Analyzing the Efficacy of Water Treatment Disinfectants as Vector Control: The Larvicidal Effects of Silver Nitrate, Copper Sulfate Pentahydrate, and Sodium Hypochlorite on Juvenile Aedes aegypti
Abstract
1. Introduction
- They prefer to bite hosts during the daytime, with peak activity occurring between dawn and dusk.
- They feed on humans relative to other vertebrate species, obtaining bloodmeals which provide the necessary nutrients required for a female mosquito’s egg production and reproduction.
- They feed on several hosts within one reproductive cycle which increases the potential for the transmission of disease.
- They prefer to lay eggs in manmade or artificial containers (e.g., household water storage containers, plant pots, tires, etc.), which is why they are commonly referred to as a container-breeding species.
2. Materials and Methods
2.1. Culturing and Rearing
2.2. Water Treatment Disinfectants
2.2.1. Silver Nitrate
2.2.2. Copper Sulfate Pentahydrate
2.2.3. Sodium Hypochlorite
2.3. Survival Bioassays: Evaluation of Dose Response to Water Treatment Disinfectants
2.3.1. Experimental Setup
- A mosquito deposits eggs in a water storage container, resulting in newly hatched larvae being exposed to a freshly applied disinfectant. This scenario evaluates whether a POUWT technology introduced into the water storage container can effectively reduce the survival and emergence of newly hatched larvae.
- Larvae are already present in the source water supplying the household water storage (HWS) system, allowing them to develop further before encountering the water storage container and disinfectant.
2.3.2. Data Analysis
3. Results and Discussion
3.1. Late Third Instar Experiments
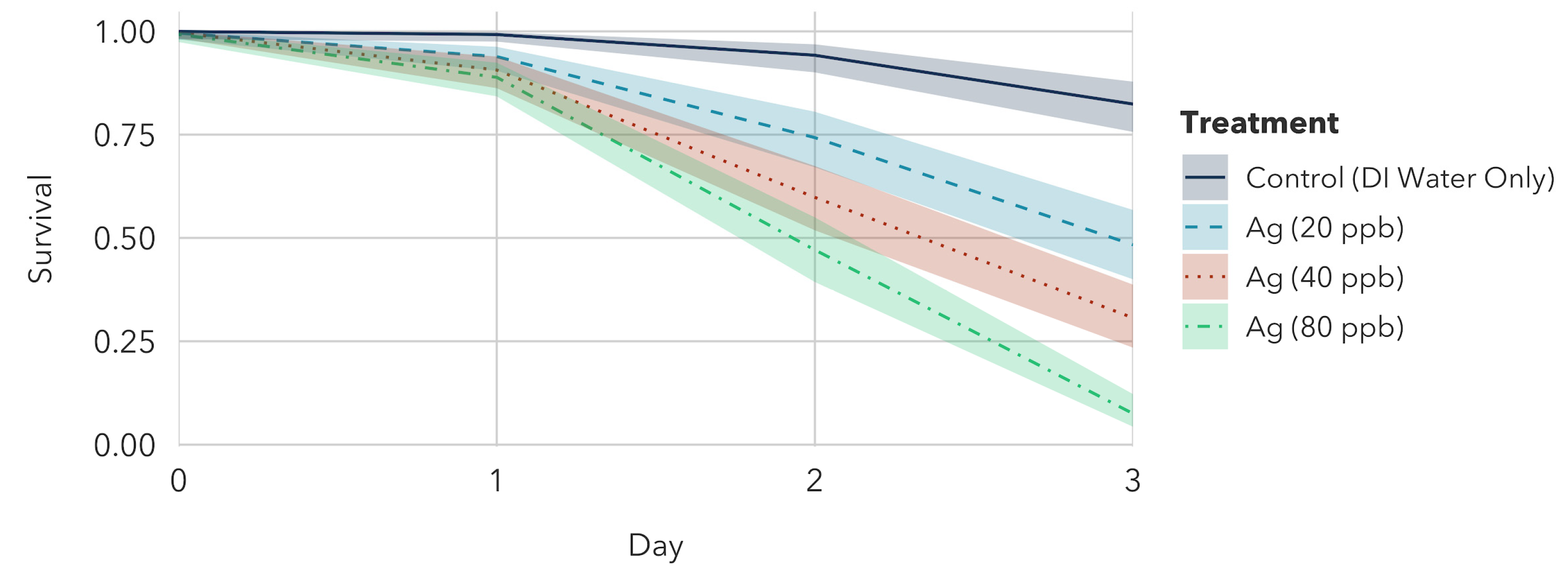
3.1.1. Silver Nitrate Exposure to Older Instar
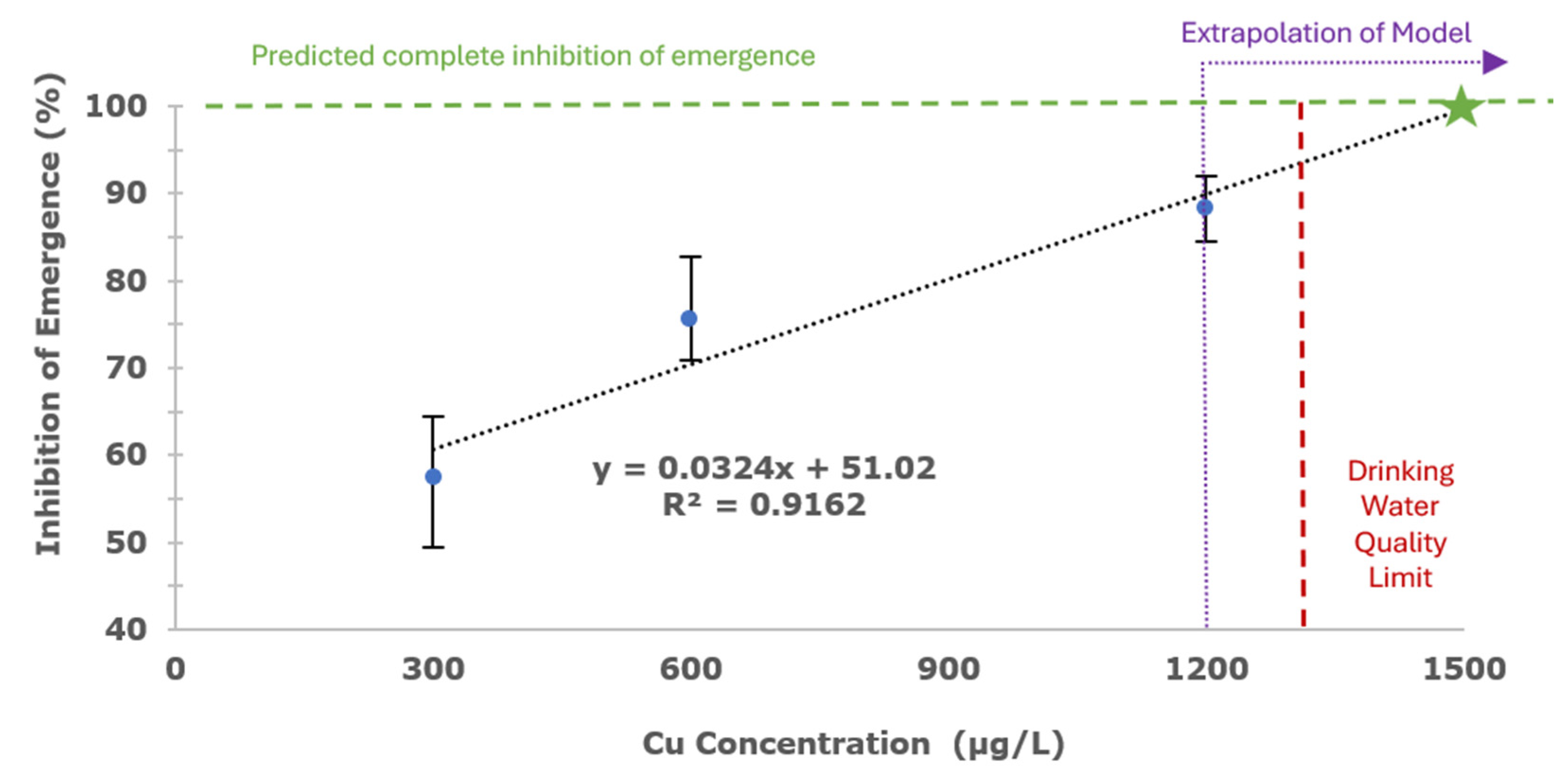
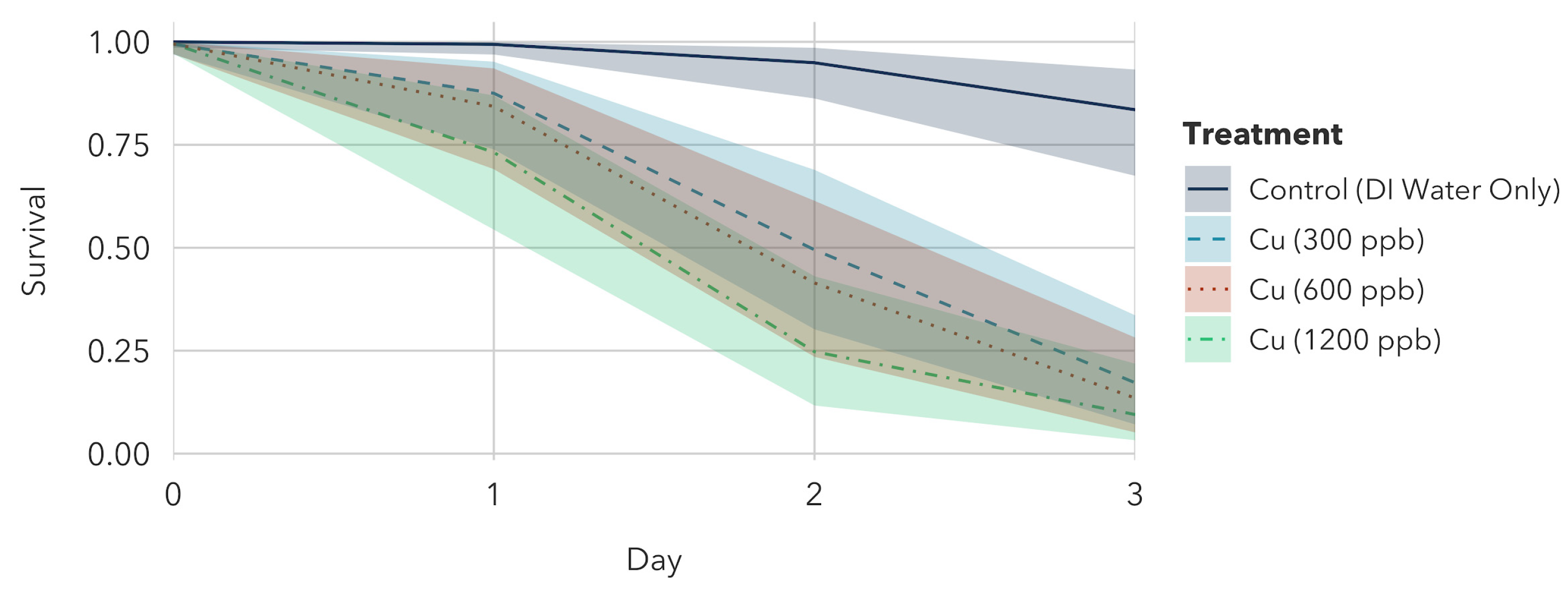
3.1.2. Copper Sulfate Pentahydrate Exposure to Older Instar
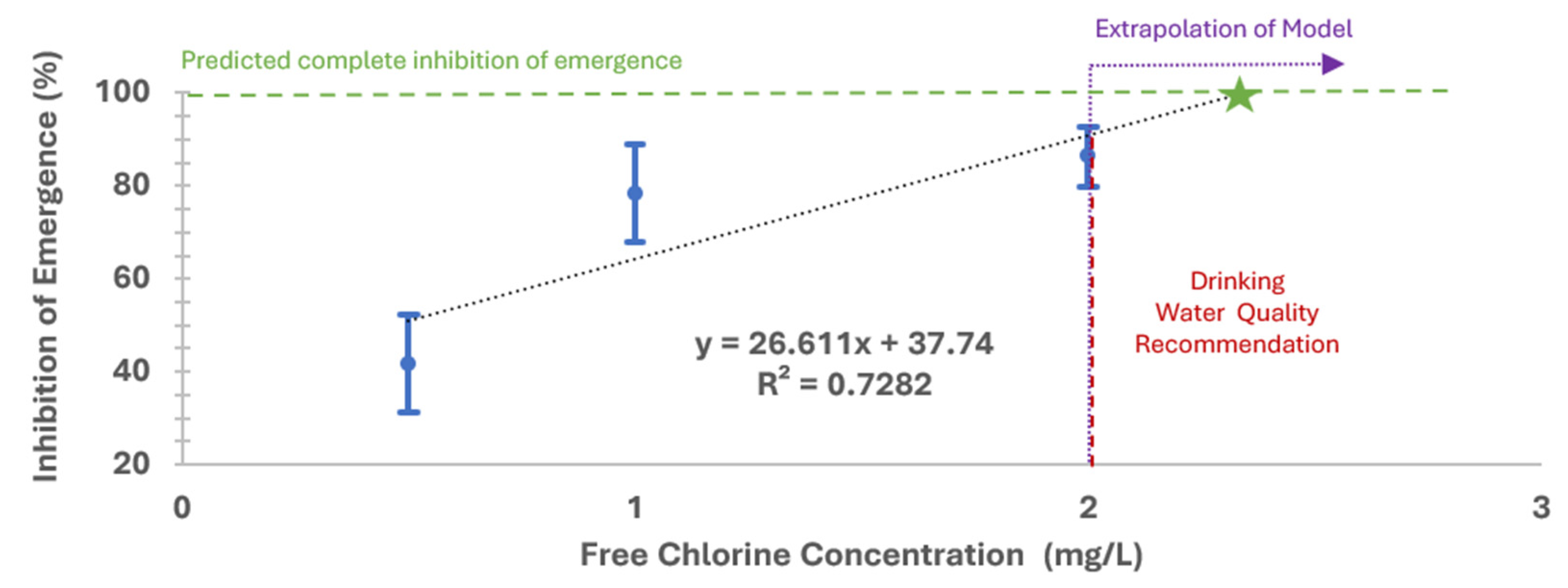
3.1.3. Sodium Hypochlorite Exposure to Older Instar
- The volatility of chlorine, which makes it less stable compared to silver and copper during long exposure periods, especially in elevated temperatures that can increase chlorine evaporation;
- Differences in the rate of free chlorine consumption within each beaker, potentially caused by larvae, external contamination sources, or variations in pH and temperature;
- The ratio of hypochlorous acid (HOCl) to hypochlorite ion (OCl−), as HOCl is the more effective disinfectant of the two forms and may also exhibit stronger larvicidal effects (ratio is influenced by the pH of the solution; higher pH values correspond to a greater concentration of OCl−).
3.2. Younger Instar Experiments
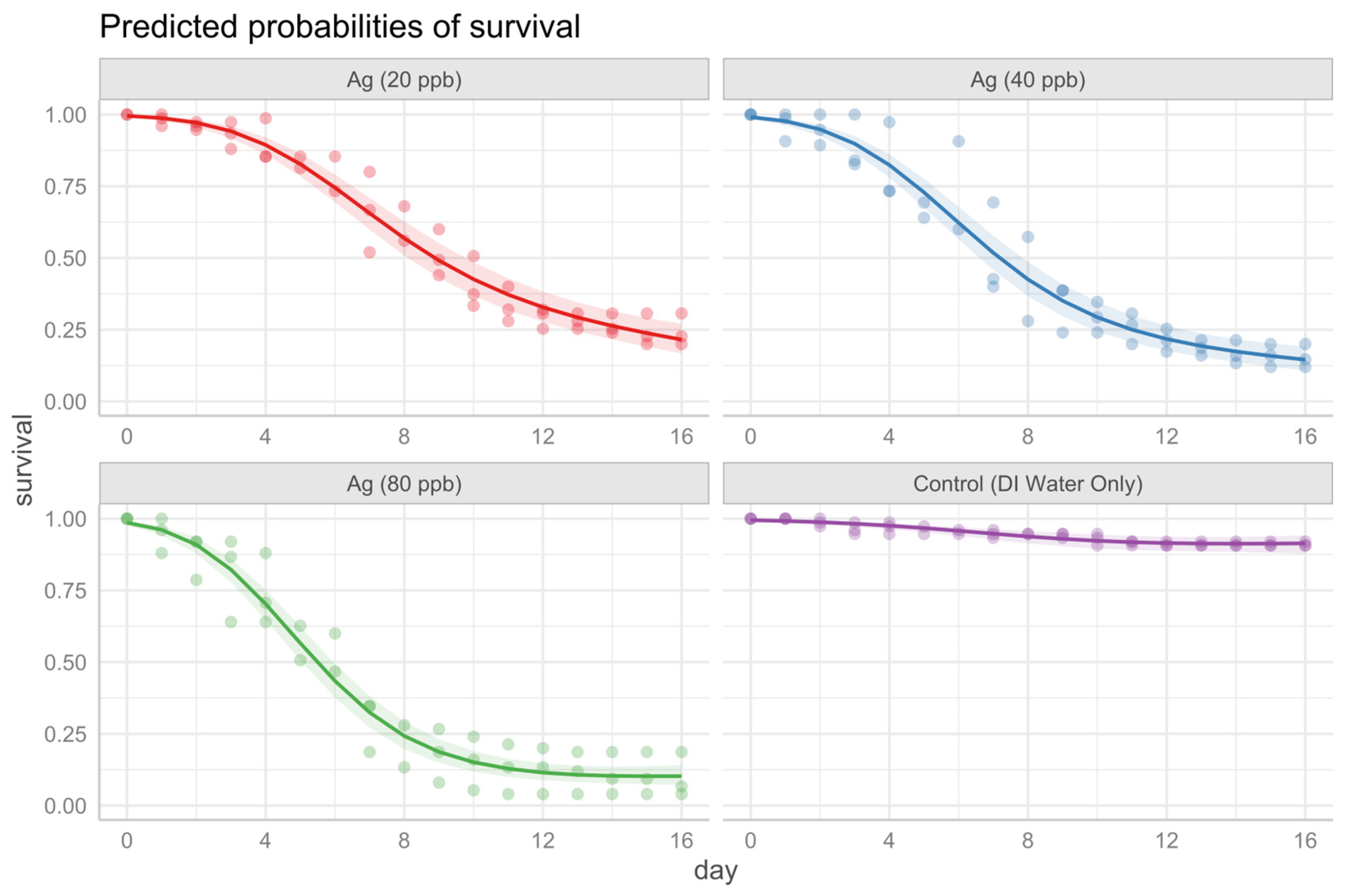
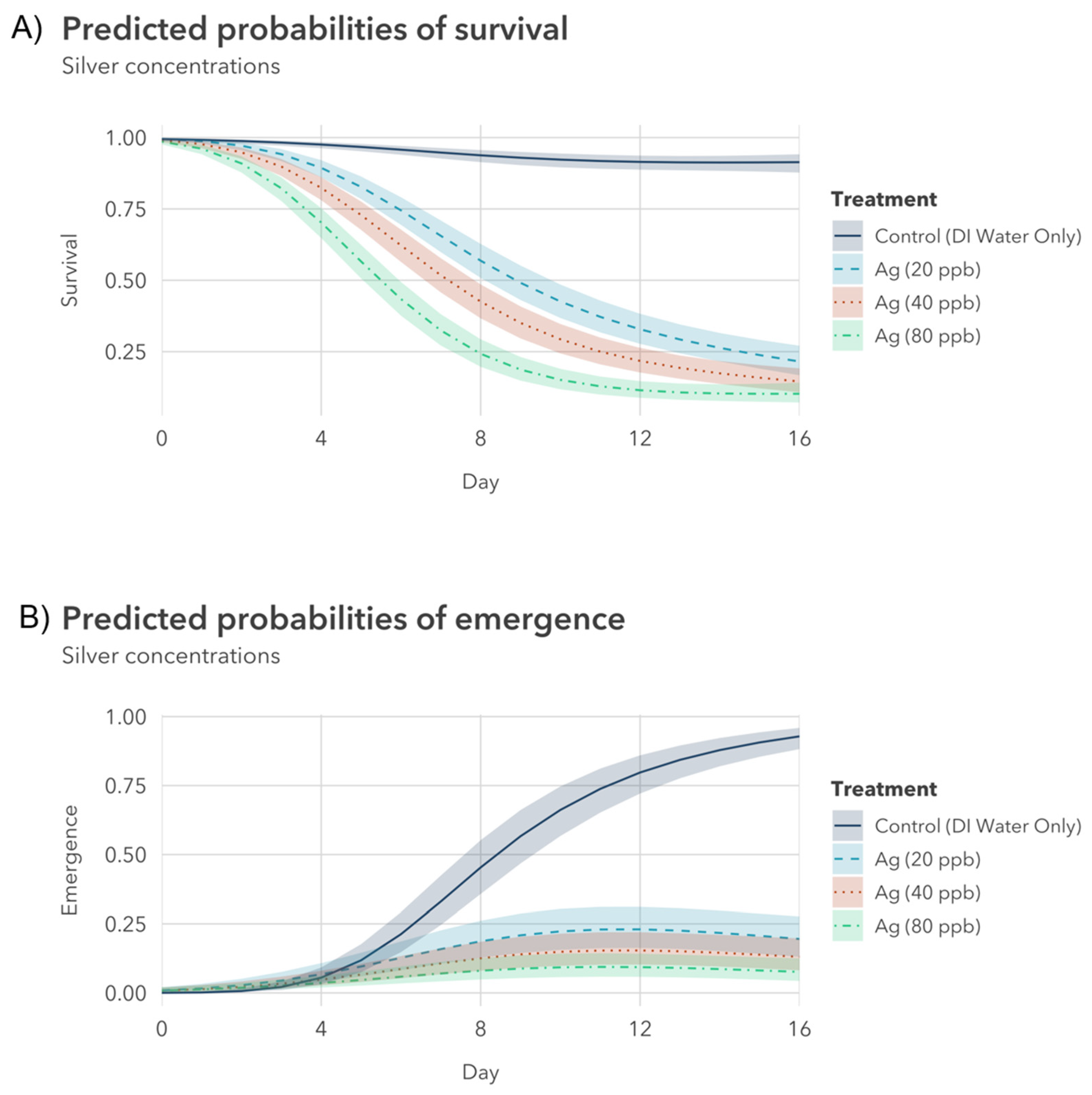
3.2.1. Silver Nitrate Exposure to Younger Instar
3.2.2. Copper Sulfate Pentahydrate Exposure to Younger Instar
