Recovery of Elemental Arsenic from Acidic As-Containing Wastewater by a Hypophosphite Reduction Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods
2.3. Analytical Methods
3. Results and Discussion
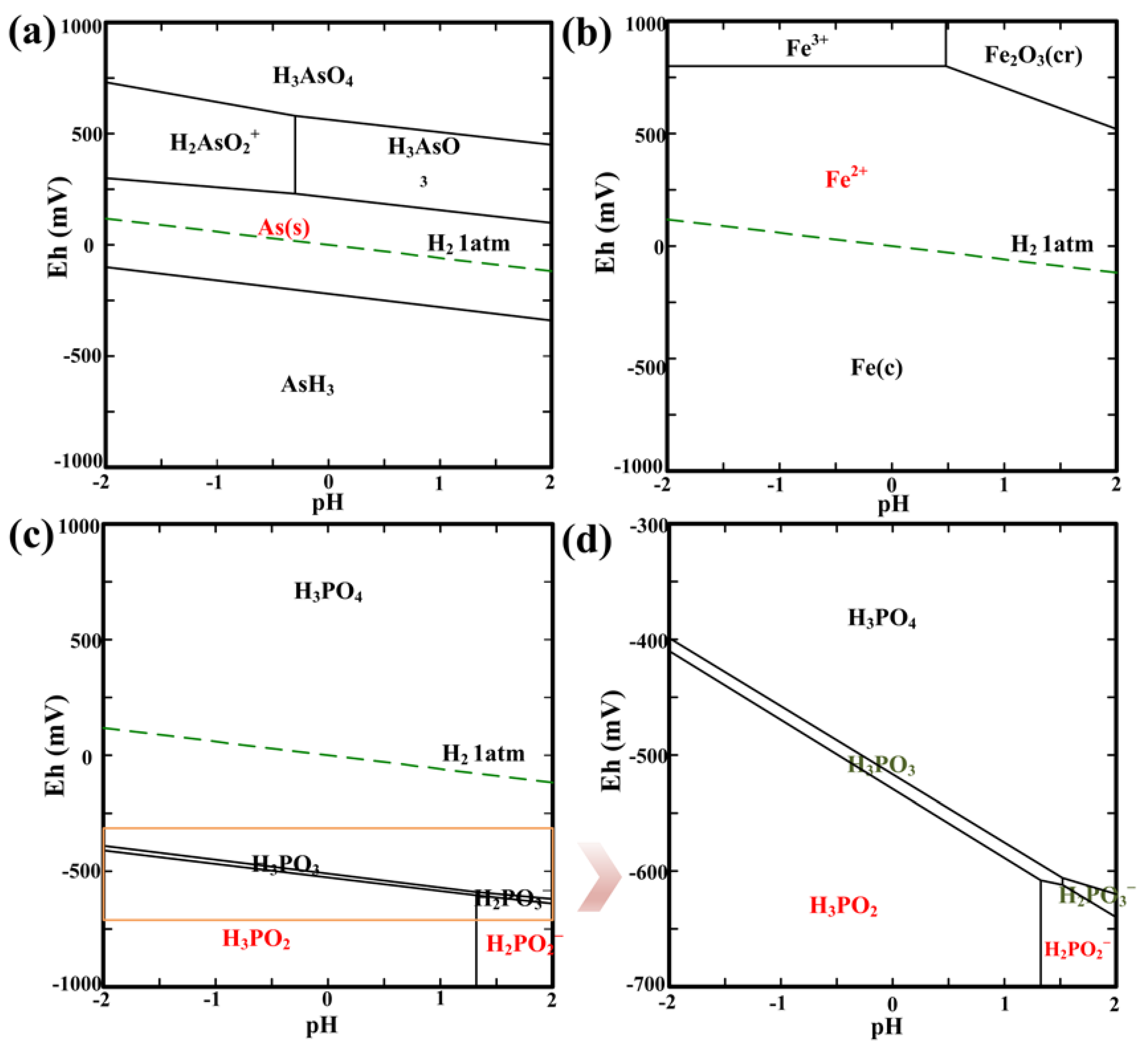
3.1. Thermodynamic Analysis
3.2. Recycling of As from the Acidic Wastewater by Hypophosphite Reduction
3.2.1. Effect of Dissolved Oxygen
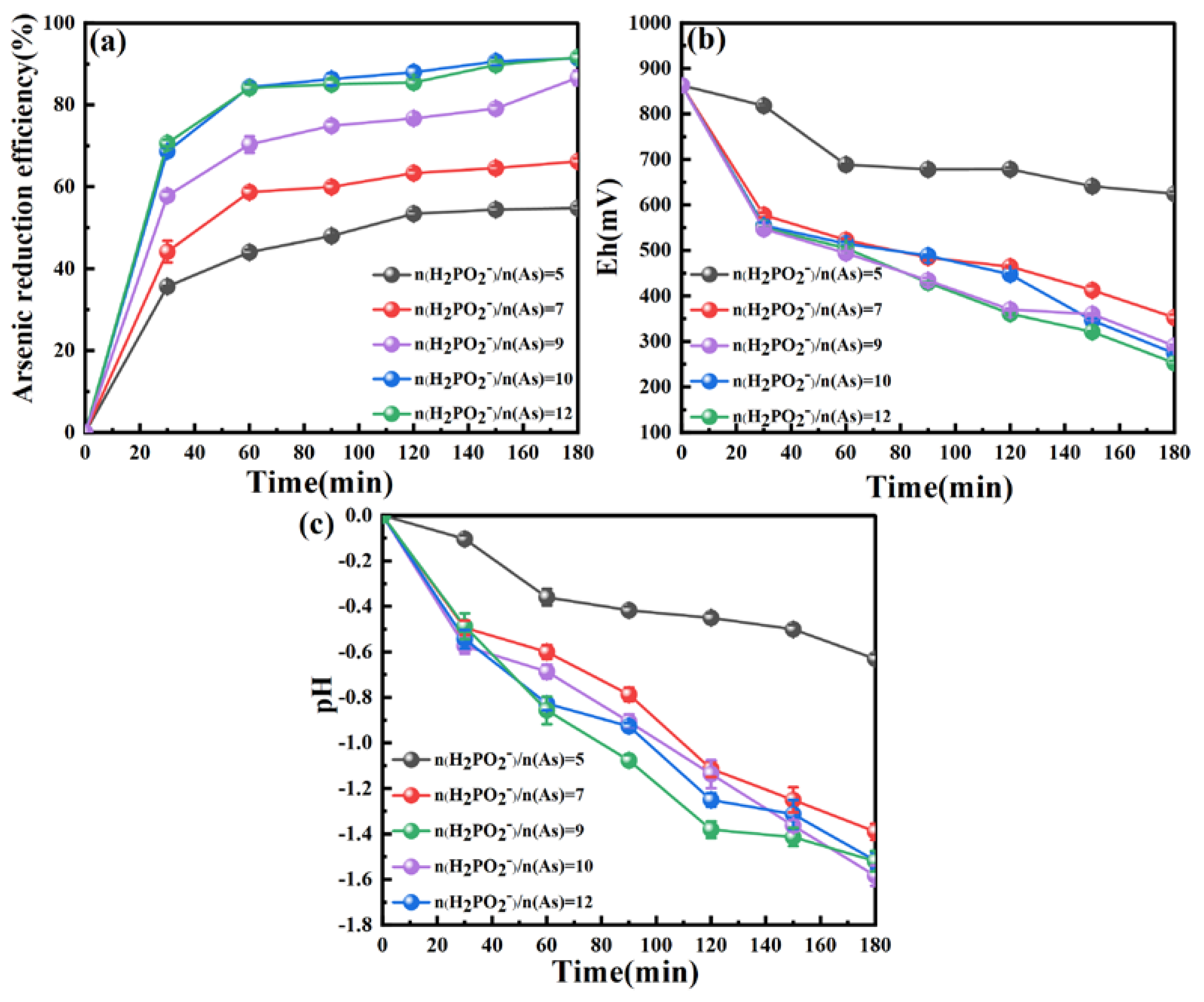
3.2.2. Effect of Hypophosphite to Arsenic Molar Ratio
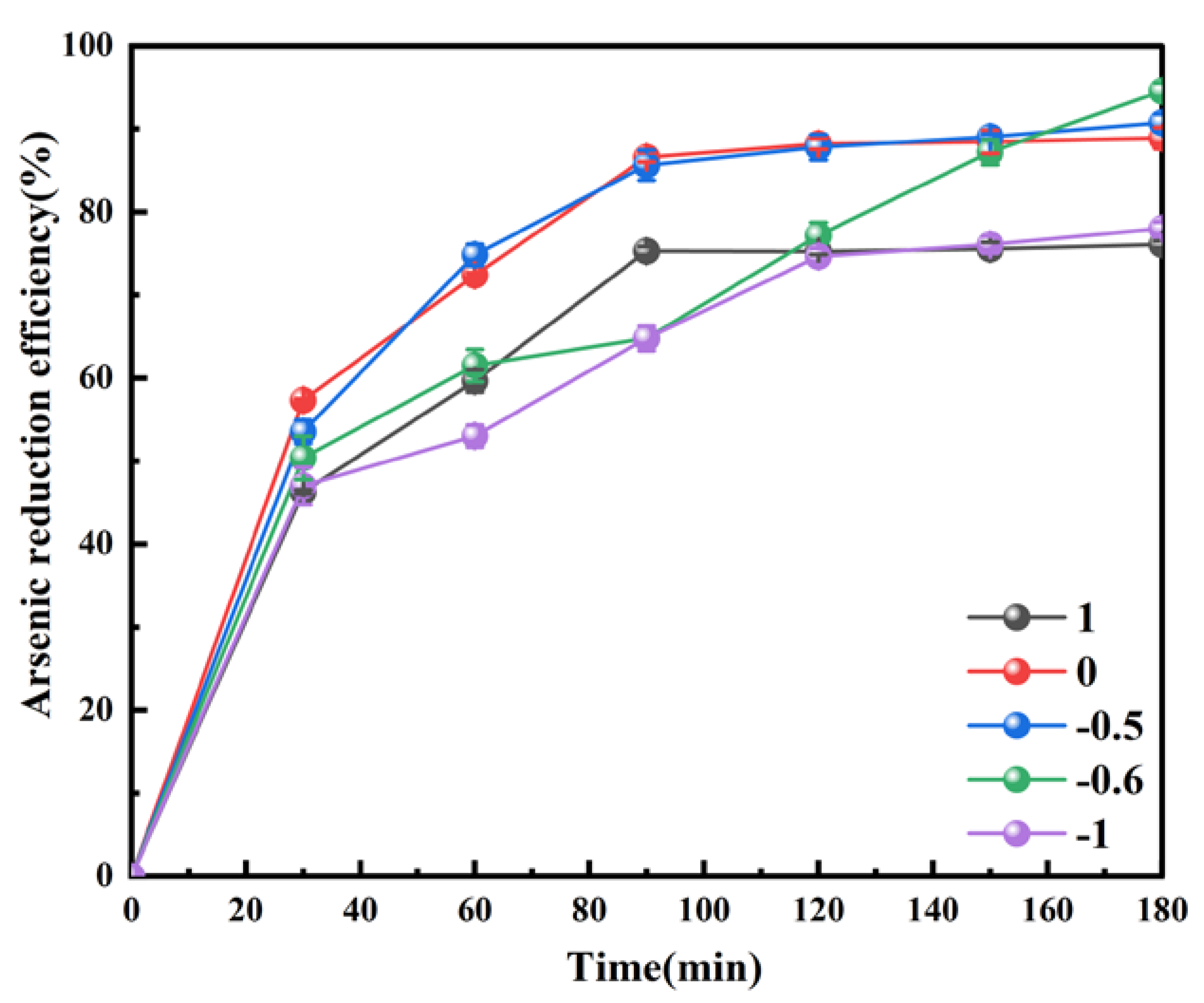
3.2.3. Effect of pH
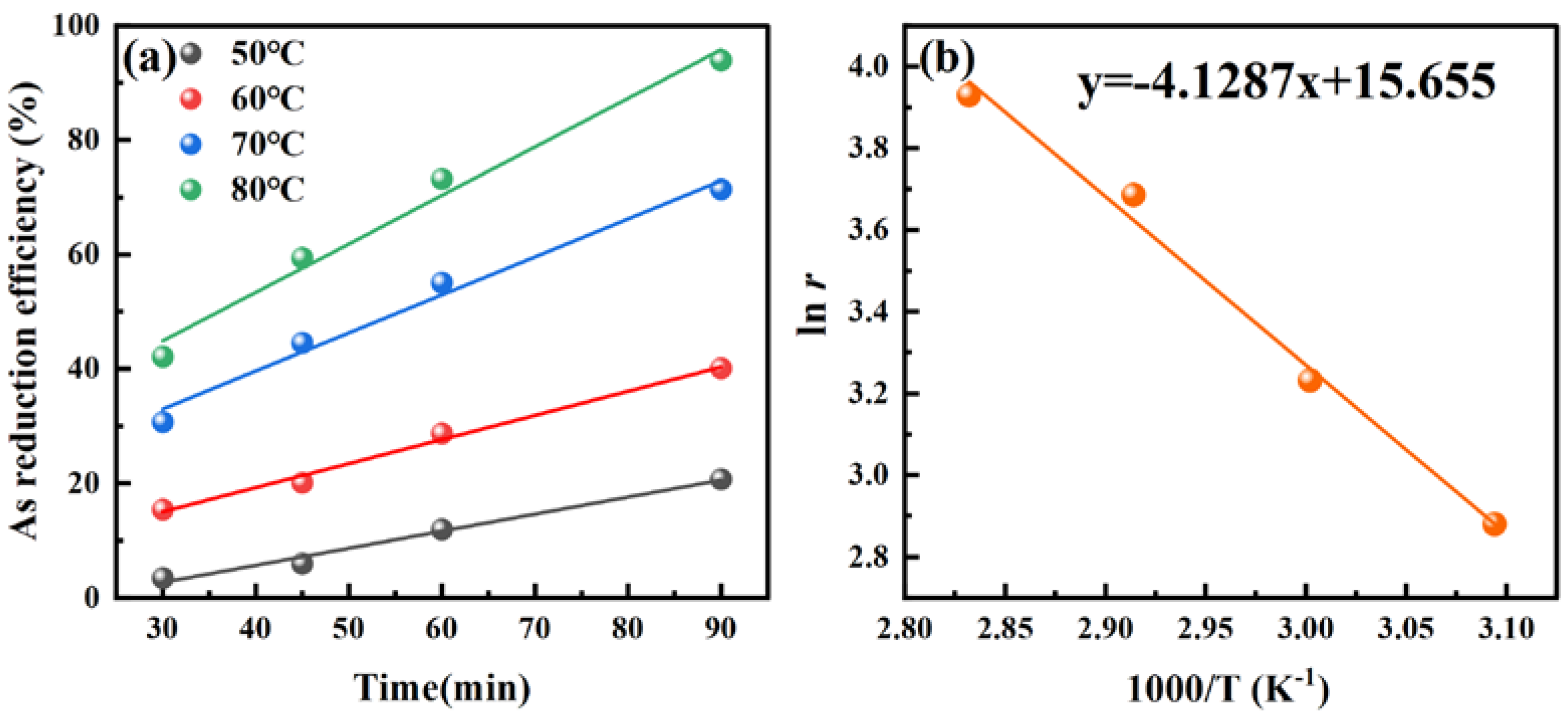
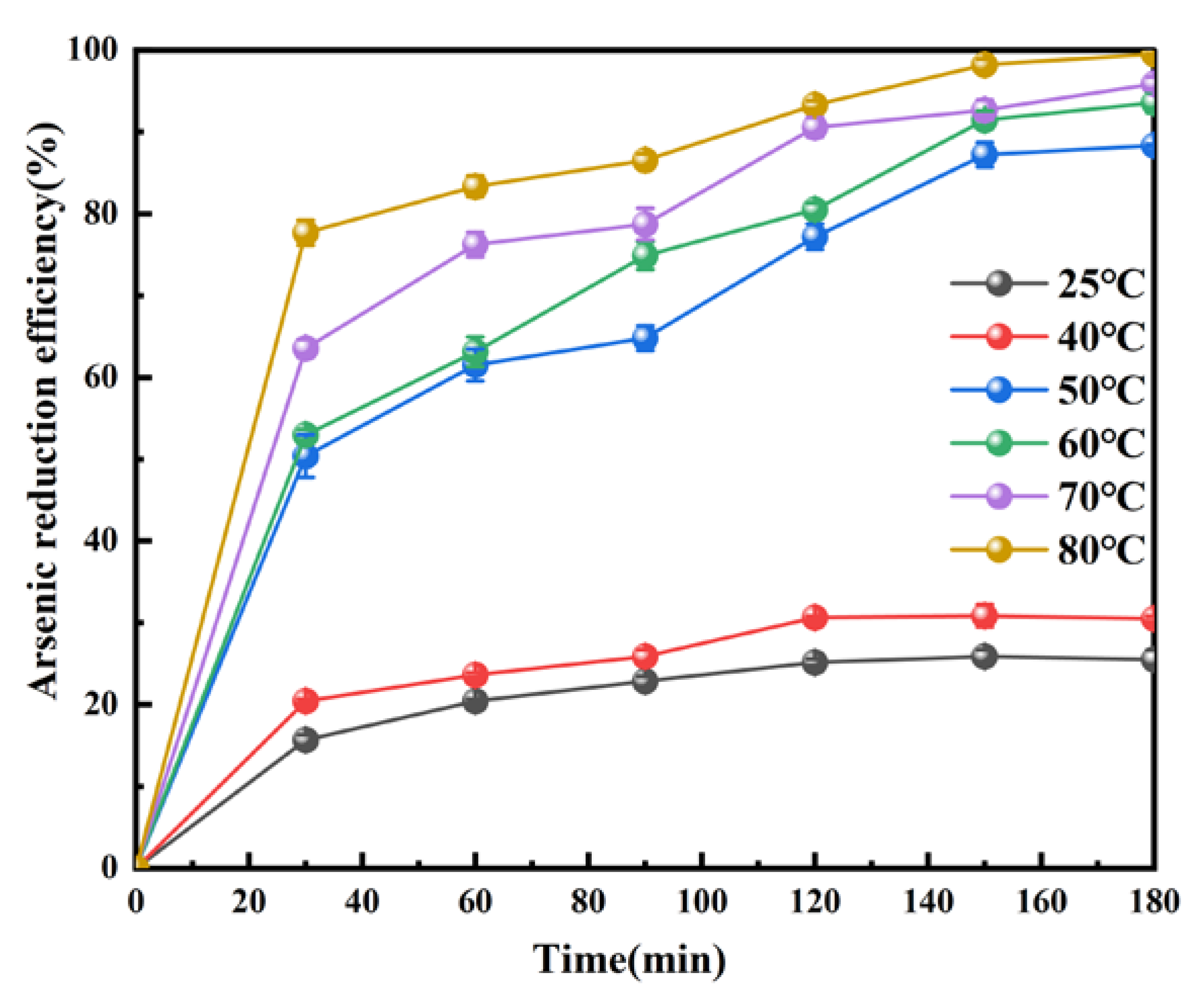
3.2.4. Effect of Temperature
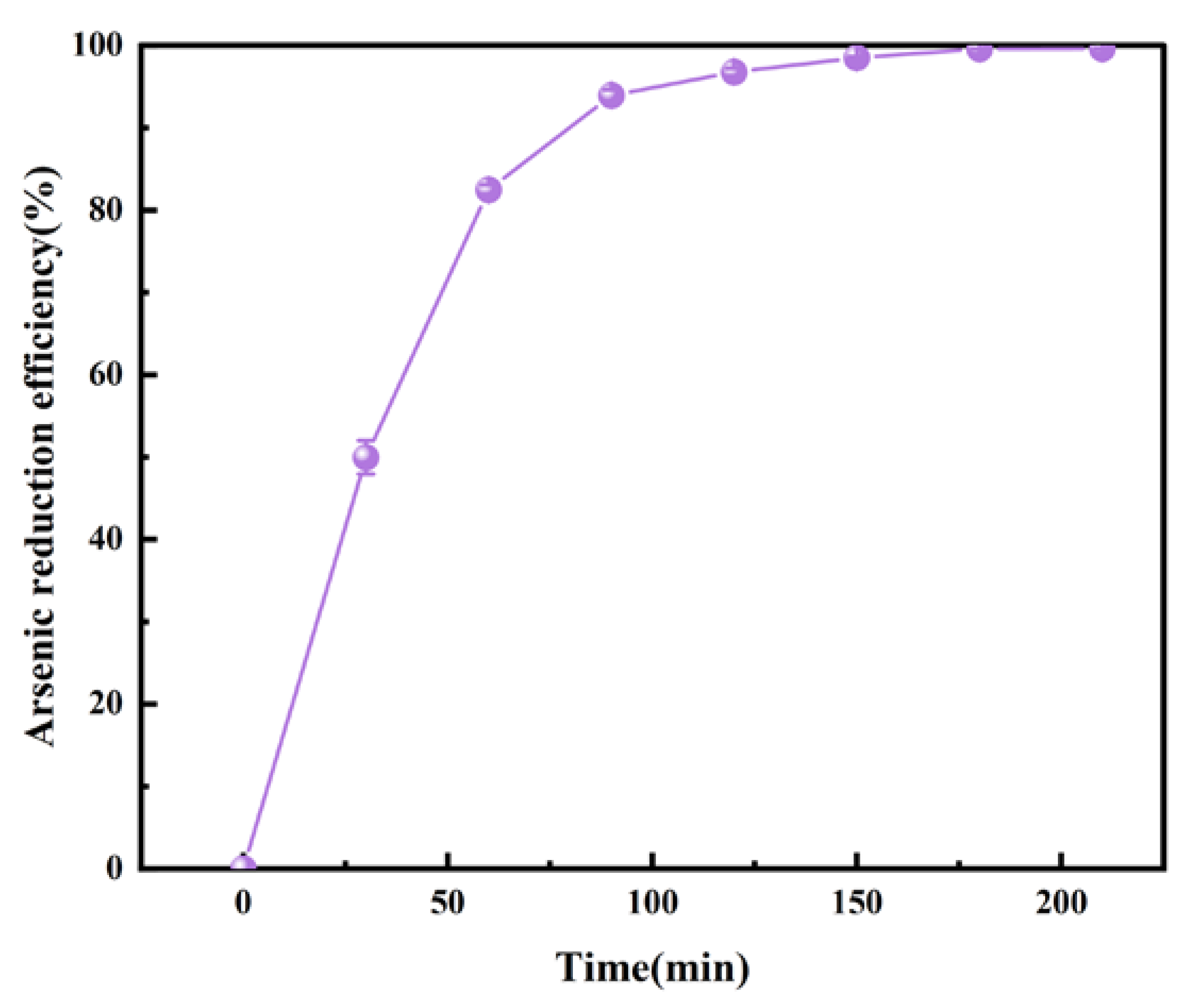
3.2.5. Effect of Time
3.3. Kinetics of the Hypophosphite Reduction Process
3.3.1. Reaction Order of Hypophosphite
3.3.2. Activation Energy
3.4. Characterization of the Elemental Arsenic Product
3.5. Reuse of the As-Containing Wastewater after Hypophosphite Reduction as Biological Culture Medium
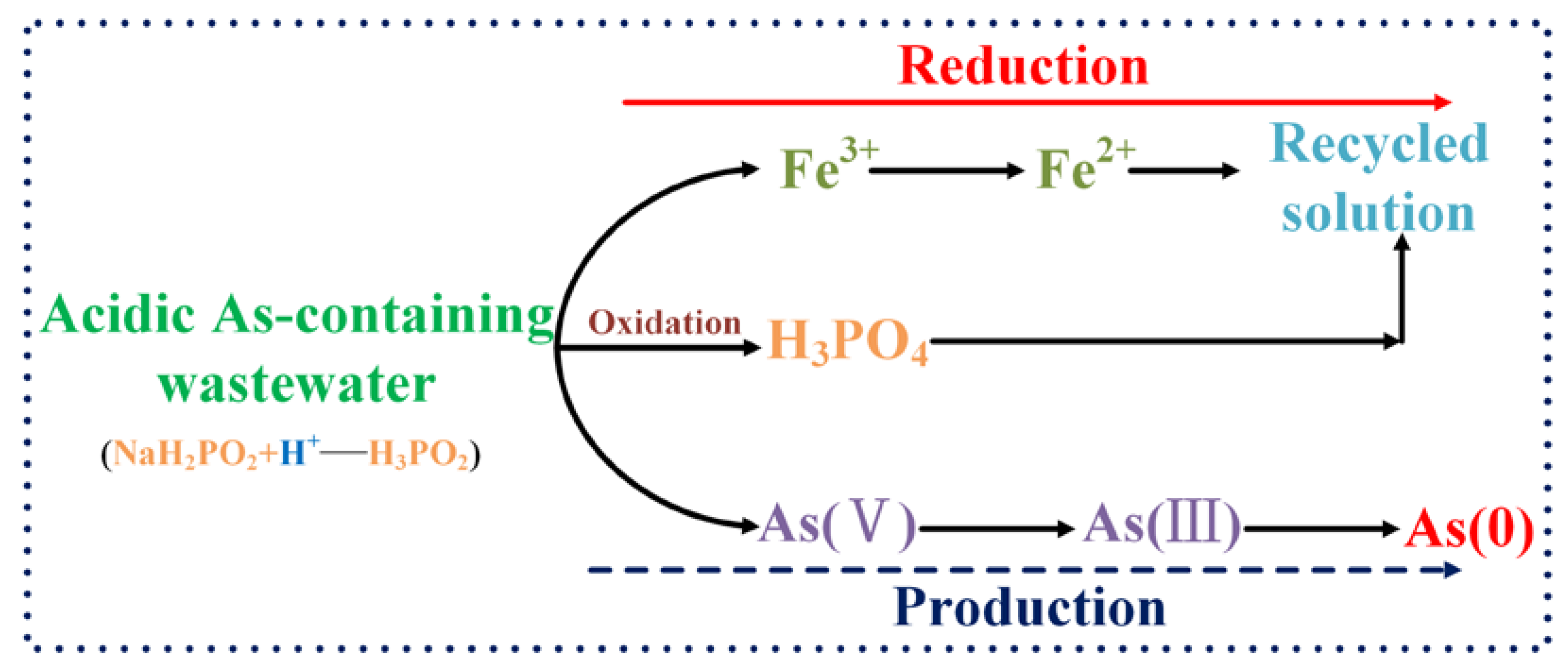
3.6. Hypophosphite Reduction Mechanisms for As Recycling from the Acidic As-Containing Wastewater
