T.G.Popova | P | eterm | st-str | - | - | - | - | b | 2.20 | - | - | - |
| Trachelomonas similis A.Stokes | P-B | - | - | - | - | i | - | b | 2.20 | - | - | - |
Notes: “-“, not found. Abbreviations: habitat (Hab) (P—planktonic, P-B—plankto-benthic, B—benthic, S—soil); temperature (T) preferences (cool—cool water, temp—temperate, eterm—eurythermic, warm—warm water); oxygenation and water movement (Oxy) (aer—aerophiles, str—streaming water, st-str—low streaming water, st—standing, H2S—sulfides); pH preference groups (pH) according to Hustedt (1957) [52] (alb—alkalibiontes; alf—alkaliphiles, ind—indifferent; acf—acidophiles, neu—neutrophiles as a part of indifferents); salinity ecological groups (Sal) according to Hustedt (1938–1939) [53] (hb—oligohalobes–halophobes, i—oligohalobes-indifferent, hl—halophiles, mh—mesohalobes, oh—oligohalobes of wide spectrum with optimum as indifferent); Index S, species-specific index saprobity according to Sládeček (1986) [54]; self-purification zone with index of saprobity (Sap) (x/0.0—xenosaprobe, x-o/0.4—xeno-oligosaprobe, o-x/0.6—oligo-xenosaprobe, x-b/0.8—xeno-betamesosaprobe, o/1.0—oligosaprobe, o-b/1.4—oligo-betamesosaprobe, b-o/1.6—beta-oligosaprobe, o-a/1.8—oligo-alphamesosaprobe, b/2.0—betamesosaprobe, b-a/2.4—beta-alphamesosaprobe, a-o/2.6—alpha-oligosaprobe, a/3.0—alphamesosaprobe, p-a/4.0—poly-alphamesosaprobe, i/>4.0—i-eusaprobe); organic pollution indicators according to Watanabe et al. (1986) [55] (D) (sx—saproxenes, es—eurysaprobes, sp—saprophiles); nitrogen uptake metabolism (Aut-Het) (Van Dam et al., 1994) [56] (ats—nitrogen-autotrophic taxa, tolerating very small concentrations of organically bound nitrogen; ate—nitrogen-autotrophic taxa, tolerating elevated concentrations of organically bound nitrogen; hne—facultative nitrogen-heterotrophic taxa, needing periodically elevated concentrations of organically bound nitrogen; hce—obligate nitrogen-heterotrophic taxa, needing continuously elevated concentrations of organically bound nitrogen); trophic state indicators (Tro) (Van Dam et al., 1994) [56] (ot—oligotraphentic, om—oligomesotraphentic, m—mesotraphentic, me—mesoeutraphentic, e—eutraphentic, o-e—oligo to hypereutraphentic).
References
- The Arctic Biodiversity Assessment (ABA) Safeguarding Arctic Biodiversity. Available online: https://arctic-council.org/explore/topics/biodiversity (accessed on 26 March 2024).
- Knights, D.; Piliouras, A.; Schwenk, J.; Hariharan, J.; Russoniello, C. Seasonal and morphological controls on nitrate retention in Arctic deltas. Geophys. Res. Lett. 2023, 50, 1–10. [Google Scholar]
- Kosinskaya, E.K. To the flora of freshwater algae of the New Siberian Islands. Proc. Bot. Inst. Russia Acad. Sci. 1956, 2, 5–32. (In Russian) [Google Scholar]
- Ushnitskaya, L.A.; Gorodnichev, R.M.; Spiridonova, I.M.; Pestryakova, L.A. Preliminary limnological characteristics of water bodies of the Faddeevsky Peninsula (New Siberian Islands). Int. J. Appl. Fundam. Res. 2013, 8, 189–192. (In Russian) [Google Scholar]
- Genkal, S.I.; Gabyshev, V.A. Diatoms (Bacillariophyta) of waterbodies and watercourses of Kotelny Island (New Siberian Islands). Bot. Zhurnal 2020, 105, 750–761. (In Russian) [Google Scholar] [CrossRef]
- Bessudova, A.Y.; Gabyshev, V.A.; Firsova, A.D.; Likhoshway, Y.V. Silica-scaled protists (Chrysophyceae. Centroplasthelida. Thaumatomonadida and Rotosphaerida) in waters bodies of Kotelny Island. Russian Arctic. Polar Biol. 2023, 46, 895–913. [Google Scholar] [CrossRef]