3.2.3. Sodium Hypochlorite Exposure to Younger Instar
3.3. Comparing and Contextualizing the Results
- Silver nitrate treatments: The 80 μg/L Ag treatment was significantly different from the 20 μg/L treatment, but not significantly different from the 40 μg/L treatment.
- Copper sulfate pentahydrate treatments: The model found that all copper treatments were statistically significant from each other.
- Free chlorine treatments: The 500 μg/L free chlorine treatment was statistically significant from the 1000 μg/L and 2000 μg/L treatments; however, the 1000 μg/L and 2000 μg/L treatments were not statistically different from each other.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Global Vector Control Response 2017–2030; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-151297-8. [Google Scholar]
- World Health Organization (WHO). Vector-Borne Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (accessed on 3 March 2022).
- Rocklöv, J.; Dubrow, R. Climate Change: An Enduring Challenge for Vector-Borne Disease Prevention and Control. Nat. Immunol. 2020, 21, 479–483. [Google Scholar] [CrossRef]
- El-Sayed, A.; Kamel, M. Climatic Changes and Their Role in Emergence and Re-Emergence of Diseases. Environ. Sci. Pollut. Res. 2020, 27, 22336–22352. [Google Scholar] [CrossRef] [PubMed]
- Githeko, A.K.; Lindsay, S.W.; Confalonieri, U.E.; Patz, J.A. Climate Change and Vector-Borne Diseases: A Regional Analysis. Bull. World Health Organ. 2000, 78, 1136–1147. [Google Scholar]
- Boisson, S.; Wohlgemuth, L.; Yajima, A.; Peralta, G.; Obiageli, N.; Matendechero, S.; Baayenda, G.; Seife, F.; Hamilton, H.; Chase, C.; et al. Building on a Decade of Progress in Water, Sanitation and Hygiene to Control, Eliminate and Eradicate Neglected Tropical Diseases. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Tidman, R.; Abela-Ridder, B.; de Castañeda, R.R. The Impact of Climate Change on Neglected Tropical Diseases: A Systematic Review. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 147–168. [Google Scholar] [CrossRef] [PubMed]
- Fazeli-Dinan, M.; Nikookar, S.H.; Azarnoosh, M.; Jafari, A.; Daneshpour, E.; Enayati, A.; Zaim, M. An Overview of Different Control Methods of Invasive Aedes Aegypti and Aedes Albopictus. J. Maz. Univ. Med. Sci. 2024, 34, 260–286. [Google Scholar]
- Facchinelli, L.; Badolo, A.; McCall, P.J. Biology and Behaviour of Aedes Aegypti in the Human Environment: Opportunities for Vector Control of Arbovirus Transmission. Viruses 2023, 15, 636. [Google Scholar] [CrossRef]
- Wilson, A.L.; Courtenay, O.; Kelly-Hope, L.A.; Scott, T.W.; Takken, W.; Torr, S.J.; Lindsay, S.W. The Importance of Vector Control for the Control and Elimination of Vector-Borne Diseases. PLoS Negl. Trop. Dis. 2020, 14, e0007831. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Dengue Guidelines, for Diagnosis, Treatment, Prevention and Control; World Health Organization: Geneva, Switzerland, 2009; ISBN 978-92-4-154787-1. [Google Scholar]
