Nejayote and Food Waste Leachate as a Medium for Scenedesmus acutus and Haematococcus pluvialis Production: A Mixture Experimental Design
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algal Strains and Cultivation Conditions
2.2. Waste Streams Source
2.3. Analytical Procedures
2.4. Statistical Analysis
3. Results and Discussion
3.1. Sample Characteristics
3.2. Biomass Growth of S. acutus and H. pluvialis in Nejayote and Food Leachate
3.3. Efficiency in Nutrient Uptake
3.3.1. Ammonium (NH4+)
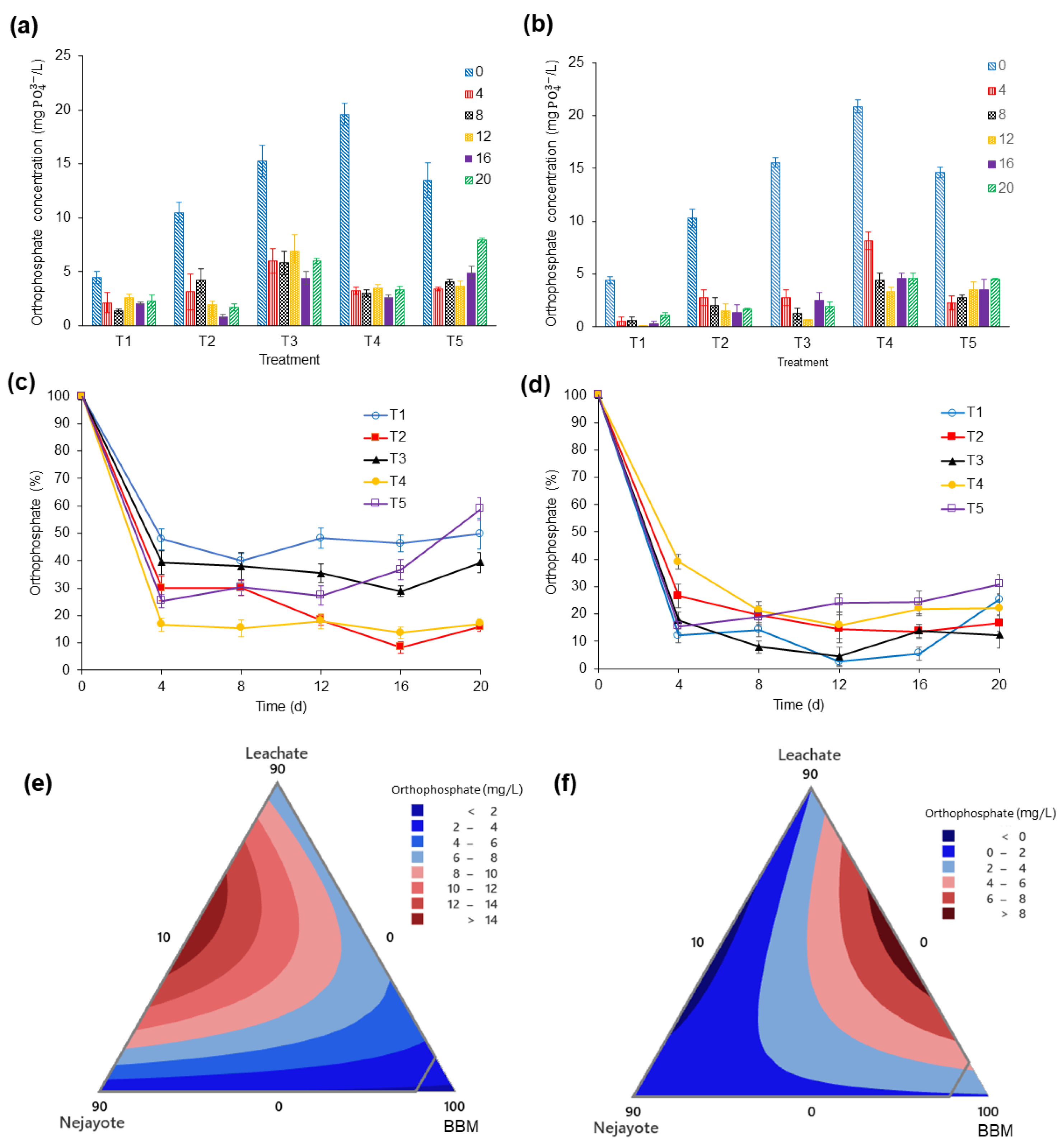
3.3.2. Orthophosphate (PO43−)
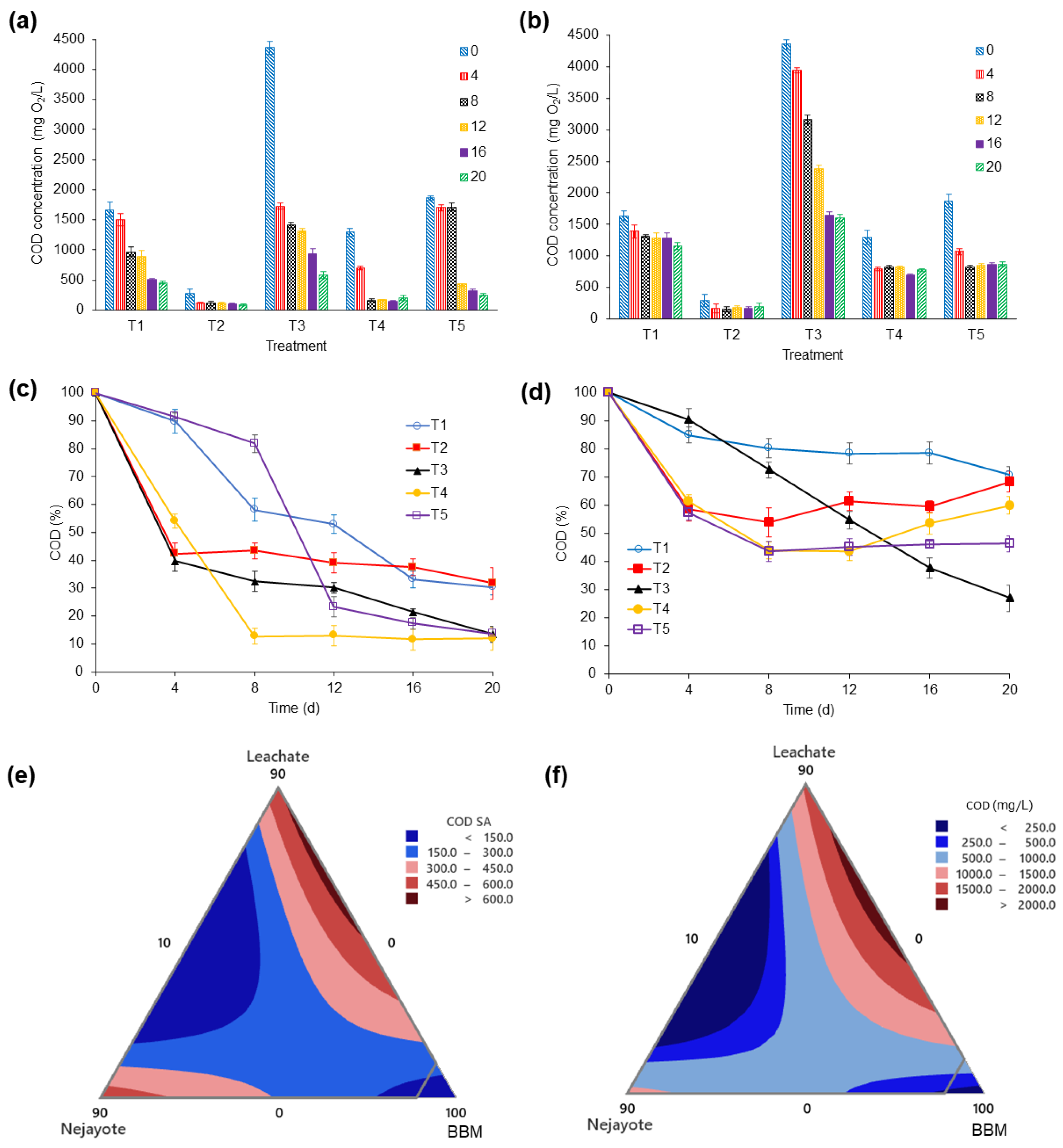
3.3.3. Soluble Chemical Oxygen Demand (COD)
3.4. Determination of the Optimal Conditions for the Growth of S. acutus and H. pluvialis and the Reduction in Nutrients
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sakr, M.; Mohamed, M.M.; Maraqa, M.A.; Hamouda, M.A.; Hassan, A.A.; Ali, J.; Jung, J. A critical review of the recent developments in micro–nano bubbles applications for domestic and industrial wastewater treatment. Alex. Eng. J. 2022, 61, 6591–6612. [Google Scholar] [CrossRef]
- Padrón-Páez, J.I.; Almaraz, S.D.L.; Román-Martínez, A. Sustainable wastewater treatment plants design through multiobjective optimization. Comput. Chem. Eng. 2020, 140, 106850. [Google Scholar] [CrossRef]
- Feng, X.; Chen, Y.; Lv, J.; Han, S.; Tu, R.; Zhou, X.; Jin, W.; Ren, N. Enhanced lipid production by Chlorella pyrenoidosa through magnetic field pretreatment of wastewater and treatment of microalgae-wastewater culture solution: Magnetic field treatment modes and conditions. Bioresour. Technol. 2020, 306, 123102. [Google Scholar] [CrossRef] [PubMed]
- Murwanashyaka, T.; Shen, L.; Yang, Z.; Chang, J.-S.; Manirafasha, E.; Ndikubwimana, T.; Chen, C.; Lu, Y. Kinetic modelling of heterotrophic microalgae culture in wastewater: Storage molecule generation and pollutants mitigation. Biochem. Eng. J. 2020, 157, 107523. [Google Scholar] [CrossRef]
- Jaiswal, K.K.; Kumar, V.; Vlaskin, M.; Sharma, N.; Rautela, I.; Nanda, M.; Arora, N.; Singh, A.; Chauhan, P. Microalgae fuel cell for wastewater treatment: Recent advances and challenges. J. Water Process Eng. 2020, 38, 101549. [Google Scholar] [CrossRef]
