Geochemical Exploration Techniques with Deep Penetration: Implications for the Exploration of Concealed Potash Deposits in the Covered Area on the Southern Margin of the Kuqa Basin
Abstract
1. Introduction
2. Geological Background
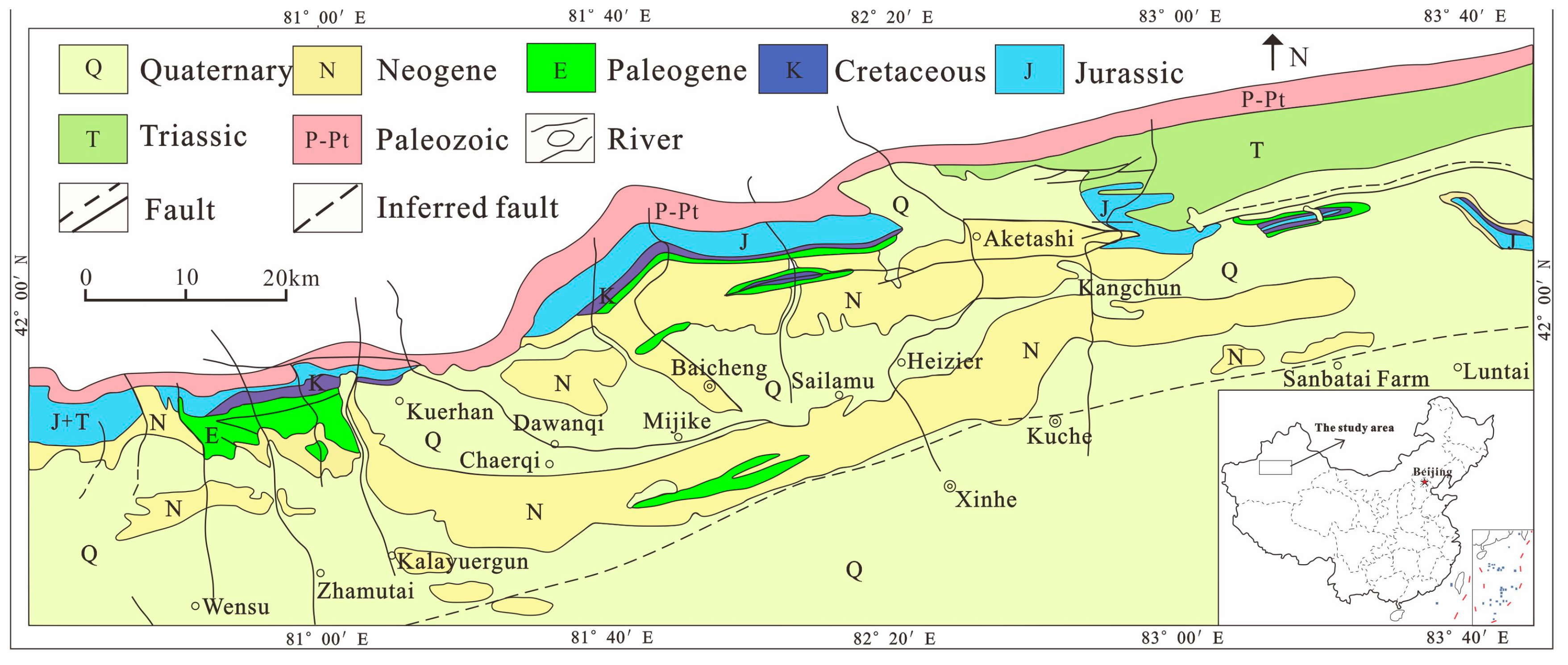
2.1. Regional Geological Background
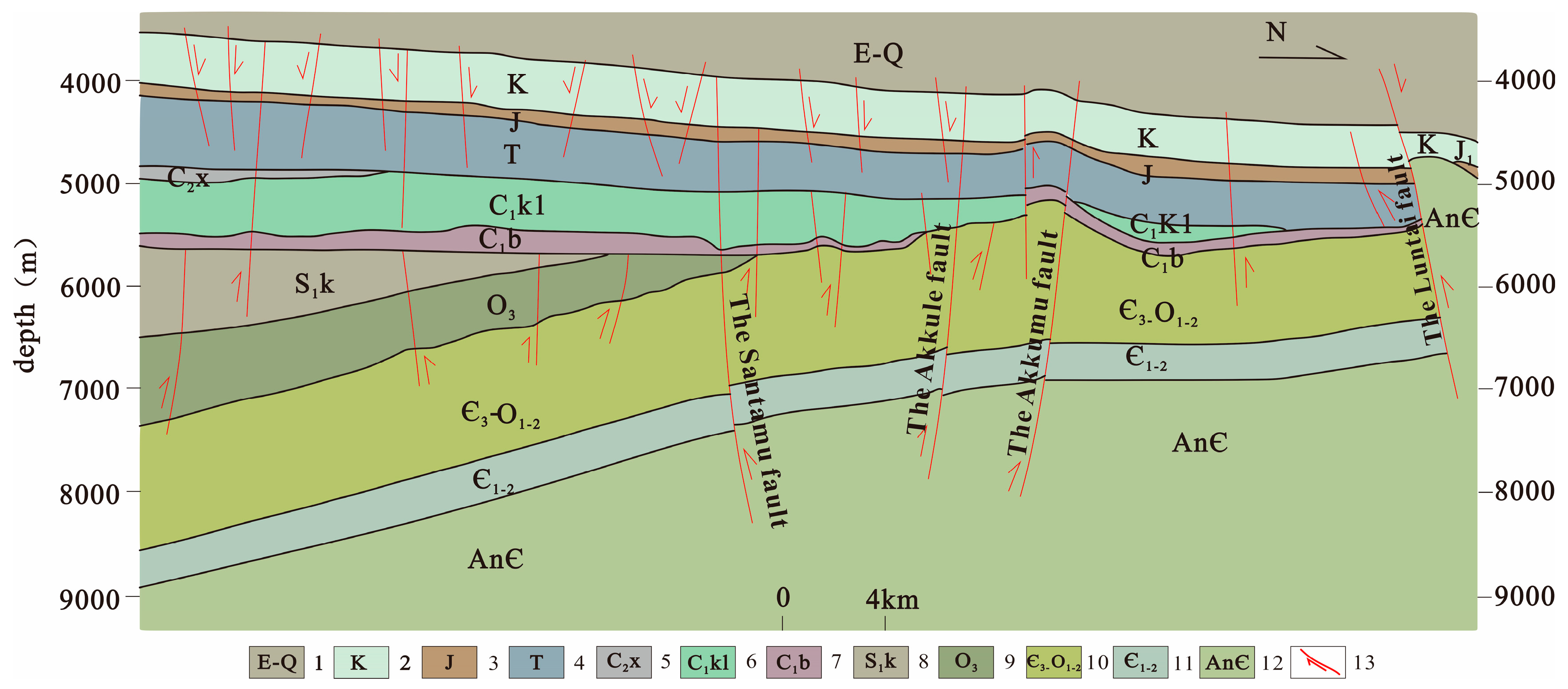
2.2. Geological Landform and Fracture Tectonic Characteristics of the Study Area
