Speciation of Trace Metals in the Bottom Sediments of the Mozhaisk Reservoir and the Moskva River
Abstract
1. Introduction
2. Objects of the Study
3. Materials and Methods of Laboratory Analysis of Bottom Sediments
4. Results and Discussion
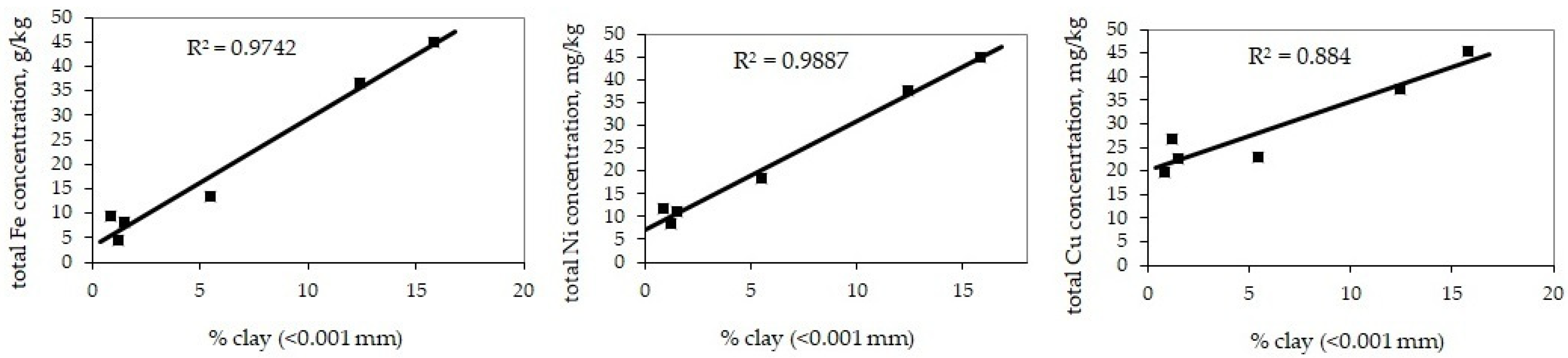
4.1. Particle Size Distribution in the Bottom Sediments
4.2. The Accumulation and Chemical Forms of Metals
5. Conclusions
- The bottom sediments of the Mozhaisk Reservoir are characterized by much higher total concentrations of the examined metals compared with the deposits in the Moskva River due to the higher relative share of clay (˂0.001 mm) and organic matter in the Mozhaisk Reservoir bottom sediments. In addition, due to the significantly higher proportion of aleurite (0.1–0.01 mm), the total metal content in the sediments of the reservoir is also higher than in the river due to a significantly higher concentration of metals in strongly bounded forms.
- Most metals in the Mozhaisk Reservoir bottom sediments are in strongly bound compounds. The high percentage of tightly bound metals (Co, Ni, Cu, Zn, Cd, Pb, and Fe) in the Mozhaisk Reservoir deposits is due to an increased aleurite (0.1–0.01 mm) fraction due to shore abrasion. The aleurite fraction carries these metals and is represented by poorly soluble primary and secondary minerals containing metals in their crystalline structures. The only exceptions are Mn and Cd, which are present in their labile forms, i.e., compounds with carbonates and hydroxides of iron and manganese.
- In the bottom sediments of the Moscow River, the strongly bound forms of silicate compounds containing Fe, Cu, Pb, and Co and Fe and Mn hydroxides containing Ni, Zn, and Cd predominate, which, together, account for 29% to 98% of the total content. However, the proportion of mobile, bioavailable forms of metals in the bottom sediments of the Moskva River is higher than in the reservoir due to their anthropogenic input. Among the loosely bonded metal compounds, there is a higher proportion of metal compounds with carbonates.
- The proportion of metals in the most mobile exchange form in the bottom sediments of the channel alluvium of the Moskva River and the reservoir bottom sediments is insignificant (1–14%). The only exceptions are Co and Cd, for which the concentration of exchange forms in the Moskva River sediments is somewhat higher and reaches 7–25%.
- Although the bottom sediments of the Mozhaisk Reservoir are richer in organic matter, the proportion of the complexes of the examined metals with organic matter is the same for the bottom sediments of both the Moskva River and the reservoir and varies from 3% to 10%.
- The Mozhaisk reservoir plays the role of a natural and anthropogenic geochemical sorption and sedimentation barrier, where the balance of granulometric fractions in bottom sediments changes and a large proportion of metals transported by the Moskva River accumulates, which is accompanied by a change in the forms of their presence in bottom sediments.