- de Almeida, J.P.; Aguiar, E.R.; Armache, J.N.; Olmo, R.P.; Marques, J.T. The Virome of Vector Mosquitoes. Curr. Opin. Virol. 2021, 49, 7–12. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control Aedes Aegypti-Factsheet for Experts. Available online: https://www.ecdc.europa.eu/en/disease-vectors/facts/mosquito-factsheets/aedes-aegypti (accessed on 6 February 2023).
- Leta, S.; Beyene, T.J.; De Clercq, E.M.; Amenu, K.; Revie, C.W.; Kraemer, M.U.G. Global Risk Mapping for Major Diseases Transmitted by Aedes Aegypti and Aedes Albopictus. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2018, 67, 25–35. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Chikungunya Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/chikungunya (accessed on 13 May 2024).
- U.S. Food and Drug Administration FDA Approves First Vaccine to Prevent Disease Caused by Chikungunya Virus. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-vaccine-prevent-disease-caused-chikungunya-virus (accessed on 13 May 2024).
- Costa, L.B.; Barreto, F.K.d.A.; Barreto, M.C.A.; dos Santos, T.H.P.; de Andrade, M.d.M.O.; Farias, L.A.B.G.; de Freitas, A.R.R.; Martinez, M.J.; Cavalcanti, L.P.d.G. Epidemiology and Economic Burden of Chikungunya: A Systematic Literature Review. Trop. Med. Infect. Dis. 2023, 8, 301. [Google Scholar] [CrossRef]
- World Health Organization Dengue and Severe Dengue. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 15 May 2024).
- Yang, X.; Quam, M.B.M.; Zhang, T.; Sang, S. Global Burden for Dengue and the Evolving Pattern in the Past 30 Years. J. Travel. Med. 2021, 28, taab146. [Google Scholar] [CrossRef]
- World Health Organization. Yellow Fever. Available online: https://www.who.int/news-room/fact-sheets/detail/yellow-fever (accessed on 15 May 2024).
- Gaythorpe, K.A.; Hamlet, A.; Jean, K.; Garkauskas Ramos, D.; Cibrelus, L.; Garske, T.; Ferguson, N. The Global Burden of Yellow Fever. eLife 2021, 10, e64670. [Google Scholar] [CrossRef]
- United States Food and Drug Administration (FDA) Yellow Fever Vaccine: YF-Vax. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/yf-vax (accessed on 15 May 2024).
- Centers for Disease Control and Prevention (CDC). What We Know About Zika. Available online: https://www.cdc.gov/zika/about/index.html (accessed on 15 May 2024).
- Guo, Z.; Jing, W.; Liu, J.; Liu, M. The Global Trends and Regional Differences in Incidence of Zika Virus Infection and Implications for Zika Virus Infection Prevention. PLoS Negl. Trop. Dis. 2022, 16, e0010812. [Google Scholar] [CrossRef]
- World Health Organization. Countries and Territories with Current or Previous Zika Virus Transmission (Data as of Feb 2022). Available online: https://www.who.int/publications/m/item/countries-and-territories-with-current-or-previous-zika-virus-transmission (accessed on 13 May 2024).