3. The Collection and Analysis of Samples
4. Analysis Results
5. Discussion
5.1. Elemental Geochemical Characteristics
5.2. The Indication of Main Trace Elements
6. Mineralization Model
7. Conclusions
- (1)
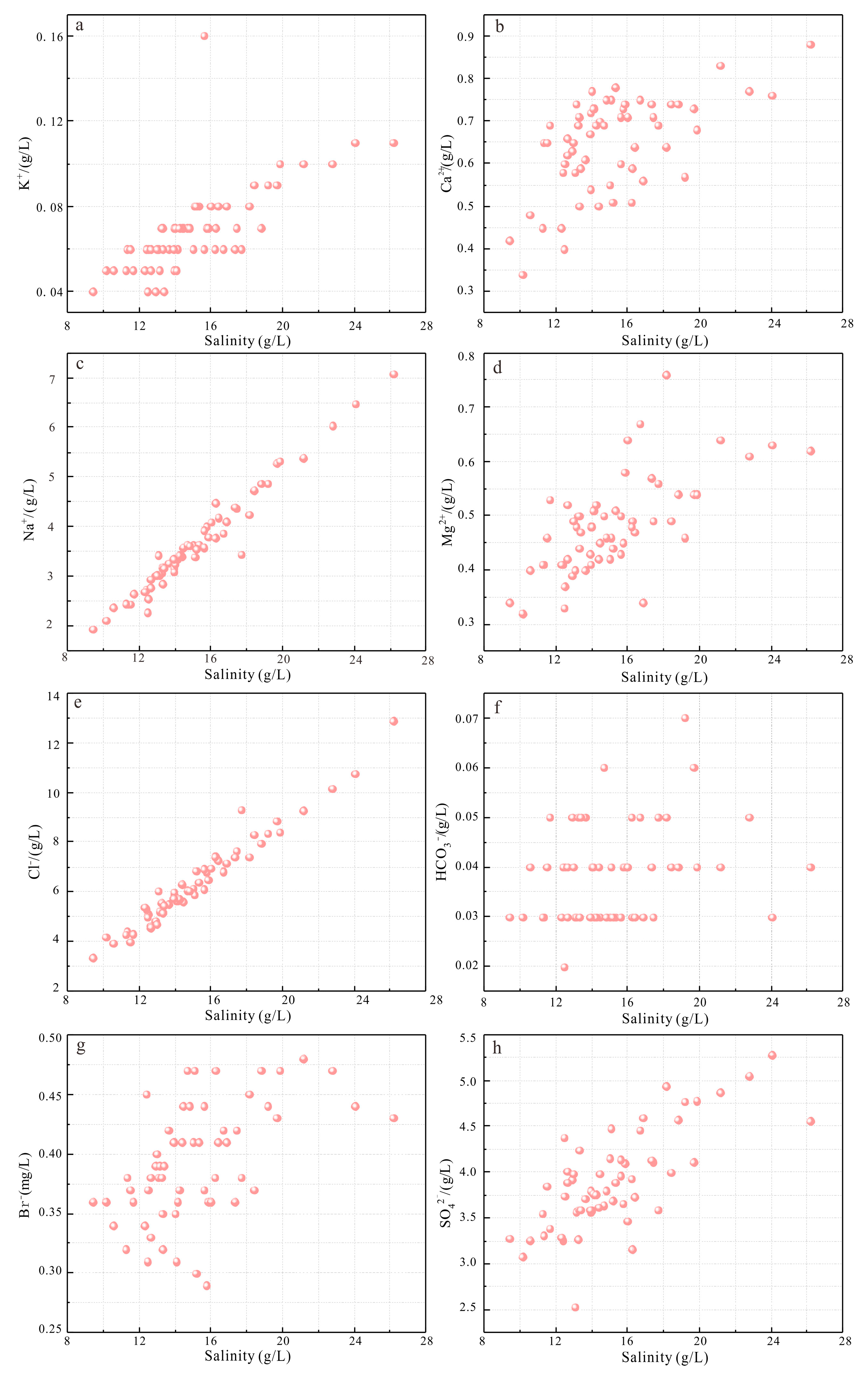
- The sample salinity ranges from 9.41 to 26.16 g/L. The potassium content is between 0.04 and 0.22 g/L, indicating potential resource value. Some sampling