Nursery Runoff Treatment by Novel Biochar-Amended Bioretention Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Porous Medium Materials
2.3. Pilot-Scale Bioretention Systems
2.4. Tracer Studies
2.5. Sample Collection and Water Quality Analysis
2.6. Data Analysis
3. Results and Discussion
3.1. Tracer Studies and Hydraulic Performance
3.2. Nitrogen Removal
3.3. Effect of Plants
3.4. Regression Model
3.5. Cost Estimation
3.6. Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hall, C.R.; Hodges, A.W.; Khachatryan, H.; Palma, M.A. Economic Contributions of the Green Industry in the United States in 2018. J. Environ. Hort. 2020, 38, 73–79. [Google Scholar]
- U.S. Environmental Protection Agency (USEPA). Numeric Nutrient Criteria for the State of Florida: Withdrawing the Federal Actions. Available online: https://www.epa.gov/sites/default/files/2015-07/documents/factsheet-withdrawl-2014.pdf (accessed on 15 November 2024).
- Wang, C.; Miao, Q.; Wei, Z.; Guo, Y.; Li, J.; Fan, Z.; Hu, Y.; Zhang, H.; Sun, J.; Cui, Z. Nutrient Runoff and Leaching under Various Fertilizer Treatments and Pedogeographic Conditions: A Case Study in Tobacco (Nicotiana tabacum L.) Fields of the Erhai Lake Basin, China. Eur. J. Agron. 2024, 156, 127170. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (USEPA). Sources and Solutions: Agriculture. Available online: https://www.epa.gov/nutrientpollution/sources-and-solutions-agriculture (accessed on 8 January 2025).
- Rahman, M.Y.A.; Richardson, N.; Nachabe, M.H.; Ergas, S.J. Treatment of Dairy Farm Runoff in Vegetated Bioretention Systems Amended with Biochar. Water 2024, 16, 1347. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (USEPA). National Strategy for the Development of Regional Nutrient Criteria. Available online: https://www.epa.gov/sites/default/files/2018-10/documents/nutrient-strategy-1998.pdf (accessed on 15 November 2024).
- U.S. Environmental Protection Agency (USEPA). Office of Water Fact Sheet: Introduction to Clean Water Act (CWA) Section 303(d) Impaired Waters Lists. Available online: https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=P1008DPU.TXT (accessed on 15 November 2024).
