Green Synthesis of Sustainable and Cost-Effective TiO2-SiO2-Fe2O3 Heterojunction Nanocomposites for Rhodamine B Dye Degradation Under Sunlight
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Synthesis of TiO2-SiO2-Fe2O3 NPs
2.3. Characterization
2.4. Photocatalytic Experiments
3. Results and Discussion
3.1. Material Characterization
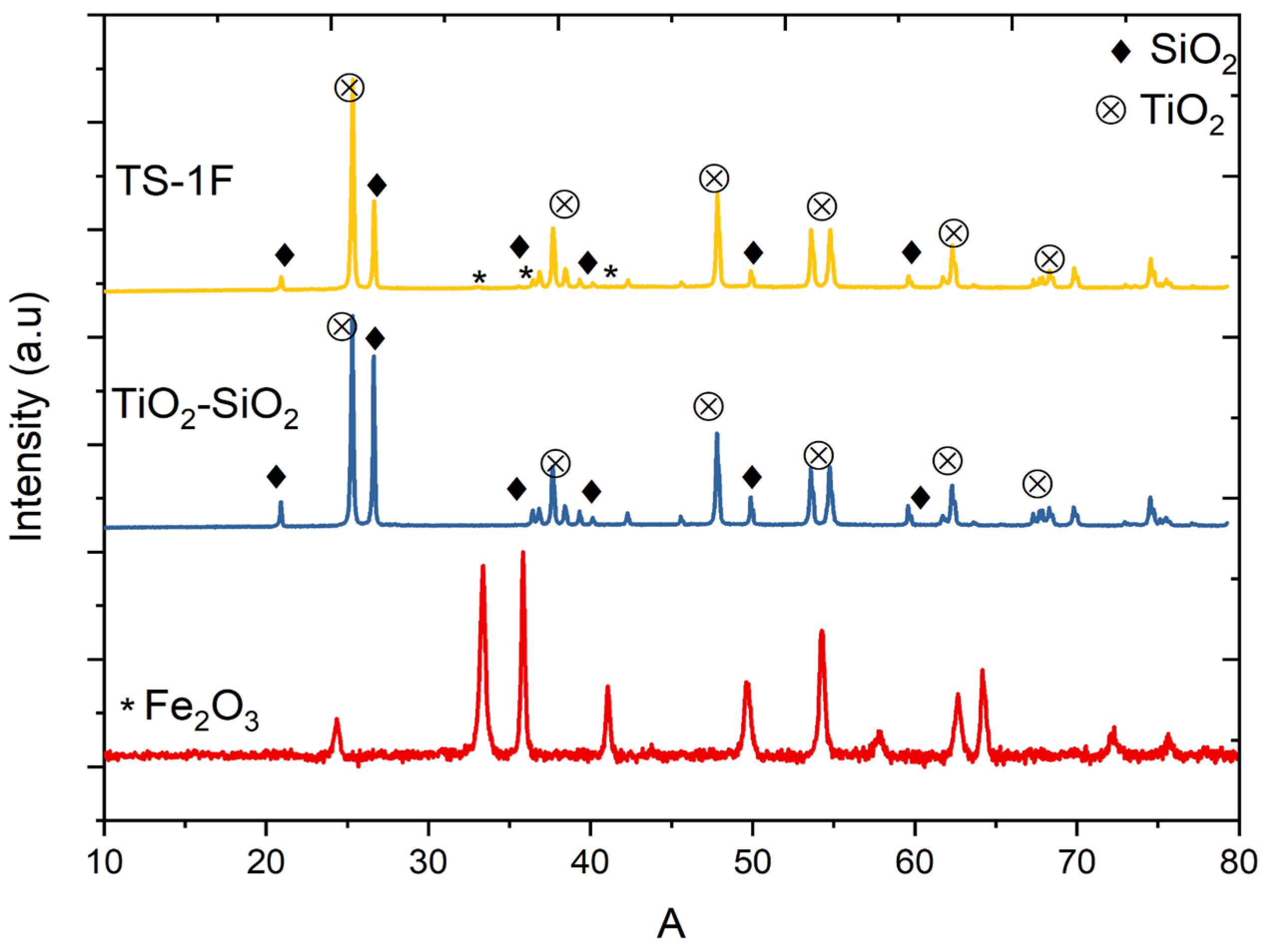
3.1.1. X-Ray Powder Diffraction
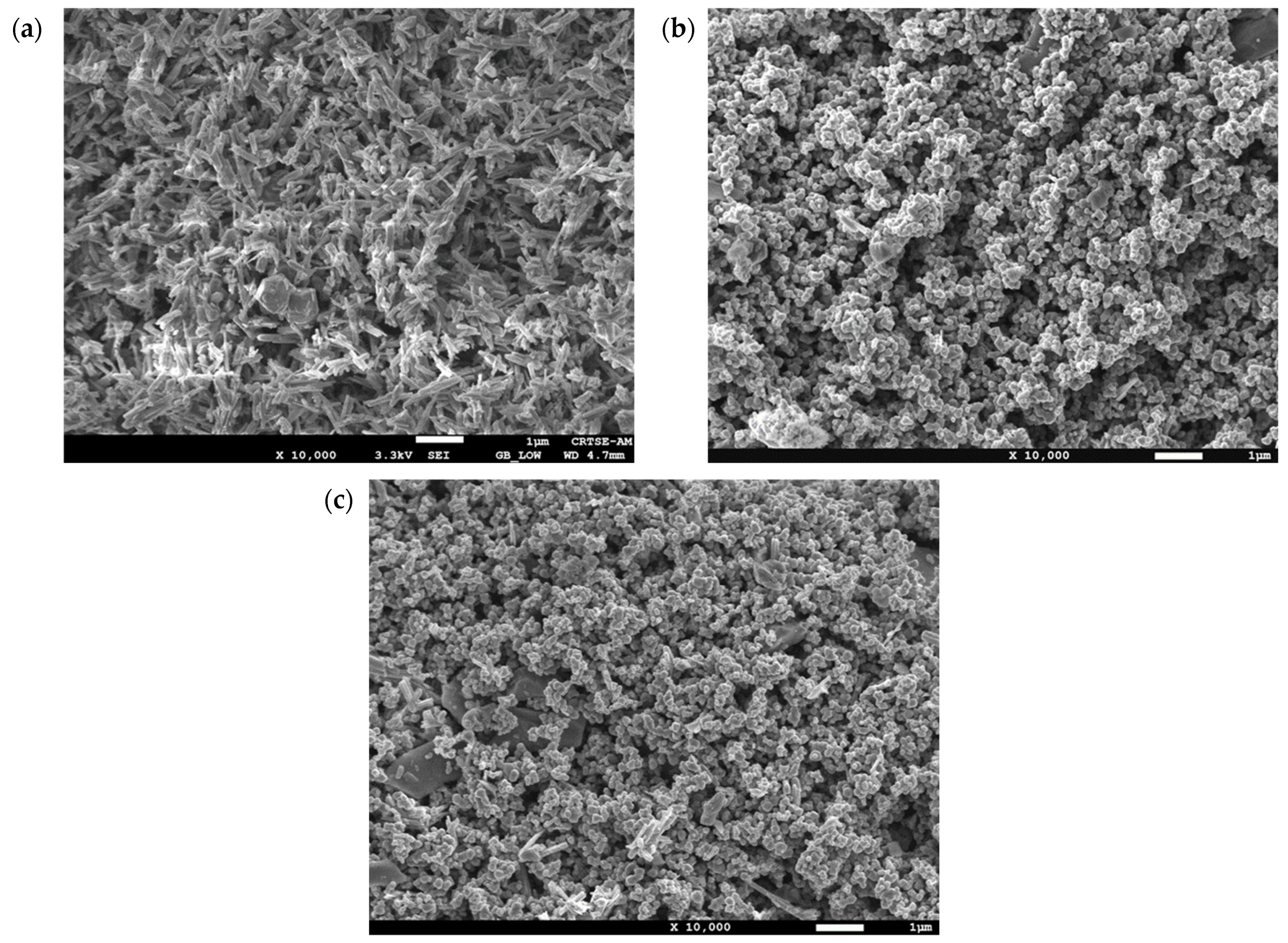
3.1.2. Scanning Electron Microscopy-Energy Dispersive Spectroscopy (EDS-SEM)
3.1.3. Fourier Transform Infrared (FTIR) Spectroscopy
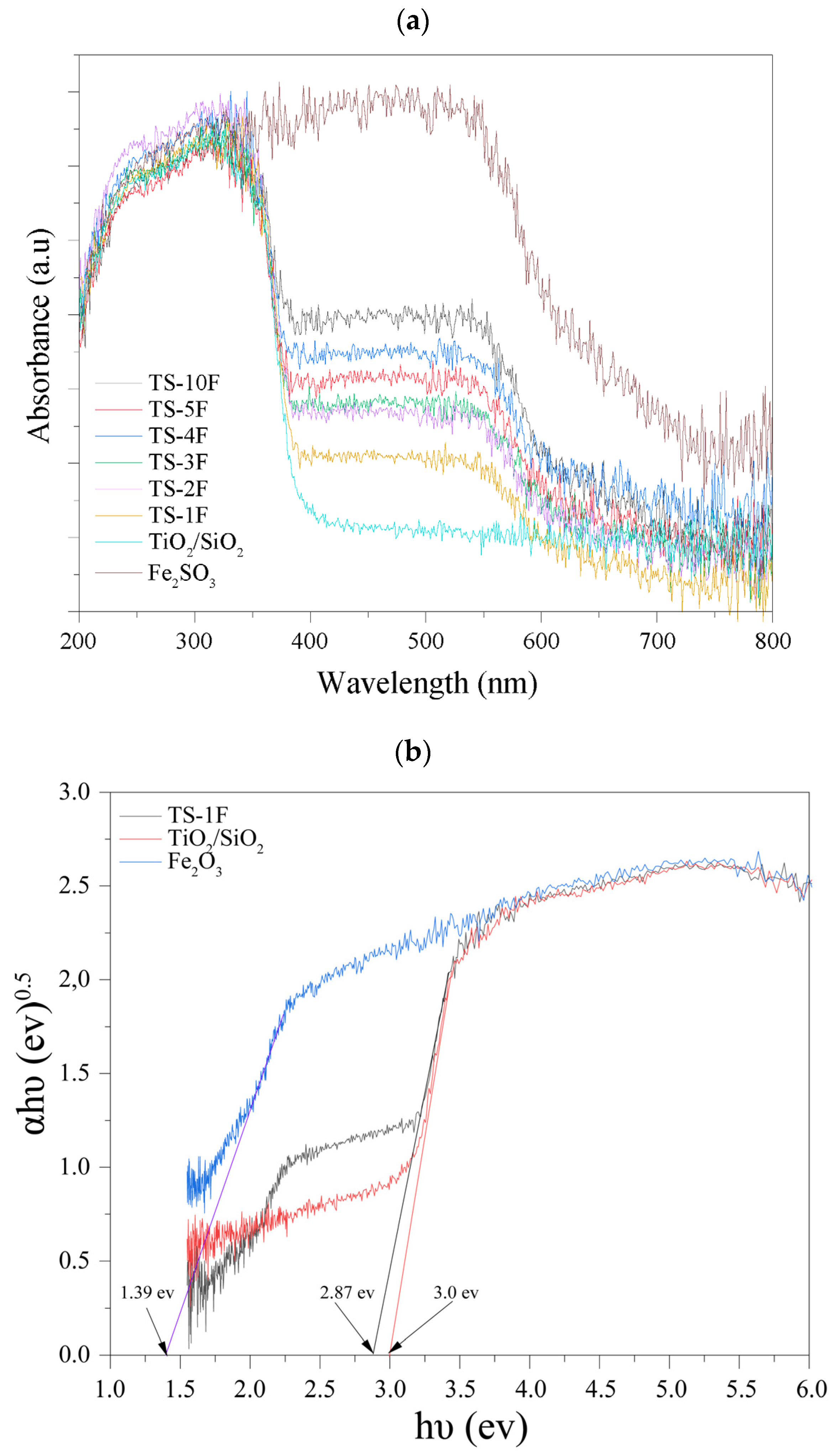
3.1.4. Optical Properties
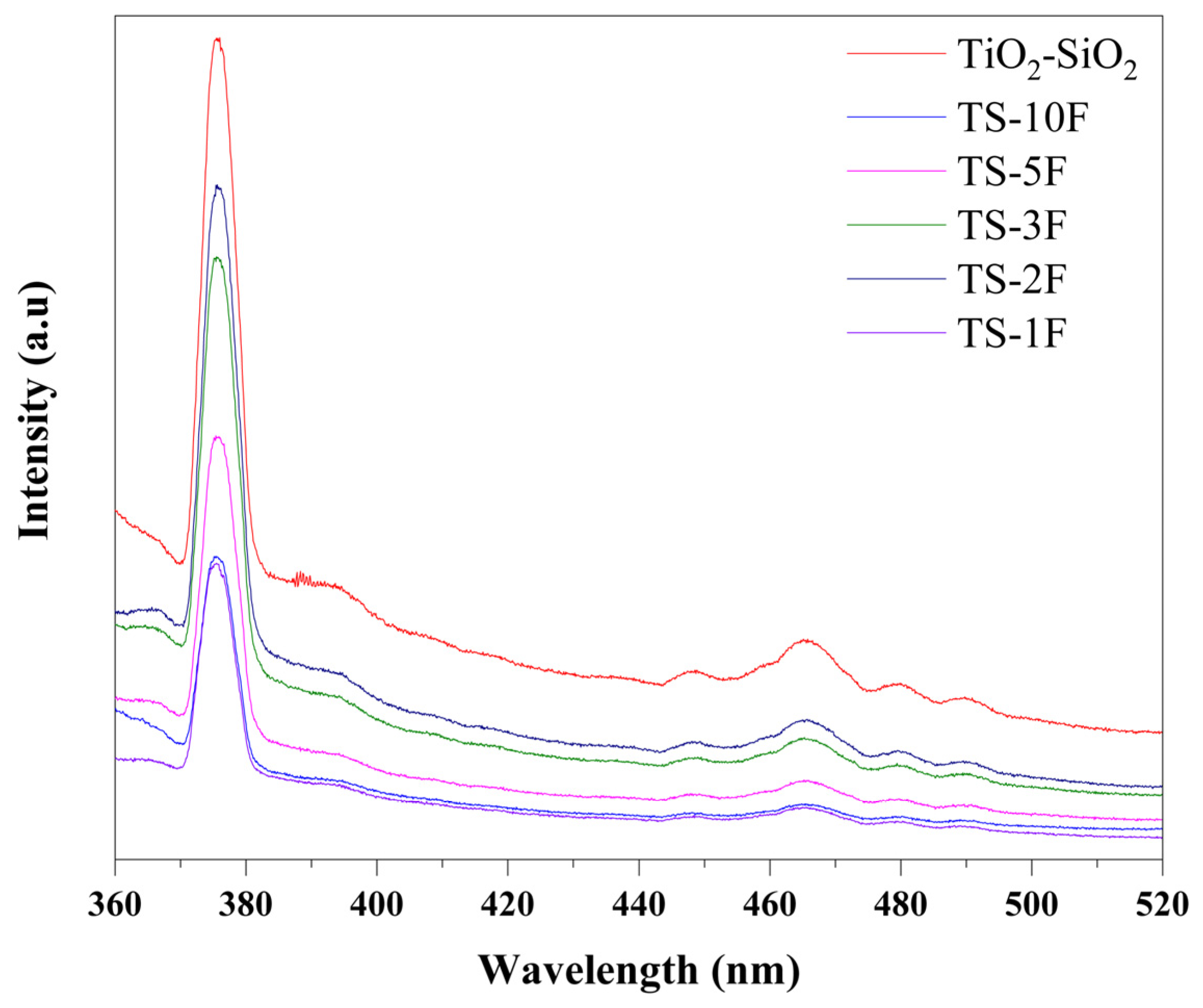
3.1.5. Photoluminescence Spectra of the Prepared Samples
3.2. Photocatalysis Tests
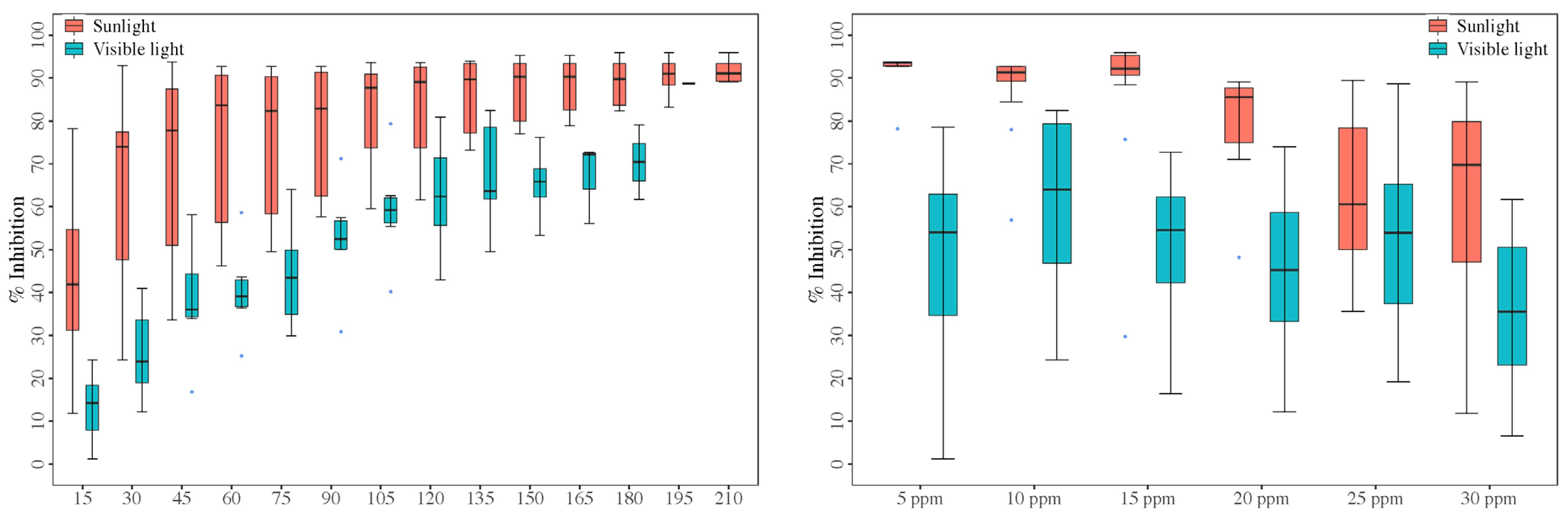
3.2.1. Under Visible Light (250 W) and Sunlight
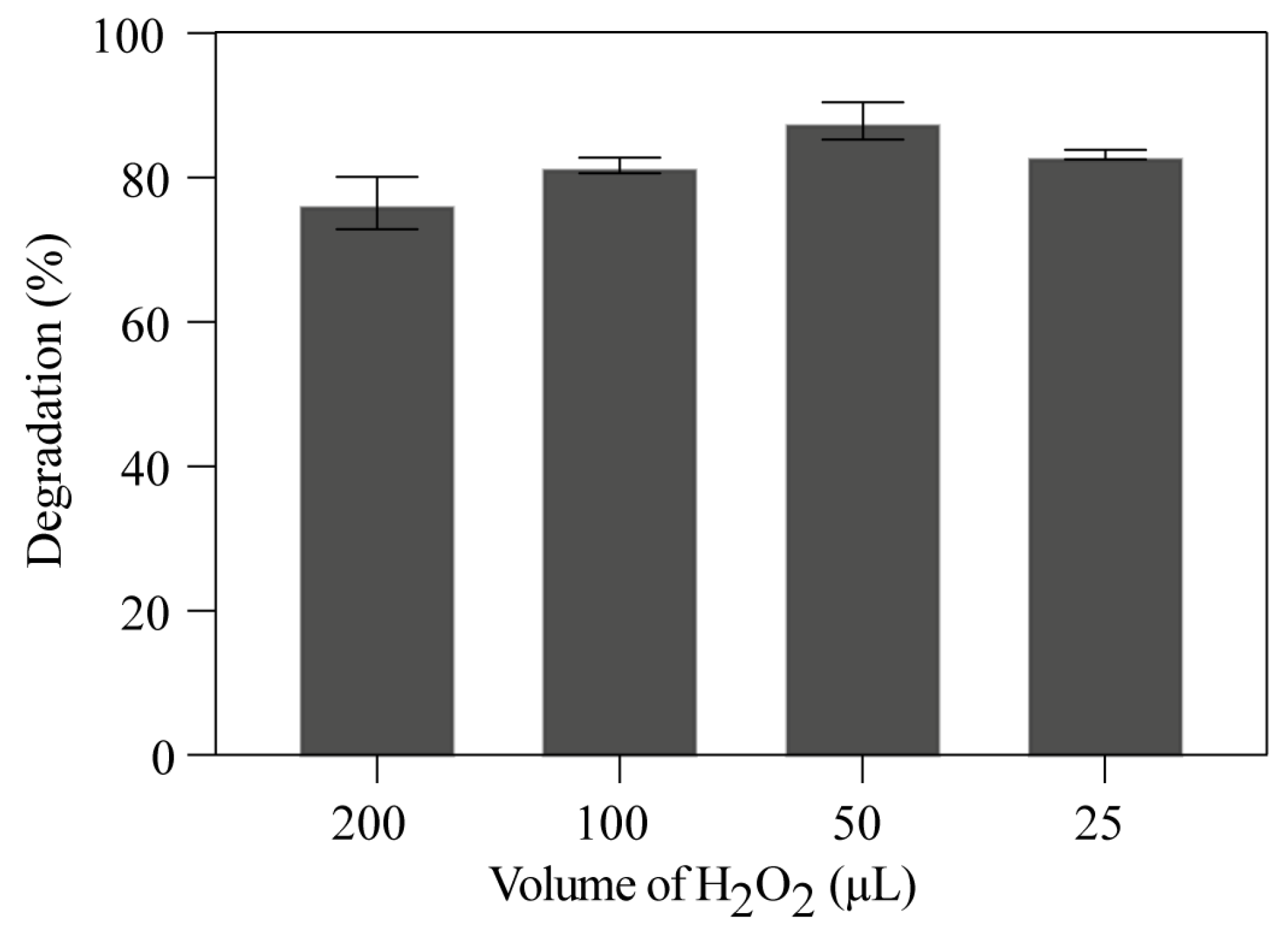
3.2.2. The Synergetic Effect of H2O2-Assisted Photocatalytic Degradation
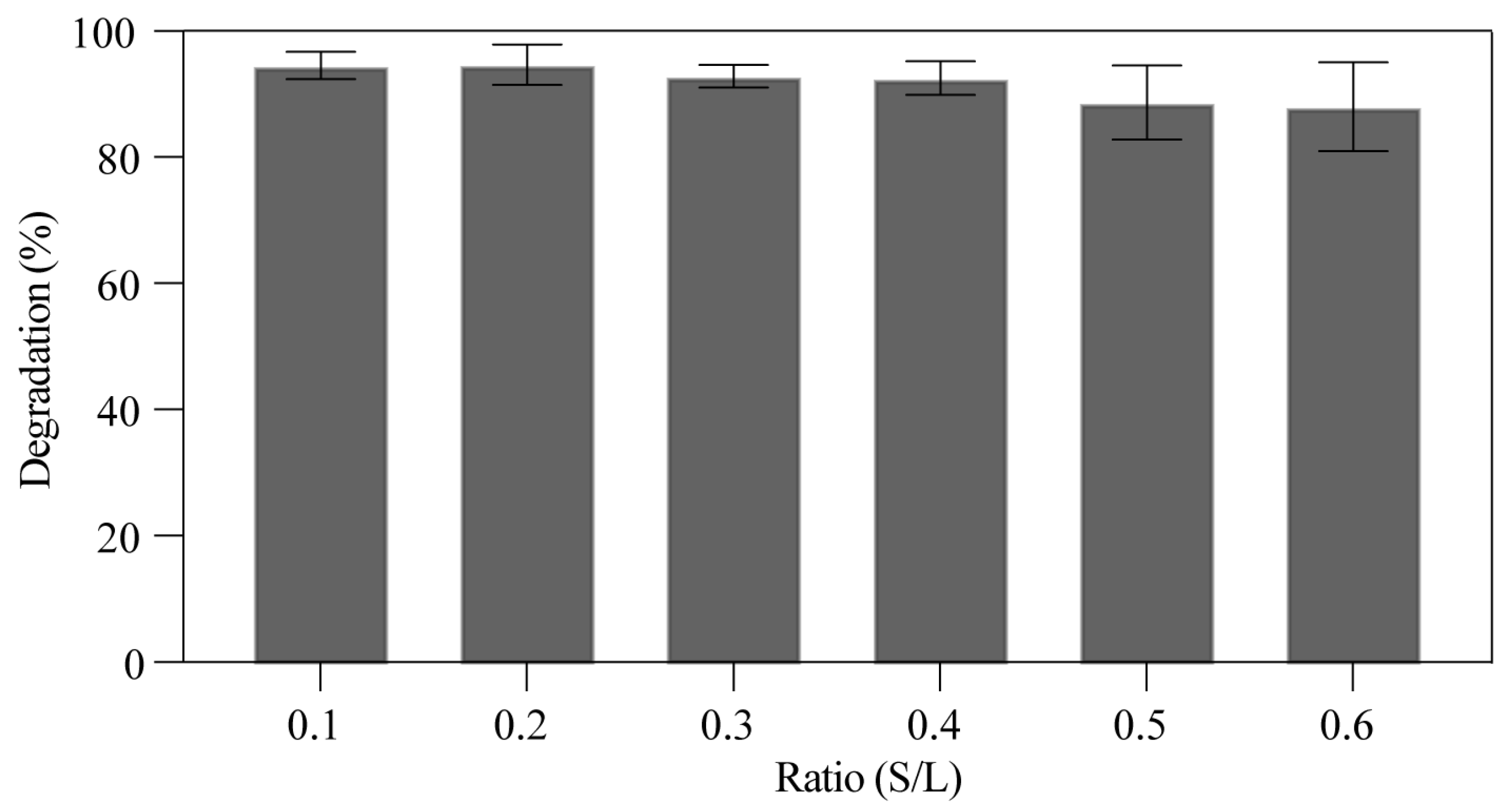
3.2.3. The Effect of TS-1F Dosage on the Degradation Rate of RhB
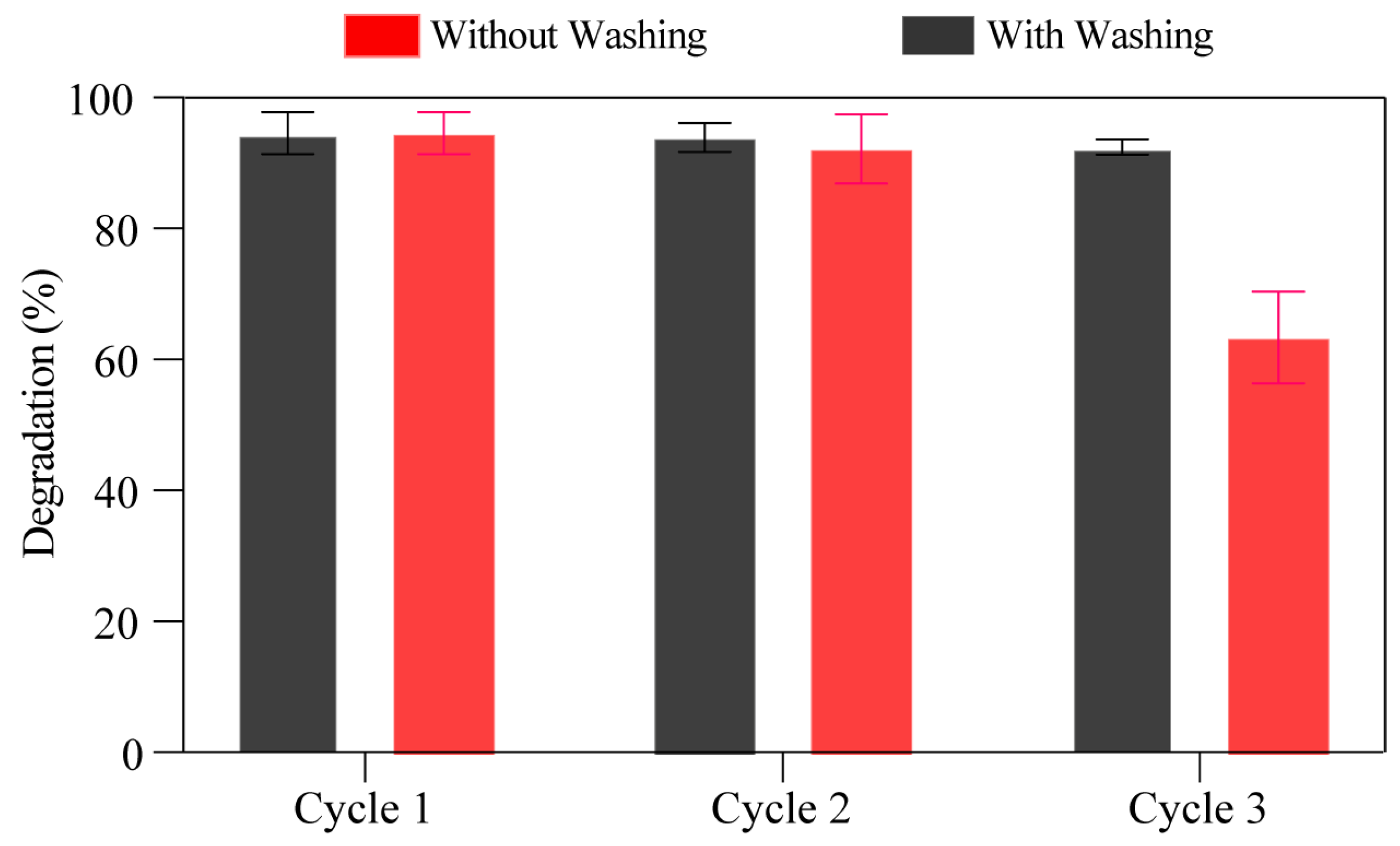
3.2.4. An Assessment of TiO2-SiO2-Fe2O3 Heterojunction Reusability and Recyclability
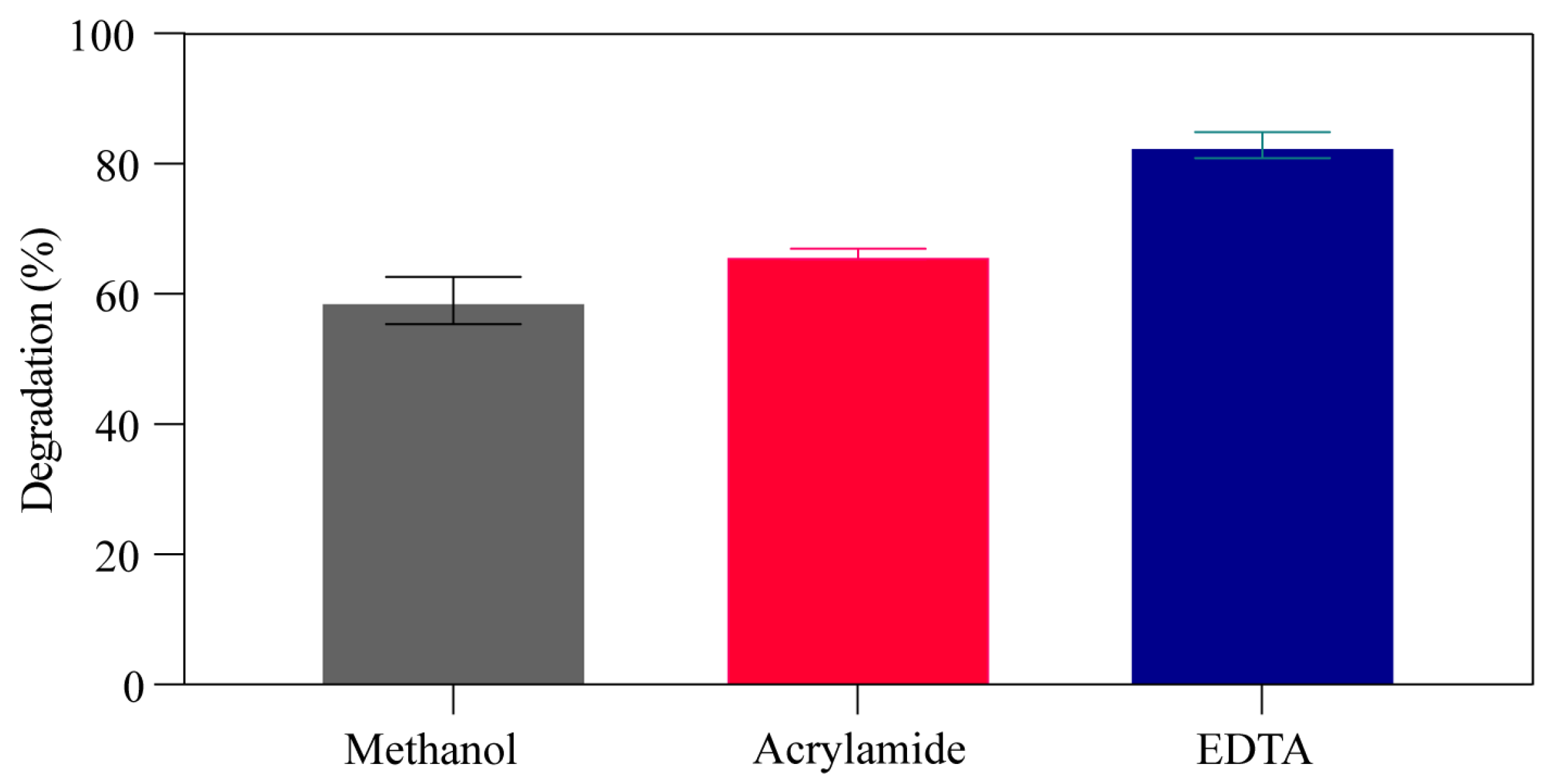
3.3. Scavenger Effect
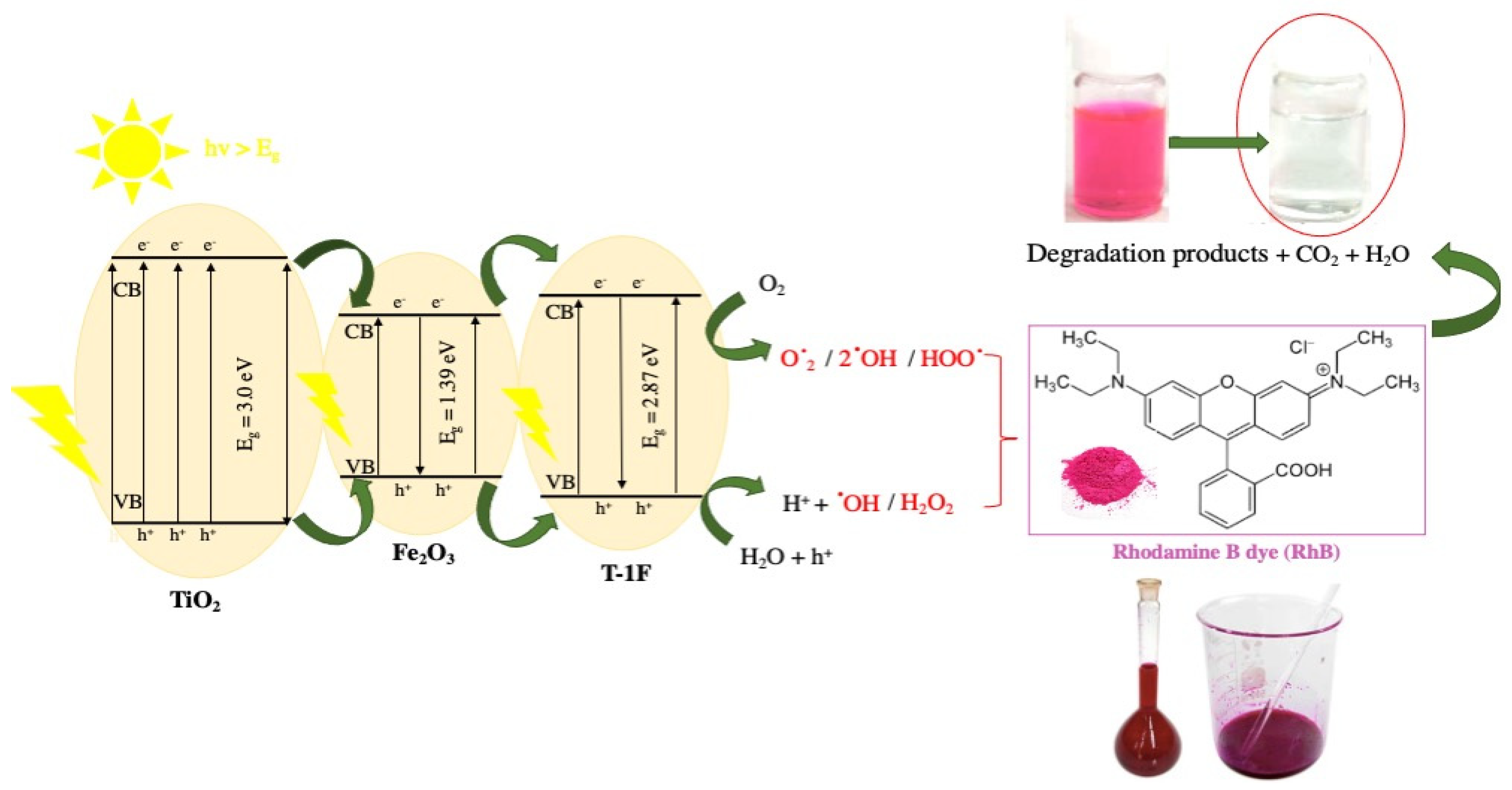
3.4. Degradation Mechanism
3.5. Cost Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prakash, O.; Maurya, S.; Tripathy, P.; Sharma, A.; Vijay, R.; Pal, S. Chapter 21—Nano- and phytoremediation technique for textile wastewater treatment and successive production of fertilizers. In Metagenomics to Bioremediation; Kumar, V., Bilal, M., Shahi, S.K., Garg, V.K., Eds.; Developments in Applied Microbiology and Biotechnology; Academic Press: Cambridge, MA, USA, 2023; pp. 537–559. ISBN 978-0-323-96113-4. [Google Scholar] [CrossRef]
- Berradi, M.; Hsissou, R.; Khudhair, M.; Assouag, M.; Cherkaoui, O.; Bachiri, A.E.; Harfi, A.E. Textile finishing dyes and their impact on aquatic environs. Heliyon 2019, 5, e02711. [Google Scholar] [CrossRef]
- Elsayed, M.; Abdel-Raouf, M.E.-S. Wastewater Treatment Methodologies, Review Article. Int. J. Environ. Agric. Sci. 2019, 3, 18. [Google Scholar]