points showed significantly higher potassium and other salt component concentrations compared to other points, indicating a potassium anomaly at depth. This suggests the possible upward migration of deep potassium–rich brine along the major deep fault segments near the sampling points, providing direct evidence for the placement of potassium well locations.
- (2)
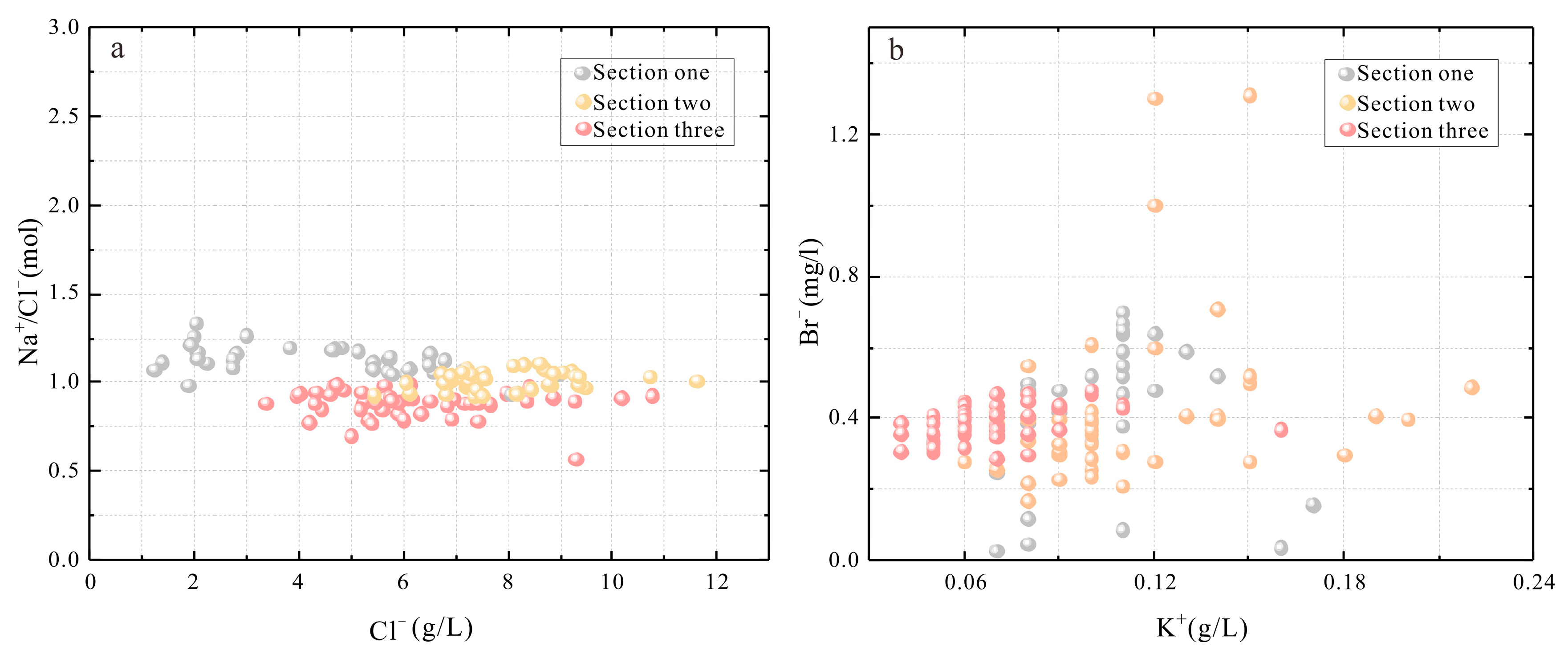