- Wang, Y.; Ho, S.-H.; Cheng, C.-L.; Guo, W.-Q.; Nagarajan, D.; Ren, N.-Q.; Lee, D.-J.; Chang, J.-S. Perspectives on the feasibility of using microalgae for industrial wastewater treatment. Bioresour. Technol. 2016, 222, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Mujtaba, G.; Memon, S.A.; Lee, K.; Rashid, N. Exploring the potential of microalgae for new biotechnology applications and beyond: A review. Renew. Sustain. Energy Rev. 2018, 92, 394–404. [Google Scholar] [CrossRef]
- Díaz-Montes, E.; Castro-Muñoz, R. Analyzing the phenolic enriched fractions from Nixtamalization wastewater (Nejayote) fractionated in a three-step membrane process. Curr. Res. Food Sci. 2022, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Serna-Saldivar, S.O. Understanding the functionality and manufacturing of nixtamalized maize products. J. Cereal Sci. 2021, 99, 103205. [Google Scholar] [CrossRef]
- Zaini, M.S.I.; Hasan, M.; Zolkepli, M.F. Urban landfills investigation for leachate assessment using electrical resistivity imaging in Johor, Malaysia. Environ. Chall. 2022, 6, 100415. [Google Scholar] [CrossRef]
- Pap, S.; Stankovits, G.J.; Gyalai-Korpos, M.; Makó, M.; Erdélyi, I.; Sekulic, M.T. Biochar application in organics and ultra-violet quenching substances removal from sludge dewatering leachate for algae production. J. Environ. Manag. 2021, 298, 113446. [Google Scholar] [CrossRef] [PubMed]
- Paiva, A.L.P.; Silva, D.G.d.F.; Couto, E. Recycling of landfill leachate nutrients from microalgae and potential applications for biomass valorization. J. Environ. Chem. Eng. 2021, 9, 105952. [Google Scholar] [CrossRef]
- Lee, P.-E.; Lee, W.-B.; Moon, H.; Kwon, J.; Namkung, H.; Lee, W.; Yoo, M.; Lee, D.-J. A feasibility study on effect of food waste leachate additions in the full-scale waste leachate treatment facility after the african swine fever outbreak in south korea. Energies 2021, 14, 8045. [Google Scholar] [CrossRef]
- Tsui, T.-H.; Wu, H.; Song, B.; Liu, S.-S.; Bhardwaj, A.; Wong, J.W. Food waste leachate treatment using an Upflow Anaerobic Sludge Bed (UASB): Effect of conductive material dosage under low and high organic loads. Bioresour. Technol. 2020, 304, 122738. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.K.; Park, J.M.; Kim, K.H.; Park, H.-S. Methane production from food waste leachate in laboratory-scale simulated landfill. Waste Manag. 2010, 30, 1502–1508. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.H.T.; Ahn, J.; Kim, J.; Lee, S.; Lee, I.; Kim, S.; Chang, S.; Chung, W. Volatile fatty acid production from food waste leachate using enriched bacterial culture and soil bacteria as co-digester. Sustainability 2021, 13, 9606. [Google Scholar] [CrossRef]