- Putnam, A.H. Water Quality/Quantity Best Management Practices for Florida Nurseries. Available online: https://www.fdacs.gov/content/download/37570/file/nurseryBMP-lores.pdf (accessed on 15 November 2024).
- Biswal, B.K.; Vijayaraghavan, K.; Adam, M.G.; Lee Tsen-Tieng, D.; Davis, A.P.; Balasubramanian, R. Biological Nitrogen Removal from Stormwater in Bioretention Cells: A Critical Review. Crit. Rev. Biotechnol. 2022, 42, 713–735. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.D.; White, S.A.; Chandler, S.L.; Klaine, S.J.; Whitwell, T. Nutrient Management of Nursery Runoff Water Using Constructed Wetland Systems. Hort. Tech. 2006, 16, 610–614. [Google Scholar] [CrossRef]
- Huett, D.O.; Morris, S.G.; Smith, G.; Hunt, N. Nitrogen and Phosphorus Removal from Plant Nursery Runoff in Vegetated and Unvegetated Subsurface Flow Wetlands. Water Res. 2005, 39, 3259–3272. [Google Scholar] [CrossRef] [PubMed]
- Spangler, J.T.; Sample, D.J.; Fox, L.J.; Owen, J.S.; White, S.A. Floating Treatment Wetland Aided Nutrient Removal from Agricultural Runoff Using Two Wetland Species. Ecol. Eng. 2019, 127, 468–479. [Google Scholar] [CrossRef]
- Mulbry, W.; Kondrad, S.; Pizarro, C.; Kebede-Westhead, E. Treatment of Dairy Manure Effluent Using Freshwater Algae: Algal Productivity and Recovery of Manure Nutrients Using Pilot-Scale Algal Turf Scrubbers. Bioresour. Technol. 2008, 99, 8137–8142. [Google Scholar] [CrossRef]
- Prosser, R.S.; Hoekstra, P.F.; Gene, S.; Truman, C.; White, M.; Hanson, M.L. A Review of the Effectiveness of Vegetated Buffers to Mitigate Pesticide and Nutrient Transport into Surface Waters from Agricultural Areas. J. Environ. Manag. 2020, 261, 110210. [Google Scholar] [CrossRef]
- Lambrechts, T.; François, S.; Lutts, S.; Muñoz-Carpena, R.; Bielders, C.L. Impact of Plant Growth and Morphology and of Sediment Concentration on Sediment Retention Efficiency of Vegetative Filter Strips: Flume Experiments and VSMOD Modeling. J. Hydrol. 2014, 511, 800–810. [Google Scholar] [CrossRef]
- Larson, R.A.; Safferman, S.I. Field Application of Farmstead Runoff to Vegetated Filter Strips: Surface and Subsurface Water Quality Assessment. J. Environ. Qual. 2012, 41, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.; Wan Yusof, K.; Takaijudin, H.; Goh, H.W.; Abdul Malek, M.; Azizan, N.A.; Ab. Ghani, A.; Sa’id Abdurrasheed, A. A Review of Nitrogen Removal for Urban Stormwater Runoff in Bioretention System. Sustainability 2019, 11, 5415. [Google Scholar] [CrossRef]