- Boudraa, R.; Talantikite-Touati, D.; Souici, A.; Djermoune, A.; Saidani, A.; Fendi, K.; Amrane, A.; Bollinger, J.-C.; Nguyen Tran, H.; Hadadi, A.; et al. Optical and photocatalytic properties of TiO2-Bi2O3-CuO supported on natural zeolite for removing Safranin-O dye from water and wastewater. J. Photochem. Photobiol. A Chem. 2023, 443, 114845. [Google Scholar] [CrossRef]
- Mouni, L.; Belkhiri, L.; Bollinger, J.-C.; Bouzaza, A.; Assadi, A.; Tirri, A.; Dahmoune, F.; Madani, K.; Remini, H. Removal of Methylene Blue from aqueous solutions by adsorption on Kaolin: Kinetic and equilibrium studies. Appl. Clay Sci. 2018, 153, 38–45. [Google Scholar] [CrossRef]
- Imessaoudene, A.; Cheikh, S.; Hadadi, A.; Hamri, N.; Bollinger, J.-C.; Amrane, A.; Tahraoui, H.; Manseri, A.; Mouni, L. Adsorption Performance of Zeolite for the Removal of Congo Red Dye: Factorial Design Experiments, Kinetic, and Equilibrium Studies. Separations 2023, 10, 57. [Google Scholar] [CrossRef]
- Merdoud, R.; Aoudjit, F.; Mouni, L.; Ranade, V.V. Degradation of methyl orange using hydrodynamic Cavitation, H2O2, and photo-catalysis with TiO2-Coated glass Fibers: Key operating parameters and synergistic effects. Ultrason. Sonochem. 2024, 103, 106772. [Google Scholar] [CrossRef]
- Hadadi, A.; Imessaoudene, A.; Bollinger, J.-C.; Cheikh, S.; Assadi, A.A.; Amrane, A.; Kebir, M.; Mouni, L. Parametrical Study for the Effective Removal of Mordant Black 11 from Synthetic Solutions: Moringa oleifera Seeds’ Extracts Versus Alum. Water 2022, 14, 4109. [Google Scholar] [CrossRef]
- Boudraa, R.; Talantikite-Touati, D.; Djermoune, A.; Souici, A.; Kebir, M.; Merzeg, F.A.; Amrane, A.; Bollinger, J.-C.; Mouni, L. Comprehensive Characterization and Unprecedented Photocatalytic Efficacy of TiO2-CuO-La2O3 and TiO2-CuO-Bi2O3 Nanocomposites: A Novel Approach to Environmental Remediation. Mater. Sci. Eng. B 2025, 312, 117863. [Google Scholar] [CrossRef]
