Uptake and Effects of Yttrium on the Seaweed Ulva sp.: A Study on the Potential Risks of Rare Earth Elements in Aquatic Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Sampling
2.3. Experimental Design
2.4. Analytical Quantification
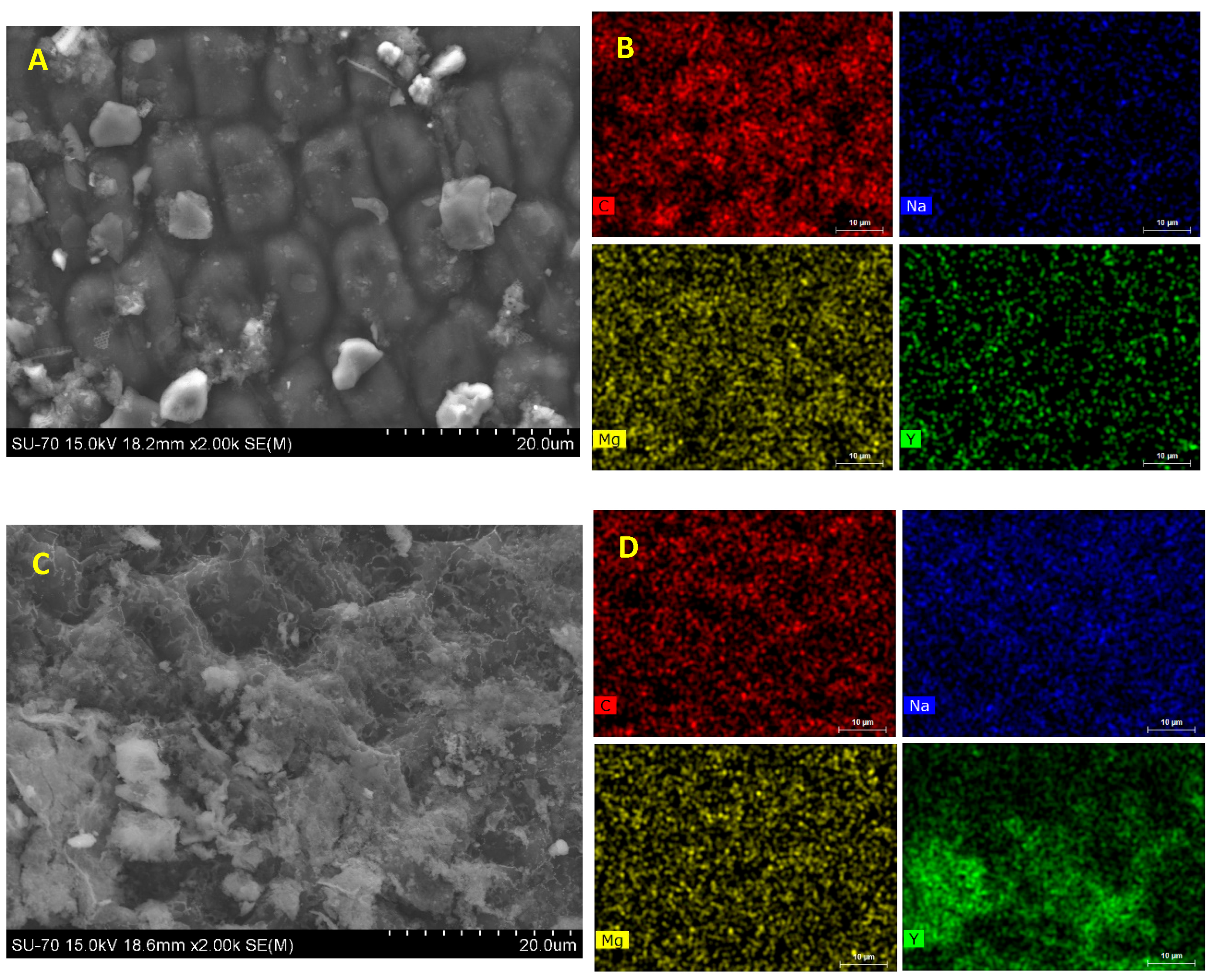
2.5. Seaweed Characterization
2.6. Partition of Y in Seaweed: Intra- vs. Extra-Cellular Concentration
2.7. Biological Responses
2.7.1. Biochemical Markers Analysis
Antioxidant and Biotransformation Enzymes
Oxidative Damage
2.7.2. Total Chlorophyll Analysis
2.7.3. Relative Growth Rate Analysis
2.8. Data Analysis
3. Results
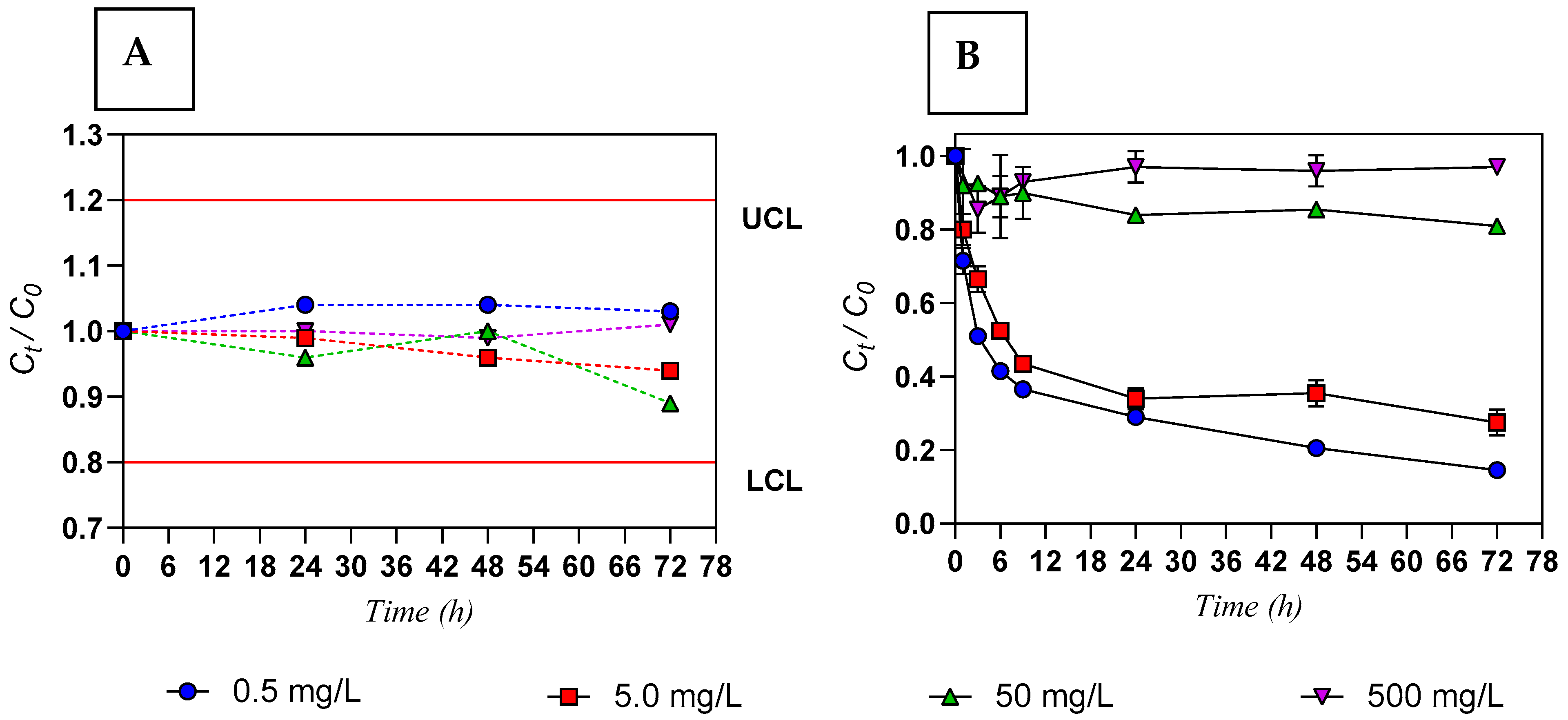
3.1. Yttrium Concentration in Water
3.2. Yttrium Concentration in Ulva sp.
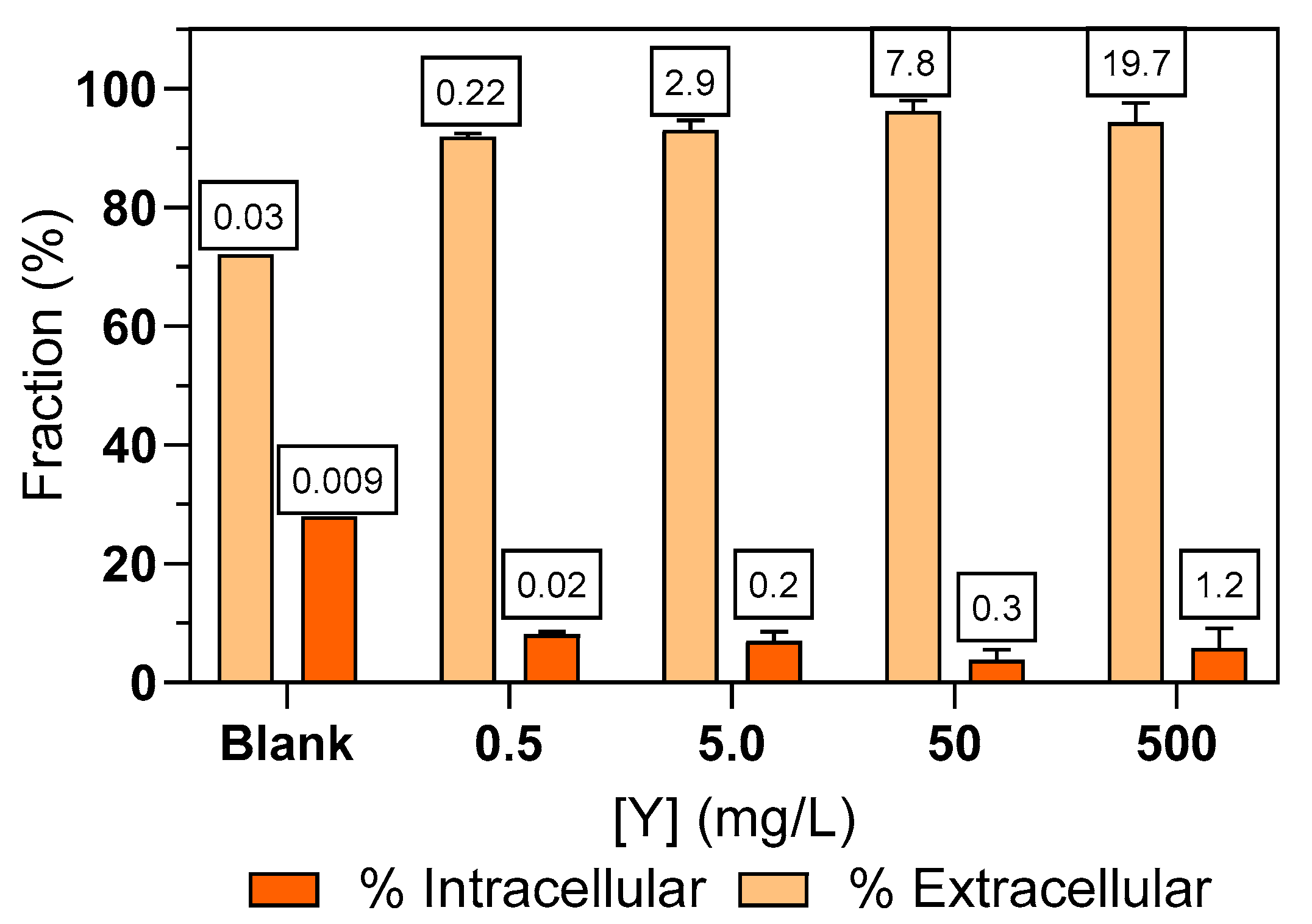
3.3. Yttrium Partition in Ulva sp. Biomass
3.4. Biological Responses
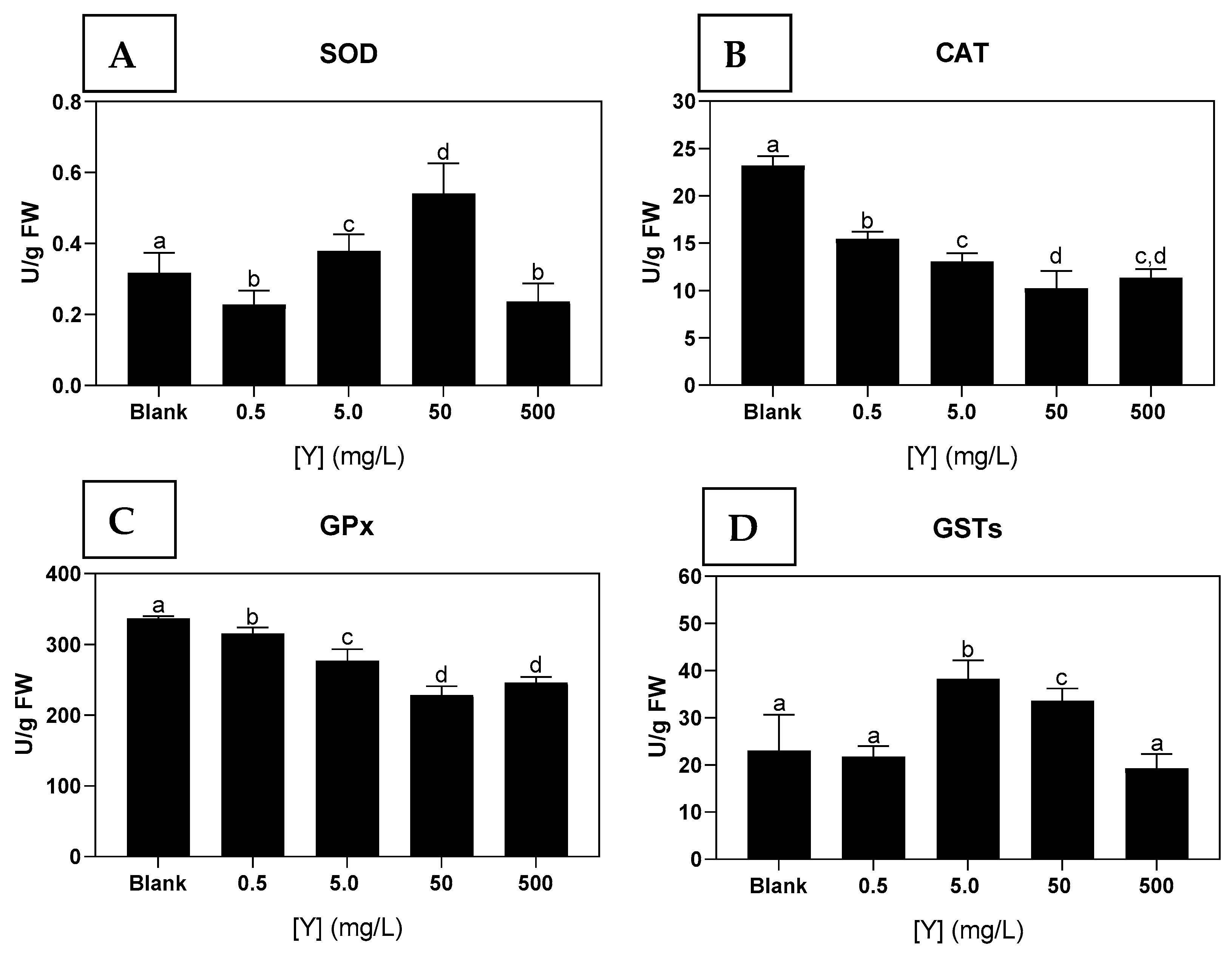
3.4.1. Antioxidant and Biotransformation Enzymes
3.4.2. Oxidative Damage
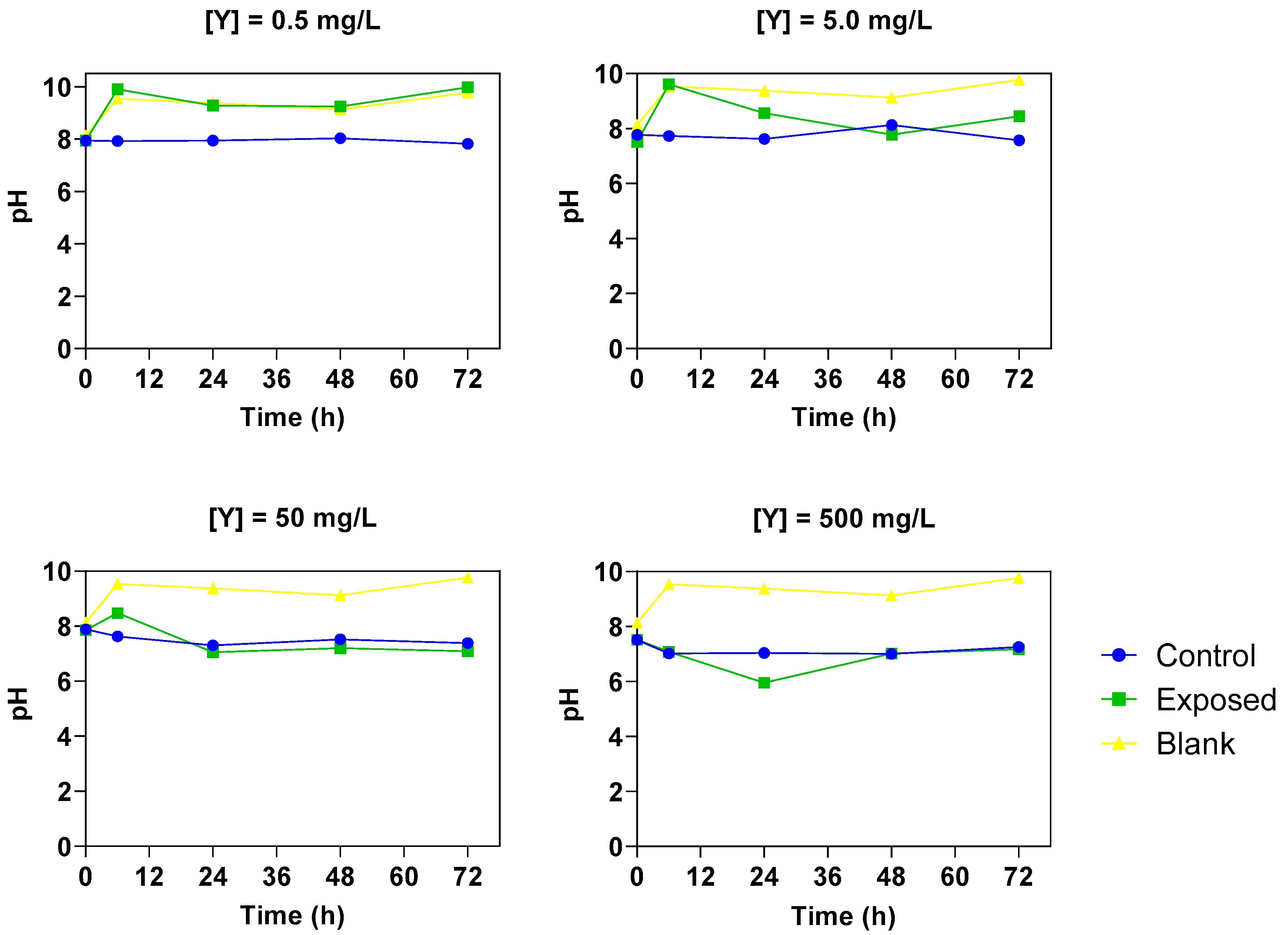
3.4.3. Total Chlorophyll, Relative Growth Rate and pH Monitoring
4. Discussion
4.1. Sorption of Y by Ulva sp.
4.2. Fractioning of Y in Ulva sp. Biomass
4.3. Biomarkers and Physiological Responses of Ulva sp. to Y Exposure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Energy Agency. Critical Minerals Market Review 2023; OECD Publishing: Paris, France, 2023. [Google Scholar] [CrossRef]
- Worlanyo, A.S.; Jiangfeng, L. Evaluating the Environmental and Economic Impact of Mining for Post-Mined Land Restoration and Land-Use: A Review. J. Environ. Manag. 2021, 279, 111623. [Google Scholar] [CrossRef] [PubMed]
- UNEP; PACE; ITU; ILO; UNIDO; UNU; UNITAR; WBSCD; WEF. A New Circular Vision for Electronics Time for a Global Reboot; World Economic Forum: Cologny, Switzerland, 2019. [Google Scholar]
- Ackah, M. Informal E-Waste Recycling in Developing Countries: Review of Metal(Loid)s Pollution, Environmental Impacts and Transport Pathways. Environ. Sci. Pollut. Res. 2017, 24, 24092–24101. [Google Scholar] [CrossRef]
- Adeel, M.; Lee, J.Y.; Zain, M.; Rizwan, M.; Nawab, A.; Ahmad, M.A.; Shafiq, M.; Yi, H.; Jilani, G.; Javed, R.; et al. Cryptic Footprints of Rare Earth Elements on Natural Resources and Living Organisms. Environ. Int. 2019, 127, 785–800. [Google Scholar] [CrossRef]
