Green Synthesis of nZVI-Modified Sludge Biochar for Cr(VI) Removal in Water: Fixed-Bed Experiments and Artificial Neural Network Model Prediction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of TP-nZVI/BC
2.3. Characterization of TP-nZVI/BC
2.4. Batch Adsorption Experiments
2.5. Fixed-Bed Experiments
2.6. Breakthrough Curve Modeling
2.7. ANN Model
3. Results and Discussion
3.1. Characterizations of TP-nZVI/BC
3.2. Adsorption Isotherms
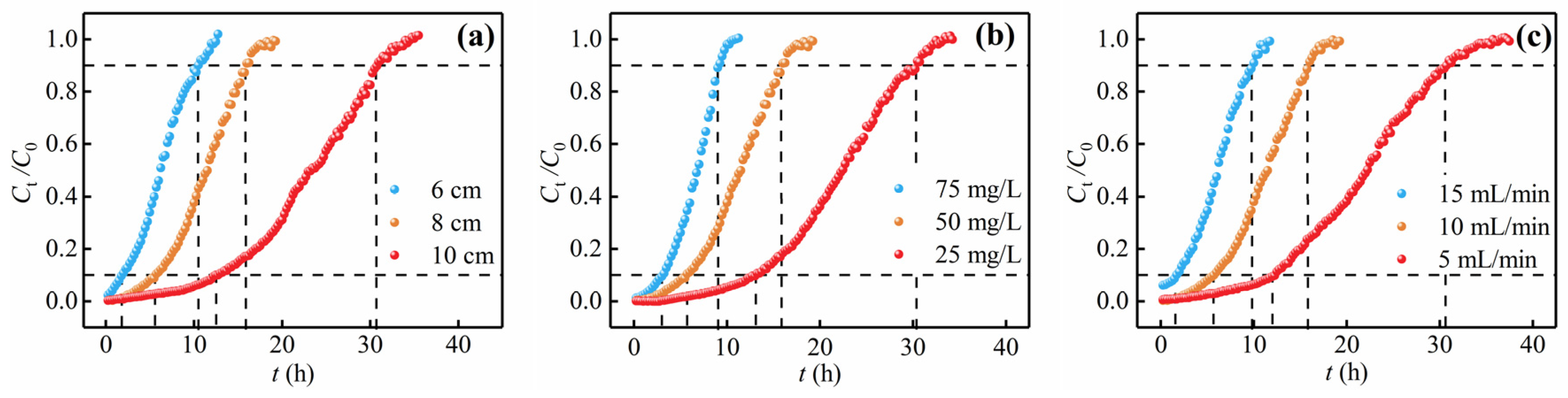
3.3. Effect of Operational Variables on Fixed-Bed Adsorption of Cr(VI)
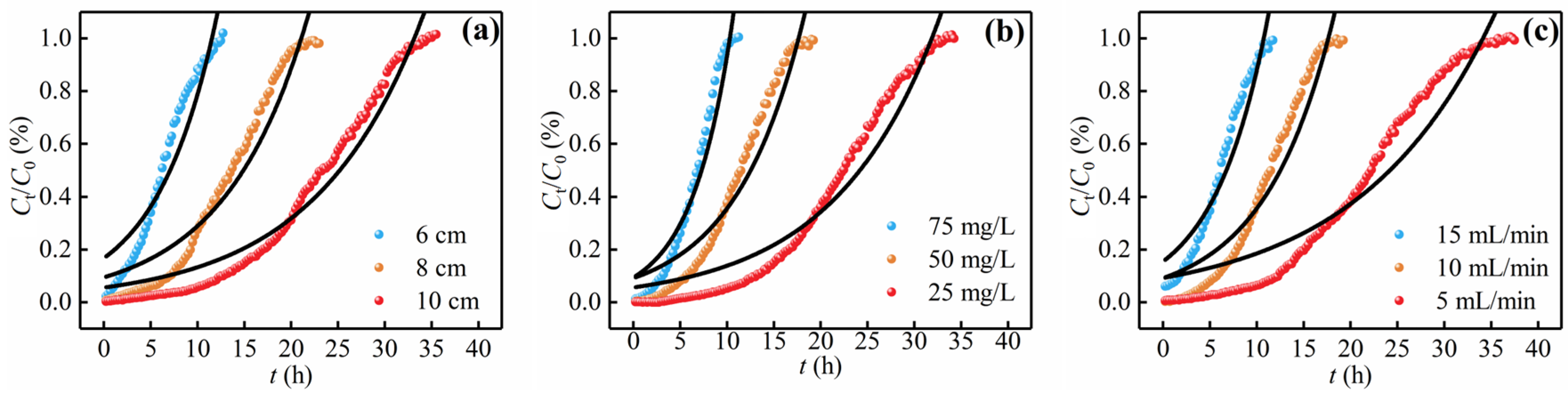
3.3.1. Bed Height
3.3.2. Influent Concentration
3.3.3. Flow Rate
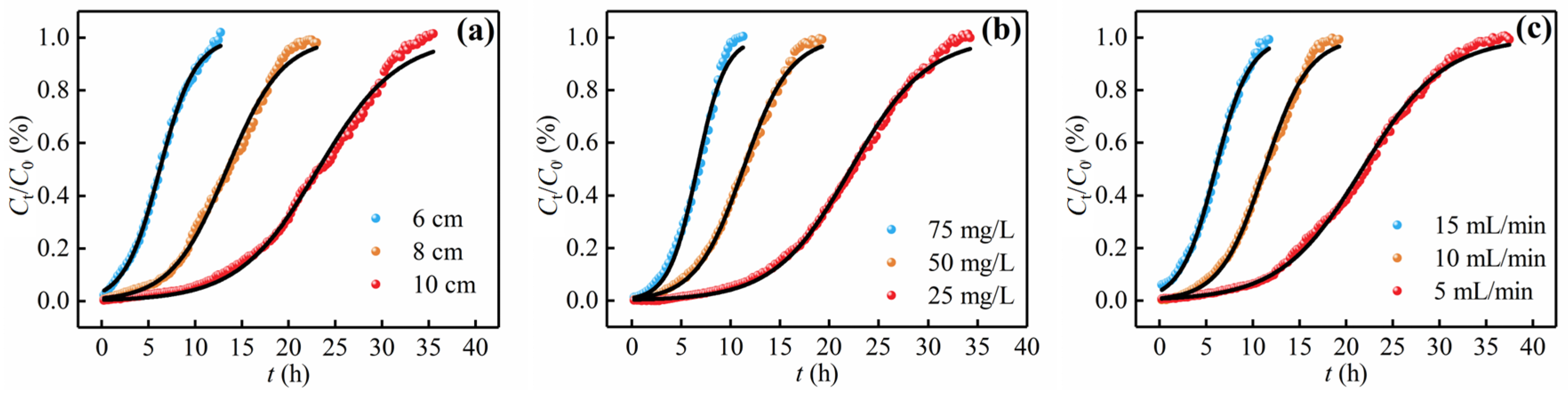
3.4. Breakthrough Curve Modeling
3.4.1. Thomas Model
3.4.2. Yoon–Nelson Model
3.4.3. Adams–Bohart Model
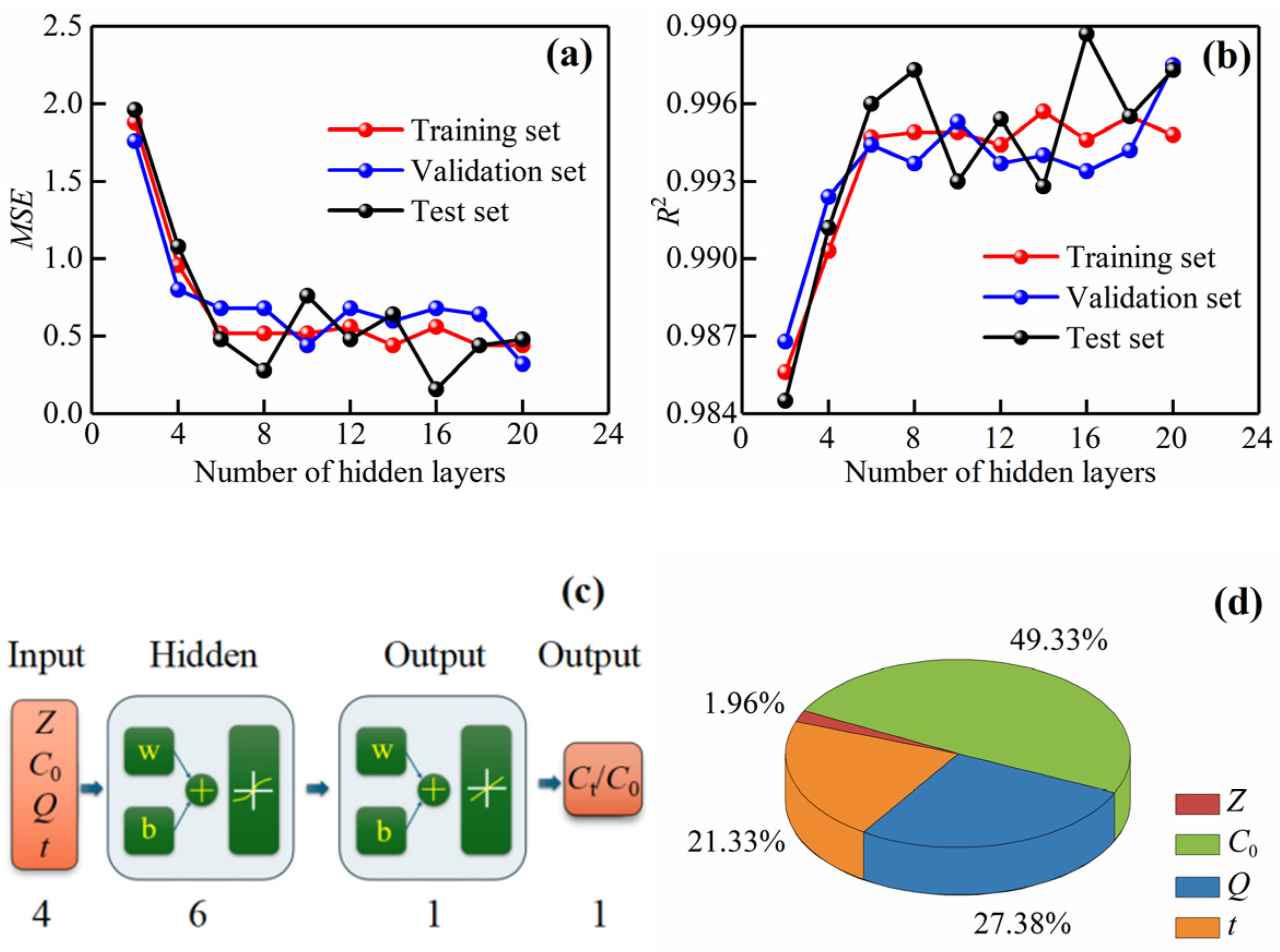
3.5. ANN Model
3.5.1. ANN Model Optimization
3.5.2. ANN Model Prediction Performance Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BC | Sludge Biochar |
| nZVI | Nano Zero-Valent Iron |
| TP | Tea Polyphenol |
| TP-nZVI/BC | Green-Synthesized Nano Zero-Valent Iron-Modified Sludge Biochar |
| ANN | Artificial Neural Network |
References
- Li, C.; Wang, H.; Liao, X.; Xiao, R.; Liu, K.; Bai, J.; Li, B.; He, Q. Heavy metal pollution in coastal wetlands: A systematic review of studies globally over the past three decades. J. Hazard. Mater. 2022, 424, 127312. [Google Scholar] [CrossRef]
- Iyer, M.; Anand, U.; Thiruvenkataswamy, S.; Babu, H.W.S.; Narayanasamy, A.; Prajapati, V.K.; Tiwari, C.K.; Gopalakrishnan, A.V.; Bontempi, E.; Sonne, C.; et al. A review of chromium (Cr) epigenetic toxicity and health hazards. Sci. Total Environ. 2023, 882, 163483. [Google Scholar] [CrossRef] [PubMed]
- Rong, K.; Li, X.; Yang, Q.; Liu, Z.; Yao, Q.; Zhang, Z.; Li, R.; Zhao, L.; Zheng, H. Removal of aqueous Cr(VI) by green synthesized sulfide iron nanoparticles loaded corn straw biochar: Performance, mechanism, and DFT calculations. Appl. Surf. Sci. 2024, 670, 160729. [Google Scholar] [CrossRef]
- Ma, L.; Liu, T.; Li, J.; Yang, Q. Interaction characteristics and mechanism of Cr(VI)/Cr(III) with microplastics: Influence factor experiment and DFT calculation. J. Hazard. Mater. 2024, 476, 134957. [Google Scholar] [CrossRef] [PubMed]