- López-Maldonado, E.A.; Oropeza-Guzmán, M.T. Nejayote biopolyelectrolytes multifunctionality (glucurono ferulauted arabinoxylans) in the separation of hazardous metal ions from industrial wastewater. Chem. Eng. J. 2021, 423, 130210. [Google Scholar] [CrossRef]
- Gutiérrez-Uribe, J.A.; Rojas-García, C.; García-Lara, S.; Serna-Saldivar, S.O. Phytochemical analysis of wastewater (nejayote) obtained after lime-cooking of different types of maize kernels processed into masa for tortillas. J. Cereal Sci. 2010, 52, 410–416. [Google Scholar] [CrossRef]
- Zayas, T.; de Gante, A.; Arvide, M.G.T.; Hernández, M.V.; Soriano-Moro, G.; Salgado, L. Treatment of nixtamalization wastewater (nejayote) using electrocoagulation and combined chemical coagulation/electrocoagulation processes. Desalin. Water Treat. 2022, 280, 44–51. [Google Scholar] [CrossRef]
- López-Maldonado, E.A.; Oropeza-Guzmán, M.T.; Suárez-Meraz, K.A. Integral Use of Nejayote: Characterization, New Strategies for Physicochemical Treatment and Recovery of Valuable By-Products; IntechOpen: London, UK, Chapter 11; pp. 239–252. [CrossRef]
- Gao, T.; Li, Y.; Xue, W.; Pan, Y.; Zhu, X. Freshwater Salinization Impacts the Interspecific Competition between Microcystis and Scenedesmus. Water 2023, 15, 1331. [Google Scholar] [CrossRef]
- Singh, D.V.; Upadhyay, A.; Singh, R. Implication of municipal wastewater on growth kinetics, biochemical profile, and defense system of Chlorella vulgaris and Scenedesmus vacuolatus. Environ. Technol. Innov. 2022, 26, 102334. [Google Scholar] [CrossRef]
- de Morais, E.G.; Murillo, A.M.; Lens, P.N.; Ferrer, I.; Uggetti, E. Selenium recovery from wastewater by the green microalgae Chlorella vulgaris and Scenedesmus sp. Sci. Total Environ. 2022, 851, 158337. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Zurano, A.; Morillas-España, A.; Gómez-Serrano, C.; Ciardi, M.; Acién, G.; Lafarga, T. Annual assessment of the wastewater treatment capacity of the microalga Scenedesmus almeriensis and optimisation of operational conditions. Sci. Rep. 2021, 11, 21651. [Google Scholar] [CrossRef] [PubMed]
- Radice, R.P.; Sansone, M.; D’arienzo, G.; Scopa, A.; Martelli, G. Bioremediation of Crude Oil by Haematococcus Pluvialis: A Preliminary Study. Processes 2022, 10, 2472. [Google Scholar] [CrossRef]