- Imessaoudene, A.; Mechraoui, O.; Aberkane, B.; Benabbas, A.; Manseri, A.; Moussaoui, Y.; Bollinger, J.-C.; Amrane, A.; Zoukel, A.; Mouni, L. Synthesis of a TiO2/zeolite composite: Evaluation of adsorption-photodegradation synergy for the removal of Malachite Green, Nano-Struct. Nano-Objects 2024, 38, 101191. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El-Nemr, M.A.; Elkatory, M.R.; Ragab, S.; Niculescu, V.-C.; El Nemr, A. Principles of Photocatalysts and Their Different Applications: A Review. Top. Curr. Chem. 2023, 381, 31. [Google Scholar] [CrossRef] [PubMed]
- Raj, K.J.A.; Smith, Y.R.; Subramanian, V.; Viswanathan, B. Structural studies of silica modified titania and its photocatalytic activity of 4-chlorophenol oxidation in aqueous medium. Indian J. Chem.-Sect. A 2010, 49, 867. [Google Scholar]
- Babyszko, A.; Wanag, A.; Sadłowski, M.; Kusiak-Nejman, E.; Morawski, A.W. Synthesis and Characterization of SiO2/TiO2 as Photocatalyst on Methylene Blue Degradation. Catalysts 2022, 12, 1372. [Google Scholar] [CrossRef]
- Ramamoorthy, V.; Kannan, K.; Joice Joseph, A.I.; Kanagaraj, T.; Thiripuranthagan, S. Photocatalytic degradation of acid orange dye using silver impregnated TiO2/SiO2 composite catalysts. J. Nanosci. Nanotechnol. 2016, 16, 9980–9986. [Google Scholar] [CrossRef]
- Chun, H.; Yizhong, W.; Hongxiao, T. Influence of adsorption on the photodegradation of various dyes using surface bond-conjugated TiO2/SiO2 photocatalyst. Appl. Catal. B Environ. 2001, 35, 95–105. [Google Scholar] [CrossRef]
- Xie, C.; Xu, Z.; Yang, Q.; Xue, B.; Du, Y.; Zhang, J. Enhanced photocatalytic activity of titania-silica mixed oxide prepared via basic hydrolyzation. Mater. Sci. Eng. B 2004, 112, 34–41. [Google Scholar] [CrossRef]
- Ding, Z.; Lu, G.Q.; Greenfield, P.F. Role of the Crystallite Phase of TiO2 in Heterogeneous Photocatalysis for Phenol Oxidation in Water. J. Phys. Chem. B 2000, 104, 4815–4820. [Google Scholar] [CrossRef]
- Joseph, C.G.; Taufiq-Yap, Y.H.; Musta, B.; Sarjadi, M.S.; Elilarasi, L. Application of Plasmonic Metal Nanoparticles in TiO2-SiO2 Composite as an Efficient Solar-Activated Photocatalyst: A Review Paper. Front. Chem. 2021, 8, 568063. [Google Scholar] [CrossRef]
- Fawzi Suleiman Khasawneh, O.; Palaniandy, P. Removal of organic pollutants from water by Fe2O3/TiO2 based photocatalytic degradation: A review. Environ. Technol. Innov. 2021, 21, 101230. [Google Scholar] [CrossRef]