- Monga, A.; Fulke, A.B.; Dasgupta, D. Recent developments in essentiality of trivalent chromium and toxicity of hexavalent chromium: Implications on human health and remediation strategies. J. Hazard. Mater. Adv. 2022, 7, 100113. [Google Scholar] [CrossRef]
- Sadeghi, P.; Kazerouni, F.; Savari, A.; Movahedinia, A.; Safahieh, A.; Ajdari, D. Application of biomarkers in Epaulet grouper (Epinephelus stoliczkae) to assess chromium pollution in the Chabahar Bay and Gulf of Oman. Sci. Total Environ. 2015, 518–519, 554–561. [Google Scholar] [CrossRef]
- Mallik, A.K.; Moktadir, M.A.; Rahman, M.A.; Shahruzzaman, M.; Rahman, M.M. Progress in surface-modified silicas for Cr(VI) adsorption: A review. J. Hazard. Mater. 2022, 423, 127041. [Google Scholar] [CrossRef]
- Wise, J.P.; Young, J.L.; Cai, J.; Cai, L. Current understanding of hexavalent chromium [Cr(VI)] neurotoxicity and new perspectives. Environ. Int. 2022, 158, 106877. [Google Scholar] [CrossRef] [PubMed]
- Pushkar, B.; Sevak, P.; Parab, S.; Nilkanth, N. Chromium pollution and its bioremediation mechanisms in bacteria: A review. J. Environ. Manag. 2021, 287, 112279. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Huang, X.; Yan, J.; Li, Y.; Zhao, Z.; Liu, Y.; Ye, J.; Wei, Y. A review of the formation of Cr(VI) via Cr(III) oxidation in soils and groundwater. Sci. Total Environ. 2021, 774, 145762. [Google Scholar] [CrossRef]
- Aihemaiti, A.; Chen, J.; Hua, Y.; Dong, C.; Wei, X.; Yan, F.; Zhang, Z. Effect of ferrous sulfate modified sludge biochar on the mobility, speciation, fractionation and bioaccumulation of vanadium in contaminated soil from a mining area. J. Hazard. Mater. 2022, 437, 129405. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Kurniawan, T.A.; Wang, Y.; Zhou, Z.; Wei, Q.; Hu, Y.; Hafiz Dzarfan Othman, M.; Wayne Chew, K.; Hwang Goh, H.; Gui, H. Applicability of magnetic biochar derived from Fe-enriched sewage sludge for chromate removal from aqueous solution. Chem. Eng. Sci. 2023, 281, 119145. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohanty, K. A review of the next-generation biochar production from waste biomass for material applications. Sci. Total Environ. 2023, 904, 167171. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Gu, L.; Chen, S.; Jiang, Y.; Huang, P.; Li, H.; Yu, H.; Xia, D. Sewage sludge derived FeCl3-activated biochars as efficient adsorbents for the treatment of toxic As(III) and Cr(VI) wastewater. J. Environ. Chem. Eng. 2022, 10, 108575. [Google Scholar] [CrossRef]