- Donaghue, A.G.; Morgan, N.; Toran, L.; McKenzie, E.R. The Impact of Bioretention Column Internal Water Storage Underdrain Height on Denitrification Under Continuous and Transient Flow. Water Res. 2022, 214, 118205. [Google Scholar] [CrossRef]
- Ergas, S.J.; Sengupta, S.; Siegel, R.; Pandit, A.; Yao, Y.; Yuan, X. Performance of Nitrogen-Removing Bioretention Systems for Control of Agricultural Runoff. J. Environ. Eng. 2010, 136, 1105–1112. [Google Scholar] [CrossRef]
- Mai, Y.; Huang, G. Hydrology and Rainfall Runoff Pollutant Removal Performance of Biochar-Amended Bioretention Facilities Based on Field-Scale Experiments in Lateritic Red Soil Regions. Sci. Total Environ. 2021, 761, 143252. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Ponnada, E.V.; Lynn, T.J.; Ergas, S.J.; Mihelcic, J.R. Long-Term Field Performance of a Conventional and Modified Bioretention System for Removing Dissolved Nitrogen Species in Stormwater Runoff. Water Res. 2020, 170, 115336. [Google Scholar] [CrossRef]
- Jiang, C.; Li, J.; Li, H.; Li, Y. Experiment and Simulation of Layered Bioretention System for Hydrological Performance. J. Water Reuse Desalin. 2019, 9, 319–329. [Google Scholar] [CrossRef]
- Anil Kumar, M.; Moradeeya, P.G.; Manikanda Bharath, K.; Jakulin Divya Mary, P.; Giridharan, K.S. Pyrolytic Conversion of Heterogenic Natural Waste Biomass from Rural Communities with Concomitant Valorization. In Circular Bioeconomy Perspectives in Sustainable Bioenergy Production; Baskar, G., Ashokkumar, V., Rokhum, S.L., Moholkar, V.S., Eds.; Energy, Environment, and Sustainability; Springer Nature: Singapore, 2024; pp. 259–287. ISBN 978-981-9725-22-9. [Google Scholar]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar Effects on Soil Biota—A Review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Omondi, M.O.; Xia, X.; Nahayo, A.; Liu, X.; Korai, P.K.; Pan, G. Quantification of Biochar Effects on Soil Hydrological Properties Using Meta-Analysis of Literature Data. Geoderma 2016, 274, 28–34. [Google Scholar] [CrossRef]