- Johngika, J.; Harun, Z. Review On the Biosynthesis of Composite TiO2—Inorganic/Metal Oxide (Fe2O3): Photocatalytic and Antibacterial Purpose Of Wastewater Treatment. Res. Prog. Mech. Manuf. Eng. 2023, 4, 504–517. [Google Scholar]
- Abbas, N.; Shao, G.N.; Haider, M.S.; Imran, S.M.; Park, S.S.; Kim, H.T. Sol–gel synthesis of TiO2-Fe2O3 systems: Effects of Fe2O3 content and their photocatalytic properties. J. Ind. Eng. Chem. 2016, 39, 112–120. [Google Scholar] [CrossRef]
- Wu, L.; Yan, H.; Xiao, J.; Li, X.; Wang, X.; Zhao, T. Characterization and photocatalytic properties of nano-Fe2O3-TiO2 composites prepared through the gaseous detonation method. Ceram. Int. 2017, 43, 14334–14339. [Google Scholar] [CrossRef]
- Baniamerian, H.; Teimoori, M.; Saberi, M. Fe2O3/TiO2/Activated Carbon Nanocomposite with Synergistic Effect of Adsorption and Photocatalysis. Chem. Eng. Technol. 2021, 44, 130–139. [Google Scholar] [CrossRef]
- Zhou, W.; Fu, H.; Pan, K.; Tian, C.; Qu, Y.; Lu, P.; Sun, C.-C. Mesoporous TiO2/α-Fe2O3: Bifunctional Composites for Effective Elimination of Arsenite Contamination through Simultaneous Photocatalytic Oxidation and Adsorption. J. Phys. Chem. C 2008, 112, 19584–19589. [Google Scholar] [CrossRef]
- Fiol, N.; Villaescusa, I. Determination of sorbent point zero charge: Usefulness in sorption studies. Environ. Chem. Lett. 2009, 7, 79–84. [Google Scholar] [CrossRef]
- Labaran, B.A.; Vohra, M.S. Photocatalytic removal of selenite and selenate species: Effect of EDTA and other process variables. Environ. Technol. 2014, 35, 1091–1100. [Google Scholar] [CrossRef]
- Boudraa, R.; Talantikite-Touati, D.; Souici, A.; Djermoune, K.; Amrane, A.; Bollinger, J.-C.; Tran, H.N.; Mouni, L. Breaking new grounds: Solid-state synthesis of TiO2-La2O3-CuO nanocomposites for degrading brilliant green dye under visible light. J. Clean. Prod. 2024, 481, 144126. [Google Scholar] [CrossRef]
- Lee, W.-G.; Chae, S.; Chung, Y.K.; Yoon, W.-S.; Choi, J.-Y.; Huh, J. Indirect-To-Direct Band Gap Transition of One-Dimensional V2Se9: Theoretical Study with Dispersion Energy Correction. ACS Omega 2019, 4, 18392–18397. [Google Scholar] [CrossRef]