- Barinova, S.; Stenina, A. Diatom diversity and ecological variables in the Arctic lakes of the Kostyanoi Nos Cape (Nenetsky Natural Reserve, Russian North). Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2013, 147, 397–410. [Google Scholar] [CrossRef]
- Barinova, S.; Gabyshev, V.; Genkal, S. Diversity of Diatom Algae in the Lena Delta Nature Reserve and the Adjacent Territory in the Specific Ecological Factors of the Arctic. Diversity 2023, 15, 802. [Google Scholar] [CrossRef]
- Kudryavtsev, V.A.; Dostovalov, V.N. General Permafrost Science; Moscow State University Publishing House: Moscow, Russia, 1967; 403p. (In Russian) [Google Scholar]
- Climate of the Yakut Autonomous Soviet Socialist Republic (Atlas); Izyumenko, S.A., Ed.; Gidrometeoizdat: Leningrad, Russia, 1968; 33p. (In Russian) [Google Scholar]
- Balonov, I.M. Preparation of diatoms and golden algae for electron microscopy. In Methods for the Study of Biogeocenoses of Inland Waterbodies; Nauka: Moscow, Russia, 1975; pp. 87–89. (In Russian) [Google Scholar]
- Lange-Bertalot, H.; Moser, G. Brachysira: Monographie der Gattung; wichtige indicator-species für das Gewässer-Monitoring und Naviculadicta nov. gen.; ein Lösungsvorschlag zu dem Problem Navicula sensu lato onhe Navicula sensu stricto. Bibl. Diatomol. 1994, 29, 1–212. [Google Scholar]
- Krammer, K. Die cymbelloiden Diatomeen. Teil 1. Allgemeines und Encyonema part. Bibl. Diatomol. 1997, 36, 1–382. [Google Scholar]
- Krammer, K. Die cymbelloiden Diatomeen. Teil 2. Encyonema part., Encyonopsis und Cymbellopsis. Bibl. Diatomol. 1997, 7, 1–469. [Google Scholar]
- Krammer, K. Pinnularia. In Diatoms of Europe; Lange-Bertalot, H., Ed.; A.R.G. Gantner Verlag K.-G.: Ruggell, Liechtenstein, 2000; Volume 1, pp. 1–703. [Google Scholar]
- Krammer, K. Cymbella. In Diatoms of Europe; Lange-Bertalot, H., Ed.; A.R.G. Gantner Verlag K.-G.: Ruggell, Liechtenstein, 2002; Volume 3, pp. 1–584. [Google Scholar]
- Krammer, K. Cymbopleura, Delicata, Navicymbula, Gomphocymbellopsis, Afrocymbella. In Diatoms of Europe; Lange-Bertalot, H., Ed.; A.R.G. Gantner Verlag K.-G.: Ruggell, Liechtenstein, 2003; Volume 4, pp. 1–530. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. Teil 1. Naviculaceae. In Die Süsswasserflora von Mitteleuropa; Bd. 2/1; Gustav Fisher Verlag: Stuttgart, Germany, 1986; pp. 1–876. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. Teil 2. Epithemiaceae, Bacillariaceae, Surirellaceae. In Die Süsswasserflora von Mitteleuropa; Bd. 2/2; Gustav Fisher Verlag: Stuttgart, Germany, 1988; pp. 1–536. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. Teil 3. Centrales, Fragilariaceae, Eunotiaceae. In Die Süsswasserflora von Mitteleuropa; Gerloff, H., Ed.; Gustav Fisher Verlag: Stuttgart, Germany, 1991; pp. 1–576. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. Teil. 4. Achnanthaceae, Kritische Erganzungen zu Navicula (Lineolatae) und Gomphonema. In Die Süsswasserflora von Mitteleuropa; Gustav Fisher Verlag: Stuttgart, Germany, 1991; pp. 1–437. [Google Scholar]
- Lange-Bertalot, H.; Genkal, S.I. Diatoms of Siberia. I. Iconogr. Diatomol. 1999, 6, 7–272. [Google Scholar]
- Reichardt, E. Zur revision der gattung Gomphonema. Iconogr. Diatomol. 1999, 8, 1–203. [Google Scholar]
- Lange-Bertalot, H. Navicula sensu stricto, 10 genera separated from Navicula sensu lato Frustulia. In Diatoms of Europe; Lange-Bertalot, H., Ed.; A.R.G. Gantner Verlag K.-G.: Ruggell, Liechtenstein, 2001; Volume 2, pp. 1–526. [Google Scholar]