- Sharifi, R.; Moore, F.; Keshavarzi, B. Geochemical Behavior and Speciation Modeling of Rare Earth Elements in Acid Drainages at Sarcheshmeh Porphyry Copper Deposit, Kerman Province, Iran. Geochemistry 2013, 73, 509–517. [Google Scholar] [CrossRef]
- Migaszewski, Z.M.; Gałuszka, A. The Use of Rare Earth Element Profiles as a Proxy for a Fractionation Source and Mine-Waste Provenance. Sci. Total Environ. 2023, 901, 166517. [Google Scholar] [CrossRef]
- Migaszewski, Z.M.; Gałuszka, A.; Dołęgowska, S. Rare Earth and Trace Element Signatures for Assessing an Impact of Rock Mining and Processing on the Environment: Wiśniówka Case Study, South-Central Poland. Environ. Sci. Pollut. Res. 2016, 23, 24943–24959. [Google Scholar] [CrossRef] [PubMed]
- Kulaksiz, S.; Bau, M. Rare Earth Elements in the Rhine River, Germany: First Case of Anthropogenic Lanthanum as a Dissolved Microcontaminant in the Hydrosphere. Environ. Int. 2011, 37, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, D.; Schmidt, K.; Klimpel, F.; Rauch, U.; Ernst, D.M.; Paul, S.A.L.; Haeckel, M.; Koschinsky, A.; Bau, M. Tracking the Distribution of Persistent and Mobile Wastewater-Derived Substances in the Southern and Central North Sea Using Anthropogenic Gadolinium from MRI Contrast Agents as a Far-Field Tracer. Mar. Pollut. Bull. 2024, 207, 116794. [Google Scholar] [CrossRef]
- Liang, C.L.; Shen, J.L. Removal of Yttrium from Rare-Earth Wastewater by Serratia Marcescens: Biosorption Optimization and Mechanisms Studies. Sci. Rep. 2022, 12, 4861. [Google Scholar] [CrossRef]
- Cardon, P.-Y.; Triffault-Bouchet, G.; Caron, A.; Rosabal, M.; Fortin, C.; Amyot, M. Toxicity and Subcellular Fractionation of Yttrium in Three Freshwater Organisms: Daphnia Magna, Chironomus Riparius, and Oncorhynchus Mykiss. ACS Omega 2019, 4, 13747–13755. [Google Scholar] [CrossRef]
- Revel, M.; van Drimmelen, C.K.E.; Weltje, L.; Hursthouse, A.; Heise, S. Effects of Rare Earth Elements in the Aquatic Environment: Implications for Ecotoxicological Testing. Crit. Rev. Environ. Sci. Technol. 2025, 55, 334–375. [Google Scholar] [CrossRef]