- Ambika, S.; Kumar, M.; Pisharody, L.; Malhotra, M.; Kumar, G.; Sreedharan, V.; Singh, L.; Nidheesh, P.V.; Bhatnagar, A. Modified biochar as a green adsorbent for removal of hexavalent chromium from various environmental matrices: Mechanisms, methods, and prospects. Chem. Eng. J. 2022, 439, 135716. [Google Scholar] [CrossRef]
- Wang, P.; Hu, J.; Liu, T.; Han, G.; Ma, W.-m.; Li, J. New insights into ball-milled zero-valent iron composites for pollution remediation: An overview. J. Clean. Prod. 2023, 385, 135513. [Google Scholar] [CrossRef]
- Zhang, X.; He, Y.; Gao, G.; Zhang, X.; Han, R.; Xu, S.; Shi, X.; Xu, C.; Yan, G.; Wang, Z.; et al. Novel core-shell structural nZVI@Fe2P for sulfadiazine (SDZ) degradation: Accelerated Fe(III)/Fe(II) dynamic cycling, boosted Fenton performance and stability. Sep. Purif. Technol. 2024, 347, 127705. [Google Scholar] [CrossRef]
- Hu, Y.-b.; Du, T.; Ma, L.; Feng, X.; Xie, Y.; Fan, X.; Fu, M.-L.; Yuan, B.; Li, X.-y. Insights into the mechanisms of aqueous Cd(II) reduction and adsorption by nanoscale zerovalent iron under different atmosphere conditions. J. Hazard. Mater. 2022, 440, 129766. [Google Scholar] [CrossRef]
- Xi, M.; Zhang, X.; Chen, G.; Zhang, L.; Jiang, Z.; Zheng, H. Synergizing carbon sequestration mechanisms during the remediation of Cr(VI) by nano zero-valent iron loaded biochar (nZVI-BC). J. Environ. Chem. Eng. 2024, 12, 114781. [Google Scholar] [CrossRef]
- Deng, J.; Yoon, S.; Pasturel, M.; Bae, S.; Hanna, K. Interactions between nanoscale zerovalent iron (NZVI) and silver nanoparticles alter the NZVI reactivity in aqueous environments. Chem. Eng. J. 2022, 450, 138406. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, Y.; Ning, P.; Lynch, I.; Guo, Z.; Zhang, P.; Wu, L. Synergetic effect of green synthesized NZVI@Chitin-modified ZSM-5 for efficient oxidative degradation of tetracycline. Environ. Res. 2024, 258, 119360. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhou, S.; Song, H.; Hu, H.; Yang, Y.; Zhang, X.; Ma, S.; Feng, X.; Pan, Y.; Gong, S.; et al. Green tea polyphenols-derived hybrid materials in manufacturing, environment, food and healthcare. Nano Today 2023, 52, 101990. [Google Scholar] [CrossRef]