- Peng, Y.; Deng, S.; Kong, Z.; Yuan, Y.; Long, H.; Fang, J.; Ma, H.; Shao, Z.; He, Q.; Chai, H. Biochar and Woodchip Amended Bioreactor Extending Reactive Volume for Enhanced Denitrification in Stormwater Runoff. J. Water Process Eng. 2022, 46, 102541. [Google Scholar] [CrossRef]
- Berger, A.W.; Valenca, R.; Miao, Y.; Ravi, S.; Mahendra, S.; Mohanty, S.K. Biochar Increases Nitrate Removal Capacity of Woodchip Biofilters During High-Intensity Rainfall. Water Res. 2019, 165, 115008. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Y.A. Biochar Amended Bioretention Systems for Nutrient and Fecal Indicator Bacteria Removal from Urban and Agricultural Runoffs. Ph.D Thesis, University of South Florida, Tampa, FL, USA, 2021. [Google Scholar]
- Glaister, B.J.; Fletcher, T.D.; Cook, P.L.M.; Hatt, B.E. Co-Optimisation of Phosphorus and Nitrogen Removal in Stormwater Biofilters: The Role of Filter Media, Vegetation and Saturated Zone. Water Sci. Technol. 2014, 69, 1961–1969. [Google Scholar] [CrossRef]
- Henderson, C.; Greenway, M.; Phillips, I. Removal of Dissolved Nitrogen, Phosphorus and Carbon from Stormwater by Biofiltration Mesocosms. Water Sci. Technol. 2007, 55, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.; Takaijudin, H.; Massoudieh, A.; Goh, H.W. Effects of Vegetation and Saturated Zone in Cascaded Bioretention on Enhancing Nutrient Removal. Environ. Eng. Res. 2022, 28, 220154. [Google Scholar] [CrossRef]
- Sarazen, J.; Hurley, S.; Faulkner, J. Nitrogen and Phosphorus Removal in a Bioretention Cell Experiment Receiving Agricultural Runoff from a Dairy Farm Production Area During Third and Fourth Years of Operation. J. Environ. Qual. 2023, 52, 149–160. [Google Scholar] [CrossRef]
- Muerdter, C.P.; Wong, C.K.; LeFevre, G.H. Emerging Investigator Series: The Role of Vegetation in Bioretention for Stormwater Treatment in the Built Environment: Pollutant Removal, Hydrologic Function, and Ancillary Benefits. Environ. Sci. Water Res. Technol. 2018, 4, 592–612. [Google Scholar] [CrossRef]
- Skorobogatov, A.; He, J.; Chu, A.; Valeo, C.; Van Duin, B. The Impact of Media, Plants and Their Interactions on Bioretention Performance: A Review. Sci. Total Environ. 2020, 715, 136918. [Google Scholar] [CrossRef]
- Kim, H.; Seagren, E.A.; Davis, A.P. Engineered Bioretention for Removal of Nitrate from Stormwater Runoff. Water Environ. Res. 2003, 75, 355–367. [Google Scholar] [CrossRef]