- Viana, T.; Almeida, R.; Figueira, P.; Rocha, L.; Neves, M.C.; Freitas, R.; Freire, M.; Henriques, B.; Pereira, E. Removal of Mercury by Silica-Supported Ionic Liquids: Efficiency and Ecotoxicological Assessment. Aquat. Toxicol. 2023, 261, 106611. [Google Scholar] [CrossRef]
- Kumar, M.; Kumari, P.; Reddy, C.R.K.; Jha, B. Salinity and Desiccation Induced Oxidative Stress Acclimation in Seaweeds. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2014; Volume 71, pp. 91–123. [Google Scholar]
- Rodrigues, M.; Oliveira, A.; Queiroga, H.; Brotas, V. Seasonal and Diurnal Water Quality and Ecological Dynamics along a Salinity Gradient (Mira Channel, Aveiro Lagoon, Portugal). Procedia Environ. Sci. 2012, 13, 899–918. [Google Scholar] [CrossRef]
- Migaszewski, Z.M.; Gałuszka, A. The Characteristics, Occurrence, and Geochemical Behavior of Rare Earth Elements in the Environment: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 429–471. [Google Scholar] [CrossRef]
- Pinto, J.; Colónia, J.; Viana, T.; Ferreira, N.; Tavares, D.; Jacinto, J.; Abdolvasei, A.; Monteiro, F.L.; Henriques, B.; Pereira, E. Potential of the Macroalga Ulva sp. for the Recovery of Yttrium Obtained from Fluorescent Lamp Waste. J. Clean. Prod. 2022, 369, 133299. [Google Scholar] [CrossRef]
- Vaz, G.V.; Pontes, F.V.M.; Salgado, L.A.B.; Carneiro, M.C.; Paulino, J.F. A Novel Method for Leaching Rare Earth Element from Fluorescent Lamp Waste via Acid Fusion. Hydrometallurgy 2025, 232, 106420. [Google Scholar] [CrossRef]
- Carvalho, L.; Reis, A.T.; Soares, E.; Tavares, C.; Monteiro, R.J.R.; Figueira, P.; Henriques, B.; Vale, C.; Pereira, E. A Single Digestion Procedure for Determination of Major, Trace, and Rare Earth Elements in Sediments. Water Air Soil Pollut. 2020, 231, 541. [Google Scholar] [CrossRef]
- Viana, T.; Henriques, B.; Ferreira, N.; Pinto, R.J.B.; Monteiro, F.L.S.; Pereira, E. Insight into the Mechanisms Involved in the Removal of Toxic, Rare Earth, and Platinum Elements from Complex Mixtures by Ulva sp. Chem. Eng. J. 2023, 453, 139630. [Google Scholar] [CrossRef]