- Ma, F.; Zhao, H.; Zheng, X.; Zhang, J.; Ding, W.; Jiao, Y.; Li, Q.; Kang, H. Green synthesis of nZVI-modified biochar significantly enhanced the removal of Cr(VI) from aqueous solution. Environ. Sci. Pollut. Res. 2024, 31, 33993–34009. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Karamalidis, A.K. Fixed-bed column adsorption of Ge(IV) using catechol-based adsorbents and aqueous complexation modeling to understand Ge(IV) selectivity. Sep. Purif. Technol. 2024, 351, 128106. [Google Scholar] [CrossRef]
- Nieto-Sandoval, J.; Morabet, F.E.; Munoz, M.; Lopez-Arago, N.; de Pedro, Z.M.; Casas, J.A. In-situ regeneration of a novel Fe3O4/GAC adsorbent for micropollutants removal in a continuous fixed-bed. J. Hazard. Mater. Adv. 2023, 10, 100267. [Google Scholar] [CrossRef]
- Bai, S.; Chen, S.; Li, J.; Ya, R.; Ao, N.; Wang, J. Effect of silicon on the mathematical model prediction and adsorption mechanism of boron removal by a fixed-bed column. J. Water Process Eng. 2022, 49, 103194. [Google Scholar] [CrossRef]
- Chatterjee, R.; Majumder, C. Application of modified graphene oxide-chitosan composite for the removal of 2-methylpyridine using fixed bed adsorption and subsequent regeneration of the adsorbent by UV photolysis. J. Water Process Eng. 2023, 53, 103654. [Google Scholar] [CrossRef]
- Diniz, V.; Rath, S. Adsorption of aqueous phase contaminants of emerging concern by activated carbon: Comparative fixed-bed column study and in situ regeneration methods. J. Hazard. Mater. 2023, 459, 132197. [Google Scholar] [CrossRef] [PubMed]
- Dalhat, M.A.; Mu’azu, N.D.; Essa, M.H. Generalized decay and artificial neural network models for fixed-Bed phenolic compounds adsorption onto activated date palm biochar. J. Environ. Chem. Eng. 2021, 9, 104711. [Google Scholar] [CrossRef]
- Vakili, M.; Mojiri, A.; Kindaichi, T.; Cagnetta, G.; Yuan, J.; Wang, B.; Giwa, A.S. Cross-linked chitosan/zeolite as a fixed-bed column for organic micropollutants removal from aqueous solution, optimization with RSM and artificial neural network. J. Environ. Manag. 2019, 250, 109434. [Google Scholar] [CrossRef] [PubMed]
- Gordanshekan, A.; Arabian, S.; Solaimany Nazar, A.R.; Farhadian, M.; Tangestaninejad, S. A comprehensive comparison of green Bi2WO6/g-C3N4 and Bi2WO6/TiO2 S-scheme heterojunctions for photocatalytic adsorption/degradation of Cefixime: Artificial neural network, degradation pathway, and toxicity estimation. Chem. Eng. J. 2023, 451, 139067. [Google Scholar] [CrossRef]