- Dietz, M.E. Modified Bioretention for Enhanced Nitrogen Removal from Agricultural Runoff. J. Environ. Eng. 2016, 142, 06016007. [Google Scholar] [CrossRef]
- Passeport, E.; Hunt, W.F.; Line, D.E.; Smith, R.A.; Brown, R.A. Field Study of the Ability of Two Grassed Bioretention Cells to Reduce Storm-Water Runoff Pollution. J. Irrig. Drain Eng. 2009, 135, 505–510. [Google Scholar] [CrossRef]
- Ullom, D.; Jayakaran, A.D.; Gardner, C.M. Nitrogen Removal in Bioretention Systems with Hydraulic Outlet Controls. J. Sustain. Water Built Environ. 2024, 10, 04024004. [Google Scholar] [CrossRef]
- Barrett, M.E.; Limouzin, M.; Lawler, D.F. Effects of Media and Plant Selection on Biofiltration Performance. J. Environ. Eng. 2013, 139, 462–470. [Google Scholar] [CrossRef]
- Richardson, N. Nutrient Removal of Biochar Amended Modified Bioretention Systems Treating Nursery Runoff. Master’s Thesis, University of South Florida, Tampa, FL, USA, 2024. [Google Scholar]
- Florida Native Plant Society (FNPS). Muhlenbergia Capillaris. Available online: https://www.fnps.org/plant/muhlenbergia-capillaris (accessed on 8 January 2025).
- Crittenden, J.C.; Trussell, R.R.; Hand, D.W.; Howe, K.J.; Tchobanoglous, G.; Ward, B.; Borchardt, J.H. (Eds.) Principles of Reactor Analysis and Mixing. In Stantec’s Water Treatment: Principles and Design; Wiley: Hoboken, NJ, USA, 2022; pp. 287–390. ISBN 978-1-119-81996-7. [Google Scholar]
- American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater, 24th ed.; Lipps, W.C., Braun-Howland, E.B., Baxter, T.E., Eds.; APHA Press: Washington, DC, USA, 2023. [Google Scholar]
- Hoaglin, D. John W. Tukey and Data Analysis. Stat. Sci. 2003, 18, 311–318. [Google Scholar] [CrossRef]
- Yazdi, M.N.; Sample, D.J.; Scott, D.; Owen, J.S.; Ketabchy, M.; Alamdari, N. Water Quality Characterization of Storm and Irrigation Runoff from a Container Nursery. Sci. Total Environ. 2019, 667, 166–178. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Q.; You, C. Biochar Effect on Water Evaporation and Hydraulic Conductivity in Sandy Soil. Pedosphere 2016, 26, 265–272. [Google Scholar] [CrossRef]
