Photo-Catalytic Reduction of Nitrate by Ag-TiO2/Formic Acid Under Visible Light: Selectivity of Nitrogen and Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of Ag-TiO2
2.3. Photocatalytic Experiments
2.4. Analytical Methods
3. Results and Discussion
3.1. Removal of Nitrate
3.2. Selectivity of Nitrogen
3.2.1. Effect of Ag-TiO2 Dosage
3.2.2. Effect of Nitrate Concentrations
3.2.3. HCOOH Concentrations
3.3. Mechanism of Nitrate Reduction
3.3.1. Choice of Hole Scavengers
3.3.2. Effective Reducing Substances
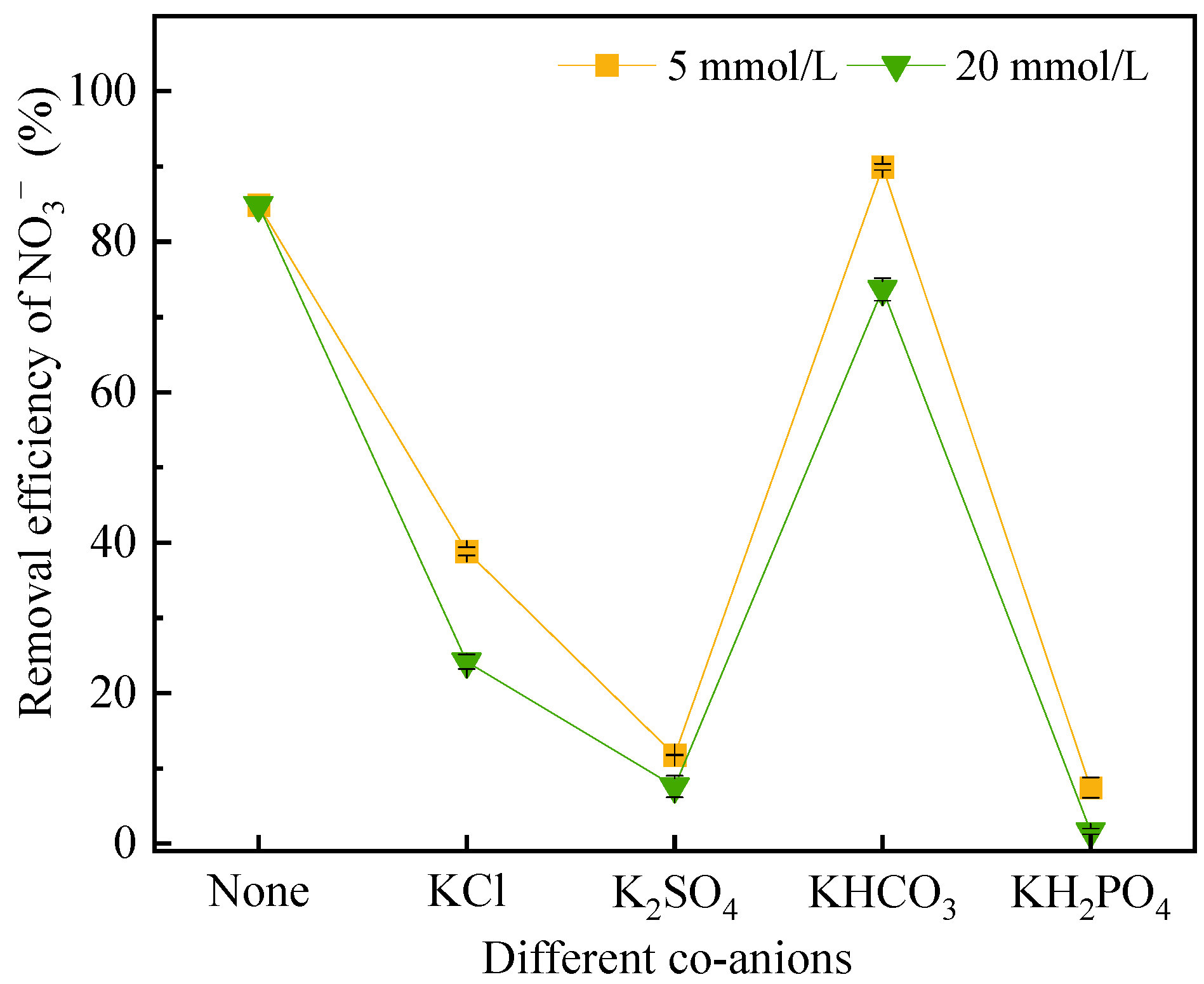
3.4. Effect of Co-Anions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Burow, K.R.; Nolan, B.T.; Rupert, M.G.; Dubrovsky, N.M. Nitrate in Groundwater of the United States, 1991–2003. Environ. Sci. Technol. 2010, 44, 4988–4997. [Google Scholar] [CrossRef] [PubMed]
- Bijay, S.; Craswell, E. Fertilizers and nitrate pollution of surface and ground water: An increasingly pervasive global problem. SN Appl. Sci. 2021, 3, 518. [Google Scholar] [CrossRef]
- Islam, M.; Patel, R. Synthesis and physicochemical characterization of Zn/Al chloride layered double hydroxide and evaluation of its nitrate removal efficiency. Desalination 2010, 256, 120–128. [Google Scholar] [CrossRef]
- Kostraba, J.N.; Gay, E.C.; Rewers, M.; Hamman, R.F. Nitrate levels in community drinking waters and risk of IDDM. An ecological analysis. Diabetes Care 1992, 15, 1505–1508. [Google Scholar] [CrossRef]
- Meng, S.; Ling, Y.; Yang, M.; Zhao, X.; Osman, A.I.; Al-Muhtaseb, A.A.H.; Rooney, D.W.; Yap, P.-S. Recent research progress of electrocatalytic reduction technology for nitrate wastewater: A review. J. Environ. Chem. Eng. 2023, 11, 109418. [Google Scholar] [CrossRef]
- Tugaoen, H.O.; Garcia-Segura, S.; Hristovski, K.; Westerhoff, P. Challenges in photocatalytic reduction of nitrate as a water treatment technology. Sci. Total Environ. 2017, 599–600, 1524–1551. [Google Scholar] [CrossRef]
- Tyagi, S.; Rawtani, D.; Khatri, N.; Tharmavaram, M. Strategies for Nitrate removal from aqueous environment using Nanotechnology: A Review. J. Water Process Eng. 2018, 21, 84–95. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Y.; Tong, S.; Zheng, M.; Zhao, Y.; Tian, C.; Liu, H.; Feng, C. Enhancement of bacterial denitrification for nitrate removal in groundwater with electrical stimulation from microbial fuel cells. J. Power Sources 2014, 268, 423–429. [Google Scholar] [CrossRef]