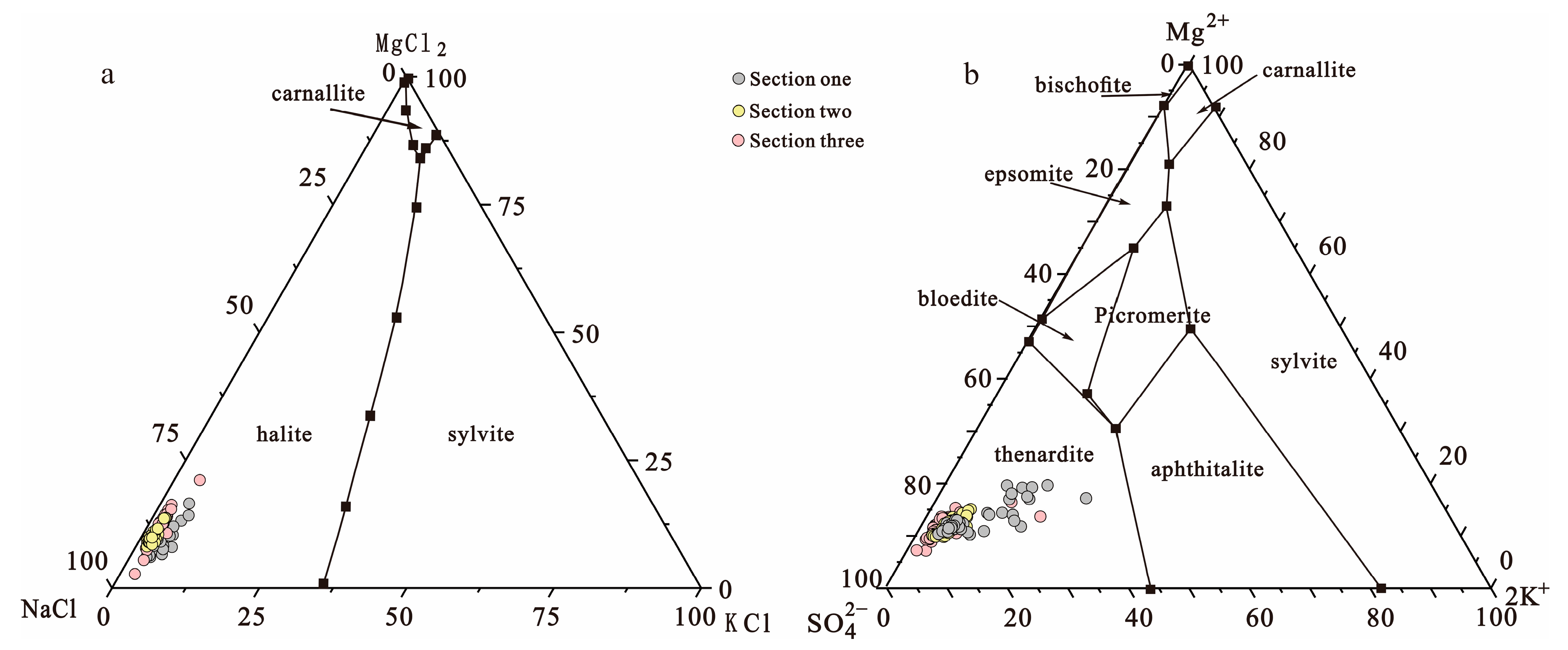
- The hydrochemical characteristic coefficients suggest that the spring water has a high nNa+/nCl− value, low K+ × 103/Cl−, and nK+/nBr− value features. According to the Quaternary system phase diagram and the biquinary system phase diagram, the brine should be rock salt leached and may suggest that the shallow brine underground is still in the initial stage of potash salt sedimentation.