- Nowicka, B. Heavy Metal–Induced Stress in Eukaryotic Algae—Mechanisms of Heavy Metal Toxicity and Tolerance with Particular Emphasis on Oxidative Stress in Exposed Cells and the Role of Antioxidant Response. Environ. Sci. Pollut. Res. 2022, 29, 16860–16911. [Google Scholar] [CrossRef]
- Ajitha, V.; Sreevidya, C.P.; Sarasan, M.; Park, J.C.; Mohandas, A.; Singh, I.S.B.; Puthumana, J.; Lee, J.-S. Effects of Zinc and Mercury on ROS-Mediated Oxidative Stress-Induced Physiological Impairments and Antioxidant Responses in the Microalga Chlorella Vulgaris. Environ. Sci. Pollut. Res. 2021, 28, 32475–32492. [Google Scholar] [CrossRef]
- Kumar, M.; Kumari, P.; Gupta, V.; Anisha, P.A.; Reddy, C.R.K.; Jha, B. Differential Responses to Cadmium Induced Oxidative Stress in Marine Macroalga Ulva lactuca (Ulvales, Chlorophyta). BioMetals 2010, 23, 315–325. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Johansson, L.H.; Håkan Borg, L.A. A Spectrophotometric Method for Determination of Catalase Activity in Small Tissue Samples. Anal. Biochem. 1988, 174, 331–336. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases. J. Biol. Chem. 1974, 22, 7130–7139. [Google Scholar] [CrossRef]
- Carregosa, V.; Velez, C.; Soares, A.M.V.M.; Figueira, E.; Freitas, R. Physiological and Biochemical Responses of Three Veneridae Clams Exposed to Salinity Changes. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2014, 177–178, 1–9. [Google Scholar] [CrossRef]
- Mcfarland, V.A.; Inouye, L.S.; Lutz, C.H.; Jarvis, A.S.; Clarke, J.U.; Mccant, D.D. Biomarkers of Oxidative Stress and Genotoxicity in Livers of Field-Collected Brown Bullhead, Ameiurus Nebulosus Environmental Contamination and Toxicology. Environ. Contam. Toxicol. 1999, 37, 236–241. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; Primer Ltd.: Plymouth, UK, 2008. [Google Scholar]
- Pinto, J.; Costa, M.; Henriques, B.; Soares, J.; Dias, M.; Viana, T.; Ferreira, N.; Vale, C.; Pinheiro-Torres, J.; Pereira, E. Competition among Rare Earth Elements on Sorption onto Six Seaweeds. J. Rare Earths 2021, 39, 734–741. [Google Scholar] [CrossRef]
- Moenne, A.; González, A.; Sáez, C.A. Mechanisms of Metal Tolerance in Marine Macroalgae, with Emphasis on Copper Tolerance in Chlorophyta and Rhodophyta. Aquat. Toxicol. 2016, 176, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Volterra, L.; Conti, M.E. Algae as Biomarkers, Bioaccumulators and Toxin Producers. Int. J. Environ. Pollut. 2000, 13, 92. [Google Scholar] [CrossRef]
