Ionic Strength and pH-Responsive Ultrafiltration Membrane to Overcome the Typical Permeability-Selectivity Tradeoff
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview
2.2. Materials
2.3. Preparation of PSf Surrogate Membrane Surfaces
2.4. Atmospheric Pressure-Induced Graft Polymerization (APPIGP)
2.5. Membrane Surface Hydrophilicity
2.6. Surface Topography
2.7. Membrane Performance Characterization
3. Results and Discussion
3.1. Overview
3.2. Surface Wettability
3.3. Ionic Strength-Responsive SNS-PAA-PSf UF Membrane Performance
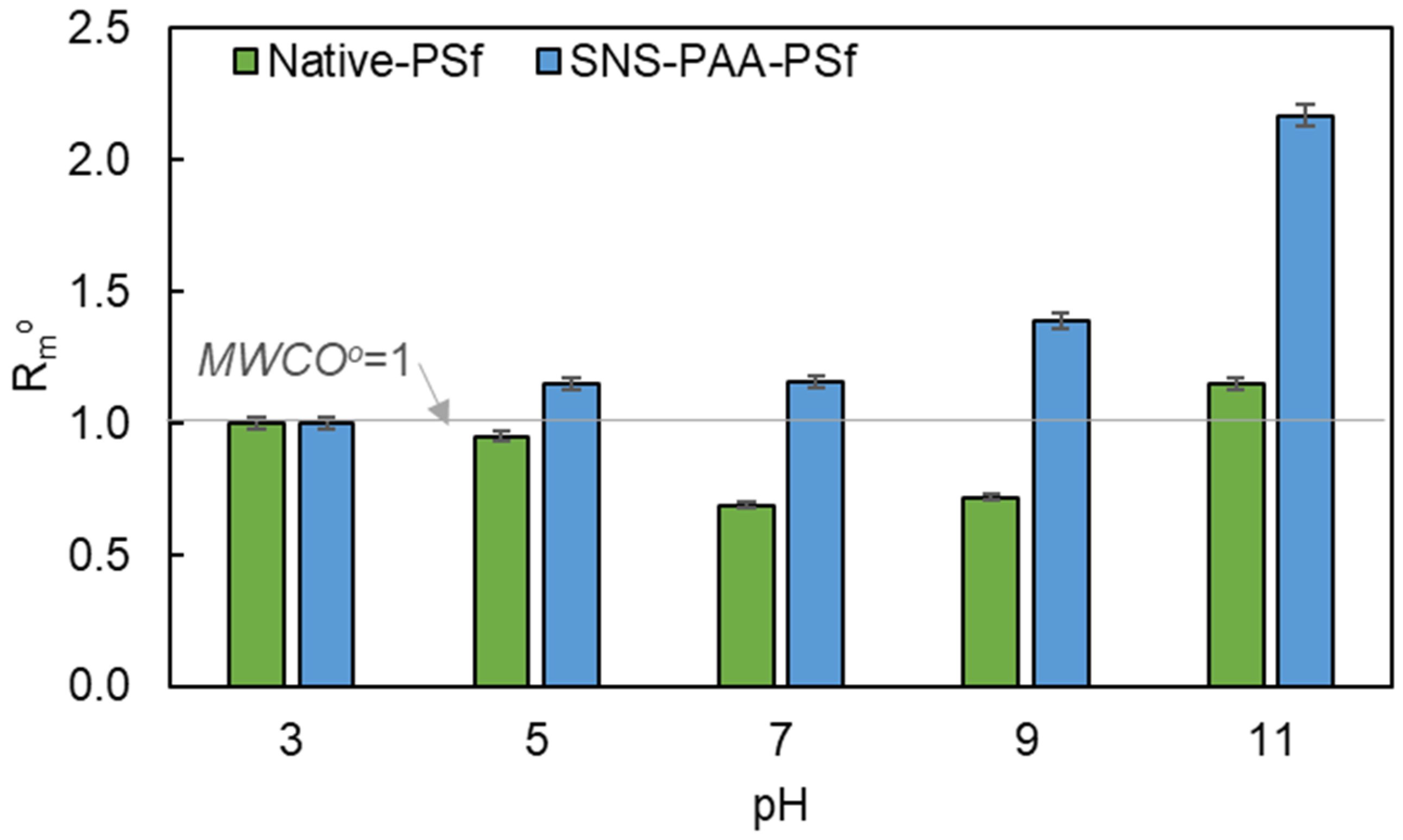
3.4. pH-Responsive SNS-PAA-PSf UF Membrane Performance
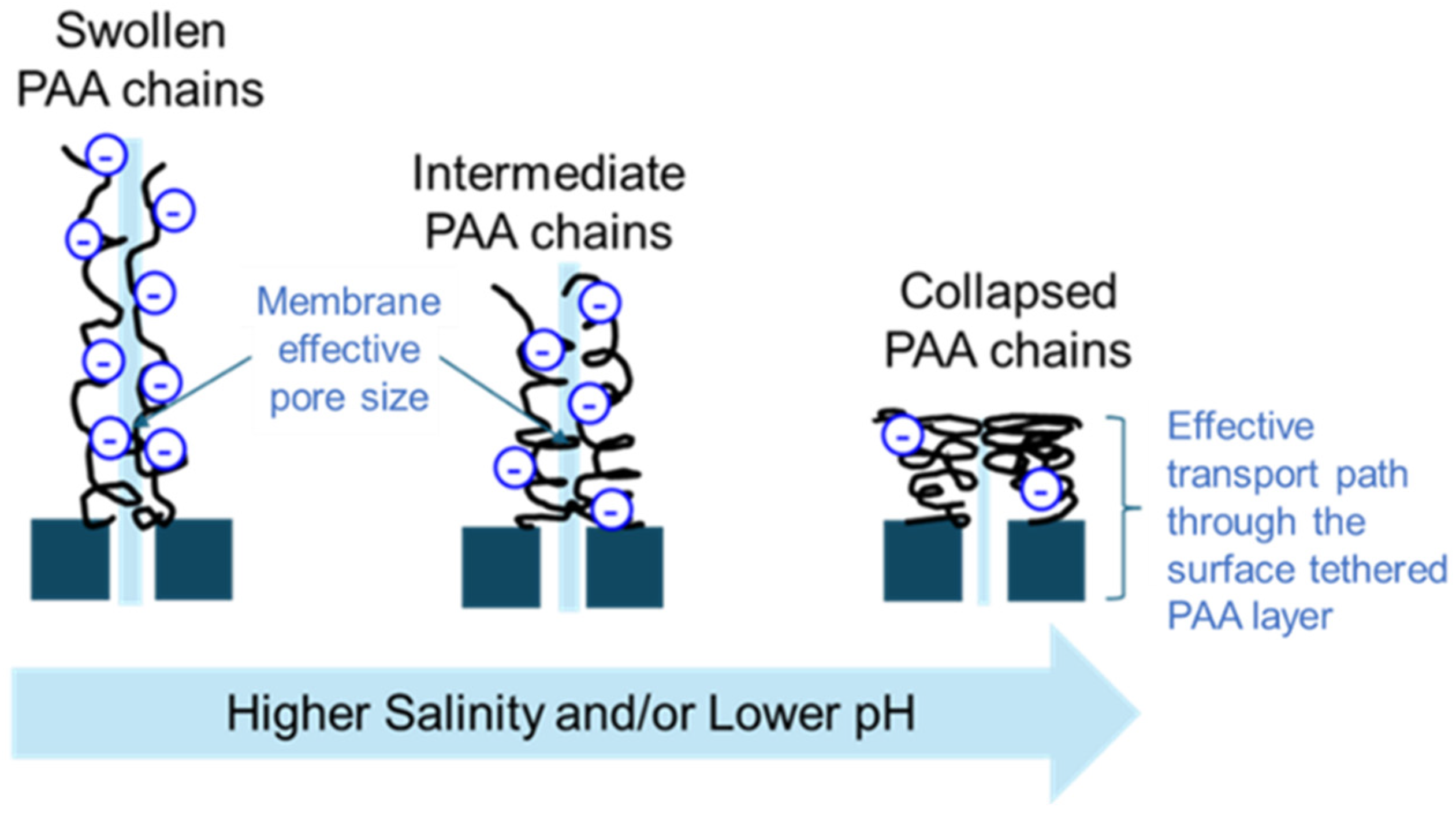
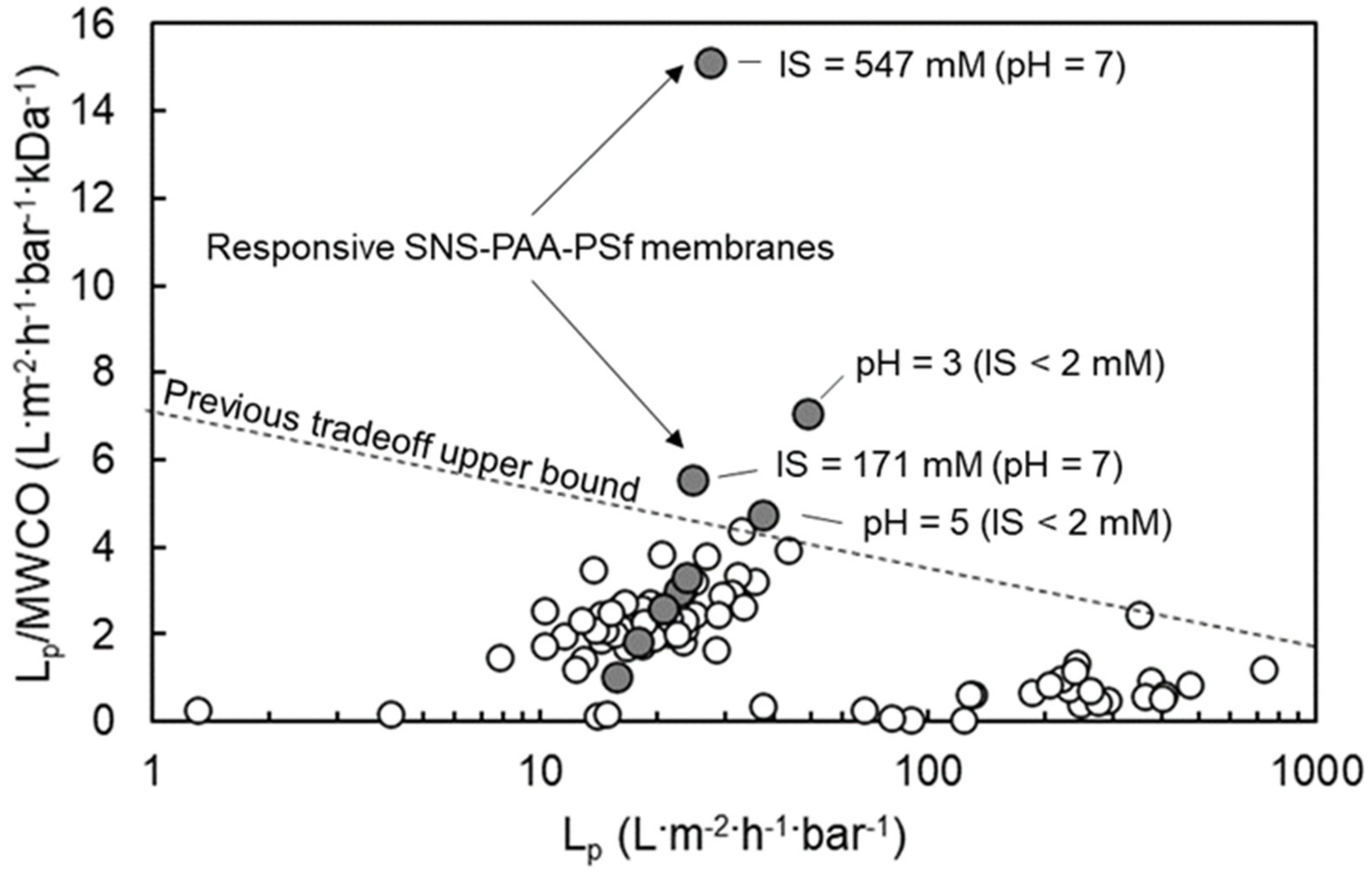
3.5. Tuning of the Responsive SNS-PAA-PSf UF Membrane Performance and Surface Wettability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, J.; Monnot, M.; Eljaddi, T.; Ercolei, L.; Simonian, L.; Moulin, P. Ultrafiltration as tertiary treatment for municipal wastewater reuse. Sep. Purif. Technol. 2021, 272, 118921. [Google Scholar] [CrossRef]
- Nikhil, M.Y.P. Ultrafiltration Market Size, Share, Competitive Landscape and Trend Analysis Report by Type (Polymeric, Ceramic), by Module (Hollow Fiber, Plate and Frame, Tubular), by Application (Municipal Treatment, Industrial Treatment): Global Opportunity Analysis and Industry Forecast, 2021–2031; Allied Market Research: Portland, OR, USA, 2022. [Google Scholar]
- Global Ultrafiltration Membrane Filtration Market by Type (Organic Membrane, Inorganic Membrane), by Application (Industrial & Municipal, Food & Beverage), by Geographic Scope and Forecast; Report ID: 78328; Verfied Market Reports: Washington, DC, USA, 2024.