- (3)
- The residual brine and salt–weathering products of the ancient salt lake undergo a “transferral mineralization” of salts, gathering in the graben–type secondary depression in the southern part of the Shaya region. At the same time, deep fluid rises to recharge the salt lake, and, after further evaporation and concentration, salt deposits are formed.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, C.L.; Lv, F.X. Brief introduction of the National 973 Project “Research on the Law and Prediction of Marine Potassium Formation in China’s Continental Blocks”. Acta Geol. Sin. 2015, 89, I0001–I0002. [Google Scholar]
- Bao, R.H.; Yan, W.D.; Jiang, Y.; Zhou, Q.Z. Risk analysis of potash supply in China. Chem. Fertil. Ind. 2018, 45, 58–62. [Google Scholar]
- Wang, X.X.; Xu, S.F.; Zhang, B.M. Deep-penetrating geochemistry for sandstone-type uranium deposits in the Turpan-Hami basin, North-Western China. Appl. Geochem. 2011, 26, 2238–2246. [Google Scholar] [CrossRef]
- Wang, X.Q.; Zhang, B.M.; Lin, X.; Xu, S.F.; Yao, W.S.; Ye, R. Geochemical challenges of diverse regolithcovered terrains for mineral exploration in China. Ore Geol. Rev. 2016, 73, 417–431. [Google Scholar]
- Ye, R.; Zhang, B.M.; Wang, Y. Mechanism of the migration of gold in desert regolith coverover a concealed gold deposit. Geochem. Explor. Environ. Anal. 2015, 15, 62–71. [Google Scholar] [CrossRef]
- Anand, R.R. Regolith-landform processes and geochemical exploration for basemetal deposits in regolith-dominated terrains of the MtIsa region, Northwest Queensland, Australia. Ore Geol. Rev. 2016, 73, 451–474. [Google Scholar] [CrossRef]
- Noble, R.R.P.; Lau, I.C.; Anand, R.R.; Pinchand, G.T. Refining fine fraction soil extraction methods and analyse is for mineral exploration. Geochmistry Explor. Environ. Anal. 2019, 20, 113–128. [Google Scholar] [CrossRef]
- Noble, R.R.P.; Morris, P.A.; Anand, R.R.; Lau, I.C.; Pinchand, G.T. Application of Ultrafine fraction soil extraction and analysis for mineral exploration. Geochem. Explor. Environ. Anal. 2020, 20, 129–154. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.Q.; Liu, H.L.; Tian, M.; Zhang, B.Y.; Li, R.H.; Yang, D.P.; Xiong, Y.X. Targeting deep-seated gold deposits: A study from the Qujia gold deposit, Shandong Province, China. Appl. Geochem. 2021, 130, 104982. [Google Scholar] [CrossRef]
- Antropova, L.V. New methods of regional exploration for blind mineralization: Application in the UssR. J. Geochem. Explor. 1992, 43, 157–166. [Google Scholar] [CrossRef]
- Zlotnicki, J.; Sasai, Y.; Toutain, J.P. Combined electromagnetic, geochemical and thermal surveys of taal volcano during the period 2005–2006. Bull. Volcanol. 2009, 71, 29–47. [Google Scholar] [CrossRef]
- Stragliani, W.M.; Doelman, P.; Salomons, W.; Schulin, R. Chemical time bombs: Predicting the unpredictable. Environment 1991, 22, 4–30. [Google Scholar] [CrossRef]
- Malmqvist, L.; Kristiansson, K. Experiment evidence for an as cending micro-flow of geogas in ground. Earth Planet. Sci. Lett. 1984, 70, 407–416. [Google Scholar] [CrossRef]
- Cameron, E.M.; Hamilton, S.M.; Leybourne, M.I.; Hall, G.E.; McClenaghan, M.B. Finding deeply-buried deposits using geochemistry. Geochem. Explor. Environ. Anal. 2004, 4, 7–32. [Google Scholar] [CrossRef]
- Ryss, Y.S.; Goldberg, I.S. The partial extraction of metals method in mineral exploration. Method Tech. 1973, 84, 5–19. [Google Scholar]
- Wang, X.Q. Deep penetration exploration geochemistry. Geophys. Geochem. Explor. 1998, 22, 166–169. [Google Scholar]
- Kristiansson, K.; Malmqvist, L. Evidence for nondiffusive transport of 222 86 Rn in the ground and a new physical model for the transport. Geophysics 1982, 47, 1444. [Google Scholar] [CrossRef]
- Clark, J.R. Enzyme-induced leaching of B-horizon soils for mineral exploration in areas of glacial overburden. Trans. Inst. Min. Metall. Sect. B Appl. Earth Sci. 1993, 102, 19–29. [Google Scholar]