- Kavitha, S.; Ranjith, R.; Jayamani, N.; Vignesh, S.; Palanivel, B.; Djellabi, R.; Bianchi, C.L.; Alharthi, F.A. Fabrication of visible-light-responsive TiO2/α-Fe2O3-heterostructured composite for rapid photo-oxidation of organic pollutants in water. J. Mater. Sci: Mater. Electron. 2022, 33, 8906–8919. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, M.; Zhao, G.; Yin, G.; Liu, B. Hierarchical Fe2O3 nanorods/TiO2 nanosheets heterostructure: Growth mechanism, enhanced visible-light photocatalytic and photoelectrochemical performances. Appl. Surf. Sci. 2019, 475, 380–388. [Google Scholar] [CrossRef]
- Banisharif, A.; Khodadadi, A.A.; Mortazavi, Y.; Anaraki Firooz, A.; Beheshtian, J.; Agah, S.; Menbari, S. Highly active Fe2O3-doped TiO2 photocatalyst for degradation of trichloroethylene in air under UV and visible light irradiation: Experimental and computational studies. Appl. Catal. B Environ. 2015, 165, 209–221. [Google Scholar] [CrossRef]
- Palanisamy, B.; Babu, C.M.; Sundaravel, B.; Anandan, S.; Murugesan, V. Sol–gel synthesis of mesoporous mixed Fe2O3/TiO2 photocatalyst: Application for degradation of 4-chlorophenol. J. Hazard. Mater. 2013, 252–253, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Mchedlov-Petrossyan, N.O.; Vodolazkaya, N.A.; Doroshenko, A.O. Ionic Equilibria of Fluorophores in Organized Solutions: The Influence of Micellar Microenvironment on Protolytic and Photophysical Properties of Rhodamine B. J. Fluoresc. 2003, 13, 235–248. [Google Scholar] [CrossRef]
- Baran, T.; Wojtyła, S.; Minguzzi, A.; Rondinini, S.; Vertova, A. Achieving efficient H2O2 production by a visible-light absorbing, highly stable photosensitized TiO2. Appl. Catal. B Environ. 2019, 244, 303–312. [Google Scholar] [CrossRef]
- Feilizadeh, M.; Attar, F.; Mahinpey, N. Hydrogen peroxide-assisted photocatalysis under solar light irradiation: Interpretation of interaction effects between an active photocatalyst and H2O2. Can. J. Chem. Eng. 2019, 97, 2009–2014. [Google Scholar] [CrossRef]