- McGregor, B.L.; Connelly, C.R. A Review of the Control of Aedes Aegypti (Diptera: Culicidae) in the Continental United States. J. Med. Entomol. 2021, 58, 10–25. [Google Scholar] [CrossRef]
- Roiz, D.; Wilson, A.L.; Scott, T.W.; Fonseca, D.M.; Jourdain, F.; Müller, P.; Velayudhan, R.; Corbel, V. Integrated Aedes Management for the Control of Aedes-Borne Diseases. PLoS Negl. Trop. Dis. 2018, 12, e0006845. [Google Scholar] [CrossRef]
- Smith, D.L.; Perkins, T.A.; Reiner, R.C.; Barker, C.M.; Niu, T.; Chaves, L.F.; Ellis, A.M.; George, D.B.; Le Menach, A.; Pulliam, J.R.C.; et al. Recasting the Theory of Mosquito-Borne Pathogen Transmission Dynamics and Control. Trans. R. Soc. Trop. Med. Hyg. 2014, 108, 185–197. [Google Scholar] [CrossRef]
- Jansen, C.C.; Beebe, N.W. The Dengue Vector Aedes Aegypti: What Comes Next. Microbes Infect. 2010, 12, 272–279. [Google Scholar] [CrossRef]
- Carvalho, F.D.; Moreira, L.A. Why Is Aedes Aegypti Linnaeus so Successful as a Species? Neotrop. Entomol. 2017, 46, 243–255. [Google Scholar] [CrossRef]
- Pruszynski, C.A.; Stenn, T.; Acevedo, C.; Leal, A.L.; Burkett-Cadena, N.D. Human Blood Feeding by Aedes Aegypti (Diptera: Culicidae) in the Florida Keys and a Review of the Literature. J. Med. Entomol. 2020, 57, 1640–1647. [Google Scholar] [CrossRef]
- Christophers, S.R. Aedes Aegypti: The Yellow Fever Mosquito: Its Life History, Bionomics and Structure; Cambridge University Press: Cambridge, MA, USA, 1960; ISBN 978-0-521-04638-1. [Google Scholar]
- Faull, K.J.; Williams, C.R. Intraspecific Variation in Desiccation Survival Time of Aedes Aegypti (L.) Mosquito Eggs of Australian Origin. J. Vector Ecol. 2015, 40, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.P.; Goulart, M.O.F.; Rolim Neto, M.L. Meta-Analysis of Studies on Chemical, Physical and Biological Agents in the Control of Aedes Aegypti. BMC Public Health 2015, 15, 858. [Google Scholar] [CrossRef] [PubMed]
- Erlanger, T.E.; Keiser, J.; Utzinger, J. Effect of Dengue Vector Control Interventions on Entomological Parameters in Developing Countries: A Systematic Review and Meta-Analysis. Med. Vet. Entomol. 2008, 22, 203–221. [Google Scholar] [CrossRef]
- Barrera, R.; Harris, A.; Hemme, R.R.; Felix, G.; Nazario, N.; Muñoz-Jordan, J.L.; Rodriguez, D.; Miranda, J.; Soto, E.; Martinez, S.; et al. Citywide Control of Aedes Aegypti (Diptera: Culicidae) during the 2016 Zika Epidemic by Integrating Community Awareness, Education, Source Reduction, Larvicides, and Mass Mosquito Trapping. J. Med. Entomol. 2019, 56, 1033–1046. [Google Scholar] [CrossRef]
- van den Berg, H.; da Silva Bezerra, H.S.; Al-Eryani, S.; Chanda, E.; Nagpal, B.N.; Knox, T.B.; Velayudhan, R.; Yadav, R.S. Recent Trends in Global Insecticide Use for Disease Vector Control and Potential Implications for Resistance Management. Sci. Rep. 2021, 11, 23867. [Google Scholar] [CrossRef]
- Metcalf, R.L. Insect Resistance to Insecticides. Pestic. Sci. 1989, 26, 333–358. [Google Scholar] [CrossRef]
- Dusfour, I.; Vontas, J.; David, J.-P.; Weetman, D.; Fonseca, D.M.; Corbel, V.; Raghavendra, K.; Coulibaly, M.B.; Martins, A.J.; Kasai, S.; et al. Management of Insecticide Resistance in the Major Aedes Vectors of Arboviruses: Advances and Challenges. PLoS Negl. Trop. Dis. 2019, 13, e0007615. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Beins, K.; Navarro Costa, D.; Coelho, G.E.; Bezerra, H.S.d.S. Patterns of Insecticide Resistance in Aedes Aegypti: Meta-Analyses of Surveys in Latin America and the Caribbean. Pest Manag. Sci. 2020, 76, 2144–2157. [Google Scholar] [CrossRef]
- Bharati, M.; Saha, D. Insecticide Resistance Status and Biochemical Mechanisms Involved in Aedes Mosquitoes: A Scoping Review. Asian Pac. J. Trop. Med. 2021, 14, 52. [Google Scholar] [CrossRef]
- Antonio, G.E.; Sánchez, D.; Williams, T.; Marina, C.F. Paradoxical Effects of Sublethal Exposure to the Naturally Derived Insecticide Spinosad in the Dengue Vector Mosquito, Aedes Aegypti. Pest Manag. Sci. 2009, 65, 323–326. [Google Scholar] [CrossRef]
- Sulaiman, S.; Fadhlina, K.; Hidayatulfathi, O. Evaluation of Pyrethrin Formulations on Dengue/Dengue Haemorrhagic Fever Vectors in the Laboratory and Sublethal Effects. J. Arthropod-Borne Dis. 2007, 1, 1–6. [Google Scholar]
- Shaalan, E.A.-S.; Canyon, D.V.; Younes, M.W.F.; Abdel-Wahab, H.; Mansour, A.-H. Effects of Sub Lethal Concentrations of Synthetic Insecticides and Callitris Glauscophylla Extracts on the Development of Aedes Aegypti. J. Vector Ecol. 2005, 30, 295–298. [Google Scholar] [PubMed]
- Silva, I.M.; Martins, G.F.; Melo, C.R.; Santana, A.S.; Faro, R.R.; Blank, A.F.; Alves, P.B.; Picanço, M.C.; Cristaldo, P.F.; Araújo, A.P.A.; et al. Alternative Control of Aedes Aegypti Resistant to Pyrethroids: Lethal and Sublethal Effects of Monoterpene Bioinsecticides. Pest Manag. Sci. 2018, 74, 1001–1012. [Google Scholar] [CrossRef]
- Salokhe, S.G.; Mukherjee, S.N.; Deshpande, S.G.; Ghule, V.P.; Mathad, J.R. Effect of Sub-Lethal Concentrations of Insect Growth Regulator, Lufenuron on Larval Growth and Development of Aedes Aegypti. Curr. Sci. 2010, 99, 1256–1259. [Google Scholar]
- da Silva, J.J.; Mendes, J.; Lomônaco, C. Effects of Sublethal Concentrations of Diflubenzuron and Methoprene on Aedes Aegypti (Diptera: Culicidae) Fitness. Int. J. Trop. Insect Sci. 2009, 29, 17–23. [Google Scholar] [CrossRef]
- Aldridge, R.L.; Alto, B.W.; Connelly, C.R.; Okech, B.; Siegfried, B.; Linthicum, K.J. Lethal and Sublethal Concentrations of Formulated Larvicides Against Susceptible Aedes Aegypti. J. Am. Mosq. Control Assoc. 2022, 38, 250–260. [Google Scholar] [CrossRef]
- Belinato, T.A.; Valle, D. The Impact of Selection with Diflubenzuron, a Chitin Synthesis Inhibitor, on the Fitness of Two Brazilian Aedes Aegypti Field Populations. PLoS ONE 2015, 10, e0130719. [Google Scholar] [CrossRef]
- Liu, W.; Todd, R.G.; Gerberg, E.J. Effect of Three Pyrethroids on Blood Feeding and Fecundity of Aedes Aegypti. J. Am. Mosq. Control Assoc. 1986, 2, 310–313. [Google Scholar]
- Agyemang-Badu, S.Y.; Awuah, E.; Oduro-Kwarteng, S.; Dzamesi, J.Y.W.; Dom, N.C.; Kanno, G.G. Environmental Management and Sanitation as a Malaria Vector Control Strategy: A Qualitative Cross-Sectional Study Among Stakeholders, Sunyani Municipality, Ghana. Environ. Health Insights 2023, 17, 11786302221146890. [Google Scholar] [CrossRef]
- Keiser, J.; Singer, B.H.; Utzinger, J. Reducing the Burden of Malaria in Different Eco-Epidemiological Settings with Environmental Management: A Systematic Review. Lancet Infect. Dis. 2005, 5, 695–708. [Google Scholar] [CrossRef]
- United Nations Children’s Fund (UNICEF). 2.1 Billion People Lack Safe Drinking Water at Home, More than Twice as Many Lack Safe Sanitation. Available online: https://www.who.int/news/item/12-07-2017-2-1-billion-people-lack-safe-drinking-water-at-home-more-than-twice-as-many-lack-safe-sanitation (accessed on 2 May 2022).
- United Nations-Water Summary Progress Update 2021: SDG 6—Water and Sanitation for All. Available online: https://www.unwater.org/publications/summary-progress-update-2021-sdg-6-water-and-sanitation-all (accessed on 10 May 2024).