- Mann, A.; Reimann, C.; De, P.; Caritat, N.; Turner, M. Mobile Metal Ion(R) analysis of European agricultural soils: Bioavailability, weathering, geogenic patterns and anthropogenic anomalies. Geochem. Explor. Environmen Anal. 2015, 15, 99–112. [Google Scholar] [CrossRef]
- Wang, Q.X.; Zhang, B.M.; Yao, W.S. Geochemical exploration: From nanoscale to global-scale patterns. Earth Sci. Front. 2014, 21, 65–74. [Google Scholar]
- Wang, Q.Y.; Cheng, H.C.; Zhu, M.H.; Yan, X.H.; Li, J. The application of geochemical soil survey to the qaganqulu Ag-Pb-Zn polymetallic ore district in Dong Ujmqn Banner lnner Mongolia. Geophys. Geochem. Explor. 2009, 33, 652–656. [Google Scholar]
- Liu, H.L.; Zhang, B.M.; Liu, D.S.; Wang, X.Q.; Zhang, Z.H.; Han, Z.X. The application of soil geochemical measurement method to the Huaniushan Pb-Zn deposit, Gansu Privince. Geophys. Geochem. Explor. 2023, 97, 291–305. [Google Scholar]
- Yang, Y.C.; Li, W.J.; He, J.P.; Zhang, J.H.; Zhou, C.X.; Lv, J.L. Application of geochemical soil survey in the Gongpoquan gold deposit at Mazongshan, Gansu Province. Geol. Explor. 2017, 53, 715–730. [Google Scholar]
- Bo, Y.; Liu, C.L.; Cao, Y.T. Fault belt geochemical deep-penetration theories and techniques for potassium prospecting. Miner. Depos. 2016, 35, 1281–1292. [Google Scholar]
- Liu, C.; Wang, L.; Yan, M.; Zhao, Y.; Cao, Y.; Fang, X.; Shen, L.; Wu, C.; Lv, F.; Ding, T. The Mesozoic-Cenozoic tectonic settings, paleogeography and evaporitic sedimentation of Tethyan blocks within China: Implications for potash formation. Ore Geol. Rev. J. Compr. Stud. Ore Genes. Ore Explor. 2018, 102, 406–425. [Google Scholar] [CrossRef]
- Liu, C.L.; Cao, Y.T.; Xu, Y.; Jiao, P.C.; Yao, F.J. Potash formation and target area prediction of Paleogene and Neogene salt lake in Kuqa basin, Xinjiang. Acta Geol. Sinca 2024, 98, 941–956. [Google Scholar]
- Liu, C.L.; Cao, Y.T.; Yang, H.J.; Jiao, P.C.; Gu, Q.W. Discussion on Paleogene-Neogene environmental change of salt lakes in Kuqa foreland basin and its potash-forming effect. Acta Geosci. Sin. 2013, 34, 547–558. [Google Scholar]
- Wang, X.Y.; Yin, H.W.; Deng, X.L.; Wei, Z. Strontium isotope characteristics and the origin of Cenozoic salt deposits in Kuqa depression. J. Nanjing Univ. 2015, 51, 1068–1074. [Google Scholar]
- Deng, X.L.; Wei, Z.; Zhao, Y.H. Discovery and geological significance of Paleogene potassium in Tarim Basin. Acta Mineral. Sin. 2013, 33, 755–756. [Google Scholar]
- Xu, Y.; Cao, Y.; Liu, C. Mid-Eocene sea surface cooling in the easternmost proto-Paratethys sea: Constraints from quantitative temperatures in halite fluid inclusions. Int. J. Earth Sci. 2021, 110, 1713–1727. [Google Scholar] [CrossRef]
- Xu, Y.; Cao, Y.; Liu, C. Whether the Middle Eocene Salt-Forming Brine in the Kuqa Basin Reached the Potash-Forming Stage: Quantitative Evidence from Halite Fluid Inclusions. Geofluids 2021, 2021, 5574772. [Google Scholar] [CrossRef]
- Liu, C.L.; Jiao, P.C.; Chen, Y.Z. Preliminary study on post-depositional potassium transfer mineralization effect in multi-stage evaporite deposits of Tarim Basin. Miner. Depos. 2010, 29, 383–384. [Google Scholar]
- Liu, C.L.; Jiao, P.C.; Wang, M.L. A tentative discussion on exploration model for potash in basins of China. Miner. Depos. 2010, 29, 581–592. [Google Scholar]
- Bally, A.W.; Snelson, S. Realms of subsidence. Can. Soc. Pet. Geol. Mem. 1980, 6, 9–94. [Google Scholar]
- Graham, S.A.; Hendrix, M.S.; Wang, L.B. Collision success or basin of western China: Impact of tectonic in heritance on sand composition. Geol. Soc. Am. Bull. 1993, 105, 323–324. [Google Scholar] [CrossRef]
- Lu, H.F.; Howell, D.G.; Jia, D.; Cai, S.; Wu, S.M.; Chen, C.M. Rejuvenation of the Kuqa fore-land basin, northern flank of the Tarim Basin, Northern China. Int. Geol. Rev. 1994, 36, 1151–1158. [Google Scholar]
- Yu, H.B.; Qi, J.F.; Yang, X.Z.; Liu, Q.Y.; Cao, S.J.; Fan, S.; Sun, T.; Yang, X.Y. The mesozoic tectonic palaeogeographic analysis in Kuqa depression of Tarim Basin. Geol. J. China Univ. 2016, 22, 657–669. [Google Scholar]