- Wehbe, N.; Jaafar, M.; Guillard, C.; Herrmann, J.-M.; Miachon, S.; Puzenat, E.; Guilhaume, N. Comparative study of photocatalytic and non-photocatalytic reduction of nitrates in water. Appl. Catal. A Gen. 2009, 368, 1–8. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, R.; Chen, D.; Liu, J.; Chen, S. Carbon dioxide radical reducing nitrate to nitrogen gas in a UV/Fe(III)-oxalate system. J. Water Process Eng. 2021, 40, 101934. [Google Scholar] [CrossRef]
- Tugaoen, H.O.N.; Herckes, P.; Hristovski, K.; Westerhoff, P. Influence of ultraviolet wavelengths on kinetics and selectivity for N-gases during TiO2 photocatalytic reduction of nitrate. Appl. Catal. B Environ. 2018, 220, 597–606. [Google Scholar] [CrossRef]
- Wu, L.; Chen, S.; Zhou, J.; Zhang, C.; Liu, J.; Luo, J.; Song, G.; Qian, G.; Song, L.; Xia, M. Simultaneous removal of organic matter and nitrate from bio-treated leachate via iron–carbon internal micro-electrolysis. RSC Adv. 2015, 5, 68356–68360. [Google Scholar] [CrossRef]
- Hérissan, A.; Meichtry, J.M.; Remita, H.; Colbeau-Justin, C.; Litter, M.I. Reduction of nitrate by heterogeneous photocatalysis over pure and radiolytically modified TiO2 samples in the presence of formic acid. Catal. Today 2017, 281, 101–108. [Google Scholar] [CrossRef]
- Zhao, F.; Xin, J.; Yuan, M.; Wang, L.; Wang, X. A critical review of existing mechanisms and strategies to enhance N2 selectivity in groundwater nitrate reduction. Water Res. 2021, 209, 117889. [Google Scholar] [CrossRef]
- Xue, S.; Wen, X.; Yun, Y. Pathway and mechanism study on improvement of N2 selectivity of catalytic denitrification. J. Saudi Chem. Soc. 2023, 27, 101577. [Google Scholar] [CrossRef]
- Chang, Y.; Meng, J.; Hu, Y.; Qi, S.; Hu, Z.; Wu, G.; Zhou, J.; Zhan, X. Unacclimated activated sludge improved nitrate reduction and N2 selectivity in iron filling/biochar systems. Sci. Total Environ. 2024, 947, 174581. [Google Scholar] [CrossRef]
- Li, F.; Zhang, W.; Zhang, P.; Gong, A.; Kexun, L. Strategies of selective electroreduction of aqueous nitrate to N2 in chloride-free system: A critical review. Green Energy Environ. 2024, 9, 198–216. [Google Scholar] [CrossRef]
- Mertah, O.; El Hajjaji, K.; El Amrani, S.; Tanji, K.; Goncharova, I.; Kherbeche, A. Visible-light Cu/TiO2@Ag3PO4 heterostructure photocatalyst for selective nitrate reduction and antimicrobial activity. Opt. Mater. 2022, 129, 112549. [Google Scholar] [CrossRef]