- Cabrera, R.I. Comparative Evaluation of Nitrogen Release Patterns from Controlled-Release Fertilizers by Nitrogen Leaching Analysis. HortScience 1997, 32, 669–673. [Google Scholar] [CrossRef]
- Tindall, J.A.; Petrusak, R.L.; McMahon, P.B. Nitrate Transport and Transformation Processes in Unsaturated Porous Media. J. Hydrol. 1995, 169, 51–94. [Google Scholar] [CrossRef]
- Lopez-Ponnada, E.V.; Lynn, T.J.; Peterson, M.; Ergas, S.J.; Mihelcic, J.R. Application of Denitrifying Wood Chip Bioreactors for Management of Residential Non-Point Sources of Nitrogen. J. Biol. Eng. 2017, 11, 16. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, X.; Chen, L.; Wang, Z.; Xia, Y.; Zhang, Y.; Wang, H.; Luo, X.; Xing, B. Enhanced Growth of Halophyte Plants in Biochar-amended Coastal Soil: Roles of Nutrient Availability and Rhizosphere Microbial Modulation. Plant Cell Environ. 2018, 41, 517–532. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Xia, J.; Yang, H.; Liu, J.; Shao, P. Biochar and Effective Microorganisms Promote Sesbania Cannabina Growth and Soil Quality in the Coastal Saline-Alkali Soil of the Yellow River Delta, China. Sci. Total Environ. 2021, 756, 143801. [Google Scholar] [CrossRef]
- Murtaza, G.; Rizwan, M.; Usman, M.; Hyder, S.; Akram, M.I.; Deeb, M.; Alkahtani, J.; AlMunqedhi, B.M.; Hendy, A.S.; Ali, M.R.; et al. Biochar Enhances the Growth and Physiological Characteristics of Medicago Sativa, Amaranthus Caudatus and Zea Mays in Saline Soils. BMC Plant Biol. 2024, 24, 304. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.; Bromm, T.; Glaser, B. Soil Organic Carbon Sequestration after Biochar Application: A Global Meta-Analysis. Agronomy 2021, 11, 2474. [Google Scholar] [CrossRef]
- Silva, I.C.B.D.; Basílio, J.J.N.; Fernandes, L.A.; Colen, F.; Sampaio, R.A.; Frazão, L.A. Biochar from Different Residues on Soil Properties and Common Bean Production. Sci. Agric. 2017, 74, 378–382. [Google Scholar] [CrossRef]
- Wang, D.; Fonte, S.J.; Parikh, S.J.; Six, J.; Scow, K.M. Biochar Additions Can Enhance Soil Structure and the Physical Stabilization of C in Aggregates. Geoderma 2017, 303, 110–117. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Yao, G.; Lin, Z.; Xu, L.; Jiang, Y.; Jin, Z.; Shan, S.; Ping, L. Advances in the Effects of Biochar on Microbial Ecological Function in Soil and Crop Quality. Sustainability 2022, 14, 10411. [Google Scholar] [CrossRef]