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, J.; Chen, S.; Liu, J.; Frost, R.L. Adsorption kinetic and species variation of arsenic for As(V) removal by biologically mackinawite (FeS). Chem. Eng. J. 2018, 354, 237–244. [Google Scholar] [CrossRef]
- Zhang, Q.; Xia, H.; Xu, Y.; Jiang, G.; Cai, W.; Zhang, L. Mechanism of removal of toxic arsenic (As) from zinc sulfate solution by ultrasonic enhanced neutralization with zinc roasting dust. Sep. Purif. Technol. 2023, 322, 124258. [Google Scholar] [CrossRef]
- Wu, J.; Li, Z.; Huang, D.; Liu, X.; Tang, C.; Parikh, S.J.; Xu, J. A novel calcium-based magnetic biochar is effective in stabilization of arsenic and cadmium co-contamination in aerobic soils. J. Hazard. Mater. 2020, 387, 122010. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.I.; Naushad, M.; Chaudhry, S.A. Promising prospects of nanomaterials for arsenic water remediation: A comprehensive review. Process Saf. Environ. Prot. 2019, 126, 60–97. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Sasaki, R.; Igarashi, T.; Park, I.; Tamoto, S.; Arima, T.; Ito, M.; Hiroyoshi, N. Simultaneous leaching of arsenite, arsenate, selenite and selenate, and their migration in tunnel-excavated sedimentary rocks: I. Column