- Deshpande, A.; Miller-Petrie, M.K.; Lindstedt, P.A.; Baumann, M.M.; Johnson, K.B.; Blacker, B.F.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; Abdollahpour, I.; et al. Mapping Geographical Inequalities in Access to Drinking Water and Sanitation Facilities in Low-Income and Middle-Income Countries, 2000–2017. Lancet Glob. Health 2020, 8, e1162–e1185. [Google Scholar] [CrossRef]
- Venkataramanan, V.; Collins, S.M.; Clark, K.A.; Yeam, J.; Nowakowski, V.G.; Young, S.L. Coping Strategies for Individual and Household-Level Water Insecurity: A Systematic Review. WIREs Water 2020, 7, e1477. [Google Scholar] [CrossRef]
- Barrera, R.; Avila, J.; González-Téllez, S. Unreliable Supply of Potable Water and Elevated Aedes Aegypti Larval Indices: A Causal Relationship? J. Am. Mosq. Control Assoc. 1993, 9, 189–195. [Google Scholar] [PubMed]
- Pinchoff, J.; Silva, M.; Spielman, K.; Hutchinson, P. Use of Effective Lids Reduces Presence of Mosquito Larvae in Household Water Storage Containers in Urban and Peri-Urban Zika Risk Areas of Guatemala, Honduras, and El Salvador. Parasites Vectors 2021, 14, 167. [Google Scholar] [CrossRef]
- Akanda, A.S.; Johnson, K.; Ginsberg, H.S.; Couret, J. Prioritizing Water Security in the Management of Vector-Borne Diseases: Lessons From Oaxaca, Mexico. GeoHealth 2020, 4, e2019GH000201. [Google Scholar] [CrossRef]
- Vannavong, N.; Seidu, R.; Stenström, T.-A.; Dada, N.; Overgaard, H.J. Effects of Socio-Demographic Characteristics and Household Water Management on Aedes Aegypti Production in Suburban and Rural Villages in Laos and Thailand. Parasites Vectors 2017, 10, 170. [Google Scholar] [CrossRef]
- Quintero, J.; Brochero, H.; Manrique-Saide, P.; Barrera-Pérez, M.; Basso, C.; Romero, S.; Caprara, A.; De Lima Cunha, J.C.; Beltrán-Ayala, E.; Mitchell-Foster, K.; et al. Ecological, Biological and Social Dimensions of Dengue Vector Breeding in Five Urban Settings of Latin America: A Multi-Country Study. BMC Infect. Dis. 2014, 14, 38. [Google Scholar] [CrossRef]
- Overgaard, H.J.; Alexander, N.; Mátiz, M.I.; Jaramillo, J.F.; Olano, V.A.; Vargas, S.; Sarmiento, D.; Lenhart, A.; Seidu, R.; Stenström, T.A. Diarrhea and Dengue Control in Rural Primary Schools in Colombia: Study Protocol for a Randomized Controlled Trial. Trials 2012, 13, 182. [Google Scholar] [CrossRef]
- George, L.; Lenhart, A.; Toledo, J.; Lazaro, A.; Han, W.W.; Velayudhan, R.; Ranzinger, S.R.; Horstick, O. Community-Effectiveness of Temephos for Dengue Vector Control: A Systematic Literature Review. PLoS Negl. Trop. Dis. 2015, 9, e0004006. [Google Scholar] [CrossRef]
- Garelli, F.M.; Espinosa, M.O.; Weinberg, D.; Trinelli, M.A.; Gürtler, R.E. Water Use Practices Limit the Effectiveness of a Temephos-Based Aedes Aegypti Larval Control Program in Northern Argentina. PLoS Negl. Trop. Dis. 2011, 5, e991. [Google Scholar] [CrossRef][Green Version]
- Tawatsin, A.; Thavara, U.; Chompoosri, J.; Bhakdeenuan, P.; Asavadachanukorn, P. Larvicidal Efficacy of New Formulations of Temephos in Non-Woven Sachets against Larvae of Aedes Aegypti (L.) (Diptera: Culicidae) in Water-Storage Containers. Southeast Asian J. Trop. Med. Public Health 2007, 38, 641–645. [Google Scholar] [PubMed]
- Chen, C.D.; Nazni, W.A.; Lee, H.L.; Sofian-Azirun, M. Weekly Variation on Susceptibility Status of Aedes Mosquitoes against Temephos in Selangor, Malaysia. Trop. Biomed. 2005, 22, 195–206. [Google Scholar] [PubMed]
- Pinheiro, V.C.S.; Tadei, W.P. Evaluation of the Residual Effect of Temephos on Aedes Aegypti (Diptera, Culicidae) Larvae in Artificial Containers in Manaus, Amazonas State, Brazil. Cad. Saude Publica 2002, 18, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Laws, E.R.; Sedlak, V.A.; Miles, J.W.; Joseph, C.R.; Lacomba, J.R.; Diaz Rivera, A. Field Study of the Safety of Abate for Treating Potable Water and Observations on the Effectiveness of a Control Programme Involving Both Abate and Malathion. Bull. World Health Organ. 1968, 38, 439–445. [Google Scholar]
- Marcombe, S.; Chonephetsarath, S.; Thammavong, P.; Brey, P.T. Alternative Insecticides for Larval Control of the Dengue Vector Aedes Aegypti in Lao PDR: Insecticide Resistance and Semi-Field Trial Study. Parasites Vectors 2018, 11, 616. [Google Scholar] [CrossRef]
- Bellinato, D.F.; Viana-Medeiros, P.F.; Araújo, S.C.; Martins, A.J.; Lima, J.B.P.; Valle, D. Resistance Status to the Insecticides Temephos, Deltamethrin, and Diflubenzuron in Brazilian Aedes Aegypti Populations. BioMed Res. Int. 2016, 2016, 8603263. [Google Scholar] [CrossRef]
- Lau, K.W.; Chen, C.D.; Lee, H.L.; Norma-Rashid, Y.; Sofian-Azirun, M. Evaluation of Insect Growth Regulators Against Field-Collected Aedes Aegypti and Aedes Albopictus (Diptera: Culicidae) from Malaysia. J. Med. Entomol. 2015, 52, 199–206. [Google Scholar] [CrossRef]
- Neto, L.; Pamplona, L.; José Soares Pontes, R.; Oliveira Lima, J.W. Influence of Water Replacement on Diflubenzuron Duration Effect in the Control of Aedes Aegypti in Simulated Field Conditions, in Northeastern Brazil. J. Health Biol. Sci. 2013, 1, 21–26. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Diflubenzuron in Drinking-Water: Use for Vector Control in Drinking-Water Sources and Containers Background Document for Development of WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Thavara, U.; Tawatsin, A.; Chansang, C.; Asavadachanukorn, P.; Zaim, M.; Mulla, M.S. Simulated Field Evaluation of the Efficacy of Two Formulations of Diflubenzuron, a Chitin Synthesis Inhibitor against Larvae of Aedes Aegypti (L.) (Diptera: Culicidae) in Water-Storage Containers. Southeast Asian J. Trop. Med. Public Health 2007, 38, 269–275. [Google Scholar]
- Juarez, J.G.; Garcia-Luna, S.M.; Roundy, C.M.; Branca, A.; Banfield, M.G.; Hamer, G.L. Susceptibility of South Texas Aedes Aegypti to Pyriproxyfen. Insects 2021, 12, 460. [Google Scholar] [CrossRef]
- Hustedt, J.C.; Boyce, R.; Bradley, J.; Hii, J.; Alexander, N. Use of Pyriproxyfen in Control of Aedes Mosquitoes: A Systematic Review. PLoS Negl. Trop. Dis. 2020, 14, e0008205. [Google Scholar] [CrossRef] [PubMed]
- Oo, S.Z.M.; Thaung, S.; Maung, Y.N.M.; Aye, K.M.; Aung, Z.Z.; Thu, H.M.; Thant, K.Z.; Minakawa, N. Effectiveness of a Novel Long-Lasting Pyriproxyfen Larvicide (SumiLarv®2MR) against Aedes Mosquitoes in Schools in Yangon, Myanmar. Parasites Vectors 2018, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Maoz, D.; Ward, T.; Samuel, M.; Müller, P.; Runge-Ranzinger, S.; Toledo, J.; Boyce, R.; Velayudhan, R.; Horstick, O. Community Effectiveness of Pyriproxyfen as a Dengue Vector Control Method: A Systematic Review. PLoS Negl. Trop. Dis. 2017, 11, e0005651. [Google Scholar] [CrossRef] [PubMed]
- Seng, C.M.; Setha, T.; Nealon, J.; Socheat, D.; Nathan, M.B. Six Months of Aedes Aegypti Control with a Novel Controlled-Release Formulation of Pyriproxyfen in Domestic Water Storage Containers in Cambodia. Southeast Asian J. Trop. Med. Public Health 2008, 39, 822–826. [Google Scholar]
- Sihuincha, M.; Zamora-Perea, E.; Orellana-Rios, W.; Stancil, J.D.; López-Sifuentes, V.; Vidal-Oré, C.; Devine, G.J. Potential Use of Pyriproxyfen for Control of Aedes Aegypti (Diptera: Culicidae) in Iquitos, Perú. J. Med. Entomol. 2005, 42, 620–630. [Google Scholar] [CrossRef]
- Ritchie, S.A.; Rapley, L.P.; Benjamin, S. Bacillus Thuringiensis Var. Israelensis (Bti) Provides Residual Control of Aedes Aegypti in Small Containers. Am. J. Trop. Med. Hyg. 2010, 82, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Setha, T.; Chantha, N.; Socheat, D. Efficacy of Bacillus Thuringiensis Israelensis, VectoBac WG and DT, Formulations against Dengue Mosquito Vectors in Cement Potable Water Jars in Cambodia. Southeast Asian J. Trop. Med. Public Health 2007, 38, 261–268. [Google Scholar]
- Mulla, M.S.; Thavara, U.; Tawatsin, A.; Chompoosri, J. Procedures for the Evaluation of Field Efficacy of Slow-Release Formulations of Larvicides against Aedes Aegypti in Water-Storage Containers. J. Am. Mosq. Control Assoc. 2004, 20, 64–73. [Google Scholar]
- Marcombe, S.; Darriet, F.; Agnew, P.; Etienne, M.; Yp-Tcha, M.-M.; Yébakima, A.; Corbel, V. Field Efficacy of New Larvicide Products for Control of Multi-Resistant Aedes Aegypti Populations in Martinique (French West Indies). Am. J. Trop. Med. Hyg. 2011, 84, 118–126. [Google Scholar] [CrossRef]
- World Health Organization. Spinosad DT in Drinking-Water: Use for Vector Control in Drinking-Water Sources and Containers Background Document for Development of WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Njoroge, T.M.; Berenbaum, M.R. Laboratory Evaluation of Larvicidal and Oviposition Deterrent Properties of Edible Plant Oils for Potential Management of Aedes Aegypti (Diptera: Culicidae) in Drinking Water Containers. J. Med. Entomol. 2019, 56, 1055–1063. [Google Scholar] [CrossRef]
- Amer, A.; Mehlhorn, H. Larvicidal Effects of Various Essential Oils against Aedes, Anopheles, and Culex Larvae (Diptera, Culicidae). Parasitol. Res. 2006, 99, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Vontas, J.; Kioulos, E.; Pavlidi, N.; Morou, E.; della Torre, A.; Ranson, H. Insecticide Resistance in the Major Dengue Vectors Aedes Albopictus and Aedes Aegypti. Pestic. Biochem. Physiol. 2012, 104, 126–131. [Google Scholar] [CrossRef]
- Saeung, M.; Ngoen-Klan, R.; Thanispong, K.; Muenworn, V.; Bangs, M.J.; Chareonviriyaphap, T. Susceptibility of Aedes Aegypti and Aedes Albopictus (Diptera: Culicidae) to Temephos in Thailand and Surrounding Countries. J. Med. Entomol. 2020, 57, 1207–1220. [Google Scholar] [CrossRef]
- Valle, D.; Bellinato, D.F.; Viana-Medeiros, P.F.; Lima, J.B.P.; Martins, A.d.J. Resistance to Temephos and Deltamethrin in Aedes Aegypti from Brazil between 1985 and 2017. Mem. Inst. Oswaldo Cruz 2019, 114, e180544. [Google Scholar] [CrossRef]
- Khan, H.A.A.; Akram, W. Resistance Status to Deltamethrin, Permethrin, and Temephos Along With Preliminary Resistance Mechanism in Aedes Aegypti (Diptera: Culicidae) From Punjab, Pakistan. J. Med. Entomol. 2019, 56, 1304–1311. [Google Scholar] [CrossRef]
- Morales, D.; Ponce, P.; Cevallos, V.; Espinosa, P.; Vaca, D.; Quezada, W. Resistance Status of Aedes Aegypti to Deltamethrin, Malathion, and Temephos in Ecuador. J. Am. Mosq. Control Assoc. 2019, 35, 113–122. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (EPA) National Primary Drinking Water Regulations. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations (accessed on 5 January 2025).