- Circuncisão, A.; Catarino, M.; Cardoso, S.; Silva, A. Minerals from Macroalgae Origin: Health Benefits and Risks for Consumers. Mar. Drugs 2018, 16, 400. [Google Scholar] [CrossRef]
- Pinto, J.; Henriques, B.; Soares, J.; Costa, M.; Dias, M.; Fabre, E.; Lopes, C.B.; Vale, C.; Pinheiro-Torres, J.; Pereira, E. A Green Method Based on Living Macroalgae for the Removal of Rare-Earth Elements from Contaminated Waters. J. Environ. Manag. 2020, 263, 110376. [Google Scholar] [CrossRef] [PubMed]
- Coelho, J.P.; Pereira, M.E.; Duarte, A.; Pardal, M.A. Macroalgae Response to a Mercury Contamination Gradient in a Temperate Coastal Lagoon (Ria de Aveiro, Portugal). Estuar. Coast. Shelf Sci. 2005, 65, 492–500. [Google Scholar] [CrossRef]
- Fu, Y.; Li, F.; Xu, T.; Cai, S.; Chu, W.; Qiu, H.; Sha, S.; Cheng, G.; Xu, Q. Bioaccumulation, Subcellular, and Molecular Localization and Damage to Physiology and Ultrastructure in Nymphoides peltata (Gmel.) O. Kuntze Exposed to Yttrium. Environ. Sci. Pollut. Res. 2014, 21, 2935–2942. [Google Scholar] [CrossRef]
- Tunali, M.; Yenigun, O. Biosorption of Ag+ and Nd3+ from Single- and Multi-Metal Solutions (Ag+, Nd3+, and Au3+) by Using Living and Dried Microalgae. J. Mater. Cycles Waste Manag. 2021, 23, 764–777. [Google Scholar] [CrossRef]
- Carreira, A.R.F.; Passos, H.; Coutinho, J.A.P. Metal Biosorption onto Non-Living Algae: A Critical Review on Metal Recovery from Wastewater. Green Chem. 2023, 25, 5775–5788. [Google Scholar] [CrossRef]
- Ordóñez, J.I.; Cortés, S.; Maluenda, P.; Soto, I. Biosorption of Heavy Metals with Algae: Critical Review of Its Application in Real Effluents. Sustainability 2023, 15, 5521. [Google Scholar] [CrossRef]
- Sager, M.; Wiche, O. Rare Earth Elements (REE): Origins, Dispersion, and Environmental Implications—A Comprehensive Review. Environments 2024, 11, 24. [Google Scholar] [CrossRef]
- Arienzo, M.; Ferrara, L.; Trifuoggi, M.; Toscanesi, M. Advances in the Fate of Rare Earth Elements, REE, in Transitional Environments: Coasts and Estuaries. Water 2022, 14, 401. [Google Scholar] [CrossRef]