- Christianson, L.E.; Collick, A.S.; Bryant, R.B.; Rosen, T.; Bock, E.M.; Allen, A.L.; Kleinman, P.J.A.; May, E.B.; Buda, A.R.; Robinson, J.; et al. Enhanced Denitrification Bioreactors Hold Promise for Mid-Atlantic Ditch Drainage. Agric. Environ. Lett. 2017, 2, 170032. [Google Scholar] [CrossRef]
- Christianson, L.E.; Cooke, R.A.; Hay, C.H.; Helmers, M.J.; Feyereisen, G.W.; Ranaivoson, A.Z.; McMaine, J.T.; McDaniel, R.; Rosen, T.R.; Pluer, W.T.; et al. Effectiveness of Denitrifying Bioreactors on Water Pollutant Reduction from Agricultural Areas. Trans. ASABE 2021, 64, 641–658. [Google Scholar] [CrossRef]
- Florida Field Guide to Low Impact Development, Bioretention Basins/Rain Gardens. Available online: https://buildgreen.ifas.ufl.edu/Fact_sheet_Bioretention_Basins_Rain_Gardens.pdf (accessed on 15 November 2024).
- Jarett, A. Rain Gardens (BioRetention Cells)—A Stormwater BMP. Available online: https://extension.psu.edu/rain-gardens-bioretention-cells-a-stormwater-bmp (accessed on 15 November 2024).
- Micah, E.; Hunt, J.; Remucal, J.; Sanchez, D.L. Biochar Carbon Credit Market Analysis: Examining the Potential for Coupled Biochar and Carbon Credit Production from Wildfire Fuel Reduction Projects in the Western U.S.; Blue Forest Conservation: San Francisco, CA, USA, 2022; p. 11. Available online: https://pacificbiochar.com/wp-content/uploads/BiocharCarbonCreditAnalysis-BFReports20221.pdf (accessed on 15 November 2024).
- Sorensen, R.B.; Lamb, M.C. Return on Investment from Biochar Application. Crop Forage Turfgrass Manag. 2018, 4, 1–6. [Google Scholar] [CrossRef]
- Crites, R.; Ogden, M. Cost of Constructed Wetlands Systems. Available online: https://brownandcaldwell.com/papers-and-reports/cost-of-constructed-wetlands-systems/ (accessed on 14 January 2025).
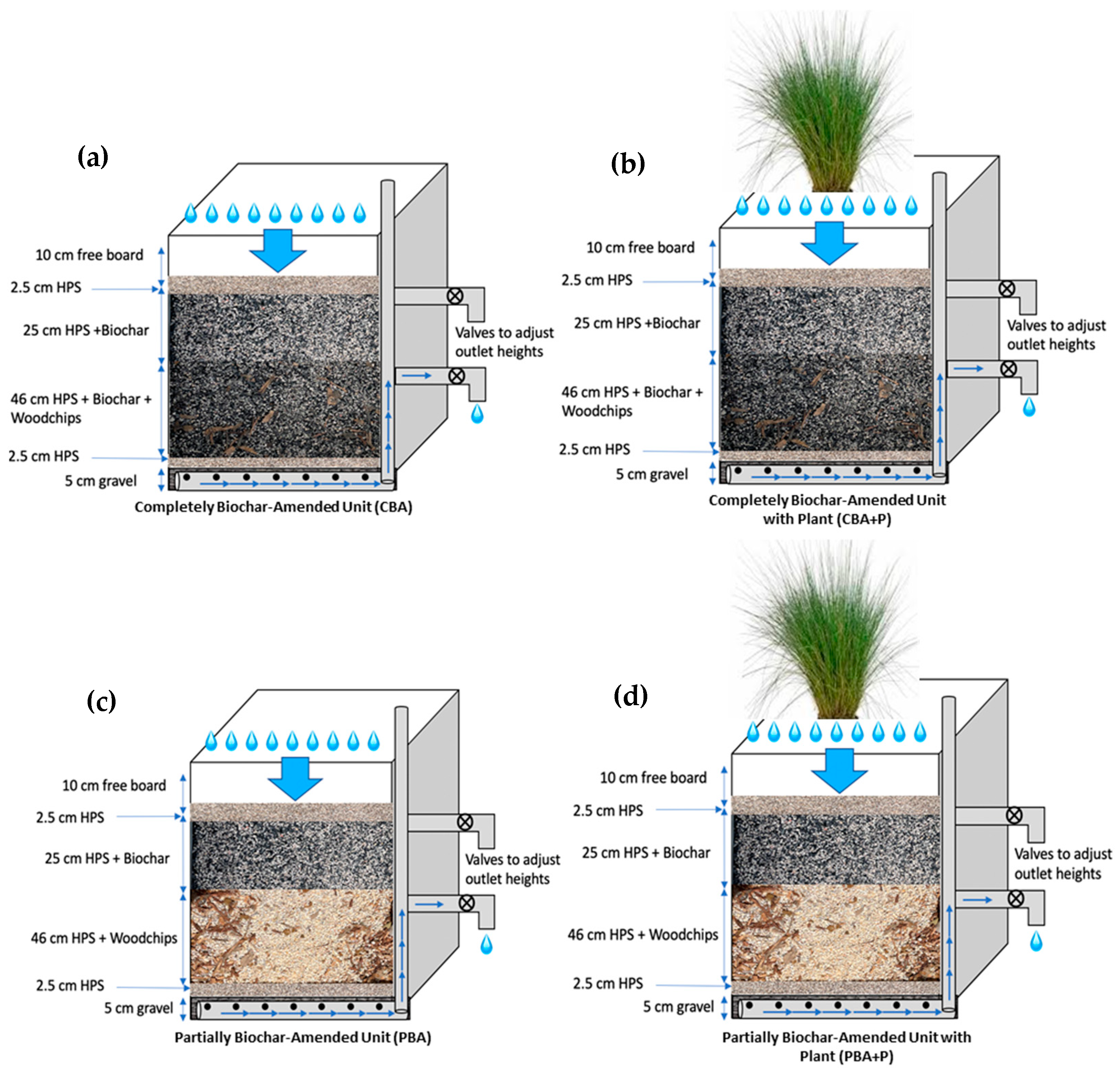

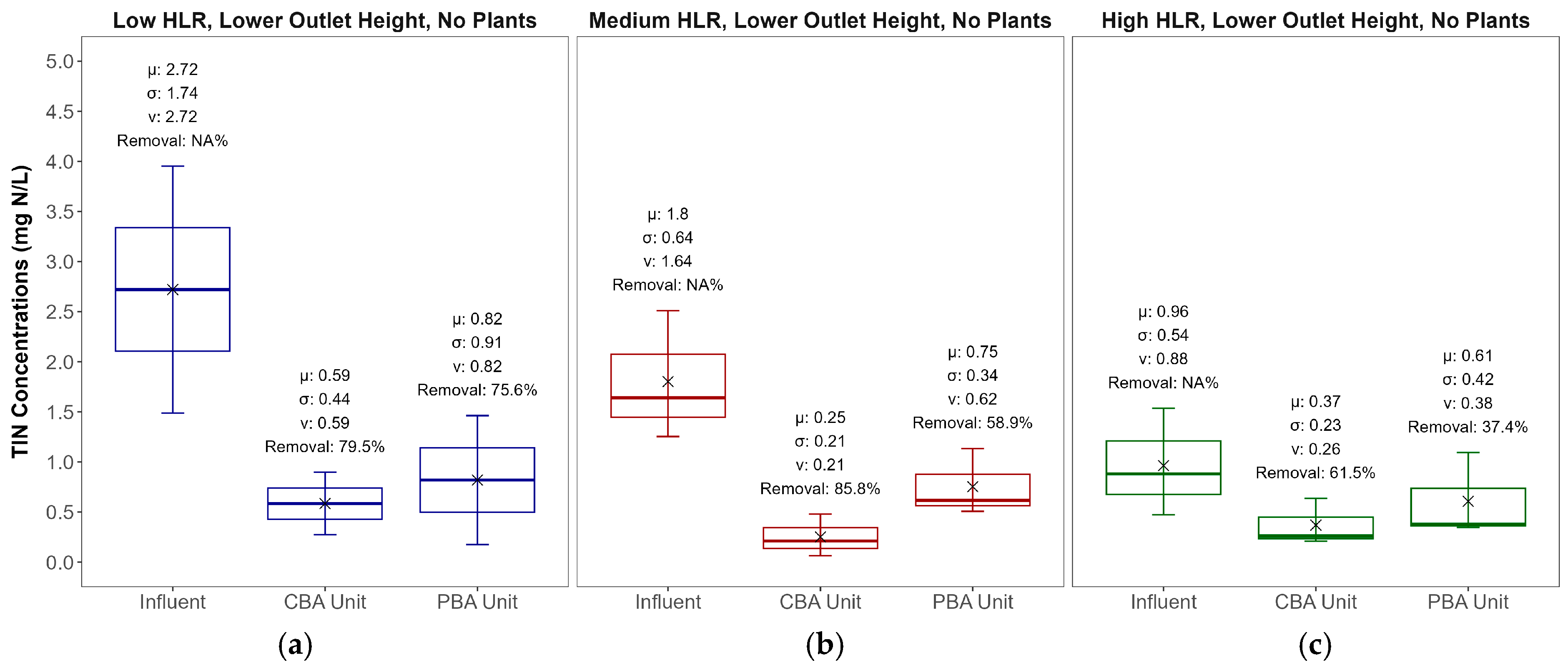
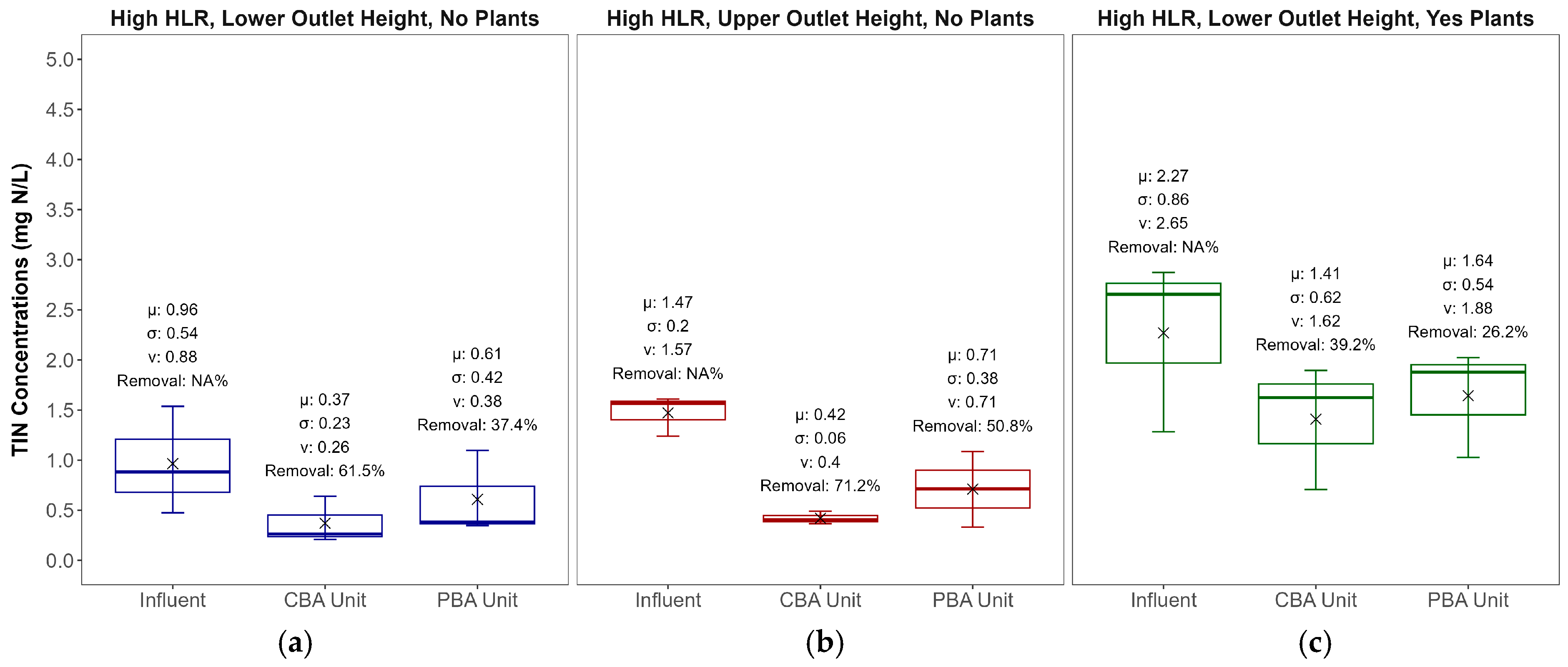


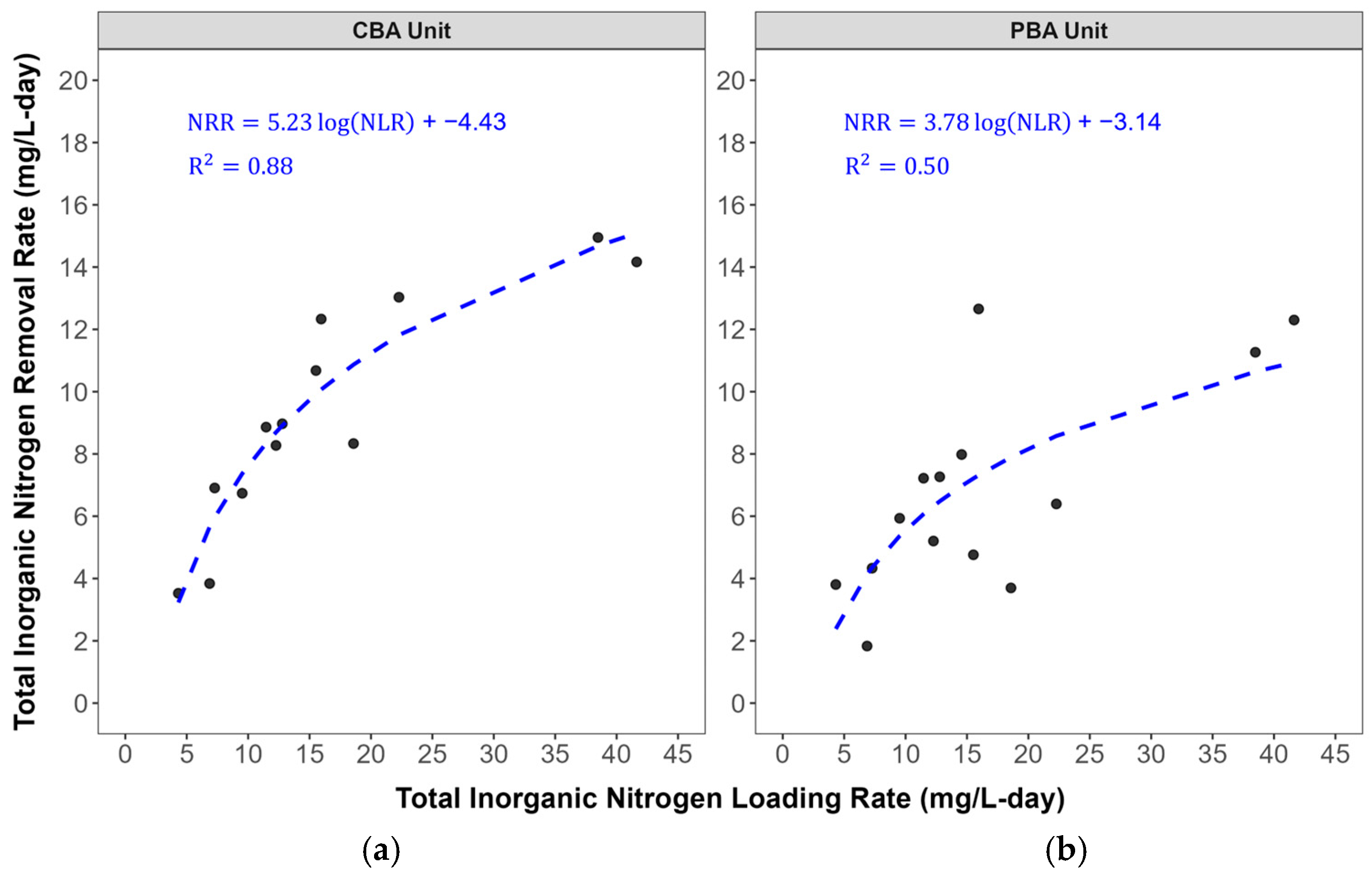


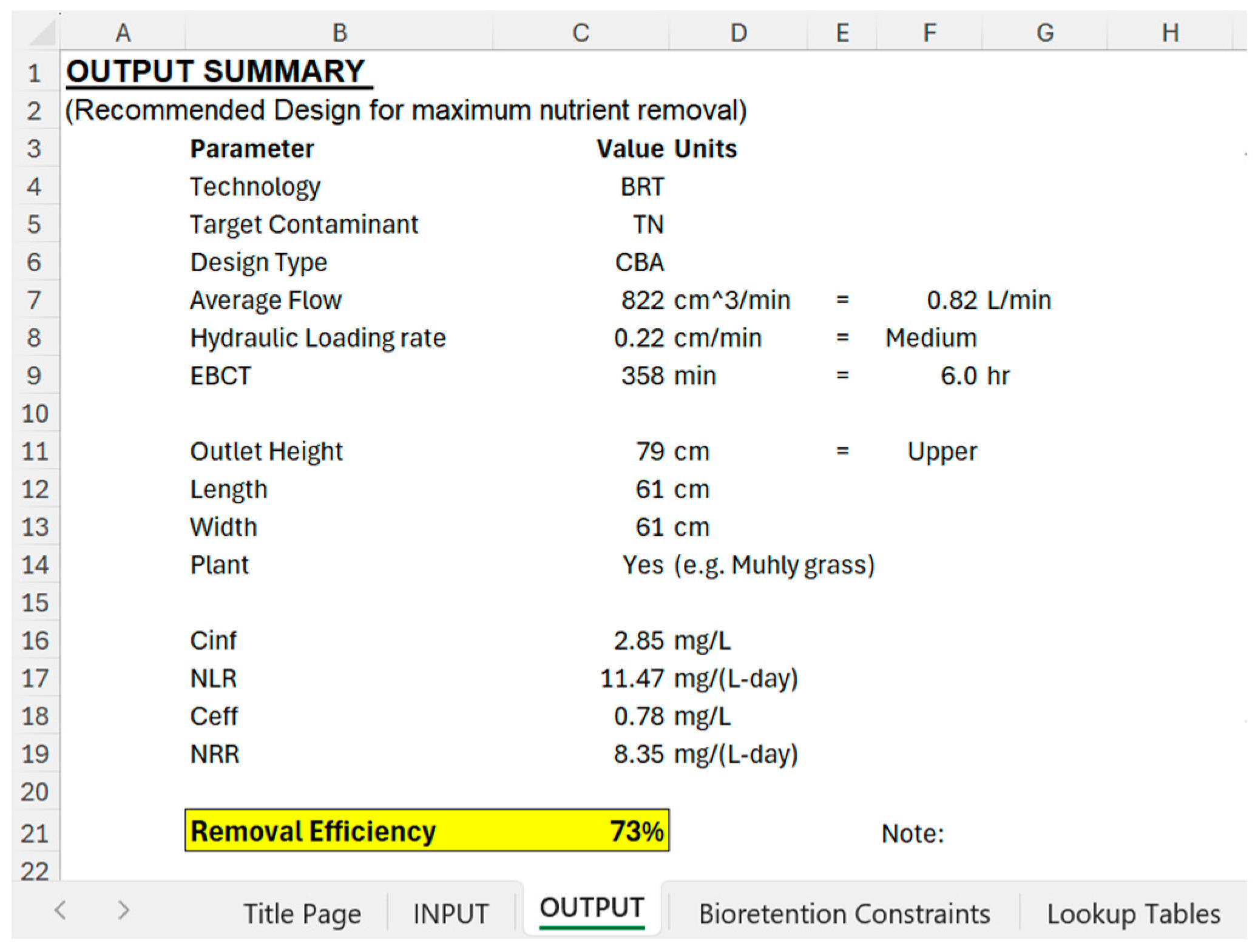
| Parameters | CBA Lower Outlet | PBA Lower Outlet | CBA Upper Outlet | PBA Upper Outlet |
|---|---|---|---|---|
| HLR (cm/min) | 0.31 | 0.29 | 0.56 | 0.50 |
| MRT (min) | 70 | 46 | 60 | 62 |