- Abbas, M.A.; Mushtaq, S.; Cheema, W.A.; Qiblawey, H.; Zhu, S.; Li, Y.; Zhang, R.; Wu, H.; Jiang, Z.; Sadiq, R. Surface Modification of TFC-PA RO Membrane by Grafting Hydrophilic pH Switchable Poly (Acrylic Acid) Brushes. Adv. Polym. Technol. 2020, 2020, 8281058. [Google Scholar] [CrossRef]
- Liu, H.; Yang, S.; Liu, Y.; Miao, M.; Zhao, Y.; Sotto, A.; Gao, C.; Shen, J. Fabricating a pH-responsive membrane through interfacial in-situ assembly of microgels for water gating and self-cleaning. J. Membr. Sci. 2019, 579, 230–239. [Google Scholar] [CrossRef]
- Huang, T.; Su, Z.; Hou, K.; Zeng, J.; Zhou, H.; Zhang, L.; Nunes, S.P. Advanced stimuli-responsive membranes for smart separation. Chem. Soc. Rev. 2023, 52, 4173–4207. [Google Scholar] [CrossRef] [PubMed]
- Wandera, D.; Wickramasinghe, S.R.; Husson, S.M. Stimuli-responsive membranes. J. Membr. Sci. 2010, 357, 6–35. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, W.; Xie, R.; Ju, X.-J.; Chu, L.-Y. Stimuli-responsive smart gating membranes. Chem. Soc. Rev. 2016, 45, 460–475. [Google Scholar] [CrossRef] [PubMed]
- Darvishmanesh, S.; Qian, X.; Wickramasinghe, S.R. Responsive membranes for advanced separations. Curr. Opin. Chem. Eng. 2015, 8, 98–104. [Google Scholar] [CrossRef]
- Uredat, S.; Gujare, A.; Runge, J.; Truzzolillo, D.; Oberdisse, J.; Hellweg, T. A review of stimuli-responsive polymer-based gating membranes. Phys. Chem. Chem. Phys. 2024, 26, 2732–2744. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Liu, Y.; Yang, S.; Zhang, C.; Ullah, Z. Recent research progress on the stimuli-responsive smart membrane: A review. Nanotechnol. Rev. 2023, 12, 20220538. [Google Scholar] [CrossRef]
- Birmod, R.P.; Lade, V.G.; Shambharkar, R.D. Responsive membranes for wastewater treatment. In Handbook of Nanomaterials for Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2021; pp. 673–697. [Google Scholar]
- Wandera, D.; Wickramasinghe, S.R.; Husson, S.M. Modification and characterization of ultrafiltration membranes for treatment of produced water. J. Membr. Sci. 2011, 373, 178–188. [Google Scholar] [CrossRef]
- Gancarz, I.; Poźniak, G.; Bryjak, M. Modification of polysulfone membranes 1. CO2 plasma treatment. Eur. Polym. J. 1999, 35, 1419–1428. [Google Scholar] [CrossRef]
- Sinha, M.; Purkait, M. Preparation and characterization of novel pegylated hydrophilic pH responsive polysulfone ultrafiltration membrane. J. Membr. Sci. 2014, 464, 20–32. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, T.; Peng, H.; Li, M.; Zhu, X.; Yao, Y. Nanostructured switchable pH-responsive membranes prepared via spherical polyelectrolyte brushes. J. Membr. Sci. 2019, 580, 117–124. [Google Scholar] [CrossRef]