- Pawar, M.; Nain, P.; Rani, S.; Sharma, B.; Kumar, S.; Majeed Khan, M.A. Structural, optical, photocatalytic and thermal behaviour of (Nb, Ta) co-doped WO3 nanoparticles and its application in photocatalytic degradation of MG and RhB dyes. Opt. Mater. 2024, 157, 116277. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, H.; Li, A.; Qiu, X.; Wang, L. Lewis acid-rich SrFexTi1−xO3/TiO2 to enhance the photocatalytic reduction of nitrate to N2. J. Hazard. Mater. 2024, 473, 198–216. [Google Scholar] [CrossRef]
- Wardman, P. Reduction Potentials of One-Electron Couples Involving Free Radicals in Aqueous Solution. J. Phys. Chem. Ref. Data 1989, 18, 1637–1755. [Google Scholar] [CrossRef]
- Li, X.; Ma, J.; Liu, G.; Fang, J.; Yue, S.; Guan, Y.; Chen, L.; Liu, X. Efficient reductive dechlorination of monochloroacetic acid by sulfite/UV process. Environ. Sci. Technol. 2012, 46, 7342–7349. [Google Scholar] [CrossRef]
- Grills, D.C.; Lymar, S.V. Radiolytic formation of the carbon dioxide radical anion in acetonitrile revealed by transient IR spectroscopy. Phys. Chem. Chem. Phys. 2018, 20, 10011–10017. [Google Scholar] [CrossRef]
- Liu, X.; Zhong, J.; Fang, L.; Wang, L.; Ye, M.; Shao, Y.; Li, J.; Zhang, T. Trichloroacetic acid reduction by an advanced reduction process based on carboxyl anion radical. Chem. Eng. J. 2016, 303, 56–63. [Google Scholar] [CrossRef]
- Berkovic, A.M.; Gonzalez, M.C.; Russo, N.; Michelini Mdel, C.; Pis Diez, R.; Martire, D.O. Reduction of mercury(II) by the carbon dioxide radical anion: A theoretical and experimental investigation. J. Phys. Chem. A 2010, 114, 12845–12850. [Google Scholar] [CrossRef]
- Vilhunen, S.; Vilve, M.; Vepsalainen, M.; Sillanpaa, M. Removal of organic matter from a variety of water matrices by UV photolysis and UV/H2O2 method. J. Hazard. Mater. 2010, 179, 776–782. [Google Scholar] [CrossRef]
- Sá, J.; Agüera, C.A.; Gross, S.; Anderson, J.A. Photocatalytic nitrate reduction over metal modified TiO2. Appl. Catal. B Environ. 2009, 85, 192–200. [Google Scholar] [CrossRef]
- Clavero, C. Plasmon-induced hot-electron generation at nanoparticle/metal-oxide interfaces for photovoltaic and photocatalytic devices. Nat. Photonics 2014, 8, 95–103. [Google Scholar] [CrossRef]