- Pauletto, P.S.; Lütke, S.F.; Dotto, G.L.; Salau, N.P.G. Forecasting the multicomponent adsorption of nimesulide and paracetamol through artificial neural network. Chem. Eng. J. 2021, 412, 127527. [Google Scholar] [CrossRef]
- Dragović, S. Artificial neural network modeling in environmental radioactivity studies—A review. Sci. Total Environ. 2022, 847, 157526. [Google Scholar] [CrossRef] [PubMed]
- Praveen, S.; Jegan, J.; Pushpa, T.B.; Gokulan, R. Artificial neural network modelling for biodecolorization of Basic Violet 03 from aqueous solution by biochar derived from agro-bio waste of groundnut hull: Kinetics and thermodynamics. Chemosphere 2021, 276, 130191. [Google Scholar] [CrossRef]
- Ahmed, Y.; Siddiqua Maya, A.A.; Akhtar, P.; Alam, M.S.; AlMohamadi, H.; Islam, M.N.; Alharbi, O.A.; Rahman, S.M. A novel interpretable machine learning and metaheuristic-based protocol to predict and optimize ciprofloxacin antibiotic adsorption with nano-adsorbent. J. Environ. Manag. 2024, 370, 122614. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Naqvi, S.R.; Ali, I.; Arshad, M.; AlMohamadi, H.; Sikandar, U. Algal-derived biochar as an efficient adsorbent for removal of Cr (VI) in textile industry wastewater: Non-linear isotherm, kinetics and ANN studies. Chemosphere 2023, 316, 137826. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Niu, X.; Zhang, D.; Li, X.; Li, H.; Wang, Z.; Lin, Z.; Fu, M. Fate and mechanistic insights into nanoscale zerovalent iron (nZVI) activation of sludge derived biochar reacted with Cr(VI). J. Environ. Manag. 2022, 319, 115771. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, X. Green synthesis of modified polyethylene packing supported tea polyphenols-NZVI for nitrate removal from wastewater: Characterization and mechanisms. Sci. Total Environ. 2022, 806, 150596. [Google Scholar] [CrossRef]
- Yang, K.; Wang, X.; Lynch, I.; Guo, Z.; Zhang, P.; Wu, L. Green construction of MBI corrosion-resistant interfaces modified NZVI@MOFs-regulated 3D PAN cryogel film to enhance Cr(VI) removal. Sep. Purif. Technol. 2024, 333, 125902. [Google Scholar] [CrossRef]