- Shi, Q.; Su, Y.; Ning, X.; Chen, W.; Peng, J.; Jiang, Z. Graft polymerization of methacrylic acid onto polyethersulfone for potential pH-responsive membrane materials. J. Membr. Sci. 2010, 347, 62–68. [Google Scholar] [CrossRef]
- Iwata, H.; Matsuda, T. Preparation and properties of novel environment-sensitive membranes prepared by graft polymerization onto a porous membrane. J. Membr. Sci. 1988, 38, 185–199. [Google Scholar] [CrossRef]
- Wang, M.; An, Q.-F.; Wu, L.-G.; Mo, J.-X.; Gao, C.-J. Preparation of pH-responsive phenolphthalein poly (ether sulfone) membrane by redox-graft pore-filling polymerization technique. J. Membr. Sci. 2007, 287, 257–263. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, R.; Chen, C.; Chen, B.; Zhu, X. High-Flux pH-Responsive Ultrafiltration Membrane for Efficient Nanoparticle Fractionation. ACS Appl. Mater. Interfaces 2021, 13, 56575–56583. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-Y.; Li, W.; Zhou, J.; Gu, J.-S.; Huang, L.; Tang, Z.-Q.; Wei, X.-W. Thermo-and pH-responsive polypropylene microporous membrane prepared by the photoinduced RAFT-mediated graft copolymerization. J. Membr. Sci. 2009, 343, 82–89. [Google Scholar] [CrossRef]
- Shim, J.K.; Lee, Y.B.; Lee, Y.M. pH-dependent permeation through polysulfone ultrafiltration membranes prepared by ultraviolet polymerization technique. J. Appl. Polym. Sci. 1999, 74, 75–82. [Google Scholar] [CrossRef]
- Fan, K.; Huang, J.; Yang, H.; Lu, R.; Sun, X.; Hu, J.; Hou, Z. pH and thermal-dependent ultrafiltration membranes prepared from poly (methacrylic acid) grafted onto polyethersulfone synthesized by simultaneous irradiation in homogenous phase. J. Membr. Sci. 2017, 543, 335–341. [Google Scholar] [CrossRef]
- Gao, K.; Kearney, L.T.; Wang, R.; Howarter, J.A. Enhanced wettability and transport control of ultrafiltration and reverse osmosis membranes with grafted polyelectrolytes. ACS Appl. Mater. Interfaces 2015, 7, 24839–24847. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Rovira-Bru, M.; Giralt, F.; Cohen, Y. Hydraulic Resistance and Protein Fouling Resistance of a Zirconia Membrane with a Tethered PVP Layer. Water 2021, 13, 951. [Google Scholar] [CrossRef]
- Welch, M.E.; Ober, C.K. Responsive and patterned polymer brushes. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1457–1472. [Google Scholar] [CrossRef]
- Zhao, Y.-F.; Zhang, P.-B.; Sun, J.; Liu, C.-J.; Zhu, L.-P.; Xu, Y.-Y. Electrolyte-responsive polyethersulfone membranes with zwitterionic polyethersulfone-based copolymers as additive. J. Membr. Sci. 2016, 510, 306–313. [Google Scholar] [CrossRef]
- Ito, Y.; Ochiai, Y.; Park, Y.S.; Imanishi, Y. pH-sensitive gating by conformational change of a polypeptide brush grafted onto a porous polymer membrane. J. Am. Chem. Soc. 1997, 119, 1619–1623. [Google Scholar] [CrossRef]