- Werum, M.; Lange-Bertalot, H. Diatoms in springs from Central Europe and elsewhere under the influence of hydrogeology and anthropogenic impacts. Iconogr. Diatomol. 2004, 13, 1–417. [Google Scholar]
- Levkov, Z. Amphora sensu lato. In Diatoms of Europe; Lange-Bertalot, H., Ed.; A.R.G. Gantner Verlag K.-G.: Ruggell, Liechtenstein, 2009; Volume 5, pp. 1–916. [Google Scholar]
- Genkal, S.I.; Bondarenko, N.A.; Shchur, L.A. Diatoms of Lakes in the South and North of Eastern Siberia; OAO “Rybinsk Press House”: Rybinsk, Russia, 2011; 72p. (In Russian) [Google Scholar]
- Lange-Bertalot, H.; Bak, M.; Witkowski, A. Eunotia and some related genera. In Diatoms of Europe; A.R.G. Gantner Verlag K.-G.: Ruggell, Liechtenstein, 2011; Volume 6, pp. 1–747. [Google Scholar]
- Kharitonov, V.G.; Genkal, S.I. Diatoms of Lake Elgygytgyn and Its Environs (Chukotka); NESC FEB RAS: Magadan, Russia, 2012; pp. 1–402. (In Russian) [Google Scholar]
- Levkov, Z.; Metzeltin, D.; Pavlov, A. Luticola, Luticolopsis. In Diatoms of Europe; Lange-Bertalot, H., Ed.; A.R.G. Gantner Verlag K.-G.: Ruggell, Liechtenstein, 2013; Volume 7, pp. 1–697. [Google Scholar]
- Levkov, Z.; Mitić-Kopanja, D.; Reichardt, E. The diatom genus Gomphonema from the Republik of Macedonia. In Diatoms of Europe; Lange-Bertalot, H., Ed.; A.R.G. Gantner Verlag K.-G.: Ruggell, Liechtenstein, 2016; Volume 8, pp. 1–552. [Google Scholar]
- Kulikovskiy, M.S.; Glushchtnko, A.M.; Genkal, S.I.; Kuznetsova, I.V. Identification Book of Diatoms from Russia; Filigran’: Yaroslavl, Russia, 2016; 804p. (In Russian) [Google Scholar]
- Lange-Bertalot, H.; Hofmann, G.; Werum, M.; Cantonati, M. Freshwater Benthic Diatoms of Central Europe; Koeltz Botanical Books: Schmitten-Oberreifenberg, Germany, 2017; 942p. [Google Scholar]
- Beauger, A.; Allain, E.; Voldoire, O.; Blavignac, C.; Rossi, S.; Wetzel, C.; Ector, L. Gomphosphenia vallei (Bacillariophyta), a new diatom species from a stream in the “Réserve Naturelle Nationale de la Vallée de Chaudefour”, Massif Central (France). Phytotaxa 2022, 542, 167–179. [Google Scholar] [CrossRef]
- Komárek, J. Heterocytous Genera. Cyanoprokaryota; Springer Spektrum: Berlin/Heidelberg, Germany, 2013; Volume 3, pp. 3–1130. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota. T. 1. Chroococcales; Gustav Fischer Verlag: Jena, Germany, 1998; 548p. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota. T. 2. Oscillatoriales; Elsevier: München, Germany, 2005; 759p. [Google Scholar]
- Hindák, F.; Wolowski, K. Atlas of Euglenophytes; VEDA: Bratislava, Slovakia, 2005; 136p. [Google Scholar]
- Vasilyeva, I.I. Freshwater Euglenids and Yellow-Green Algae of Waterbodies of Yakutia; Nauka: Leningrad, Russia, 1987; 265p. (In Russian) [Google Scholar]
- Popovský, J.; Pfiester, L.A. Dinophyceae (Dinoflagellida); Gustav Fischer Verlag: Jena, Stuttgart, Germany, 1990; 272p. [Google Scholar]
- Tsarenko, P.M. Brief Identification Book of Chlorococcal Algae of the Ukrainian SSR; Naukova Dumka: Kyiv, Russia, 1990; 208p. (In Russian) [Google Scholar]
- Palamar-Mordvintseva, G.M. Green Algae. Class Conjugates. Identification Book of Freshwater Algae of the USSR; Nauka: Leningrad, Russia, 1982; Volume 11, 483p. (In Russian) [Google Scholar]
- Starmach, K. Chrysophyceae und Haptophyceae; VEB Gustav Fischer Verlag: Jena, Germany, 1985; 515p. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase World-Wide Electronic Publication. National University of Ireland, Galway. Available online: http://www.algaebase.org (accessed on 15 January 2024).