- Zhang, L.J.; Li, D.L.; Sun, Y.S.; Chen, M. Analysis characteristics of sedimenyary reservorir between cretaceous and palaeogene in the Western part of the Kuqa depression. Natueal Gas Geosci. 2006, 17, 355–360. [Google Scholar]
- Tan, H.B.; Ma, W.D.; Ma, H.Z.; Zhang, X.Y.; Cao, C.D. Hydrochemical Characteristics of brines and Application to locating potassium in western Tarim Basin. Geochimica 2004, 33, 152–158. [Google Scholar]
- Bo, Y.; Cao, Y.T.; Liu, C.L.; Gao, C.; Jiao, P.C. Chemical characteristics and origin of saline springs and their significance to potash prospecting in the Kuqa Basin, Xinjiang. Acta Geol. Sin. 2015, 89, 1936–1944. [Google Scholar]
- Liu, C.L. Characteristics and formation of potash deposits in continental rift basins: A review. Acta Geosci. Sin. 2013, 34, 515–527. [Google Scholar]
- Xu, Y.; Cao, Y.T.; Liu, C.L.; Jiao, P.C. Provenance and degree of evaporation and concentration of eocene salt lake in the Kuqa Basin. Acta Geol. Sin. 2018, 92, 1617–1629. [Google Scholar]
- Xu, Y.; Liu, C.L.; Jiao, P.C.; Chen, Y.Z.; Cao, Y.T. Geochemical characteristics and potash formation analysis of paleocene-eocene evaporites in Kuqa depression of Xinjiang: A case study of borehole KL4. Acta Pet. Mineral. 2017, 36, 755–764. [Google Scholar]
- Xu, Y.; Zhang, Y.M.; Cao, Y.T. Chlorine isotopic characteristics of the Middle Eocene rock salt in the Kuqa basin and their implications for potash formation. Acta Geol. Sin. 2021, 95, 3492–3500. [Google Scholar]
- Hu, P.; Yan, Q.S.; Lao, W.C. The Kuqa Basin rock salt and brine geochemical characteristics and metallogenic potential. Sichuan Nonferrous Met. 2012, 4, 38–42. [Google Scholar]
- Li, H.Y.; Han, J.; Chen, P.; Li, Y.; Bo, X.Q. Deformation and favorable area evaluation of Shunbei No. 4 Strikr-Slip fault zone in Tarim Basin. Xinjiang Pet. Geol. 2023, 44, 127–135. [Google Scholar]
- Liu, H.L.; Wang, X.Q.; Zhang, B.M.; Zhang, B.J.; Wang, Q.; Yang, D.P.; Xiong, Y.X.; Zhou, J. Indication of deep-penetrating geochemistry for hidden gold deposit in alluvial soil area in Jiaodong. Acta Geosci. Sin. 2023, 97, 1683–1700. [Google Scholar]
- Lu, Y.X.; Zhang, B.M.; Liu, H.L.; Chi, Q.H.; Dou, B.; Liu, F.T.; Wang, Q.; Xie, M.J.; Hu, Y.Y.Y. Indication of deep-penetrating geochemical exploration techniques for concealed rare metal ore: A case study of X03 vein in Jiajika lithium ore. Acta Geol. Sin. 2023, 97, 291–305. [Google Scholar]
- Lowenstein, T.K.; Risacher, F. Closed Basin Brine Evolution and the Influence of Ca-Cl Inflow Waters: Death Valley and Bristol Dry Lake California, Qaidam Basin, China, and Salar de Atacama, Chile. Aquat. Geochem. 2009, 15, 71–94. [Google Scholar] [CrossRef]
- Lowenstein, T.K.; Spencer, R.J.; Zhang, P.X. Origin of ancient potash evaporites-clues from the modern nonmarine Qaidam Basin of western China. Science 1989, 245, 1090–1092. [Google Scholar] [CrossRef]
- Fan, Q.S.; Ma, H.Z.; Tan, H.B.; Li, Y.W.; Xu, J.X. Characteristics of brines and potassium-prospecting researches in western Qaidam basin. Acta Geosci. Sin. 2007, 28, 446–455. [Google Scholar]
- Bo, Y.; Liu, C.L.; Jiao, P.C.; Chen, Y.Z.; Cao, Y.T. Saline spring hydrochemical characteristics and indicators for potassium exploration in southwestern and northern Tarim Basin, Xinjiang. Acta Geosci. Sin. 2013, 34, 594–602. [Google Scholar]
- Shan, J.J.; Wang, M.X.; Li, J.S.; Shan, F.S.; Fan, X.S.; Wei, H.C.; Qin, Z.J.; Yuan, Q.; Zhang, X.R.; Li, Q.K. Hydrochemical characteristics of potassium-rich saline spring and its implications for sylvine deposits in the Kuqa basin, Xinjiang. Acta Geol. Sin. 2019, 93, 1180–1188. [Google Scholar]
- Drever, J.I. The Geochemistry of Natural Waters, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1988. [Google Scholar]