- Zhao, N.; Zhao, C.; Liu, K.; Zhang, W.; Tsang, D.C.W.; Yang, Z.; Yang, X.; Yan, B.; Morel, J.L.; Qiu, R. Experimental and DFT investigation on N-functionalized biochars for enhanced removal of Cr(VI). Environ. Pollut. 2021, 291, 118244. [Google Scholar] [CrossRef]
- Puccia, V.; Avena, M.J. On the use of the Dubinin-Radushkevich equation to distinguish between physical and chemical adsorption at the solid-water interface. Colloid Interface Sci. Commun. 2021, 41, 100376. [Google Scholar] [CrossRef]
- Ordonez, D.; Podder, A.; Valencia, A.; Sadmani, A.H.M.A.; Reinhart, D.; Chang, N.-B. Continuous fixed-bed column adsorption of perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) from canal water using zero-valent Iron-based filtration media. Sep. Purif. Technol. 2022, 299, 121800. [Google Scholar] [CrossRef]
- Bacelo, H.; Santos, S.C.R.; Ribeiro, A.; Boaventura, R.A.R.; Botelho, C.M.S. Antimony removal from water by pine bark tannin resin: Batch and fixed-bed adsorption. J. Environ. Manag. 2022, 302, 114100. [Google Scholar] [CrossRef]
- Albayati, T.M.; Kalash, K.R. Polycyclic aromatic hydrocarbons adsorption from wastewater using different types of prepared mesoporous materials MCM-41in batch and fixed bed column. Process Saf. Environ. Prot. 2020, 133, 124–136. [Google Scholar] [CrossRef]
- Feizi, F.; Sarmah, A.K.; Rangsivek, R. Adsorption of pharmaceuticals in a fixed-bed column using tyre-based activated carbon: Experimental investigations and numerical modelling. J. Hazard. Mater. 2021, 417, 126010. [Google Scholar] [CrossRef]
- de Araujo, C.M.B.; Ghislandi, M.G.; Rios, A.G.; da Costa, G.R.B.; do Nascimento, B.F.; Ferreira, A.F.P.; da Motta Sobrinho, M.A.; Rodrigues, A.E. Wastewater treatment using recyclable agar-graphene oxide biocomposite hydrogel in batch and fixed-bed adsorption column: Bench experiments and modeling for the selective removal of organics. Colloids Surf. A Physicochem. Eng. Asp. 2022, 639, 128357. [Google Scholar] [CrossRef]
- Chu, K.H.; Hashim, M.A. Removal of antibiotics through fixed bed adsorption: Comparison of different breakthrough curve models. J. Water Process Eng. 2023, 56, 104512. [Google Scholar] [CrossRef]