- Ciobanu, A.-A.; Lucaci, A.-R.; Bulgariu, L. Efficient Metal Ions Biosorption on Red and Green Algae Biomass: Isotherm, Kinetic and Thermodynamic Study. J. Appl. Phycol. 2024, 36, 3809–3827. [Google Scholar] [CrossRef]
- Wei, Z.; Hong, F.; Yin, M.; Li, H.; Hu, F.; Zhao, G.; Woonchungwong, J. Subcellular and Molecular Localization of Rare Earth Elements and Structural Characterization of Yttrium Bound Chlorophyll a in Naturally Grown Fern Dicranopteris Dichotoma. Microchem. J. 2005, 80, 1–8. [Google Scholar] [CrossRef]
- Regoli, F.; Giuliani, M.E.; Benedetti, M.; Arukwe, A. Molecular and Biochemical Biomarkers in Environmental Monitoring: A Comparison of Biotransformation and Antioxidant Defense Systems in Multiple Tissues. Aquat. Toxicol. 2011, 105, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Ling, N.; Tian, H.; Guo, C.; Wang, Q. Toxicity, Physiological Response, and Biosorption Mechanism of Dunaliella Salina to Copper, Lead, and Cadmium. Front. Microbiol. 2024, 15, 1374275. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Gill, S.S.; Corpas, F.J.; Ortega-Villasante, C.; Hernandez, L.E.; Tuteja, N.; Sofo, A.; Hasanuzzaman, M.; Fujita, M. Editorial: Recent Insights Into the Double Role of Hydrogen Peroxide in Plants. Front. Plant Sci. 2022, 13, 843274. [Google Scholar] [CrossRef]
- Qi, Y.; Li, X.; Guo, S.; He, F.; Liu, R. Insights into the Potential Toxicity of Zn(II) to Catalase and Their Binding Mechanisms. J. Mol. Liq. 2024, 394, 123760. [Google Scholar] [CrossRef]
- Park, H.; Kim, H.-S.; Abassi, S.; Bui, Q.T.N.; Ki, J.-S. Two Novel Glutathione S-Transferase (GST) Genes in the Toxic Marine Dinoflagellate Alexandrium Pacificum and Their Transcriptional Responses to Environmental Contaminants. Sci. Total Environ. 2024, 915, 169983. [Google Scholar] [CrossRef]
- Rahhou, A.; Layachi, M.; Akodad, M.; El Ouamari, N.; Rezzoum, N.E.; Skalli, A.; Oudra, B.; El Bakali, M.; Kolar, M.; Imperl, J.; et al. The Bioremediation Potential of Ulva lactuca (Chlorophyta) Causing Green Tide in Marchica Lagoon (NE Morocco, Mediterranean Sea): Biomass, Heavy Metals, and Health Risk Assessment. Water 2023, 15, 1310. [Google Scholar] [CrossRef]