- Lee, K.H.; Chun, Y.; Lee, J.H.; Park, C.; Yoo, H.Y.; Kwak, H.S. Improved Productivity of Astaxanthin from Photosensitive Haematococcus pluvialis Using Phototaxis Technology. Mar. Drugs 2022, 20, 220. [Google Scholar] [CrossRef] [PubMed]
- de Souza, L.; Lima, A.S.; Matos, P.; Wheeler, R.M.; Bork, J.A.; Cubas, A.L.V.; Moecke, E.H.S. Biopolishing sanitary landfill leachate via cultivation of lipid-rich Scenedesmus microalgae. J. Clean. Prod. 2021, 303, 127094. [Google Scholar] [CrossRef]
- Wang, F.; Gao, B.; Wu, M.; Huang, L.; Zhang, C. A novel strategy for the hyper-production of astaxanthin from the newly isolated microalga Haematococcus pluvialis JNU35. Algal Res. 2019, 39, 101466. [Google Scholar] [CrossRef]
- Lu, Z.; Zheng, L.; Liu, J.; Dai, J.; Song, L. A novel fed-batch strategy to boost cyst cells production based on the understanding of intracellular carbon and nitrogen metabolism in Haematococcus pluvialis. Bioresour. Technol. 2019, 289, 121744. [Google Scholar] [CrossRef]
- Wu, K.; Ying, K.; Zhou, J.; Liu, D.; Liu, L.; Tao, Y.; Hanotu, J.; Zhu, X.; Cai, Z. Optimizing the growth of Haematococcus pluvialis based on a novel microbubble-driven photobioreactor. iScience 2021, 24, 103461. [Google Scholar] [CrossRef]
- Salbitani, G.; Carfagna, S. Ammonium utilization in microalgae: A sustainable method for wastewater treatment. Sustainability 2021, 13, 956. [Google Scholar] [CrossRef]
- Pham, T.L.; Bui, M.H. Removal of Nutrients from Fertilizer Plant Wastewater Using Scenedesmus sp.: Formation of Bioflocculation and Enhancement of Removal Efficiency. J. Chem. 2020, 2020, 8094272. [Google Scholar] [CrossRef]
- Zheng, H.; Wu, X.; Zou, G.; Zhou, T.; Liu, Y.; Ruan, R. Cultivation of Chlorella vulgaris in manure-free piggery wastewater with high-strength ammonium for nutrients removal and biomass production: Effect of ammonium concentration, carbon/nitrogen ratio and pH. Bioresour. Technol. 2019, 273, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Zhu, X.; Pan, G.; Angelidak, I. Integrated valorization system for simultaneous high strength organic wastewater treatment and astaxanthin production from Haematococcus pluvialis. Bioresour. Technol. 2021, 326, 124761. [Google Scholar] [CrossRef] [PubMed]