- Juela, D.; Vera, M.; Cruzat, C.; Astudillo, A.; Vanegas, E. A new approach for scaling up fixed-bed adsorption columns for aqueous systems: A case of antibiotic removal on natural adsorbent. Process Saf. Environ. Prot. 2022, 159, 953–963. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, K.; Hu, X.; He, Q.; Yan, J.; Xue, Y. Cadmium removal by MgCl2 modified biochar derived from crayfish shell waste: Batch adsorption, response surface analysis and fixed bed filtration. J. Hazard. Mater. 2021, 408, 124860. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Chen, H. A review on machine learning forecasting growth trends and their real-time applications in different energy systems. Sustain. Cities Soc. 2020, 54, 102010. [Google Scholar] [CrossRef]
- Kavitha, B.; Deepa, R.; Sivakumar, S. Evolvulus alsinoides plant mediated synthesis of Ag2O nanoparticles for the removal of Cr(VI) ions from aqueous solution: Modeling of experimental data using artificial neural network. Mater. Today Sustain. 2022, 18, 100124. [Google Scholar] [CrossRef]
- Hou, J.; Bao, W.; Zhang, J.; Yu, J.; Chen, L.; Di, G.; Zhou, Q.; Li, X. Characteristics and mechanisms of sulfamethoxazole adsorption onto modified biochars with hierarchical pore structures: Batch, predictions using artificial neural network and fixed bed column studies. J. Water Process Eng. 2023, 54, 103975. [Google Scholar] [CrossRef]
- Yang, C.; Liu, K.; Yang, S.; Zhu, W.; Tong, L.; Shi, J.; Wang, Y. Prediction of metformin adsorption on subsurface sediments based on quantitative experiment and artificial neural network modeling. Sci. Total Environ. 2023, 899, 165666. [Google Scholar] [CrossRef]

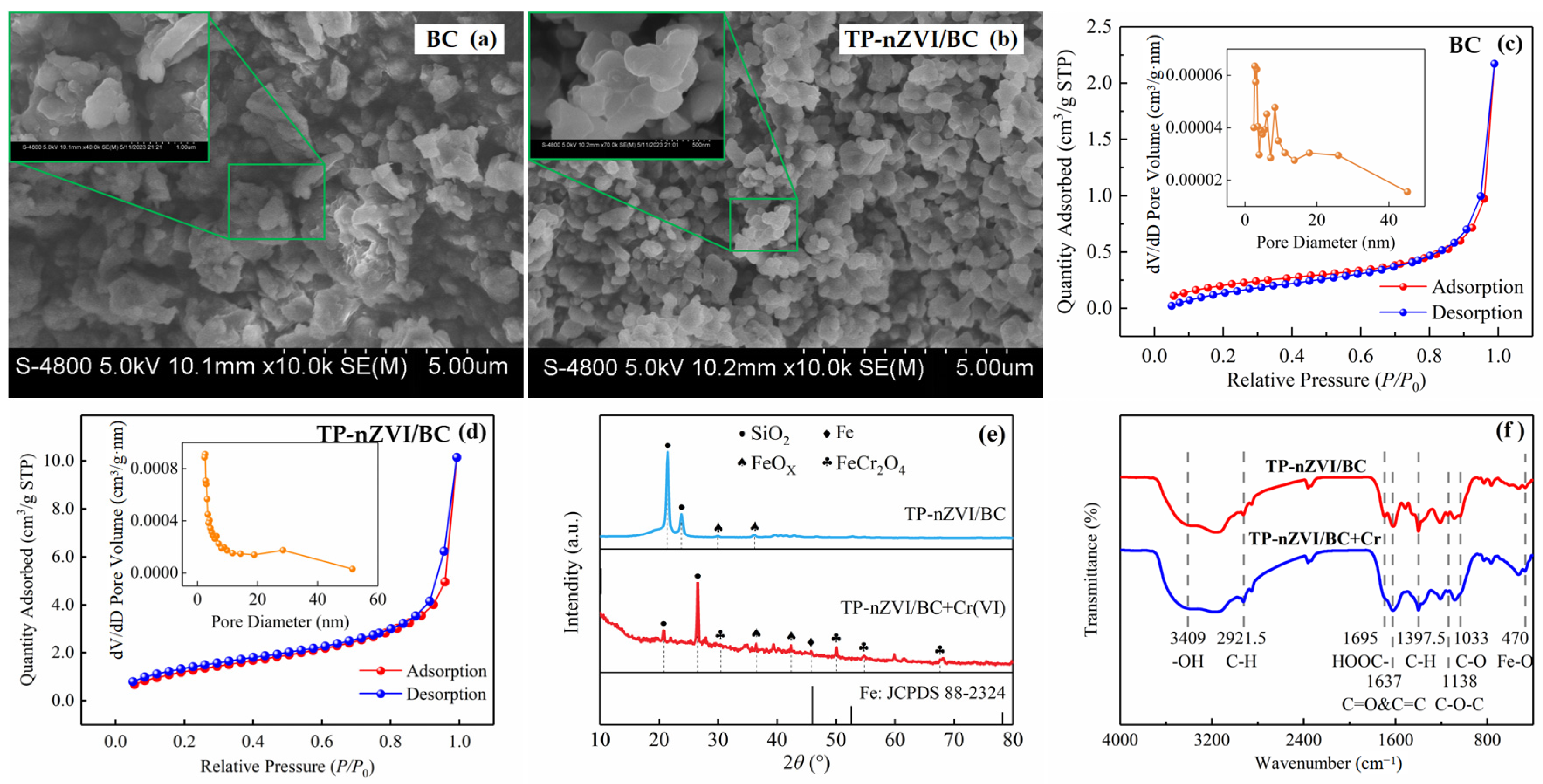
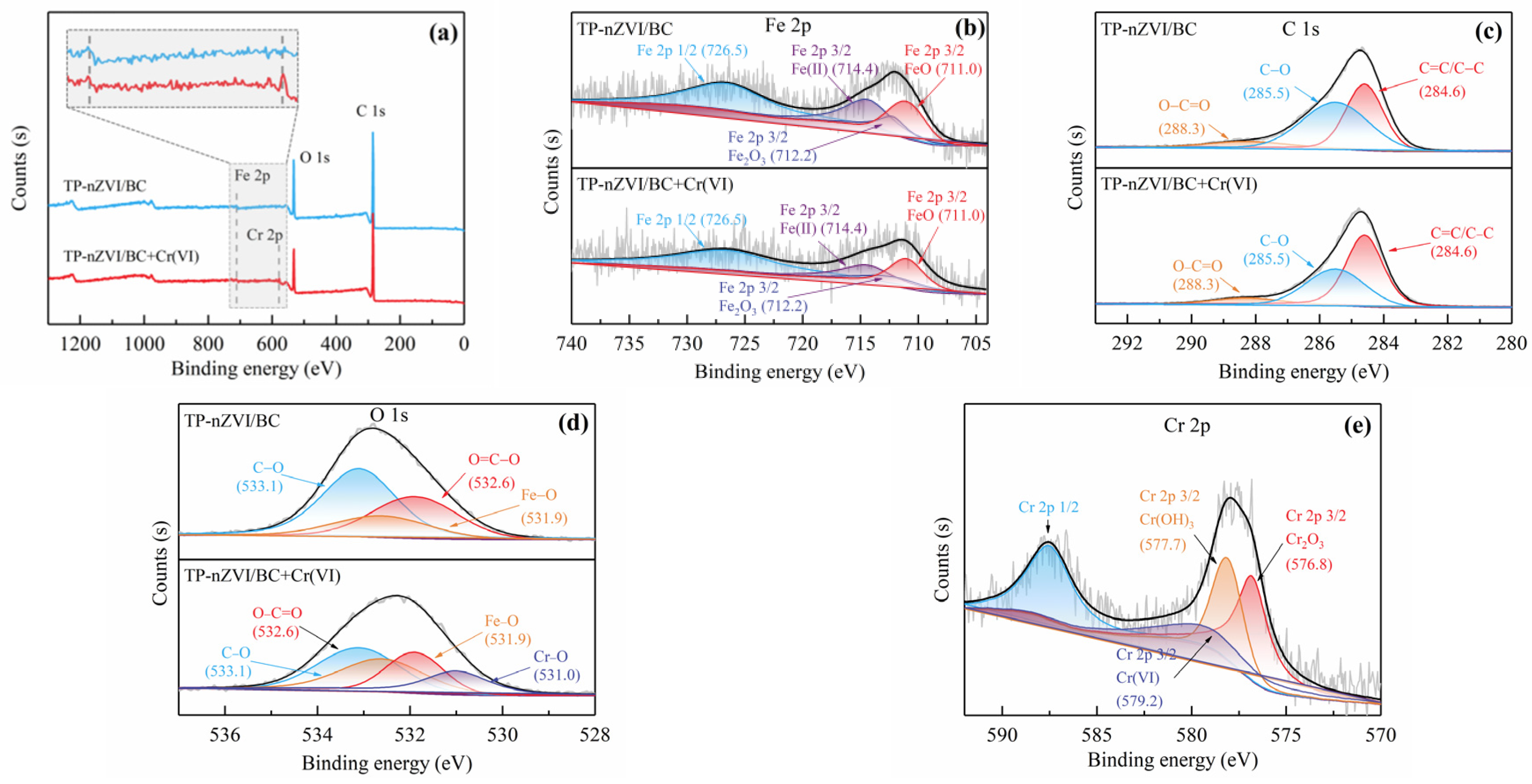






| Type | Specific Surface Area (m2/g) | Porosity (cm3/g) | Aperture (nm) |
|---|---|---|---|
| BC | 0.88 | 0.003 | 21.50 |
| TP-nZVI/BC | 4.87 | 0.015 | 15.20 |
| Model | Parameter 1 | Parameter 2 | R2 |
|---|---|---|---|
| Langmuir | KL = 0.004 | Qm = 105.64 | 0.9834 |
| Freundlich | KF = 2.97 | N = 1.91 | 0.9915 |
| Temkin | A = 19.78 | Kt = 0.07 | 0.9326 |
| Dubinin Radushkevich | qm = 17.11 | E = 48.34 | 0.8070 |
| Z | C0 | Q | Thomas | Yoon–Nelson | Adams–Bohart | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| kTH | Q0 | R2 | kYN | τ | R2 | kAB | N0 | R2 | |||
| cm | mg/L | mL/min | mL/mg·min | mg/g | mL/mg·min | min | mL/mg·min | mg/L | |||
| 6 | 50 | 10 | 0.0099 | 7.228 | 0.998 | 0.53 | 6.23 | 0.997 | 0.0031 | 70.74 | 0.897 |
| 8 | 50 | 10 | 0.0094 | 10.881 | 0.996 | 0.42 | 11.26 | 0.990 | 0.0027 | 110.27 | 0.922 |