- Duan, L.; Lin, Q.; Peng, H.; Lu, C.; Shao, C.; Wang, D.; Rao, S.; Cao, H.; Lv, W. The catalytic reduction mechanisms of metal-doped TiO2 for nitrate produced from non-thermal discharge plasma: The interfacial photogenerated electron transfer and reduction process. Appl. Catal. A Gen. 2023, 650, 118995. [Google Scholar] [CrossRef]
- Nain, P.; Pawar, M.; Rani, S.; Sharma, B.; Kumar, S.; Majeed Khan, M.A. (Ce, Nd) co-doped TiO2 NPs via hydrothermal route:Structural, optical, photocatalytic and thermal behavior. Mater. Sci. Eng. B 2024, 309, 117648. [Google Scholar] [CrossRef]
- Khan, M.A.M.; Ansari, A.A.; Choudhary, P.; Ahmed, J.; Kumar, S.; Hussain, S. Reduced graphene oxide supported Ag-loaded brookite TiO2 nanowires: Enhanced photocatalytic degradation performance and electrochemical energy storage applications. Diam. Relat. Mater. 2023, 139, 110397. [Google Scholar] [CrossRef]
- Khan, M.A.M.; Nain, P.; Kumar, S.; Ansari, A.A.; Ahamed, M.; Shahabuddin, M. Improved photocatalytic and electrochemical activities of (Nd3+, Yb3+) co-doped TiO2 nanoparticles synthesized by hydrothermal protocol. J. Mater. Sci. Mater. Electron. 2024, 35, 2149. [Google Scholar] [CrossRef]
- Xu, R.; Guan, Y.; Cao, L.; Yu, X.; Li, L.; Ma, B.; Liu, C. Boosting light harvesting and charge separation in Au nanoparticles and g-C3N4 co-modified TiO2 nanotube arrays for efficient photoelectrocatalytic reduction of nitrate in water. J. Water Process Eng. 2024, 68, 106316. [Google Scholar] [CrossRef]
- Ghafourian, N.; Lashanizadegan, M.; Hosseini, S.N. Ag/TiO2/EP: A low-cost and floating plasmonic photocatalyst for degrading furfural under visible light irradiation. Int. J. Environ. Sci. Technol. 2017, 14, 2721–2732. [Google Scholar] [CrossRef]
- Yang, W.; Wang, J.; Chen, R.; Xiao, L.; Shen, S.; Li, J.; Dong, F. Reaction mechanism and selectivity regulation of photocatalytic nitrate reduction for wastewater purification: Progress and challenges. J. Mater. Chem. A 2022, 10, 17357–17376. [Google Scholar] [CrossRef]
- Ko, S.; Banerjee, C.K.; Sankar, J. Photochemical synthesis and photocatalytic activity in simulated solar light of nanosized Ag doped TiO2 nanoparticle composite. Compos. Part B Eng. 2011, 42, 579–583. [Google Scholar] [CrossRef]
- Adewuyi, Y.G.; Sakyi, N.Y.; Arif Khan, M. Simultaneous removal of NO and SO2 from flue gas by combined heat and Fe2+ activated aqueous persulfate solutions. Chemosphere 2018, 193, 1216–1225. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Zhou, J.; Chen, D. Reductive removal of nitrate by carbon dioxide radical with high product selectivity to form N2 in a UV/H2O2/HCOOH system. J. Water Process Eng. 2020, 33, 101097. [Google Scholar] [CrossRef]