- Khanzada, Z.T. Phosphorus removal from landfill leachate by microalgae. Biotechnol. Rep. 2020, 25, e00419. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, H.-Y.; Gan, K.; Sun, Y.-X. Effects of different nitrogen and phosphorus concentrations on the growth, nutrient uptake, and lipid accumulation of a freshwater microalga Scenedesmus sp. Bioresour. Technol. 2010, 101, 5494–5500. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Nagare, H.; Huynh, T.N.C.; Komatsu, H. Development of a new wastewater treatment process for resource recovery of carotenoids. Water Sci. Technol. 2015, 72, 1191–1197. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Nguyen, T.P.; Pham, T.H.; Duong, T.T.; Van Do, M.; Van Trinh, T.; Nguyen, Q.T.X.; Trinh, V.M. Removal of Nutrients and COD in Wastewater from Vietnamese Piggery Farm by the Culture of Chlorella vulgaris in a Pilot-Scaled Membrane Photobioreactor. Water 2022, 14, 3645. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Nguyen, T.-T.; Binh, Q.A.; Bui, X.-T.; Ngo, H.H.; Vo, H.N.P.; Lin, K.-Y.A.; Vo, T.-D.; Guo, W.; Lin, C.; et al. Co-culture of microalgae-activated sludge for wastewater treatment and biomass production: Exploring their role under different inoculation ratios. Bioresour. Technol. 2020, 314, 123754. [Google Scholar] [CrossRef] [PubMed]
- López-Pacheco, I.Y.; Castillo-Vacas, E.I.; Castañeda-Hernández, L.; Gradiz-Menjivar, A.; Rodas-Zuluaga, L.I.; Castillo-Zacarías, C.; Sosa-Hernández, J.E.; Barceló, D.; Iqbal, H.M.; Parra-Saldívar, R. CO2 biocapture by Scenedesmus sp. grown in industrial wastewater. Sci. Total Environ. 2021, 790, 148222. [Google Scholar] [CrossRef] [PubMed]
- El Ouaer, M.; Kallel, A.; Kasmi, M.; Hassen, A.; Trabelsi, I. Tunisian landfill leachate treatment using Chlorella sp.: Effective factors and microalgae strain performance. Arab. J. Geosci. 2017, 10, 457. [Google Scholar] [CrossRef]
- Lee, S.-A.; Lee, N.; Oh, H.-M.; Ahn, C.-Y. Enhanced and balanced microalgal wastewater treatment (COD, N, and P) by interval inoculation of activated sludge. J. Microbiol. Biotechnol. 2019, 29, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Fernando, J.S.R.; Premaratne, M.; Dinalankara, D.M.S.D.; Perera, G.L.N.J.; Ariyadasa, T.U. Cultivation of microalgae in palm oil mill effluent (POME) for astaxanthin production and simultaneous phycoremediation. J. Environ. Chem. Eng. 2021, 9, 105375. [Google Scholar] [CrossRef]



| OrderEst | Run Order | TipoPt | Nejayote (%) | Leachate (%) | BBM (%) |
|---|---|---|---|---|---|
| 11 | 1 | 1 | 0 | 90 | 10 |
| 8 | 2 | 1 | 90 | 0 | 10 |
| 7 | 3 | 1 | 0 | 10 | 90 |
| 2 | 4 | 1 | 0 | 10 | 90 |
| 1 | 5 | 1 | 0 | 90 | 10 |