- It looks like the discovered patterns in the microelement distributions in their chemical forms in the Mozhaisk Reservoir bottom sediments are typical of the valley reservoirs of central Russia.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moiseenko, T.I.; Kudryavtseva, L.P.; Gashkina, N.A. Trace Elements in Continental Surface Waters: Technophilicity, Bioaccumulation, and Ecotoxicology; Nauka: Moscow, Russia, 2006; p. 261. (In Russian) [Google Scholar]
- Ferraro, A.; Siciliano, A.; Spampinato, M.; Morello, R.; Trancone, G.; Race, M.; Guida, M.; Fabbricino, M.; Spasiano, D.; Fratino, U. A multi-disciplinary approach based on chemical characterization of foreshore sediments, ecotoxicity assessment and statistical analyses for environmental monitoring of marine-coastal areas. Marine Env. Res. 2024, 202, 106780. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.K.; Favas, P.; Rakshit, D.; Satpathy, K.K. Geochemical speciation and risk assessment of heavy metals in soils and sediments. Environmental Risk Assessment of Soil Contamination; Hernandez-Soriano, M.C., Ed.; IntechOpen: London, UK, 2014; pp. 723–757. [Google Scholar] [CrossRef]
- Gleyzes, C.; Tellier, S.; Astruc, M. Fractionation studies of trace elements in contaminated soils and sediments: A review of sequential extraction procedures. Trends Anal. Chem. 2002, 21, 451–467. [Google Scholar] [CrossRef]
- Keshavarzifard, M.; Moore, F.; Sharifi, R. The influence of physicochemical parameters on bioavailability and bioaccessibility of heavy metals in sediments of the intertidal zone of Asaluyeh region, Persian Gulf, Iran. Geochemistry 2019, 79, 178–187. [Google Scholar] [CrossRef]
- Ferraro, A.; Marino, E.; Trancone, G.; Race, M.; Mali, M.; Pontoni, L.; Fabbricino, M.; Spasiano, D.; Fratino, U. Assessment of environmental parameters effect on potentially toxic elements mobility in foreshore sediments to support marine-coastal contamination prediction. Marine Poll. Bull. 2023, 194, 115338. [Google Scholar] [CrossRef] [PubMed]
- Sahuquillo, A.; Rigol, A.; Rauret, G. Overview of the use of leaching/extraction tests for risk assessment of trace metals in contaminated soils and sediments. Trends Anal. Chem. 2003, 22, 152–159. [Google Scholar] [CrossRef]
- Papina, T.S. Transport and Peculiarities of Heavy Metals Distribution in the Row: Water—Suspended Substance—River Ecosystems Sludge: Analytical Review; GPNTB SO RAN: Novosibirsk, Russia, 2001; p. 58. (In Russian) [Google Scholar]
- Eggleton, J.; Thomas, K.V. A review of factors affecting the release and bioavailability of contaminants during sediment disturbance events. Environ. Intern. 2004, 30, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Pardo, R.; Barrado, E.; Perez, L.; Vega, M. Determination and association of heavy metals in sediments of the Pisuerga River. Water Res. 1990, 24, 373–379. [Google Scholar] [CrossRef]
- Jardo, C.P.; Nickless, G. Chemical association of Zn, Cd, Pb and Cu in soils and sediments determined by the sequential extraction technique. Environ. Sci. Technol. Lett. 1989, 10, 743–752. [Google Scholar] [CrossRef]
- Rauret, G. Extraction procedures for the determination of heavy metals in contaminated soil and sediment. Talanta 1998, 46, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.P.; Mohan, D.; Singh, V.K.; Malik, A. Studies on distribution and fractionation of heavy metals in Gomti River sediments—A tributary of the Ganges India. J. Hydrol. 2005, 312, 14–27. [Google Scholar] [CrossRef]
- Moore, J.W.; Ramamoorthy, S. Heavy Metals in Natural Waters; Springer: New York, NY, USA, 1984; p. 268. [Google Scholar]
- Vinogradova, N.N. Formation and distribution of bed soils in the Mozhaisk Reservoir. Vestn. Mosk. Univ. Ser. 5 Geogr. 1969, 6, 37–40. [Google Scholar]
- Khrustaleva, M.A.; Zadorozhnaya, E.A.; Korenevskaya, V.E.; Bykova, N.I. Microelement concentrations in the Mozhaisk Reservoir. In Integrated Studies of Water Reservoirs; Issue 2; Bykov, V.D., Vazhnov, A.N., Eds.; MSU: Moscow, Russia, 1973; pp. 76–84. (In Russian) [Google Scholar]