experiments under intermittent and unsaturated flow. Chemosphere 2017, 186, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, T.; Herrera, P.S.; Uchiyama, H.; Miyamae, H.; Iyatomi, N.; Hashimoto, K.; Tabelin, C.B. The two-step neutralization ferrite-formation process for sustainable acid mine drainage treatment: Removal of copper, zinc and arsenic, and the influence of coexisting ions on ferritization. Sci. Total Environ. 2020, 715, 136877. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.-C.; Chio, C.-P.; Liao, C.-M. Assessing airborne PM-bound arsenic exposure risk in semiconductor manufacturing facilities. J. Hazard. Mater. 2009, 167, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Melkonian, S.; Argos, M.; Pierce, B.L.; Chen, Y.; Islam, T.; Ahmed, A.; Syed, E.H.; Parvez, F.; Graziano, J.; Rathouz, P.J.; et al. A Prospective Study of the Synergistic Effects of Arsenic Exposure and Smoking, Sun Exposure, Fertilizer Use, and Pesticide Use on Risk of Premalignant Skin Lesions in Bangladeshi Men. Am. J. Epidemiol. 2011, 173, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Alka, S.; Shahir, S.; Ibrahim, N.; Ndejiko, M.J.; Vo, D.-V.N.; Abd Manan, F. Arsenic removal technologies and future trends: A mini review. J. Clean. Prod. 2021, 278, 123805. [Google Scholar] [CrossRef]