- World Health Organization (WHO). Alternative Drinking-Water Disinfectants: Bromine, Iodine and Silver; World Health Organization (WHO): Geneva, Switzerland, 2018; ISBN 978-92-4-151369-2. [Google Scholar]
- World Health Organization (WHO). Copper in Drinking-Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality. Available online: https://cdn.who.int/media/docs/default-source/wash-documents/wash-chemicals/copper.pdf?sfvrsn=194c0f12_4s://www.who.int/teams/environment-climate-change-and-health/water-sanitation-and-health/chemical-hazards-in-drinking-water/copper (accessed on 12 April 2023).
- World Health Organization (WHO). Silver in Drinking-Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality. Available online: https://cdn.who.int/media/docs/default-source/wash-documents/wash-chemicals/silver.pdf?sfvrsn=195cf8b3_4 (accessed on 12 April 2023).
- Estrella-You, A.; Harris, J.; Singh, R.; Smith, J. Inactivation of Waterborne Pathogens by Copper and Silver Ions, Free Chlorine, and N-Chloramines in Point-of-Use Technology: A Review. In Water Purification: Processes, Applications and Health Effects; LeBlanc, P., Ed.; Water Purification; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2022; pp. 1–88. ISBN 978-1-68507-622-1. [Google Scholar]
- Arnold, B.F.; Colford, J.M. Treating Water with Chlorine at Point-of-Use to Improve Water Quality and Reduce Child Diarrhea in Developing Countries: A Systematic Review and Meta-Analysis. Am. J. Trop. Med. Hyg. 2007, 76, 354–364. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Benigna, I.; Sorlini, S.; Torretta, V. Overview of the Main Disinfection Processes for Wastewater and Drinking Water Treatment Plants. Sustainability 2018, 10, 86. [Google Scholar] [CrossRef]
- Crider, Y.; Sultana, S.; Unicomb, L.; Davis, J.; Luby, S.P.; Pickering, A.J. Can You Taste It? Taste Detection and Acceptability Thresholds for Chlorine Residual in Drinking Water in Dhaka, Bangladesh. Sci. Total Environ. 2018, 613–614, 840–846. [Google Scholar] [CrossRef]
- Albert, J.; Luoto, J.; Levine, D. End-User Preferences for and Performance of Competing POU Water Treatment Technologies among the Rural Poor of Kenya. Environ. Sci. Technol. 2010, 44, 4426–4432. [Google Scholar] [CrossRef]
- Kim, J.; Chung, Y.; Shin, D.; Kim, M.; Lee, Y.; Lim, Y.; Lee, D. Chlorination By-Products in Surface Water Treatment Process. Desalination 2003, 151, 1–9. [Google Scholar] [CrossRef]
- Thurman, R.B.; Gerba, C.P.; Bitton, G. The Molecular Mechanisms of Copper and Silver Ion Disinfection of Bacteria and Viruses. Crit. Rev. Environ. Control 1989, 18, 295–315. [Google Scholar] [CrossRef]
- Ngwenya, N.; Ncube, E.J.; Parsons, J. Recent Advances in Drinking Water Disinfection: Successes and Challenges. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer: New York, NY, USA, 2013; pp. 111–170. ISBN 978-1-4614-4717-7. [Google Scholar]
- Lucier, K.J.; Dickson-Anderson, S.E.; Schuster-Wallace, C.J. Effectiveness of Silver and Copper Infused Ceramic Drinking Water Filters in Reducing Microbiological Contaminants. J. Water Supply Res. Technol.-AQUA 2017, 66, 528–536. [Google Scholar] [CrossRef]
- Pooi, C.K.; Ng, H.Y. Review of Low-Cost Point-of-Use Water Treatment Systems for Developing Communities. Npj Clean Water 2018, 1, 1–8. [Google Scholar] [CrossRef]
- Singh, R.; Edokpayi, J.N.; Odiyo, J.O.; Smith, J.A. E. Coli Inactivation by Metals and Effects of Changes in Water Chemistry. J. Environ. Eng. 2019, 145, 04018136. [Google Scholar] [CrossRef]
- Ehdaie, B.; Su, Y.-H.; Swami, N.S.; Smith, J.A. Protozoa and Virus Disinfection by Silver- and Copper-Embedded Ceramic Tablets for Water Purification. J. Environ. Eng. 2020, 146, 04020015. [Google Scholar] [CrossRef]
- Ndebele, N.; Edokpayi, J.N.; Odiyo, J.O.; Smith, J.A. Field Investigation and Economic Benefit of a Novel Method of Silver Application to Ceramic Water Filters for Point-Of-Use Water Treatment in Low-Income Settings. Water 2021, 13, 285. [Google Scholar] [CrossRef]
- Estrella-You, A.; Smith, J.A. Synergistic Bacterial Inactivation by Silver Ions and Free Chlorine in Natural Waters. J. Environ. Eng. 2022, 148, 04022072. [Google Scholar] [CrossRef]
- Hill, C.L.; Harris, J.D.; Turner, S.S.; Wason, K.L.; Gaylord, A.P.; Hatley, M.G.; Hardcastle, L.T.; Roberts, I.T.; You, J.Y.; Renneker, K.O.; et al. Field and Laboratory Assessment of a New Electrolytic Point-of-Use Water Treatment Technology. Water 2022, 14, 1077. [Google Scholar] [CrossRef]
- Alherek, M.; Basu, O.D. Impact of Low Levels of Silver, Zinc and Copper Nanoparticles on Bacterial Removal and Potential Synergy in Water Treatment Applications. J. Chem. Technol. Biotechnol. 2023, 98, 1137–1146. [Google Scholar] [CrossRef]
- Harris, J.D.; Homola, L.M.J.; Estrella-You, A.; Smith, J.A. Enhancing Microbial Disinfection in Household Water Treatment by Combining a Silver–Ceramic Tablet with Copper and Chlorine Technologies. J. Environ. Eng. 2024, 150, 04024026. [Google Scholar] [CrossRef]
- Silvestry-Rodriguez, N.; Sicairos-Ruelas, E.E.; Gerba, C.P.; Bright, K.R. Silver as a Disinfectant. In Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 2007; pp. 23–45. ISBN 978-0-387-69163-3. [Google Scholar]
- Ehdaie, B.; Rento, C.T.; Son, V.; Turner, S.S.; Samie, A.; Dillingham, R.A.; Smith, J.A. Evaluation of a Silver-Embedded Ceramic Tablet as a Primary and Secondary Point-of-Use Water Purification Technology in Limpopo Province, S. Africa. PLoS ONE 2017, 12, e0169502. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.M.; Sobsey, M.D.; Casanova, L.M. Disinfection of Escherichia Coli and Pseudomonas Aeruginosa by Copper in Water. J. Water Health 2016, 14, 424–432. [Google Scholar] [CrossRef] [PubMed][Green Version]
- World Health Organization. Guidelines for Drinking-Water Quality, 4th Edition, Incorporating the 1st Addendum; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-154995-0. [Google Scholar]
- World Health Organization. Guidelines for Laboratory and Field Testing of Mosquito Larvicides; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Ngonzi, A.J.; Muyaga, L.L.; Ngowo, H.; Urio, N.; Vianney, J.-M.; Lwetoijera, D.W. Susceptibility Status of Major Malaria Vectors to Novaluron, an Insect Growth Regulator South-Eastern Tanzania. Pan Afr. Med. J. 2022, 41, 273. [Google Scholar] [CrossRef]
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Bliss, C.I. The Method of Probits. Science 1934, 79, 38–39. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis; Cambridge University Press: Cambridge, MA, USA; References-Scientific Research Publishing: Wuhan, China, 1971; 333p, Available online: https://www.scirp.org/(S(i43dyn45teexjx455qlt3d2q))/reference/ReferencesPapers.aspx?ReferenceID=1493267 (accessed on 4 April 2023).