- Lin, B.; Sun, P.; Zhou, Y.; Jiang, S.; Gao, B.; Chen, Y. Interstratified nanohybrid assembled by alternating cationic layered double hydroxide nanosheets and anionic layered titanate nanosheets with superior photocatalytic activity. J. Hazard. Mater. 2014, 280, 156–163. [Google Scholar] [CrossRef]
- Sowmya, A.; Meenakshi, S. Photocatalytic reduction of nitrate over Ag–TiO2 in the presence of oxalic acid. J. Water Process Eng. 2015, 8, e23–e30. [Google Scholar] [CrossRef]
- Chen, H.; Shao, Y.; Xu, Z.; Wan, H.; Wan, Y.; Zheng, S.; Zhu, D. Effective catalytic reduction of Cr(VI) over TiO2 nanotube supported Pd catalysts. Appl. Catal. B Environ. 2011, 105, 255–262. [Google Scholar] [CrossRef]
- Nishio, J.; Tokumura, M.; Znad, H.T.; Kawase, Y. Photocatalytic decolorization of azo-dye with zinc oxide powder in an external UV light irradiation slurry photoreactor. J. Hazard. Mater. 2006, 138, 106–115. [Google Scholar] [CrossRef]
- Shankar, M.V.; Nelieu, S.; Kerhoas, L.; Einhorn, J. Photo-induced degradation of diuron in aqueous solution by nitrites and nitrates: Kinetics and pathways. Chemosphere 2007, 66, 767–774. [Google Scholar] [CrossRef]
- Ward, M.H.; Jones, R.R.; Brender, J.D.; de Kok, T.M.; Weyer, P.J.; Nolan, B.T.; Villanueva, C.M.; van Breda, S.G. Drinking Water Nitrate and Human Health: An Updated Review. Int. J. Environ. Res. Public Health 2018, 15, 1557. [Google Scholar] [CrossRef]
- Guo, J.; Jiang, L.; Liang, J.; Xu, W.; Yu, H.; Zhang, J.; Ye, S.; Xing, W.; Yuan, X. Photocatalytic degradation of tetracycline antibiotics using delafossite silver ferrite-based Z-scheme photocatalyst: Pathways and mechanism insight. Chemosphere 2021, 270, 128651. [Google Scholar] [CrossRef]
- Xu, L.; Yang, L.; Johansson, E.M.J.; Wang, Y.; Jin, P. Photocatalytic activity and mechanism of bisphenol a removal over TiO2−x/rGO nanocomposite driven by visible light. Chem. Eng. J. 2018, 350, 1043–1055. [Google Scholar] [CrossRef]
- Harbour, J.R.; Hair, M.L. Spin trapping of the ·CO2− radical in aqueous medium. Can. J. Chem. 1979, 57, 1150–1152. [Google Scholar] [CrossRef]
- Kobwittaya, K.; Sirivithayapakorn, S. Photocatalytic reduction of nitrate over TiO2 and Ag-modified TiO2. J. Saudi Chem. Soc. 2014, 18, 291–298. [Google Scholar] [CrossRef]