- Birkner, M.; Ulbricht, M. Ultrafiltration membranes with markedly different pH-and ion-responsivity by photografted zwitterionic polysulfobetain or polycarbobetain. J. Membr. Sci. 2015, 494, 57–67. [Google Scholar] [CrossRef]
- Gajda, M.; Ulbricht, M. Capillary pore membranes with grafted diblock copolymers showing reversibly changing ultrafiltration properties with independent response to ions and temperature. J. Membr. Sci. 2016, 514, 510–517. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, D.; Wu, J.; Protsak, I.; Mao, S.; Ma, C.; Ma, M.; Zhong, M.; Tan, J.; Yang, J. Novel Salt-Responsive SiO2@ Cellulose Membranes Promote Continuous Gradient and Adjustable Transport Efficiency. ACS Appl. Mater. Interfaces 2020, 12, 42169–42178. [Google Scholar] [CrossRef]
- Yang, Q.; Adrus, N.; Tomicki, F.; Ulbricht, M. Composites of functional polymeric hydrogels and porous membranes. J. Mater. Chem. 2011, 21, 2783–2811. [Google Scholar] [CrossRef]
- Ito, Y.; Park, Y.S.; Imanishi, Y. Nanometer-sized channel gating by a self-assembled polypeptide brush. Langmuir 2000, 16, 5376–5381. [Google Scholar] [CrossRef]
- Kostaras, C.; Kati, D.; Christoulaki, A.; Spiliopoulos, N.; Anastassopoulos, D.; Vradis, A.; Toprakcioglu, C.; Priftis, G. Stimuli-responsive polymer brushes in nanoconfined geometry. In Proceedings of the 10th Panhellenic Chemical Engineering Scientific Conference, Patras, Greece, 4–6 June 2015. [Google Scholar]
- Chen, Y.; Kim, S.; Cohen, Y. Tuning the hydraulic permeability and molecular weight cutoff (MWCO) of surface nano-structured ultrafiltration membranes. J. Membr. Sci. 2021, 629, 119180. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Cohen, Y. Fouling resistant and performance tunable ultrafiltration membranes via surface graft polymerization induced by atmospheric pressure air plasma. Sep. Purif. Technol. 2022, 286, 120490. [Google Scholar] [CrossRef]
- Chen, Y.; Kim, S.; Kim, Y.; Walker, J.S.; Wolfe, T.; Coleman, K.; Cohen, Y. Scale up of polyamide reverse osmosis membranes surface modification with tethered poly (acrylic acid) for fabrication of low fouling spiral-wound elements. Desalination 2022, 536, 115762. [Google Scholar] [CrossRef]
- Chen, Y. Performance Tuning of Ultrafiltration and Reverse Osmosis Membranes Surface Nano-structured with Tethered Poly (Acrylic Acid) Chains; UCLA: Los Angeles, CA, USA, 2022. [Google Scholar]
- Kim, S.; Cohen, Y.; Moses, K.J.; Sharma, S.; Bilal, M. Polysulfone surface nano-structured with tethered polyacrylic acid. Appl. Surf. Sci. 2019, 470, 411–422. [Google Scholar] [CrossRef]
- Chen, Y.; Cohen, Y. RO membrane with a surface tethered polymer brush layer for enhanced rejection of nitrate, boron, and arsenic. J. Membr. Sci. Lett. 2023, 3, 100062. [Google Scholar] [CrossRef]