- Cermikli, E.; Sen, F.; Altiok, E.; Wolska, J.; Cyganowski, P.; Kabay, N.; Bryjak, M.; Arda, M.; Yuksel, M. Performances of novel chelating ion exchange resins for boron and arsenic removal from saline geothermal water using adsorption-membrane filtration hybrid process. Desalination 2020, 491, 114504. [Google Scholar] [CrossRef]
- Sanchez, J.; Butter, B.; Chavez, S.; Riffo, L.; Basaez, L.; Rivas, B.L. Quaternized hydroxyethyl cellulose ethoxylate and membrane separation techniques for arsenic removal. Desalination Water Treat. 2016, 57, 25161–25169. [Google Scholar] [CrossRef]
- Zhu, N.; Yan, T.; Qiao, J.; Cao, H. Adsorption of arsenic, phosphorus and chromium by bismuth impregnated biochar: Adsorption mechanism and depleted adsorbent utilization. Chemosphere 2016, 164, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zhang, M.; Lu, Y.; Zhang, H.; Von Lau, E. New insights for improving low-rank coal flotation performance via emulsified waste fried oil collector. Fuel 2024, 357, 129925. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Peng, X.; He, M.; Hu, X.; Zhao, J.; Zhu, F.; Yang, X.; Kong, L. Reductive removal of As(V) and As(III) from aqueous solution by the UV/ sulfite process: Recovery of elemental arsenic. Water Res. 2022, 223, 118981. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Ning, Y.; Yang, S.; Yu, J.; Ouyang, W.; Li, Y. A novel strategy for arsenic removal from acid wastewater via strong reduction processing. Environ. Sci. Pollut. Res. 2023, 30, 43886–43900. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Tian, Y.; Zhen, C.; Li, Z. Thermodynamic analysis and experiment of arsenic recovery from waste liquid of biological oxidation gold extraction. Environ. Chem. 2011, 30, 851–855. [Google Scholar]
- Chen, H.; Ding, L.; Zhang, K.; Chen, Z.; Lei, Y.; Zhou, Z.; Hou, R. Preparation of chemically reduced graphene using hydrazine hydrate as the reduction agent and its NO2 sensitivity at room temperature. Int. J. Electrochem. Sci. 2020, 15, 10231–10242. [Google Scholar] [CrossRef]
- Qian, D.-W.; Yang, J.; Wang, G.-W.; Yang, S.-D. Nickel-Catalyzed Sodium Hypophosphite-Participated Direct Hydrophosphonylation of Alkyne toward H-Phosphinates. J. Org. Chem. 2023, 88, 3539–3554. [Google Scholar] [CrossRef] [PubMed]