- Postelnicu, T. Probit Analysis. In International Encyclopedia of Statistical Science; Lovric, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1128–1131. ISBN 978-3-642-04898-2. [Google Scholar]
- Ong, S.-Q.; Jaal, Z. Larval Age and Nutrition Affect the Susceptibility of Culex Quinquefasciatus (Diptera: Culicidae) to Temephos. J. Insect Sci. 2018, 18, 38. [Google Scholar] [CrossRef]
- Nartey, R.; Owusu-Dabo, E.; Kruppa, T.; Baffour-Awuah, S.; Annan, A.; Oppong, S.; Becker, N.; Obiri-Danso, K. Use of Bacillus Thuringiensis Var Israelensis as a Viable Option in an Integrated Malaria Vector Control Programme in the Kumasi Metropolis, Ghana. Parasites Vectors 2013, 6, 116. [Google Scholar] [CrossRef]
- Patil, C.D.; Borase, H.P.; Patil, S.V.; Salunkhe, R.B.; Salunke, B.K. Larvicidal Activity of Silver Nanoparticles Synthesized Using Pergularia Daemia Plant Latex against Aedes Aegypti and Anopheles Stephensi and Nontarget Fish Poecillia Reticulata. Parasitol. Res. 2012, 111, 555–562. [Google Scholar] [CrossRef]
- Perez, M.H.; Noriega, F.G. Aedes Aegypti Pharate 1st Instar Quiescence Affects Larval Fitness and Metal Tolerance. J. Insect Physiol. 2012, 58, 824–829. [Google Scholar] [CrossRef]
- Miah, M.A. Effect of Copper Sulphate Pentahydrate on Mosquito Larval Aedes Aegypti, Culex Quinquefasciatus, and Anopheles Quadrimaculatus in Laboratory and under Semi-Field Conditions. J. Fla. Mosq. Control. Assoc. 2021, 68, 79–85. [Google Scholar] [CrossRef]
- Ratte, H.T. Bioaccumulation and Toxicity of Silver Compounds: A Review. Environ. Toxicol. Chem. 1999, 18, 89–108. [Google Scholar] [CrossRef]
- Shanmugasundaram, T.; Balagurunathan, R. Mosquito Larvicidal Activity of Silver Nanoparticles Synthesised Using Actinobacterium, Streptomyces Sp. M25 against Anopheles Subpictus, Culex Quinquefasciatus and Aedes Aegypti. J. Parasites Dis. Off. Organ Indian Soc. Parasitol. 2015, 39, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Velayutham, K.; Ramanibai, R.; Umadevi, M. Green Synthesis of Silver Nanoparticles Using Manihot Esculenta Leaves against Aedes Aegypti and Culex Quinquefasciatus. J. Basic Appl. Zool. 2016, 74, 37–40. [Google Scholar] [CrossRef][Green Version]
- Rayms-Keller, A.; Olson, K.E.; McGaw, M.; Oray, C.; Carlson, J.O.; Beaty, B.J. Effect of Heavy Metals onAedes Aegypti (Diptera:Culicidae) Larvae. Ecotoxicol. Environ. Saf. 1998, 39, 41–47. [Google Scholar] [CrossRef]
- Reza, M.; Ilmiawati, C. Laboratory Testing of Very Low-Copper-Treated Water to Prolong Pupation and Emerging Time of Mosquito Larvae: An Alternative Method to Delay Mosquito Breeding Capability. PLoS ONE 2020, 15, e0226859. [Google Scholar] [CrossRef]
- Miranda, F.R.; Fernandes, K.M.; Farder-Gomes, C.F.; Bernardes, R.C.; de Oliveira, A.H.; Arthidoro de Castro, M.B.; Dourado, L.A.; Oliveira, L.L.; Martins, G.F.; Serrão, J.E. Exposure to Copper Sulfate Impairs Survival, Post-Embryonic Midgut Development and Reproduction in Aedes Aegypti. Infect. Genet. Evol. 2022, 97, 105185. [Google Scholar] [CrossRef]
- Neff, E.; Dharmarajan, G. The Direct and Indirect Effects of Copper on Vector-Borne Disease Dynamics. Environ. Pollut. 2021, 269, 116213. [Google Scholar] [CrossRef]
- Reza, M.; Yamamoto, D.S.; Matsuoka, H. Larvicidal and Ovipositional Preference Test of Copper Solution for Mosquitoes. Med. Entomol. Zool. 2014, 65, 147–150. [Google Scholar] [CrossRef]
- El-Sheikh, E.-S.M.Y.; Fouda, M.A.; Hassan, M.I.; Abd-Elghaphar, A.-E.A.; Hasaballah, A.I. Toxicological Effects of Some Heavy Metal Ions on Culex Pipiens L. (Diptera: Culicidae). Egypt. Acad. J. Biol. Sci. F Toxicol. Pest Control 2010, 2, 63–76. [Google Scholar] [CrossRef]
- Salunkhe, R.B.; Patil, S.V.; Patil, C.D.; Salunke, B.K. Larvicidal Potential of Silver Nanoparticles Synthesized Using Fungus Cochliobolus Lunatus against Aedes Aegypti (Linnaeus, 1762) and Anopheles Stephensi Liston (Diptera; Culicidae). Parasitol. Res. 2011, 109, 823–831. [Google Scholar] [CrossRef]
- Sherman, C.; Fernandez, E.A.; Chan, A.S.; Lozano, R.C.; Leontsini, E.; Winch, P.J. La Untadita: A Procedure for Maintaining Washbasins and Drums Free of Aedes Aegypti Based on Modification of Existing Practices. Am. J. Trop. Med. Hyg. 1998, 58, 257–262. [Google Scholar] [CrossRef]
- Shahen, M.; El-Wahsh, H.; Ramadan, H.; Hegazi, M.; Al-Sharkawi, I.; Seif, A. A Comparison Of The Toxicity Of Calcium And Sodium Hypochlorite Against Culex Pipiens (Diptera: Culicidae) Larvae. Environ. Sci. Curr. Res. 2020, 3, 1–9. [Google Scholar] [CrossRef]
- Barrera, R.; Amador, M.; Clark, G.G. The Use of Household Bleach to Control Aedes Aegypti. J. Am. Mosq. Control Assoc. 2004, 20, 444–448. [Google Scholar]
- Brown, M.D.; Walker, D.O.; Hendrikz, J.K.; Cabral, C.P.; Araujo, D.B.; Ribeiro, Z.M.; Kay, B.H. Chlorine Tolerance of Mesocyclops (Cyclopoida: Cyclopidae) Copepods and Three Container-Breeding Species of Mosquitoes. Environ. Entomol. 1994, 23, 1245–1249. [Google Scholar] [CrossRef]
- Muttkowski, R.A. Copper: Its Occurrence and Rôle in Insects and Other Animals. Trans. Am. Microsc. Soc. 1921, 40, 144–157. [Google Scholar] [CrossRef]









| Vector-Borne Disease | Common Symptoms | Global Burden | Location of Prevalence | Vaccine for Prevention |
|---|---|---|---|---|
| Chikungunya (CHIKV) | Fever, severe joint pain, joint swelling, muscle pain [15] | Since 2023, 5+ million cases have been reported in last 15 yrs [16] | Detected in >100 countries as of 2021, circulating mainly in Africa, Asia, South America, and regions of the Pacific Ocean [17]. | IXCHIQ (manufactured by Valenva) [16] |
| Dengue | High fever (40 °C/104 °F), severe headache, pain behind the eyes, muscle and joint pains, nausea, vomiting, swollen glands, rash [18] | In 2019, age-standardized incidence rate (ASIR) (A statistical measurement that compares the number of new cases of a disease in a population to a standard population. It’s used to compare health metrics across populations with different age distributions.) estimated to be 7.40 per 1000 [19]. An estimated 100–400 million infections occur each year [18]. | Endemic in >100 countries in WHO regions of Africa, the Americas, the Eastern Mediterranean, Southeast Asia and the Western Pacific. The Americas, Southeast Asia, and Western Pacific regions are most significantly affected [18]. | Dengvaxia® (CYD-TDV), developed by Sanofi Pasteur, Qdenga® (TAK-003), developed by Takeda [18]. |
| Yellow Fever | Fever, muscle pain, headache, loss of appetite, nausea or vomiting, jaundice, dark urine, abdominal pain [20] | In 2018, estimated 109,000 severe infections and 51,000 deaths in Africa and South America [21]. | Thirty-four countries in Africa and thirteen countries in Central and South America are either endemic for, or have regions that are endemic for, yellow fever as of 2023 [20]. | YF-VAX®, manufactured by Sanofi Pasteur [22] |
| Zika virus (ZIKV) | Fever, rash, headache, joint pain, conjunctivitis (red eye), muscle pains. Virus can be passed through sex and from a pregnant woman to her fetus [23] | In 2019, ASIR estimated to be 3.44 per 100,000 [24] | Eighty-nine countries and territories have documented evidence of current or previous transmission as of February 2022, circulating primarily in the Americas, South Asia, and the Pacific Islands [24,25]. | No [24]. |