- Cohen, Y.; Lin, N.; Varin, K.J.; Chien, D.; Hicks, R.F. Membrane surface nanostructuring with terminally anchored polymer chains. In Functional Nanostructured Materials and Membranes for Water Treatment; Wiley: Hoboken, NJ, USA, 2013; pp. 85–124. [Google Scholar]
- Levy, R.; Maaloum, M. Measuring the spring constant of atomic force microscope cantilevers: Thermal fluctuations and other methods. Nanotechnology 2001, 13, 33. [Google Scholar] [CrossRef]
- Yushkin, A.; Borisov, R.; Volkov, V.; Volkov, A. Improvement of MWCO determination by using branched PEGs and MALDI method. Sep. Purif. Technol. 2019, 211, 108–116. [Google Scholar] [CrossRef]
- Toh, Y.S.; Lim, F.; Livingston, A. Polymeric membranes for nanofiltration in polar aprotic solvents. J. Membr. Sci. 2007, 301, 3–10. [Google Scholar]
- Hilal, N.; Al-Abri, M.; Al-Hinai, H. Characterization and retention of UF membranes using PEG, HS and polyelectrolytes. Desalination 2007, 206, 568–578. [Google Scholar] [CrossRef]
- Milner, S.T. Polymer brushes. Science 1991, 251, 905–914. [Google Scholar] [CrossRef]
- Jin, X.; Hsieh, Y.-L. pH-responsive swelling behavior of poly (vinyl alcohol)/poly (acrylic acid) bi-component fibrous hydrogel membranes. Polymer 2005, 46, 5149–5160. [Google Scholar] [CrossRef]
- Bryjak, M.; Gancarz, I.; Poźniak, G. Surface evaluation of plasma-modified polysulfone (Udel P-1700) films. Langmuir 1999, 15, 6400–6404. [Google Scholar] [CrossRef]
- Chen, T.; Ferris, R.; Zhang, J.; Ducker, R.; Zauscher, S. Stimulus-responsive polymer brushes on surfaces: Transduction mechanisms and applications. Prog. Polym. Sci. 2010, 35, 94–112. [Google Scholar] [CrossRef]
- Van Oss, C.J. Interfacial Forces in Aqueous Media; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Hosseinzadeh, A.; Ranjbar, P.R.; Bozorg, A. pH-responsive P (AA-b-SBMA)-grafted magnetic GO high-performance ultrafiltration membrane with improved antifouling tendency and cleaning efficiency. Desalination 2024, 574, 117299. [Google Scholar] [CrossRef]
- Zhao, X.; Su, Y.; Chen, W.; Peng, J.; Jiang, Z. pH-responsive and fouling-release properties of PES ultrafiltration membranes modified by multi-functional block-like copolymers. J. Membr. Sci. 2011, 382, 222–230. [Google Scholar] [CrossRef]
- Ma, R.; Lu, X.; Wu, C.; Zhang, S.; Zheng, S.; Ren, K.; Gu, J.; Wang, H.; Shen, H. Performance design of a highly anti-fouling porous membrane with dual pH-responsiveness. J. Membr. Sci. 2022, 660, 120886. [Google Scholar] [CrossRef]
- Zhao, X.; Qin, A.; Liu, D.; He, C. Tuning the antifouling property of PVDF ultrafiltration membrane with surface anchored polyelectrolyte complexes for sewage treatment. RSC Adv. 2015, 5, 63580–63587. [Google Scholar] [CrossRef]
- Bouranene, S.; Szymczyk, A.; Fievet, P.; Vidonne, A. Influence of inorganic electrolytes on the retention of polyethyleneglycol by a nanofiltration ceramic membrane. J. Membr. Sci. 2007, 290, 216–221. [Google Scholar] [CrossRef]