| 10 | 50 | 10 | 0.0047 | 16.437 | 0.997 | 0.23 | 23.17 | 0.992 | 0.0017 | 201.18 | 0.956 |
| 8 | 25 | 10 | 0.0103 | 12.035 | 0.997 | 0.26 | 22.16 | 0.997 | 0.0018 | 199.2 | 0.942 |
| 8 | 50 | 10 | 0.0094 | 10.881 | 0.996 | 0.42 | 11.26 | 0.990 | 0.0027 | 110.27 | 0.922 |
| 8 | 75 | 10 | 0.0084 | 9.434 | 0.992 | 0.70 | 6.6 | 0.993 | 0.0046 | 64.46 | 0.933 |
| 8 | 50 | 5 | 0.0046 | 11.744 | 0.997 | 0.23 | 21.73 | 0.996 | 0.0014 | 213.25 | 0.915 |
| 8 | 50 | 10 | 0.0092 | 10.881 | 0.996 | 0.42 | 11.26 | 0.990 | 0.0027 | 110.27 | 0.922 |
| 8 | 50 | 15 | 0.0109 | 8.226 | 0.997 | 0.55 | 6.01 | 0.997 | 0.0035 | 67.21 | 0.922 |
| n1 | n2 | n3 | n4 | n5 | n6 | |
|---|---|---|---|---|---|---|
| Bed height (cm) | −3.7112 | −5.1146 | 0.2323 | 1.1503 | −2.9781 | −5.1545 |
| Influent concentration (mg/L) | 2.9673 | −22.5589 | 8.9515 | −1.9446 | 5.4000 | 3.1561 |
| Inlet flow rate (mL/min) | 3.7202 | 3.6071 | 3.4719 | −3.1030 | 1.1774 | 2.4647 |
| Running time (h) | −7.4896 | −10.6434 | 1.7755 | −3.5541 | 3.1830 | 1.5486 |
| Hidden layer to output layer weights | −0.3011 | −0.5723 | −0.2447 | −0.2916 | 0.3641 | 0.1648 |
| Hide layer bias items | −8.5007 | 16.5916 | −6.9992 | −1.0295 | 1.1103 | 3.2168 |
| Output layer bias term | −0.2876 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Ma, F.; Ren, X.; Zhao, B.; Jiang, Y.; Zhang, J. Green Synthesis of nZVI-Modified Sludge Biochar for Cr(VI) Removal in Water: Fixed-Bed Experiments and Artificial Neural Network Model Prediction. Water 2025, 17, 341. https://doi.org/10.3390/w17030341
Zhao H, Ma F, Ren X, Zhao B, Jiang Y, Zhang J. Green Synthesis of nZVI-Modified Sludge Biochar for Cr(VI) Removal in Water: Fixed-Bed Experiments and Artificial Neural Network Model Prediction. Water. 2025; 17(3):341. https://doi.org/10.3390/w17030341
Chicago/Turabian StyleZhao, Hao, Fengfeng Ma, Xuechang Ren, Baowei Zhao, Yufeng Jiang, and Jian Zhang. 2025. "Green Synthesis of nZVI-Modified Sludge Biochar for Cr(VI) Removal in Water: Fixed-Bed Experiments and Artificial Neural Network Model Prediction" Water 17, no. 3: 341. https://doi.org/10.3390/w17030341
APA StyleZhao, H., Ma, F., Ren, X., Zhao, B., Jiang, Y., & Zhang, J. (2025). Green Synthesis of nZVI-Modified Sludge Biochar for Cr(VI) Removal in Water: Fixed-Bed Experiments and Artificial Neural Network Model Prediction. Water, 17(3), 341. https://doi.org/10.3390/w17030341