- Lee, H.; Kim, G.; Depuydt, S.; Shin, K.; Han, T.; Park, J. Metal Toxicity across Different Thallus Sections of the Green Macroalga, Ulva Australis. Toxics 2023, 11, 548. [Google Scholar] [CrossRef]
- Figueiredo, C.; Grilo, T.F.; Oliveira, R.; Ferreira, I.J.; Gil, F.; Lopes, C.; Brito, P.; Ré, P.; Caetano, M.; Diniz, M.; et al. A Triple Threat: Ocean Warming, Acidification, and Rare Earth Elements Exposure Triggers a Superior Antioxidant Response and Pigment Production in the Adaptable Ulva Rigida. Environ. Adv. 2022, 8, 100235. [Google Scholar] [CrossRef]
- Henriques, B.; Morais, T.; Cardoso, C.E.D.; Freitas, R.; Viana, T.; Ferreira, N.; Fabre, E.; Pinheiro-Torres, J.; Pereira, E. Can the Recycling of Europium from Contaminated Waters Be Achieved through Living Macroalgae? Study on Accumulation and Toxicological Impacts under Realistic Concentrations. Sci. Total Environ. 2021, 786, 147176. [Google Scholar] [CrossRef]
- Long, A.; Zhang, J.; Yang, L.-T.; Ye, X.; Lai, N.-W.; Tan, L.-L.; Lin, D.; Chen, L.-S. Effects of Low PH on Photosynthesis, Related Physiological Parameters, and Nutrient Profiles of Citrus. Front. Plant Sci. 2017, 8, 185. [Google Scholar] [CrossRef]
- Alp, F.N.; Arikan, B.; Ozfidan-Konakci, C.; Gulenturk, C.; Yildiztugay, E.; Turan, M.; Cavusoglu, H. Hormetic Activation of Nano-Sized Rare Earth Element Terbium on Growth, PSII Photochemistry, Antioxidant Status and Phytohormone Regulation in Lemna Minor. Plant Physiol. Biochem. 2023, 194, 361–373. [Google Scholar] [CrossRef]
- Cheng, J.; Du, X.; Long, H.; Zhang, H.; Ji, X. The Effects of Exogenous Cerium on Photosystem II as Probed by in Vivo Chlorophyll Fluorescence and Lipid Production of Scenedesmus Obliquus XJ002. Biotechnol. Appl. Biochem. 2020, 68, 1216–1226. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, X.; Chen, Z. Effects of Rare Earth Elements and REE-Binding Proteins on Physiological Responses in Plants. Protein Pept. Lett. 2012, 19, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Zicari, M.A.; D’Aquino, L.; Paradiso, A.; Mastrolitti, S.; Tommasi, F. Effect of Cerium on Growth and Antioxidant Metabolism