| 14 | 6 | 1 | 10 | 0 | 90 |
| 10 | 7 | 0 | 25 | 25 | 50 |
| 5 | 8 | 0 | 25 | 25 | 50 |
| 15 | 9 | 0 | 25 | 25 | 50 |
| 6 | 10 | 1 | 0 | 90 | 10 |
| 3 | 11 | 1 | 90 | 0 | 10 |
| 4 | 12 | 1 | 10 | 0 | 90 |
| 9 | 13 | 1 | 10 | 0 | 90 |
| 13 | 14 | 1 | 90 | 0 | 10 |
| 12 | 15 | 1 | 0 | 10 | 90 |
| Parameter | Units | Food Leachate | Nejayote |
|---|---|---|---|
| Total dissolved solids | (g/L) | 1.97 ± 0.05 | 9.66 ± 0.08 |
| Total solids | (g/L) | 2.16 ± 0.09 | 10.04 ± 0.18 |
| Total volatile solids | (g/L) | 1.31 ± 0.02 | 7.02 ± 0.21 |
| Total fixed solids | (g/L) | 0.86 ± 0.01 | 3.03 ± 0.14 |
| Volatile dissolved solids | (g/L) | 1.73 ± 0.03 | 6.75± 0.08 |
| Fixed dissolved solids | (g/L) | 0.24 ± 0.01 | 2.90± 0.10 |
| Ammonium | (mg NH4-N/L) | 290 ± 8.16 | 23.91 ± 2.66 |
| Nitrate | (mg NO3-N/L) | 260 ± 23.15 | 146.25 ± 17.78 |
| Nitrite | (mg NO2-N/L) | 1446 ± 37.48 | 1927.73 ± 59.44 |
| COD | (mg O2/L) | 4788 ± 68.29 | 1798.33 ± 43.03 |
| Orthophosphates | (mg PO43−/L) | 17.8 ± 0.32 | 8.9 ± 0.03 |
| Electric conductivity | (S/m) | 5.65 ± 0.08 | 3.03 ± 0.03 |
| pH | 9.1 | 8.6 | |
| Sulfates | (mg SO42−/L) | 40.05 ± 1.64 | 473.39 ± 14.77 |
| Turbidity | NTU | 2390 ± 86.74 | 944 ± 47.09 |
| Alkalinity | (mg CaCO3/L) | 731 ± 17.03 | 2466.66 ± 400.04 |
| Hardness | (mg CaCO3/L) | 640 ± 20.78 | 2050.00 ± 173.20 |
| Chloride | (mg Cl−/L) | 0.251 ± 0.02 | 0.127 ± 0.01 |
| Treatment | Period (d) | Biomass Concentration (g/L) | Maximum Specific Growth Rate (1/d) | Maximum Cell Productivity (g/L/d) | R2 | Model Equation |
|---|---|---|---|---|---|---|
| Scenedesmus acutus | ||||||
| BBM | 0–6 | 0.65 ± 0.16 | 0.29 | 0.09 | 0.92 | |
| 0–20 | 2.54 ± 0.09 | 0.12 | 0.12 | 0.95 | ||
| T1 | 0–6 | 1.82 ± 0.05 | 0.31 | 0.25 | 0.67 | |
| 0–20 | 4.09 ± 0.23 | 0.01 | 0.19 | 0.91 | ||
| T2 | 0–6 | 1.80 ± 0.17 | 0.37 | 0.26 | 0.98 | |
| 0–20 | 4.45 ± 0.26 | 0.15 | 0.21 | 0.98 | ||
| T3 | 0–6 | 3.25 ± 0.12 | 0.39 | 0.48 | 0.96 | |
| 0–20 | 4.59 ± 0.13 | 0.13 | 0.21 | 0.75 | ||
| T4 | 0–6 | 2.17 ± 0.04 | 0.38 | 0.32 | 0.82 | |
| 0–20 | 5.34 ± 0.16 | 0.16 | 0.25 | 0.95 | ||
| T5 | 0–6 | 2.92 ± 0.14 | 0.39 | 0.44 | 0.99 | |
| 0–20 | 1.79 ± 0.11 | 0.09 | 0.13 | 0.06 | ||
| Haematococcus pluvialis | ||||||
| BBM | 0–6 | 1.19 ± 0.18 | 0.19 | 0.13 | 0.88 | |
| 0–20 | 3.30 ± 0.12 | 0.10 | 0.14 | 0.95 | ||
| T1 | 0–6 | 2.53 ± 0.18 | 0.35 | 0.31 | 0.89 | |
| 0–20 | 4.23 ± 0.12 | 0.19 | 0.11 | 0.89 | ||