- Castellanos, R.M.; Paulo Bassin, J.; Dezotti, M.; Boaventura, R.A.R.; Vilar, V.J.P. Tube-in-tube membrane reactor for heterogeneous TiO2 photocatalysis with radial addition of H2O2. Chem. Eng. J. 2020, 395, 124998. [Google Scholar] [CrossRef]
- Lachheb, H.; Puzenat, E.; Houas, A.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic degradation of various types of dyes (Alizarin S, Crocein Orange G, Methyl Red, Congo Red, Methylene Blue) in water by UV-irradiated titania. Appl. Catal. B Environ. 2002, 39, 75–90. [Google Scholar] [CrossRef]
- Chaudhuri, M.; Elmolla, E.S.; Othman, R.B. Removal of reactive dyes from aqueous solution by adsorption on coconut coir activated carbon. In Proceedings of the 2nd International Conference on Engineering Technology 2009 (ICET 2009), Kuala Lumpur, Malaysia, 8–10 December 2009. [Google Scholar]
- Zheng, R.; Yang, D.; Chen, Y.; Bian, Z.; Li, H. Fe2O3/TiO2/reduced graphene oxide-driven recycled visible-photocatalytic Fenton reactions to mineralize organic pollutants in a wide pH range. J. Environ. Sci. 2023, 134, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Trenczek-Zajac, A.; Synowiec, M.; Zakrzewska, K.; Zazakowny, K.; Kowalski, K.; Dziedzic, A.; Radecka, M. Scavenger-Supported Photocatalytic Evidence of an Extended Type I Electronic Structure of the TiO2@Fe2O3 Interface. ACS Appl. Mater. Interfaces 2022, 14, 38255–38269. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Pignatello, J.J. Evidence for a surface dual hole-radical mechanism in the titanium dioxide photocatalytic oxidation of 2,4-D. Environ. Sci. Technol. 1995, 29, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Maurino, V.; Minella, M.; Sordello, F.; Minero, C. A proof of the direct hole transfer in photocatalysis: The case of melamine. Appl. Catal. A Gen. 2016, 521, 57–67. [Google Scholar] [CrossRef]
- Malika, M.; Sonawane, S.S. The sono-photocatalytic performance of a Fe2O3 coated TiO2 based hybrid nanofluid under visible light via RSM. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128545. [Google Scholar] [CrossRef]
- Arora, I.; Chawla, H.; Chandra, A.; Sagadevan, S.; Garg, S. Advances in the strategies for enhancing the photocatalytic activity of TiO2: Conversion from UV-light active to visible-light active photocatalyst. Inorg. Chem. Commun. 2022, 143, 109700. [Google Scholar] [CrossRef]