- Zhou, Z.; Murdoch, W.J.; Shen, Y. Synthesis of an esterase-sensitive degradable polyester as facile drug carrier for cancer therapy. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 507–515. [Google Scholar] [CrossRef]
- Hoek, E.M.; Ghosh, A.K.; Huang, X.; Liong, M.; Zink, J.I. Physical–chemical properties, separation performance, and fouling resistance of mixed-matrix ultrafiltration membranes. Desalination 2011, 283, 89–99. [Google Scholar] [CrossRef]
- Dang, H.T.; Narbaitz, R.M.; Matsuura, T. Double-pass casting: A novel technique for developing high performance ultrafiltration membranes. J. Membr. Sci. 2008, 323, 45–52. [Google Scholar] [CrossRef]
- Lai, G.; Lau, W.; Goh, P.; Ismail, A.; Yusof, N.; Tan, Y. Graphene oxide incorporated thin film nanocomposite nanofiltration membrane for enhanced salt removal performance. Desalination 2016, 387, 14–24. [Google Scholar] [CrossRef]
- Qasm, N.A.; Generous, M.M.; Qureshi, B.A.; Zubair, S.M. A Comprehensive Review of Saline Water Correlations and Data: Part II—Thermophysical Properties. Arab. J. Sci. Eng. 2021, 46, 1941–1979. [Google Scholar] [CrossRef]
- Nayar, K.; Panchanathan, D.; McKinley, G.; Lienhard, J. Surface tension of seawater. J. Phys. Chem. Ref. Data 2014, 43, 043103. [Google Scholar] [CrossRef]
- Beattie, J.K.; Djerdjev, A.M.; Gray-Weale, A.; Kallay, N.; Lützenkirchen, J.; Preočanin, T.; Selmani, A. pH and the surface tension of water. J. Colloid Interface Sci. 2014, 422, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Lü, Y.; Wei, B. Second inflection point of water surface tension. Appl. Phys. Lett. 2006, 89, 164106. [Google Scholar] [CrossRef]
- Fu, D.; Lu, J.-F.; Bao, T.-Z.; Li, Y.-G. Investigation of surface tension and interfacial tension in surfactant solutions by SAFT. Ind. Eng. Chem. Res. 2000, 39, 320–327. [Google Scholar] [CrossRef]
- Goncalves, F.; Kestin, J.; Sengers, J. Surface-tension effects in suspended-level capillary viscometers. Int. J. Thermophys. 1991, 12, 1013–1028. [Google Scholar] [CrossRef]
- Nikitas, P.; Pappa-Louisi, A. Thermodynamic and modelistic study of surface solutions: Aqueous solutions containing 2-butanol. J. Phys. Chem. 1990, 94, 361–370. [Google Scholar] [CrossRef]


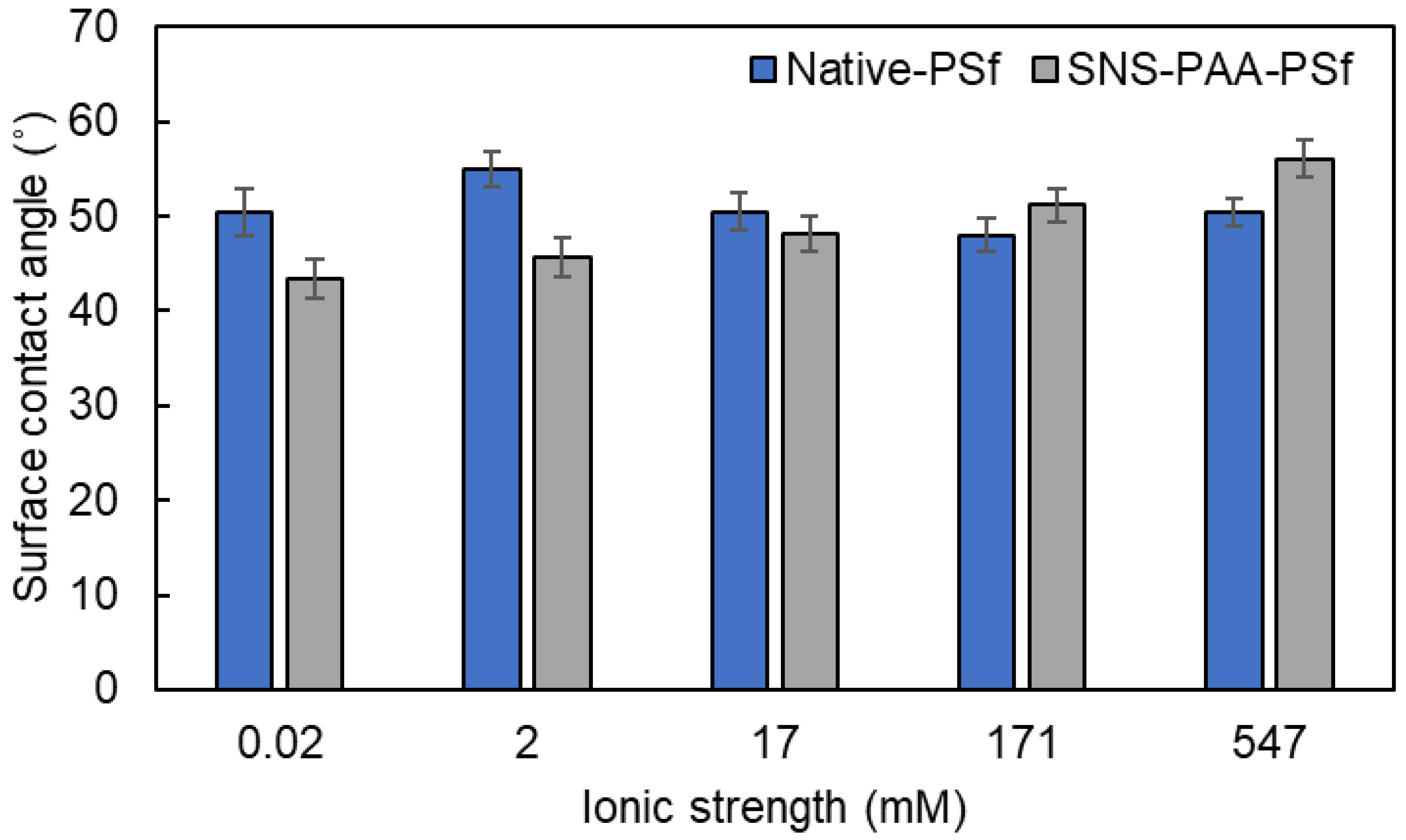
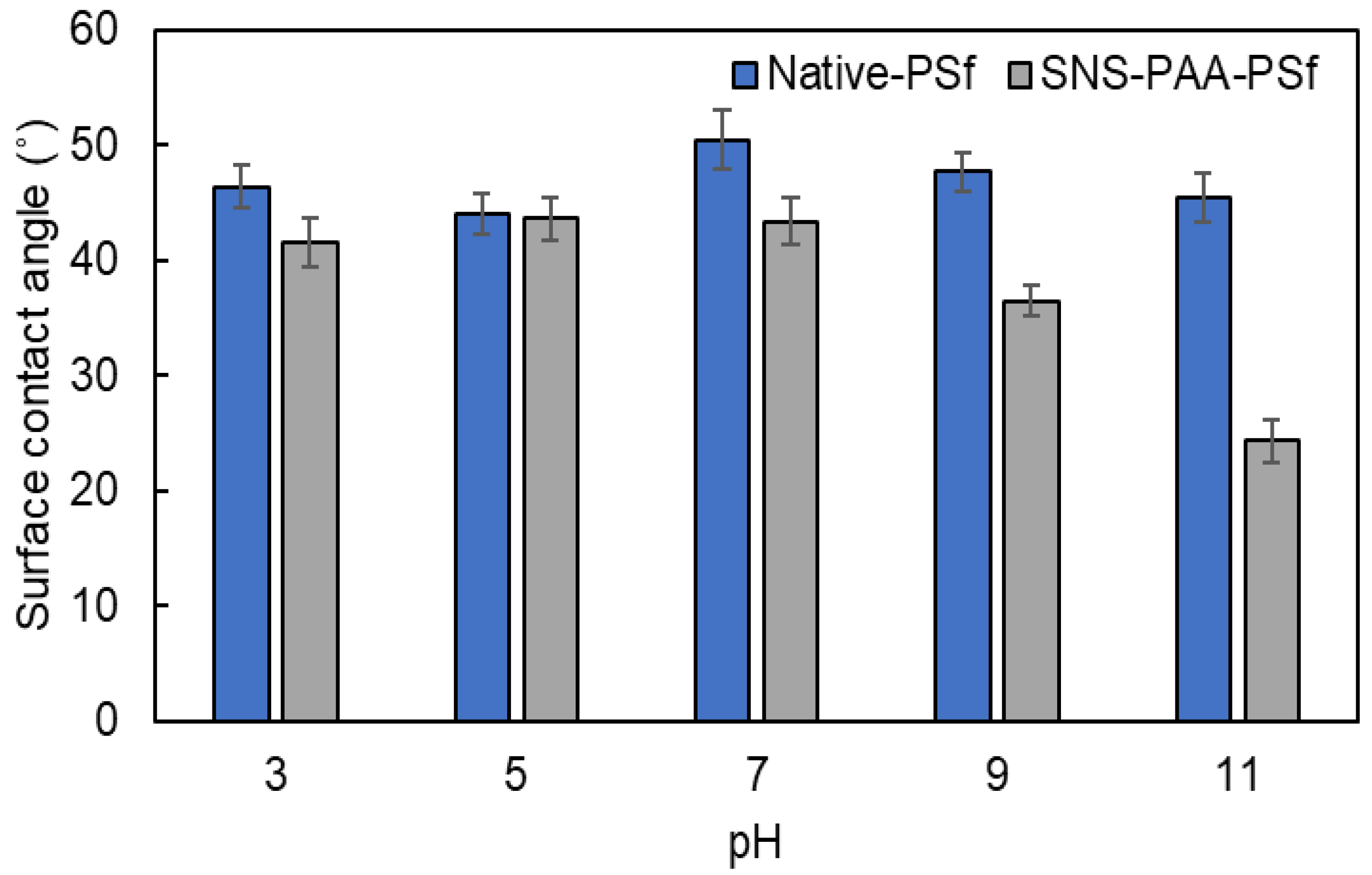


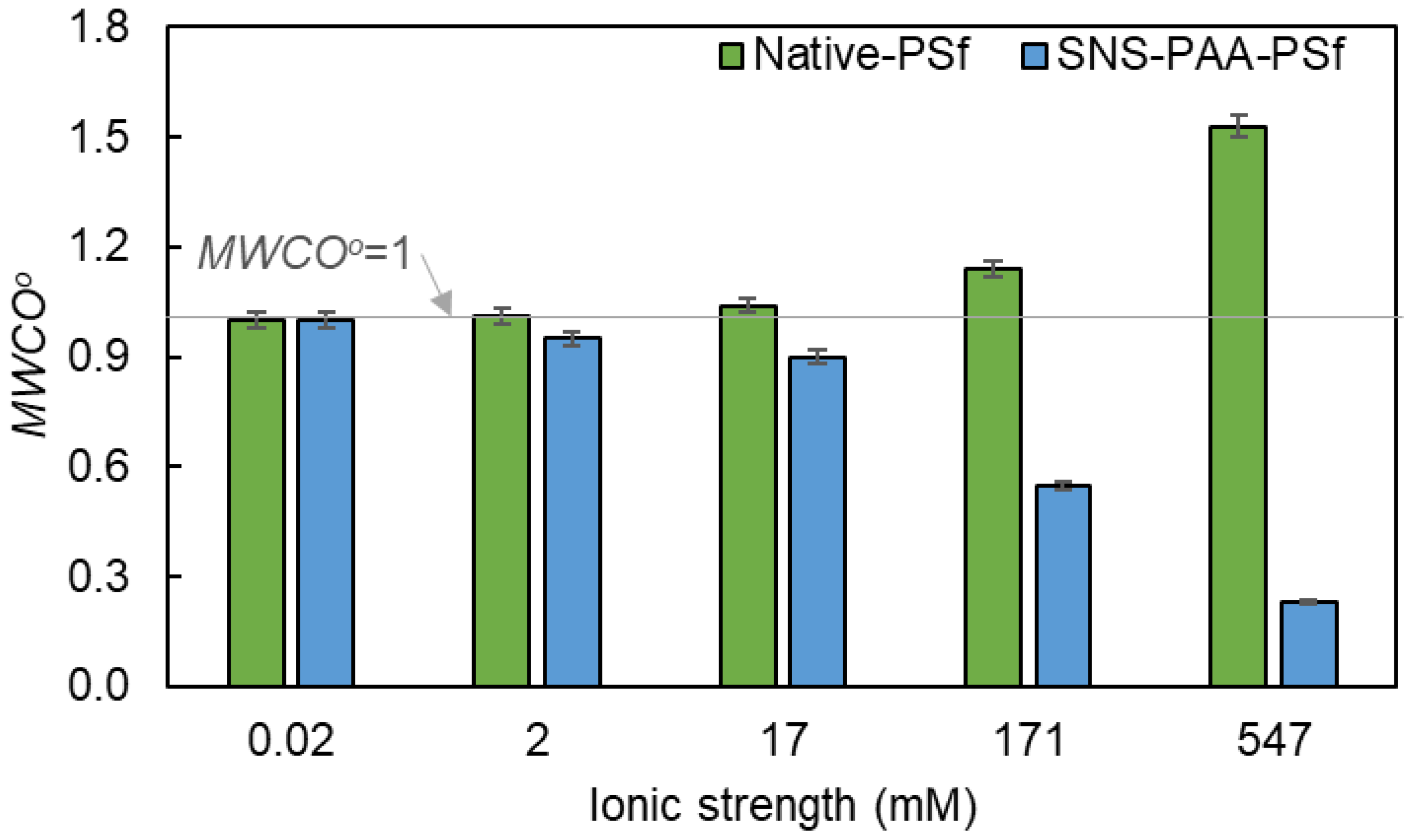


| Aqueous Environment | Surface Tension a (mN·m−1) | Native-PSf Membrane b | SNS-PAA-PSf Membrane | ||
|---|---|---|---|---|---|
| CB Contact Angle (°) | Surface Energy c (mJ·m−2) | CB Contact Angle (°) | Surface Energy c (mJ·m−2) | ||
| Ionic Strength (mM) | |||||
| ~0.02 | 72.8 | 50.5 ± 2.5 | −119.1 ± 2.4 | 43.4 ± 2.0 | −125.7 ± 1.7 |
| 2 | 72.8 | 55.0 ± 1.9 | −114.6 ± 2.0 | 45.6 ± 2.1 | −123.8 ± 1.9 |