- Boudouresque, C.F.; Caumette, P.; Bertrand, J.C.; Normand, P.; Sime-Ngando, T. Systematic and evolution of microorganisms: General concepts. In Environmental Microbiology: Fundamentals and Applications. Microbial Ecology; Bertrand, J.C., Caumette, P., Lebaron, P., Matheron, R., Normand, P., Sime-Ngando, T., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 107–144. [Google Scholar]
- Boudouresque, C.F. Taxonomy and phylogeny of unicellular eukaryotes. In Environmental Microbiology: Fundamentals and Applications. Microbial Ecology; Bertrand, J.C., Caumette, P., Lebaron, P., Matheron, R., Normand, P., Sime-Ngando, T., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 191–257. [Google Scholar]
- Barinova, S. Essential and practical bioindication methods and systems for the water quality assessment. Int. J. Environ. Sci. Nat. Resour. 2017, 2, 555588. [Google Scholar] [CrossRef]
- Barinova, S.S.; Medvedeva, L.A.; Anissimova, O.V. Diversity of Algal Indicators in Environmental Assessment; Pilies Studio Publisher: Tel Aviv, Israel, 2006; 498p. (In Russian) [Google Scholar]
- Barinova, S.S.; Bilous, O.P.; Tsarenko, P.M. Algal Indication of Water Bodies in Ukraine: Methods and Prospects; Publishing House of Haifa University: Haifa, Israel, 2019; 367p. (In Russian) [Google Scholar]
- Love, J.; Selker, R.; Marsman, M.; Jamil, T.; Dropmann, D.; Verhagen, J.A.; Ly, A.; Gronau, F.Q.; Smira, M.; Epskamp, S.; et al. JASP: Graphical statistical software for common statistical designs. J. Stat. Softw. 2019, 88, 1–17. [Google Scholar] [CrossRef]
- McAleece, N.; Gage, J.D.G.; Lambshead, P.J.D.; Paterson, G.L.J. BioDiversity Professional Statistics Analysis Software; Jointly Developed by the Scottish Association for Marine Science and the Natural History Museum London; Informer Technologies, Inc.: Los Angeles, CA, USA; The Scottish Association for Marine Science: Oban, UK, 1997. [Google Scholar]
- Hustedt, F. Die Diatomeenflora des Flußsystems der Weser im Gebiet der Hansestadt Bremen. Abh. Naturwissenschaftlichen Ver. Brem. 1957, 34, 181–440. [Google Scholar]
- Hustedt, F. Systematisch und Ökologische Untersuchungen über die Diatomeenflora von Java, Bali und Sumatra. Archiv Hydrobiol. Suppl. 1938, 15, 131–177, 393–506, 638–790, 1939, 16, 1–155, 274–394. [Google Scholar]
- Sládeček, V. Diatoms as indicators of organic pollution. Acta Hydrochim. Hydrobiol. 1986, 14, 555–566. [Google Scholar] [CrossRef]
- Watanabe, T.; Asai, K.; Houki, A. Numerical estimation of organic pollution of flowing water by using the epilithic diatom assemblage—Diatom Assemblage Index (DAIpo). Sci. Total Environ. 1986, 55, 209–218. [Google Scholar] [CrossRef]
- Van Dam, H.; Mertens, A.; Sinkeldam, J. A coded checklist and ecological indicator values of freshwater diatoms from the Netherlands. Neth. J. Aquat. Ecol. 1994, 28, 117–133. [Google Scholar] [CrossRef]
- Vasilyeva, I.I. Algae of the New Siberian Islands. In Proceedings of the XI All-Union Symposium Biological Problems of the North, Yakutsk, Russia, 25 September 1986; Volume 2, pp. 39–40. (In Russian). [Google Scholar]
- Anisimov, M.A.; Ivanova, V.V.; Pushina, Z.V.; Pitulko, V.V. Lagoon deposits of the Zhokhov Island: Age, formation conditions and significance for paleogeographic reconstruction of the New Siberian Islands region. Izv. RAS. Geogr. Ser. 2009, 5, 107–119. (In Russian) [Google Scholar]
- Palagushkina, O.V.; Wetterich, S.; Schirrmeister, L.; Nazarova, L.B. Modern and Fossil Diatom Assemblages from Bol’shoy Lyakhovsky Island (New Siberian Archipelago. Arctic Siberia). Contemp. Probl. Ecol. 2017, 10, 380–394. [Google Scholar] [CrossRef]
- Global Monthly Climate Data on an OpenLayers Map. Available online: https://climatemaps.romgens.com (accessed on 26 March 2024).
- Barinova, S.; Gabyshev, V.; Genkal, S.; Gabysheva, O. Diatoms of Small Water Bodies as Bioindicators in the Assessment of Climatic and Anthropogenic Impacts on the Coast of Tiksi Bay, Russian Arctic. Water 2023, 15, 1533. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).