of Lemna minor L. Ecotoxicol. Environ. Saf. 2018, 163, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, Y.; Feng, Z.; Wu, M.; Xu, T.; Qiao, S.; Wang, W.; Ma, J.; Xu, J. Photosynthetic Physiological Response of Porphyra Yezoensis to Light Change at Different CO2 Concentrations. Water 2023, 15, 781. [Google Scholar] [CrossRef]
- Zerveas, S.; Mente, M.S.; Tsakiri, D.; Kotzabasis, K. Microalgal Photosynthesis Induces Alkalization of Aquatic Environment as a Result of H+ Uptake Independently from CO2 Concentration—New Perspectives for Environmental Applications. J. Environ. Manag. 2021, 289, 112546. [Google Scholar] [CrossRef]

| Ulva sp. | |||||
|---|---|---|---|---|---|
| [Y] (mg/L) | Blank | 0.5 | 5.0 | 50 | 500 |
| qreal (mg/g) | <0.02 | 0.237 ± 0.098 | 3.11 ± 0.17 | 8.12 ± 0.35 | 20.9 ± 1.3 |
| BCF (L/kg) | - | 474 ± 197 | 621 ± 33 | 162 ± 7.0 | 42 ± 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viana, T.; Colónia, J.; Tavares, D.S.; Andrade, M.; Ferreira, N.; Freitas, R.; Pereira, E.; Henriques, B. Uptake and Effects of Yttrium on the Seaweed Ulva sp.: A Study on the Potential Risks of Rare Earth Elements in Aquatic Environments. Water 2025, 17, 3023. https://doi.org/10.3390/w17203023
Viana T, Colónia J, Tavares DS, Andrade M, Ferreira N, Freitas R, Pereira E, Henriques B. Uptake and Effects of Yttrium on the Seaweed Ulva sp.: A Study on the Potential Risks of Rare Earth Elements in Aquatic Environments. Water. 2025; 17(20):3023. https://doi.org/10.3390/w17203023
Chicago/Turabian StyleViana, Thainara, João Colónia, Daniela S. Tavares, Madalena Andrade, Nicole Ferreira, Rosa Freitas, Eduarda Pereira, and Bruno Henriques. 2025. "Uptake and Effects of Yttrium on the Seaweed Ulva sp.: A Study on the Potential Risks of Rare Earth Elements in Aquatic Environments" Water 17, no. 20: 3023. https://doi.org/10.3390/w17203023
APA StyleViana, T., Colónia, J., Tavares, D. S., Andrade, M., Ferreira, N., Freitas, R., Pereira, E., & Henriques, B. (2025). Uptake and Effects of Yttrium on the Seaweed Ulva sp.: A Study on the Potential Risks of Rare Earth Elements in Aquatic Environments. Water, 17(20), 3023. https://doi.org/10.3390/w17203023