- Dechdacho, P.; Howard, S.; Hershey, R.L.; Parashar, R.; Perez, L.J. Effective removal of arsenic from contaminated groundwater using an iron-based metal-organic framework. Environ. Technol. Innov. 2023, 32, 103406. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Liu, X.; Yin, H.; Yang, Y.; Xu, B.; Jiang, T.; He, Y. The catalytic effect of copper ion in the bioleaching of arsenopyrite by Acidithiobacillus ferrooxidans in 9K culture medium. J. Clean. Prod. 2020, 256, 120391. [Google Scholar] [CrossRef]
- Deng, S.; Gu, G.; Xu, B.; Li, L.; Wu, B. Surface characterization of arsenopyrite during chemical and biological oxidation. Sci. Total Environ. 2018, 626, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-R.; Zhang, D.-R.; Li, Q.; Nie, Z.-Y.; Pakostova, E. Release and fate of As mobilized via bio-oxidation of arsenopyrite in acid mine drainage: Importance of As/Fe/S speciation and As(III) immobilization. Water Res. 2022, 223, 118957. [Google Scholar] [CrossRef] [PubMed]
- Karamanev, D.G.; Nikolov, L.N.; Mamatarkova, V. Rapid simultaneous quantitative determination of ferric and ferrous ions in drainage waters and similar solutions. Miner. Eng. 2002, 15, 341–346. [Google Scholar] [CrossRef]