| Disinfectant | Drinking Water Quality Guideline (DWQG) | Concentrations Tested (μg/L) | ||
|---|---|---|---|---|
| High (80–95% of DWQG) | Mid (40–50% of DWQG) | Low (20–25% of DWQG) | ||
| Silver (Ag): AgNO3 | EPA and WHO: 100 μg/L | 20 | 40 | 80 |
| Copper (Cu): CuSO4.5H2O | EPA: 1300 μg/L WHO: 2000 μg/L | 300 | 600 | 1200 |
| Chlorine (OCl−/HOCl): NaOCl | EPA: 4000 μg/L(EPA) WHO: 2000 μg/L free chlorine dose for clear water (<10 NTU) and 4000 μg/L for turbid water (≥10 NTU) for POUWT | 500 | 1000 | 2000 |
| Observed Data | ||||||||||||
| Silver Treatment | 20 μg/L Ag | 40 μg/L Ag | 80 μg/L Ag | Control | ||||||||
| Variable | Mean | St. Dev | SEM | Mean | St. Dev | SEM | Mean | St. Dev | SEM | Mean | St. Dev | SEM |
| Survival (%) | 26.85 | 6.25 | 3.61 | 17.09 | 4.58 | 2.64 | 10.75 | 8.64 | 4.99 | 91.11 | 0.77 | 0.44 |
| Emergence (%) | 25.01 | 6.44 | 3.72 | 16.69 | 5.24 | 3.02 | 10.29 | 8.94 | 5.16 | 90.67 | 1.33 | 0.77 |
| IE (%) | 72.40 | 7.16 | 4.13 | 81.56 | 5.86 | 3.39 | 88.66 | 9.87 | 5.70 | |||
| Copper Treatment | 300 μg/L Cu | 600 μg/L Cu | 1200 μg/L Cu | Control | ||||||||
| Variable | Mean | St. Dev | SEM | Mean | St. Dev | SEM | Mean | St. Dev | SEM | Mean | St. Dev | SEM |
| Survival (%) | 42.55 | 7.13 | 4.12 | 25.80 | 10.91 | 6.30 | 11.92 | 7.50 | 4.33 | 89.78 | 0.77 | 0.44 |
| Emergence (%) | 40.30 | 5.38 | 3.11 | 24.88 | 9.94 | 5.74 | 11.44 | 7.51 | 4.34 | 89.33 | 0.00 | 0.00 |
| IE (%) | 54.89 | 6.02 | 3.48 | 72.15 | 11.12 | 6.42 | 87.19 | 8.41 | 4.86 | |||
| Chlorine Treatment | 500 μg/L Free Cl | 1000 μg/L Free Cl | 2000 μg/L Free Cl | Control | ||||||||
| Variable | Mean | St. Dev | SEM | Mean | St. Dev | SEM | Mean | St. Dev | SEM | Mean | St. Dev | SEM |
| Survival (%) | 58.40 | 21.88 | 12.63 | 24.75 | 12.85 | 7.42 | 17.39 | 14.70 | 8.49 | 90.67 | 2.67 | 1.54 |
| Emergence (%) | 56.53 | 20.75 | 11.98 | 24.98 | 12.99 | 7.50 | 17.59 | 14.95 | 8.63 | 89.78 | 2.78 | 1.60 |
| IE (%) | 37.44 | 21.35 | 12.32 | 72.46 | 13.77 | 7.95 | 80.65 | 16.04 | 9.26 | |||
| Probit Regression Model | ||||
| Silver Treatment | 20 μg/L Ag | 40 μg/L Ag | 80 μg/L Ag | Control |
| Survival (%) | 23.57 [18.10, 28.57] | 15.91 [11.49, 20.01] | 11.21 [8.25, 14.29] | 91.39 [87.75, 94.15] |
| Emergence (%) | 21.04 [15.48, 29.37] | 14.10 [8.79, 20.55] | 8.20 [4.81, 11.96] | 92.84 [88.22, 95.93] |
| IE (%) | 78.96 [71.87, 85.19] | 85.90 [78.13, 90.51] | 91.80 [86.72, 94.84] | |
| Copper Treatment | 300 μg/L Cu | 600 μg/L Cu | 1200 μg/L Cu | Control |
| Survival (%) | 42.56 [34.02, 53.57] | 27.50 [19.17, 37.88] | 12.52 [7.05, 20.42] | 90.52 [84.18, 94.76] |
| Emergence (%) | 42.65 [36.46, 49.15] | 24.43 [18.71, 30.17] | 11.77 [7.96, 15.68] | 93.36 [90.36, 95.58] |
| IE (%) | 57.35 [49.48, 64.47] | 75.57 [70.91, 82.82] | 88.23 [84.47, 91.90] | |
| Chlorine Treatment | 500 μg/L Free Cl | 1000 μg/L Free Cl | 2000 μg/L Free Cl | Control |
| Survival (%) | 57.53 [8.14, 17.24] | 19.77 [14.32, 26.56] | 12.19 [7.64, 18.30] | 91.63 [86.98, 94.90] |
| Emergence (%) | 58.34 [45.76, 69.53] | 21.57 [14.00, 34.51] | 13.73 [7.55, 21.99] | 92.85 [86.58, 96.58] |
| IE (%) | 41.66 [31.12, 54.55] | 78.43 [67.94, 86.38] | 86.27 [79.95, 92.99] | |
| Silver Treatment | 20 μg/L Ag | 40 μg/L Ag | 80 μg/L Ag | Control | ||||||||
| Time (h) | Mean | St. Dev | SEM | Mean | St. Dev | SEM | Mean | St. Dev | SEM | Mean | St. Dev | SEM |
| 24 | 92.78 | 4.47 | 2.58 | 89.70 | 1.92 | 1.11 | 86.06 | 7.46 | 4.31 | 99.11 | 1.54 | 0.89 |
| 48 | 81.13 | 11.95 | 6.90 | 65.55 | 2.36 | 1.36 | 54.11 | 15.69 | 9.06 | 94.22 | 2.04 | 1.18 |
| 72 | 57.73 | 12.30 | 7.10 | 36.44 | 19.45 | 11.23 | 7.95 | 11.10 | 6.41 | 82.22 | 2.04 | 1.18 |
| Copper Treatment | 300 μg/L Cu | 600 μg/L Cu | 1200 μg/L Cu | Control | ||||||||
| Time (h) | Mean | St. Dev | SEM | Mean | St. Dev | SEM | Mean | St. Dev | SEM | Mean | St. Dev | SEM |
| 24 | 82.59 | 14.96 | 8.64 | 79.47 | 14.77 | 8.53 | 68.31 | 24.64 | 14.23 | 99.11 | 1.54 | 0.89 |
| 48 | 57.24 | 25.06 | 14.47 | 49.21 | 25. 64 | 14.80 | 32.41 | 33.08 | 19.10 | 94.22 | 2.04 | 1.18 |
| 72 | 21.85 | 23.74 | 13.71 | 17.71 | 12.43 | 7.18 | 12.81 | 15.23 | 8.79 | 82.22 | 2.04 | 1.18 |
| Chlorine Treatment | 500 μg/L Free Cl | 1000 μg/L Free Cl | 2000 μg/L Free Cl | Control | ||||||||
| Time (h) | Mean | St. Dev | SEM | Mean | St. Dev | SEM | Mean | St. Dev | SEM | Mean | St. Dev | SEM |
| 24 | 76.67 | 6.55 | 3.78 | 72.56 | 12.53 | 7.23 | 36.91 | 11.13 | 6.43 | 93.78 | 5.39 | 3.11 |
| 48 | 25.67 | 3.79 | 2.19 | 14.72 | 17.20 | 9.93 | 6.13 | 5.36 | 3.10 | 81.33 | 4.00 | 2.31 |
| 72 | 15.40 | 1.20 | 0.69 | 8.60 | 11.93 | 6.89 | 0.00 | 0.00 | 0.00 | 75.11 | 3.36 | 1.94 |
| Silver Treatment | 20 μg/L Ag | 40 μg/L Ag | 80 μg/L Ag | Control |
| t = 24 h | 94.75 [91.50, 96.92] | 91.51 [87.19, 94.63] | 89.85 [85.30, 93.27] | 99.24 [97.52, 99.81] |
| t = 48 h | 79.20 [72.29, 84.97] | 63.89 [55.72, 71.47] | 50.09 [41.91, 58.26] | 94.24 [90.01, 96.91] |
| t = 72 h | 58.66 [49.92, 66.99] | 36.87 [28.85, 45.52] | 8.38 [4.90, 13.45] | 82.43 [75.48, 87.98] |
| Copper Treatment | 300 μg/L Cu | 600 μg/L Cu | 1200 μg/L Cu | Control |
| t = 24 h | 88.71 [75.01, 95.97] | 85.46 [69.89, 94.42] | 74.39 [54.91, 88.25] | 99.42 [96.88, 99.93] |
| t = 48 h | 53.40 [32.80, 73.11] | 44.86 [25.47, 65.60] | 26.94 [12.57, 46.72] | 95.02 [85.93, 98.66] |
| t = 72 h | 20.67 [8.66, 39.21] | 16.82 [6.59, 33.90] | 11.38 [3.92, 25.69] | 83.63 [66.76, 93.64] |
| Chlorine Treatment | 500 μg/L Free Cl | 1000 μg/L Free Cl | 2000 μg/L Free Cl | Control |
| t = 24 h | 77.29 [71.79, 82.13] | 72.55 [66.53, 77.97] | 41.02 [35.19, 47.05] | 94.55 [91.65, 96.59] |
| t = 48 h | 25.37 [20.28, 31.05] | 14.57 [10.53, 19.56] | 3.34 [2.02, 5.29] | 80.39 [75.26, 84.82] |
| t = 72 h | 15.24 [11.04, 20.38] | 8.92 [5.75, 13.23] | 0.80 [0.18, 2.87] | 75.44 [69.54, 80.66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turner, S.S.; Smith, J.A.; Howle, S.L.; Hancock, P.I.; Brett, K.; Davis, J.; Bruno, L.M.; Cecchetti, V.; Ford, C. Analyzing the Efficacy of Water Treatment Disinfectants as Vector Control: The Larvicidal Effects of Silver Nitrate, Copper Sulfate Pentahydrate, and Sodium Hypochlorite on Juvenile Aedes aegypti. Water 2025, 17, 348. https://doi.org/10.3390/w17030348
Turner SS, Smith JA, Howle SL, Hancock PI, Brett K, Davis J, Bruno LM, Cecchetti V, Ford C. Analyzing the Efficacy of Water Treatment Disinfectants as Vector Control: The Larvicidal Effects of Silver Nitrate, Copper Sulfate Pentahydrate, and Sodium Hypochlorite on Juvenile Aedes aegypti. Water. 2025; 17(3):348. https://doi.org/10.3390/w17030348
Chicago/Turabian StyleTurner, Sydney S., James A. Smith, Sophie L. Howle, Patrick I. Hancock, Karin Brett, Julia Davis, Lorin M. Bruno, Victoria Cecchetti, and Clay Ford. 2025. "Analyzing the Efficacy of Water Treatment Disinfectants as Vector Control: The Larvicidal Effects of Silver Nitrate, Copper Sulfate Pentahydrate, and Sodium Hypochlorite on Juvenile Aedes aegypti" Water 17, no. 3: 348. https://doi.org/10.3390/w17030348
APA StyleTurner, S. S., Smith, J. A., Howle, S. L., Hancock, P. I., Brett, K., Davis, J., Bruno, L. M., Cecchetti, V., & Ford, C. (2025). Analyzing the Efficacy of Water Treatment Disinfectants as Vector Control: The Larvicidal Effects of Silver Nitrate, Copper Sulfate Pentahydrate, and Sodium Hypochlorite on Juvenile Aedes aegypti. Water, 17(3), 348. https://doi.org/10.3390/w17030348