| MDI (t90/t10) | 3.5 | 6.7 | 3.0 | 2.4 |
| t50/MRT | 0.89 | 0.74 | 0.85 | 0.89 |
| Standard Deviation (min) | 32 | 33 | 27 | 23 |
| TIS n | 5.8 | 3.0 | 6.1 | 8.4 |
| Mass Recovered (%) | 99.9 | 98.0 | 104 | 102 |
| CBA | PBA | 1 p-Value | ||||
|---|---|---|---|---|---|---|
| Parameter | Average Influent | Average Effluent | Average % Removal | Average Effluent | Average % Removal | CBA vs. PBA Δ |
| TIN (mg/L) | 2.84 ± 2.51 | 0.71 ± 0.48 | 70.15 ± 17.58 | 1.63 ± 1.38 | 54.59 ± 20.73 | 0.031 * |
| NH4+-N (mg/L) | 0.18 ± 0.14 | 0.30 ± 0.17 | −150.70 ± 252.48 | 0.13 ± 0.08 | −58.62 ± 141.28 | NS |
| NO2−N (mg/L) | 0.06 ± 0.04 | 0.11 ± 0.06 | −121.13 ± 190.27 | 0.22 ± 0.23 | −191.78 ± 167.54 | NS |
| NO3−N (mg/L) | 2.55 ± 2.52 | 0.30 ± 0.33 | 85.33 ± 15.58 | 1.28 ± 1.19 | 61.96 ± 18.52 | 0.002 ** |
| 2 NOx−N (mg/L) | 2.62 ± 2.42 | 0.42 ± 0.36 | 78.93 ± 17.11 | 1.50 ± 0.08 | 57.41 ± 18.29 | NS |
| COD (mg/L) | 32.55 ± 6.37 | 27.61 ± 5.07 | 13.16 ± 11.80 | 33.52 ± 6.38 | −21.89 ± 44.72 | 0.032 * |
| Alkalinity (mg/L as CaCO3) | 123.46 ± 18.20 | 152.80 ± 50.05 | −20.36 ± 19.69 | 124.10 ± 30.19 | −17.46 ± 11.48 | NS |
| pH | 7.81 ± 0.17 | 7.20 ± 0.20 | 7.76 ± 2.78 | 7.25 ± 0.24 | 7.39 ± 3.27 | NS |
| 3 DO (mg/L) | 4.97 ± 0.82 | 0.78 ± 0.20 | 83.46 ± 2.80 | 0.35 ± 0.60 | 77.55 ± 7.04 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richardson, N.; Luangphairin, N.; Bhattacharjee, A.S.; Nachabe, M.H.; Ergas, S.J. Nursery Runoff Treatment by Novel Biochar-Amended Bioretention Systems. Water 2025, 17, 330. https://doi.org/10.3390/w17030330
Richardson N, Luangphairin N, Bhattacharjee AS, Nachabe MH, Ergas SJ. Nursery Runoff Treatment by Novel Biochar-Amended Bioretention Systems. Water. 2025; 17(3):330. https://doi.org/10.3390/w17030330
Chicago/Turabian StyleRichardson, Nicholas, Natchaya Luangphairin, Ananda S. Bhattacharjee, Mahmood H. Nachabe, and Sarina J. Ergas. 2025. "Nursery Runoff Treatment by Novel Biochar-Amended Bioretention Systems" Water 17, no. 3: 330. https://doi.org/10.3390/w17030330
APA StyleRichardson, N., Luangphairin, N., Bhattacharjee, A. S., Nachabe, M. H., & Ergas, S. J. (2025). Nursery Runoff Treatment by Novel Biochar-Amended Bioretention Systems. Water, 17(3), 330. https://doi.org/10.3390/w17030330