- Lin, Y.T.; Yao, Y.C.; Kang, Z.H.; Wang, N.J. Study on the geochemical characteristics and resource significance of the highmineralized potassium-rich brine in the Sichuan Xuan da salt basin. J. Salt Lake Res. 2004, 12, 8–17. [Google Scholar]
- Fan, Q.S.; Ma, H.B.; Tan, H.B.; Xu, J.X.; Li, Y.W. Characteristics and origin of brines in Western Qaidam Bssin. Geochimica 2007, 36, 601–611. [Google Scholar]
- Cai, C.F.; Mei, B.W.; Li, W. The hydrogeochemistry of oil-fields in Tarim basin. Geochimica 1996, 6, 614–623. [Google Scholar]
- Chen, Y.H. Sequence of salt separation and regularity of some trace elements distribution during isothermal evaporation (25 °C) of the Huanghai sea water. Acta Geosci. Sin. 1983, 57, 379–390. [Google Scholar]
- Tan, H.B.; Ma, H.Z.; Ma, W.Z.; Dong, Y.P.; Zhang, X.Y.; Xu, J.X. Research on geological and geochemical characteristics of Paleo-Salt rock and its potash ore formation in Western Tarim Basin. Bull. Mineral. Petrol. Geochem. 2004, 23, 194–199. [Google Scholar]
- Tang, L.J.; Qi, L.X.; Xiu, H.J.; Yu, L.; Li, M.; Xie, D.Q.; Yang, Y.; Wan, G.M. Poly-phase differential fault movement and hydrocarbon accumlation of the Tarim Basin, NW China. Acta Petrol. Sin. 2012, 28, 2569–2583. [Google Scholar]






| Sample Number | K+ (g/L) | Na+ (g/L) | Ca2+ (g/L) | Mg2+ (g/L) | Cl− (g/L) | SO42− (g/L) | Br− (mg/L) |
|---|---|---|---|---|---|---|---|
| Maximum value | 0.17 | 7.44 | 0.47 | 0.60 | 11.32 | 4.59 | 0.70 |
| Minimum value | 0.06 | 0.85 | 0.02 | 0.08 | 1.19 | 0.28 | 0.03 |
| Mean value | 0.10 | 3.44 | 0.20 | 0.35 | 4.87 | 2.45 | 0.42 |
| Standard deviation | 0.02 | 1.65 | 0.14 | 0.16 | 2.48 | 1.36 | 0.20 |
| Sample number | 43 | 43 | 43 | 43 | 43 | 43 | 43 |
| Sample Number | K+ (g/L) | Na+ (g/L) | Ca2+ (g/L) | Mg2+ (g/L) | Cl− (g/L) | SO42− (g/L) | Br− (mg/L) |
|---|---|---|---|---|---|---|---|
| Maximum value | 0.22 | 7.63 | 0.81 | 0.73 | 11.60 | 5.70 | 1.31 |
| Minimum value | 0.06 | 3.29 | 0.46 | 0.45 | 5.44 | 2.97 | 0.17 |
| Mean value | 0.11 | 5.12 | 0.64 | 0.57 | 7.83 | 4.23 | 0.43 |
| Standard deviation | 0.04 | 0.88 | 0.11 | 0.07 | 1.17 | 0.65 | 0.23 |
| Sample number | 47 | 47 | 47 | 47 | 47 | 47 | 47 |
| Sample Number | K+ (g/L) | Na+ (g/L) | Ca2+ (g/L) | Mg2+ (g/L) | Cl− (g/L) | SO42− (g/L) | HCO3− (g/L) | TDS (g/L) | Br− (mg/L) |
|---|---|---|---|---|---|---|---|---|---|
| Maximum value | 0.16 | 7.08 | 0.88 | 0.76 | 12.87 | 5.28 | 0.07 | 26.16 | 0.48 |
| Minimum value | 0.04 | 1.94 | 0.34 | 0.32 | 3.36 | 2.53 | 0.02 | 9.41 | 0.29 |
| Mean value | 0.07 | 3.64 | 0.65 | 0.48 | 6.37 | 3.90 | 0.04 | 15.14 | 0.39 |
| Standard deviation | 0.02 | 1.04 | 0.11 | 0.09 | 1.78 | 0.53 | 0.01 | 3.28 | 0.05 |
| Sample number | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zhou, Y.; Liu, C.; Zhang, S.; Yao, F.; Yang, G.; Hou, W. Geochemical Exploration Techniques with Deep Penetration: Implications for the Exploration of Concealed Potash Deposits in the Covered Area on the Southern Margin of the Kuqa Basin. Water 2025, 17, 298. https://doi.org/10.3390/w17030298
Li J, Zhou Y, Liu C, Zhang S, Yao F, Yang G, Hou W. Geochemical Exploration Techniques with Deep Penetration: Implications for the Exploration of Concealed Potash Deposits in the Covered Area on the Southern Margin of the Kuqa Basin. Water. 2025; 17(3):298. https://doi.org/10.3390/w17030298
Chicago/Turabian StyleLi, Junyang, Yu Zhou, Chengling Liu, Songyuang Zhang, Fujun Yao, Guoliang Yang, and Wenbin Hou. 2025. "Geochemical Exploration Techniques with Deep Penetration: Implications for the Exploration of Concealed Potash Deposits in the Covered Area on the Southern Margin of the Kuqa Basin" Water 17, no. 3: 298. https://doi.org/10.3390/w17030298
APA StyleLi, J., Zhou, Y., Liu, C., Zhang, S., Yao, F., Yang, G., & Hou, W. (2025). Geochemical Exploration Techniques with Deep Penetration: Implications for the Exploration of Concealed Potash Deposits in the Covered Area on the Southern Margin of the Kuqa Basin. Water, 17(3), 298. https://doi.org/10.3390/w17030298