- Lovley, D.R.; Phillips, E.J. Rapid assay for microbially reducible ferric iron in aquatic sediments. Appl. Environ. Microbiol. 1987, 53, 1536–1540. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Zhao, J.; Hu, X.; Zhu, F.; Peng, X. Reductive Removal and Recovery of As(V) and As(III) from Strongly Acidic Wastewater by a UV/Formic Acid Process. Environ. Sci. Technol. 2022, 56, 9732–9743. [Google Scholar] [CrossRef] [PubMed]
- Gu, K.; Li, W.; Han, J.; Liu, W.; Qin, W.; Cai, L. Arsenic removal from lead-zinc smelter ash by NaOH-H2O2 leaching. Sep. Purif. Technol. 2019, 209, 128–135. [Google Scholar] [CrossRef]
- Ostermeyer, P.; Bonin, L.; Folens, K.; Verbruggen, F.; Garcia-Timermans, C.; Verbeken, K.; Rabaey, K.; Hennebel, T. Effect of speciation and composition on the kinetics and precipitation of arsenic sulfide from industrial metallurgical wastewater. J. Hazard. Mater. 2021, 409, 124418. [Google Scholar] [CrossRef]
- Xue, J.; Long, D.; Zhong, H.; Wang, S.; Liu, L. Comprehensive recovery of arsenic and antimony from arsenic-rich copper smelter dust. J. Hazard. Mater. 2021, 413, 125365. [Google Scholar] [CrossRef]