| 17 | 72.8 | 50.5 ± 1.9 | −119.1 ± 1.9 | 48.1 ± 1.9 | −121.4 ± 1.8 |
| 171 | 73.0 | 48.0 ± 1.7 | −121.9 ± 1.6 | 51.2 ± 1.8 | −118.8 ± 1.8 |
| 547 | 73.5 | 50.4 ± 1.5 | −120.4 ± 1.5 | 56.1 ± 1.9 | −114.5 ± 2.0 |
| pH | |||||
| 3 | 72.8 | 46.4 ± 1.9 | −123.0 ± 1.7 | 41.6 ± 2.1 | −127.3 ± 1.8 |
| 5 | 72.8 | 44.0 ± 1.8 | −125.2 ± 1.6 | 43.6 ± 1.9 | −125.5 ± 1.7 |
| 7 | 72.8 | 50.5 ± 2.5 | −119.1 ± 2.5 | 43.4 ± 2.0 | −125.7 ± 1.8 |
| 9 | 72.8 | 47.7 ± 1.7 | −121.8 ± 1.6 | 36.5 ± 1.4 | −131.3 ± 1.0 |
| 11 | 72.8 | 45.4 ± 2.1 | −123.9 ± 1.9 | 24.3 ± 1.8 | −139.2 ± 1.0 |
| Native-PSf Membrane (a) | SNS-PAA-PSf Membrane (b) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lp (L·m−2·h−1·bar−1) (c) | Rm (m−1) (d) | Rmo (e) | MWCO (kDa) (f) | MWCOo (e) | Pore Size (nm) | Lp (L·m−2·h−1·bar−1) | Rm (m−1) | Rmo (e) | MWCO (kDa) (f) | MWCOo (e) | Pore Size (nm) | |
| Ionic strength (mM) (g) | ||||||||||||
| ~0.02 | 23.2 | 1.55 × 1013 | 1.00 | 10.0 | 1.00 | 2.9 | 20.7 | 1.74 × 1013 | 1.00 | 8.0 | 1.00 | 2.7 |
| 2 | 23.2 | 1.55 × 1013 | 1.00 | 10.1 | 1.01 | 2.9 | 22.7 | 1.59 × 1013 | 0.91 | 7.6 | 0.95 | 2.6 |
| 17 | 23.1 | 1.55 × 1013 | 1.00 | 10.4 | 1.04 | 2.9 | 23.8 | 1.51 × 1013 | 0.87 | 7.2 | 0.90 | 2.6 |
| 171 | 22.3 | 1.59 × 1013 | 1.02 | 11.4 | 1.14 | 3.0 | 24.5 | 1.44 × 1013 | 0.83 | 4.4 | 0.55 | 2.2 |
| 547 | 21.1 | 1.61 × 1013 | 1.04 | 15.3 | 1.53 | 3.3 | 27.2 | 1.24 × 1013 | 0.72 | 1.8 | 0.23 | 1.6 |
| pH (h) | ||||||||||||
| 3 | 26.2 | 1.37 × 1013 | 1.00 | 14.6 | 1.00 | 3.3 | 48.7 | 7.38 × 1012 | 1.00 | 6.9 | 1.00 | 2.5 |
| 5 | 25.1 | 1.44 × 1013 | 1.05 | 13.8 | 0.95 | 3.2 | 37.4 | 9.62 × 1012 | 1.30 | 7.9 | 1.15 | 2.7 |
| 7 | 23.2 | 1.55 × 1013 | 1.13 | 10.0 | 0.69 | 2.9 | 20.7 | 1.74 × 1013 | 2.36 | 8.0 | 1.16 | 2.7 |
| 9 | 23.8 | 1.51 × 1013 | 1.10 | 10.5 | 0.72 | 2.9 | 17.7 | 2.03 × 1013 | 2.75 | 9.6 | 1.39 | 2.8 |
| 11 | 24.9 | 1.45 × 1013 | 1.05 | 16.8 | 1.15 | 3.4 | 15.7 | 2.29 × 1013 | 3.11 | 15.0 | 2.17 | 3.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Cohen, Y. Ionic Strength and pH-Responsive Ultrafiltration Membrane to Overcome the Typical Permeability-Selectivity Tradeoff. Water 2025, 17, 254. https://doi.org/10.3390/w17020254
Chen Y, Cohen Y. Ionic Strength and pH-Responsive Ultrafiltration Membrane to Overcome the Typical Permeability-Selectivity Tradeoff. Water. 2025; 17(2):254. https://doi.org/10.3390/w17020254
Chicago/Turabian StyleChen, Yian, and Yoram Cohen. 2025. "Ionic Strength and pH-Responsive Ultrafiltration Membrane to Overcome the Typical Permeability-Selectivity Tradeoff" Water 17, no. 2: 254. https://doi.org/10.3390/w17020254
APA StyleChen, Y., & Cohen, Y. (2025). Ionic Strength and pH-Responsive Ultrafiltration Membrane to Overcome the Typical Permeability-Selectivity Tradeoff. Water, 17(2), 254. https://doi.org/10.3390/w17020254