- Du, S.; Lian, J.; Zhang, F. Visible Light-Responsive N-Doped TiO2 Photocatalysis: Synthesis, Characterizations, and Applications. Trans. Tianjin Univ. 2022, 28, 33–52. [Google Scholar] [CrossRef]





| Type | Category | NB Time | Min | Max | Mean | Median | SD | CV |
|---|---|---|---|---|---|---|---|---|
| C/C0 | ||||||||
| Sunlight | 5 ppm | 14 | 0.063 | 0.218 | 0.077 | 0.064 | 0.011 | 52.913 |
| 10 ppm | 14 | 0.073 | 0.431 | 0.124 | 0.087 | 0.026 | 78.969 | |
| 15 ppm | 14 | 0.041 | 0.703 | 0.126 | 0.078 | 0.047 | 138.134 | |
| 20 ppm | 14 | 0.108 | 0.518 | 0.201 | 0.145 | 0.030 | 55.522 | |
| 25 ppm | 14 | 0.106 | 0.644 | 0.363 | 0.394 | 0.049 | 50.324 | |
| 30 ppm | 14 | 0.109 | 0.882 | 0.394 | 0.302 | 0.065 | 61.276 | |
| Visible light | 5 ppm | 10 | 0.215 | 0.988 | 0.523 | 0.460 | 0.079 | 47.843 |
| 10 ppm | 9 | 0.175 | 0.757 | 0.396 | 0.360 | 0.070 | 52.636 | |
| 15 ppm | 11 | 0.273 | 0.836 | 0.490 | 0.455 | 0.048 | 32.726 | |
| 20 ppm | 8 | 0.261 | 0.879 | 0.552 | 0.548 | 0.072 | 36.621 | |
| 25 ppm | 13 | 0.113 | 0.809 | 0.478 | 0.461 | 0.060 | 45.062 | |
| 30 ppm | 12 | 0.383 | 0.935 | 0.646 | 0.645 | 0.052 | 27.993 | |
| % Inhibition | ||||||||
| Sunlight | 5 ppm | 14 | 78.182 | 93.709 | 92.288 | 93.636 | 1.091 | 4.422 |
| 10 ppm | 14 | 56.881 | 92.661 | 87.615 | 91.284 | 2.614 | 11.163 | |
| 15 ppm | 14 | 29.730 | 95.946 | 87.404 | 92.230 | 4.650 | 19.908 | |
| 20 ppm | 14 | 48.193 | 89.157 | 79.865 | 85.542 | 2.988 | 13.998 | |
| 25 ppm | 14 | 35.577 | 89.454 | 63.670 | 60.577 | 4.886 | 28.715 | |
| 30 ppm | 14 | 11.777 | 89.096 | 60.634 | 69.759 | 6.447 | 39.782 | |
| Visible light | 5 ppm | 10 | 1.181 | 78.543 | 47.658 | 54.035 | 7.919 | 52.547 |
| 10 ppm | 9 | 24.318 | 82.463 | 60.414 | 63.991 | 6.946 | 34.490 | |
| 15 ppm | 11 | 16.361 | 72.732 | 51.004 | 54.536 | 4.835 | 31.438 | |
| 20 ppm | 8 | 12.152 | 73.928 | 44.780 | 45.203 | 7.150 | 45.159 | |
| 25 ppm | 13 | 19.139 | 88.715 | 52.249 | 53.927 | 5.968 | 41.183 | |
| 30 ppm | 12 | 6.533 | 61.708 | 35.441 | 35.527 | 5.217 | 50.992 | |