- Zhang, F.; Jin, R.; Chen, J.; Shao, C.; Gao, W.; Li, L.; Guan, N. High photocatalytic activity and selectivity for nitrogen in nitrate reduction on Ag/TiO2 catalyst with fine silver clusters. J. Catal. 2005, 232, 424–431. [Google Scholar] [CrossRef]
- Kormann, C. Photolysis of Chloroform and Other Organic Molecules in Aqueous TiO2 Suspensions. Env. Sci. Technol. 1991, 25, 494–500. [Google Scholar] [CrossRef]
- Freire, J.M.A.; Matos, M.A.F.; Abreu, D.S.; Becker, H.; Diógenes, I.C.N.; Valentini, A.; Longhinotti, E. Nitrate photocatalytic reduction on TiO2: Metal loaded, synthesis and anions effect. J. Environ. Chem. Eng. 2020, 8, 103844. [Google Scholar] [CrossRef]
- Sheng, H.; Li, Q.; Ma, W.; Ji, H.; Chen, C.; Zhao, J. Photocatalytic degradation of organic pollutants on surface anionized TiO2: Common effect of anions for high hole-availability by water. Appl. Catal. B Environ. 2013, 138–139, 212–218. [Google Scholar] [CrossRef]




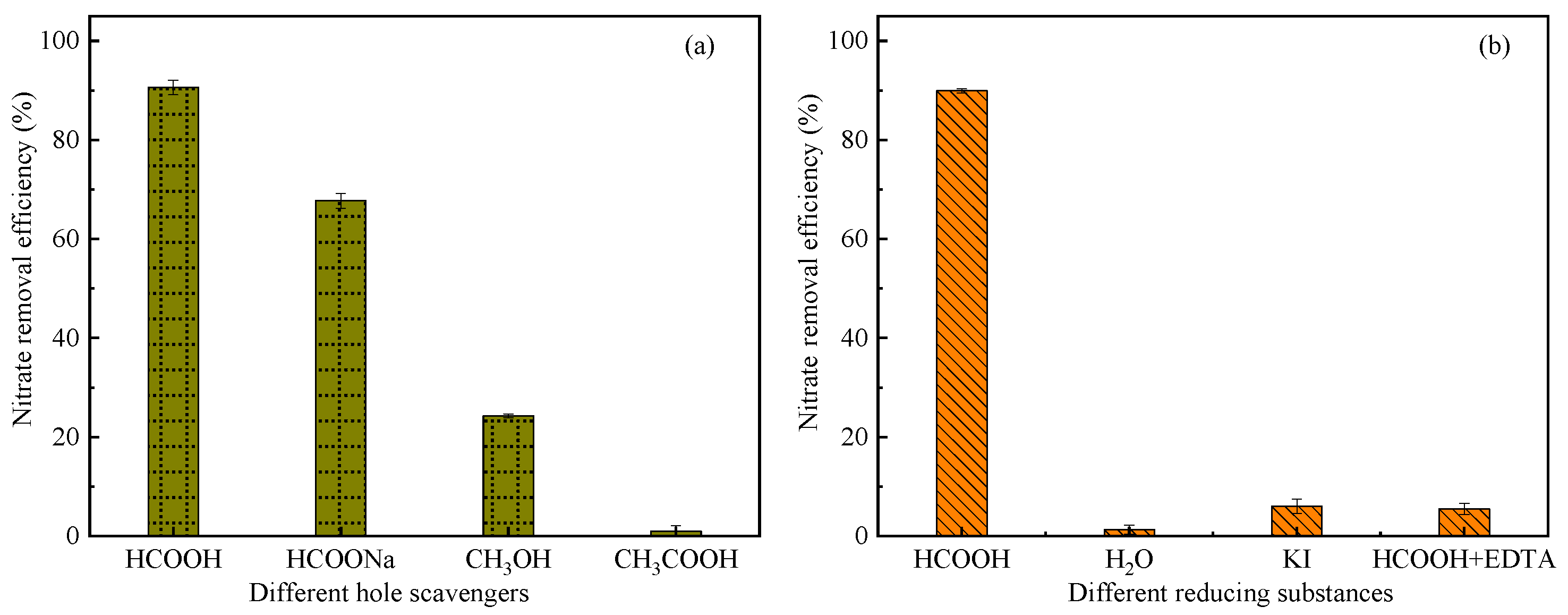

| Hole Scavengers | HCOOH | HCOONa | CH3OH | CH3COOH |
|---|---|---|---|---|
| Initial pH | 2.7 | 6.6 | 5.2 | 3.5 |
| Hole Scavenger | Main Reducing Substances |
|---|---|
| HCOOH | ·CO2−, photo-generated e−, HCOO−, ·OH |
| H2O | ·OH |
| KI | Photo-generated e− |
| EDTA+HCOOH | Photo-generated e−, HCOO− |
| KI (mmol/L) | Removal Efficiency of NO3− (%) |
|---|---|
| 1 | 6.8 |
| 2 | 7.1 |
| 20 | 7.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Xie, Y.; Xia, J.; Zhang, X.; Cheng, H.; Chen, J. Photo-Catalytic Reduction of Nitrate by Ag-TiO2/Formic Acid Under Visible Light: Selectivity of Nitrogen and Mechanism. Water 2025, 17, 155. https://doi.org/10.3390/w17020155
Shi Y, Xie Y, Xia J, Zhang X, Cheng H, Chen J. Photo-Catalytic Reduction of Nitrate by Ag-TiO2/Formic Acid Under Visible Light: Selectivity of Nitrogen and Mechanism. Water. 2025; 17(2):155. https://doi.org/10.3390/w17020155
Chicago/Turabian StyleShi, Yuanyuan, Yi Xie, Jun Xia, Xiaolin Zhang, Hui Cheng, and Jialin Chen. 2025. "Photo-Catalytic Reduction of Nitrate by Ag-TiO2/Formic Acid Under Visible Light: Selectivity of Nitrogen and Mechanism" Water 17, no. 2: 155. https://doi.org/10.3390/w17020155
APA StyleShi, Y., Xie, Y., Xia, J., Zhang, X., Cheng, H., & Chen, J. (2025). Photo-Catalytic Reduction of Nitrate by Ag-TiO2/Formic Acid Under Visible Light: Selectivity of Nitrogen and Mechanism. Water, 17(2), 155. https://doi.org/10.3390/w17020155