| Element | Fe2+ | Fe3+ | As3+ | As5+ |
| Content (g/L) | 0.231 | 5.363 | 1.647 | 0.876 |
| Element | Fe2+ | Fe3+ | As3+ | As5+ | PO43− |
| Content | 5.591 | 0.003 | 0.003 | 0.007 | 24.367 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Zhao, S.; Zhang, Y.; Li, Y.; Liu, X.; Yang, Y. Recovery of Elemental Arsenic from Acidic As-Containing Wastewater by a Hypophosphite Reduction Process. Water 2024, 16, 1301. https://doi.org/10.3390/w16091301
Li Q, Zhao S, Zhang Y, Li Y, Liu X, Yang Y. Recovery of Elemental Arsenic from Acidic As-Containing Wastewater by a Hypophosphite Reduction Process. Water. 2024; 16(9):1301. https://doi.org/10.3390/w16091301
Chicago/Turabian StyleLi, Qian, Shiyu Zhao, Yan Zhang, Yong Li, Xiaoliang Liu, and Yongbin Yang. 2024. "Recovery of Elemental Arsenic from Acidic As-Containing Wastewater by a Hypophosphite Reduction Process" Water 16, no. 9: 1301. https://doi.org/10.3390/w16091301
APA StyleLi, Q., Zhao, S., Zhang, Y., Li, Y., Liu, X., & Yang, Y. (2024). Recovery of Elemental Arsenic from Acidic As-Containing Wastewater by a Hypophosphite Reduction Process. Water, 16(9), 1301. https://doi.org/10.3390/w16091301