| T2 | 0–6 | 2.12 ± 0.08 | 0.30 | 0.33 | 0.99 | |
| 0–20 | 4.44 ± 0.12 | 0.20 | 0.13 | 0.82 | ||
| T3 | 0–6 | 4.73 ± 0.31 | 0.71 | 0.40 | 0.95 | |
| 0–20 | 4.62 ± 0.23 | 0.20 | 0.11 | 0.49 | ||
| T4 | 0–6 | 2.15 ± 0.11 | 0.30 | 0.33 | 0.92 | |
| 0–20 | 3.04 ± 0.17 | 0.13 | 0.11 | 0.72 | ||
| T5 | 0–6 | 3.48 ± 0.05 | 0.51 | 0.36 | 0.95 | |
| 0–20 | 5.50 ± 0.33 | 0.25 | 0.13 | 0.89 | ||
| Procedure | Mixture Composition (%) | Predicted Responses | |||||
|---|---|---|---|---|---|---|---|
| Nejayote | Food Waste Leachate | BBM | Biomass Growth (g/L) | NH4+ (mg/L) | PO43− (mg/L) | COD (mg/L) | |
| Scenedesmus acutus | |||||||
| Biomass growth | 0 | 46 | 54 | 6.64 | ― | ― | ― |
| Nutrient uptake | 10 | 0 | 90 | ― | 10.6 | 1.7 | 89 |
| Biomass growth and nutrient uptake | 10 | 0 | 90 | 4.45 | 10.6 | 1.7 | 89 |
| Haematococcus pluvialis | |||||||
| Biomass growth | 25 | 25 | 50 | 5.5 | ― | ― | ― |
| Nutrient uptake | 10 | 0 | 90 | ― | 3.24 | 1.7 | 196.66 |
| Biomass growth and nutrient uptake | 10 | 0 | 90 | 4.44 | 3.24 | 1.7 | 196.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garza-Valverde, E.; García-Gómez, C.; Nápoles-Armenta, J.; Samaniego-Moreno, L.; Martínez-Orozco, E.; De La Mora-Orozco, C. Nejayote and Food Waste Leachate as a Medium for Scenedesmus acutus and Haematococcus pluvialis Production: A Mixture Experimental Design. Water 2024, 16, 1314. https://doi.org/10.3390/w16091314
Garza-Valverde E, García-Gómez C, Nápoles-Armenta J, Samaniego-Moreno L, Martínez-Orozco E, De La Mora-Orozco C. Nejayote and Food Waste Leachate as a Medium for Scenedesmus acutus and Haematococcus pluvialis Production: A Mixture Experimental Design. Water. 2024; 16(9):1314. https://doi.org/10.3390/w16091314
Chicago/Turabian StyleGarza-Valverde, Elizabeth, Celestino García-Gómez, Juan Nápoles-Armenta, Luis Samaniego-Moreno, Edgardo Martínez-Orozco, and Celia De La Mora-Orozco. 2024. "Nejayote and Food Waste Leachate as a Medium for Scenedesmus acutus and Haematococcus pluvialis Production: A Mixture Experimental Design" Water 16, no. 9: 1314. https://doi.org/10.3390/w16091314
APA StyleGarza-Valverde, E., García-Gómez, C., Nápoles-Armenta, J., Samaniego-Moreno, L., Martínez-Orozco, E., & De La Mora-Orozco, C. (2024). Nejayote and Food Waste Leachate as a Medium for Scenedesmus acutus and Haematococcus pluvialis Production: A Mixture Experimental Design. Water, 16(9), 1314. https://doi.org/10.3390/w16091314