| pH Value | k (10−4 min−1) | R Squared COD | Adj. R-Squared |
|---|---|---|---|
| 2 | 37.7± 2.76 | 0.94 | 0.93 |
| 6 | 96.7± 6.11 | 0.96 | 0.96 |
| 8 | 100 ± 2.41 | 0.99 | 0.99 |
| 10 | 143.7± 5.45 | 0.99 | 0.98 |
| Chemicals Used | Amount per Experiment | Cost (€) | Total Cost (€) |
| TiO2/SiO2 | 0.05 g | 0.65 | 0.75 |
| Fe2O3 | 0.05 g | 0.10 | |
| HCl/NaOH | Drops | - | |
| H2O2 | 25–200 μL | 0.01 | |
| Equipment | Energy Consumed | Cost (€) | Total Cost (€) |
| Calcination oven (1200 W) | 6 h (for the whole study) | 1.16 | 1.95 |
| Magnetic stirrer (610 W) | 3 h | 0.29 | |
| Refrigerated centrifuge (720 W) | 30 min in total | 0.11 | |
| Water purificator | 3 L | 0.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tebbi, S.O.; Amrane, A.; Boudraa, R.; Bollinger, J.-C.; Salvestrini, S.; Kanjal, M.I.; Tiri, A.; Belkhiri, L.; Alharthi, M.N.; Mouni, L. Green Synthesis of Sustainable and Cost-Effective TiO2-SiO2-Fe2O3 Heterojunction Nanocomposites for Rhodamine B Dye Degradation Under Sunlight. Water 2025, 17, 168. https://doi.org/10.3390/w17020168
Tebbi SO, Amrane A, Boudraa R, Bollinger J-C, Salvestrini S, Kanjal MI, Tiri A, Belkhiri L, Alharthi MN, Mouni L. Green Synthesis of Sustainable and Cost-Effective TiO2-SiO2-Fe2O3 Heterojunction Nanocomposites for Rhodamine B Dye Degradation Under Sunlight. Water. 2025; 17(2):168. https://doi.org/10.3390/w17020168
Chicago/Turabian StyleTebbi, Sara Oumenoune, Abdeltif Amrane, Reguia Boudraa, Jean-Claude Bollinger, Stefano Salvestrini, Muhammad Imran Kanjal, Ammar Tiri, Lazhar Belkhiri, Maymounah N. Alharthi, and Lotfi Mouni. 2025. "Green Synthesis of Sustainable and Cost-Effective TiO2-SiO2-Fe2O3 Heterojunction Nanocomposites for Rhodamine B Dye Degradation Under Sunlight" Water 17, no. 2: 168. https://doi.org/10.3390/w17020168
APA StyleTebbi, S. O., Amrane, A., Boudraa, R., Bollinger, J.-C., Salvestrini, S., Kanjal, M. I., Tiri, A., Belkhiri, L., Alharthi, M. N., & Mouni, L. (2025). Green Synthesis of Sustainable and Cost-Effective TiO2-SiO2-Fe2O3 Heterojunction Nanocomposites for Rhodamine B Dye Degradation Under Sunlight. Water, 17(2), 168. https://doi.org/10.3390/w17020168