- Martynova, M.V. Manganese concentration in the silts of the Mozhaisk Reservoir. Water Resour. 2012, 39, 223–228. [Google Scholar] [CrossRef]
- Koronkevich, N.I.; Melnik, K.S. Anthropogenic Impacts on the Moskva River Runoff; Maks Press: Moskva, Russia, 2015; p. 168. (In Russian) [Google Scholar]
- Alekseevskii, N.I.; Reteyum, K.F. World Rivers and Lakes; Entsiklopedia: Moscow, Russia, 2012; p. 924. (In Russian) [Google Scholar]
- Kremenetskaya, E.R.; Lomova, D.V.; Sokolov, D.I.; Edelshtein, K.K. Sedimentation of suspension in the Mozhaisk Reservoir. Water Resour. 2011, 38, 522–529. [Google Scholar] [CrossRef]
- Bykov, V.D.; Edelshtein, K.K. Integrated Studies of Reservoirs; Issue 3; In Mozhaisk Reservoir; MSU: Moscow, Russia, 1979; p. 467. (In Russian) [Google Scholar]
- Edelshtein, K.K. Reservoirs of Russia: Environmental Problems, Ways to Their Solution; GEOS: Moscow, Russia, 1998; p. 277. (In Russian) [Google Scholar]
- Bykov, V.D.; Sokolova, N.Y.; Edelshtein, K.K. Reservoirs of the Moskvoretskaya Water System; MSU: Moscow, Russia, 1985; p. 266. (In Russian) [Google Scholar]
- Sokolov, D.I.; Erina, O.N.; Tereshina, M.A.; Puklakov, V.V. Impact of Mozhaisk Dam on the Moscow River sediment transport. Geogr. Environ. Sustain. 2020, 13, 24–31. [Google Scholar] [CrossRef]
- GOST-12536-2014; Soils—Methods of Laboratory Determination of Granulometric (Grain) and Microaggregate Composition. Euro-Asian Council for Standardization, Metrology and Certification: Moscow, Russia, 2015.
- GOST 26213-2021; Soils—Methods for the determination of organic matter. Euro-Asian Council for Standardization, Metrology and Certification: Moscow, Russia, 2021.
- Bychkova, Y.V.; Nikolaeva, I.Y.; Ermina, O.S.; Tskhovrebova, A.R.; Shubin, I.I.; Stennikov, A.V. The Details of a Method for the Preparation of Solid Geological Samples for ICP-MS Multielement Analysis. Mos. Univ. Geol. Bull. 2018, 73, 520–526. [Google Scholar] [CrossRef]
- Berkovits, L.A.; Lukashin, V.N. Three marine sediment reference samples: SDO-1, SDO-2 and SDO-3. Geostand. Newsl. 1984, 8, 51–56. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Trofimov, V.T.; Korolev, V.A.; Voznesenskii, E.A.; Golodkovskaya, E.A.; Vasilchuk, Y.K.; Ziangirov, R.S. Soil Science; MSU: Moscow, Russia, 2005; p. 1024. (In Russian) [Google Scholar]
- Shepeleva, E.S. Ecological-Geochemical Studies of the Behavior of Heavy Metals in Aquatic and Terrestrial Ecosystems of the Ivankovo Reservoir; Extended Abstract of Cand. Sci. (Geol.-Miner.) Dissertation; Moscow State University: Moscow, Russia, 2004. [Google Scholar]
- Yanin, E.P. Technogenic Silts in Rivers in Moscow Region (Geochemical Features and Ecological Estimate); IMGRE: Moscow, Russia, 2004; p. 95. (In Russian) [Google Scholar]
- Grishantseva, E.S.; Safronova, N.S. Ecological-geochemical assessment of the state of the Volga source of water supply to Moscow. Water Resour. 2012, 39, 305–321. [Google Scholar] [CrossRef]
- Kolomiitsev, N.V.; Tolkachev, G.Y.; Korzhenevskii, B.I.; Il’ina, T.A. Patterns of Pollution of Bottom Sediments of Water Bodies with Heavy Metals; Kostyakov VNIIGiM: Moscow, Russia, 2023; p. 180. (In Russian) [Google Scholar]
- Minkina, T.M.; Motuzova, G.V.; Nazarenko, O.G. Composition of Heavy Metal Compounds in Soils; Everest Publishing House: Rostov-on-Don, Russia, 2009; p. 206. (In Russian) [Google Scholar]
- Strakhov, N.M. Fundamentals of Lithogenesis Theory; Nauka: Moscow, Russia, 1962; p. 212. (In Russian) [Google Scholar]
- Bourg, A.C.M.; Loch, J.P.G. Mobilization of heavy metals as affected by pH and redox conditions. In Biogeodynamics of Pollutants in Soils and Sediments; Salomons, W., Stigliani, W.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; pp. 87–102. [Google Scholar]
- Lipatnikova, O.A.; Grichuk, D.V.; Grigorieva, I.L.; Hasanova, A.I.; Shestakova, T.I.; Bychkov, A.U.; Ilina, S.M.; Puhov, V.V. Features of different forms of trace elements in bottom sediments of Ivan’kovskoe Reservoir. Geoekol. Inzh. Geol. Gidrogeol. Geokriol. 2014, 1, 37–48. (In Russian) [Google Scholar]
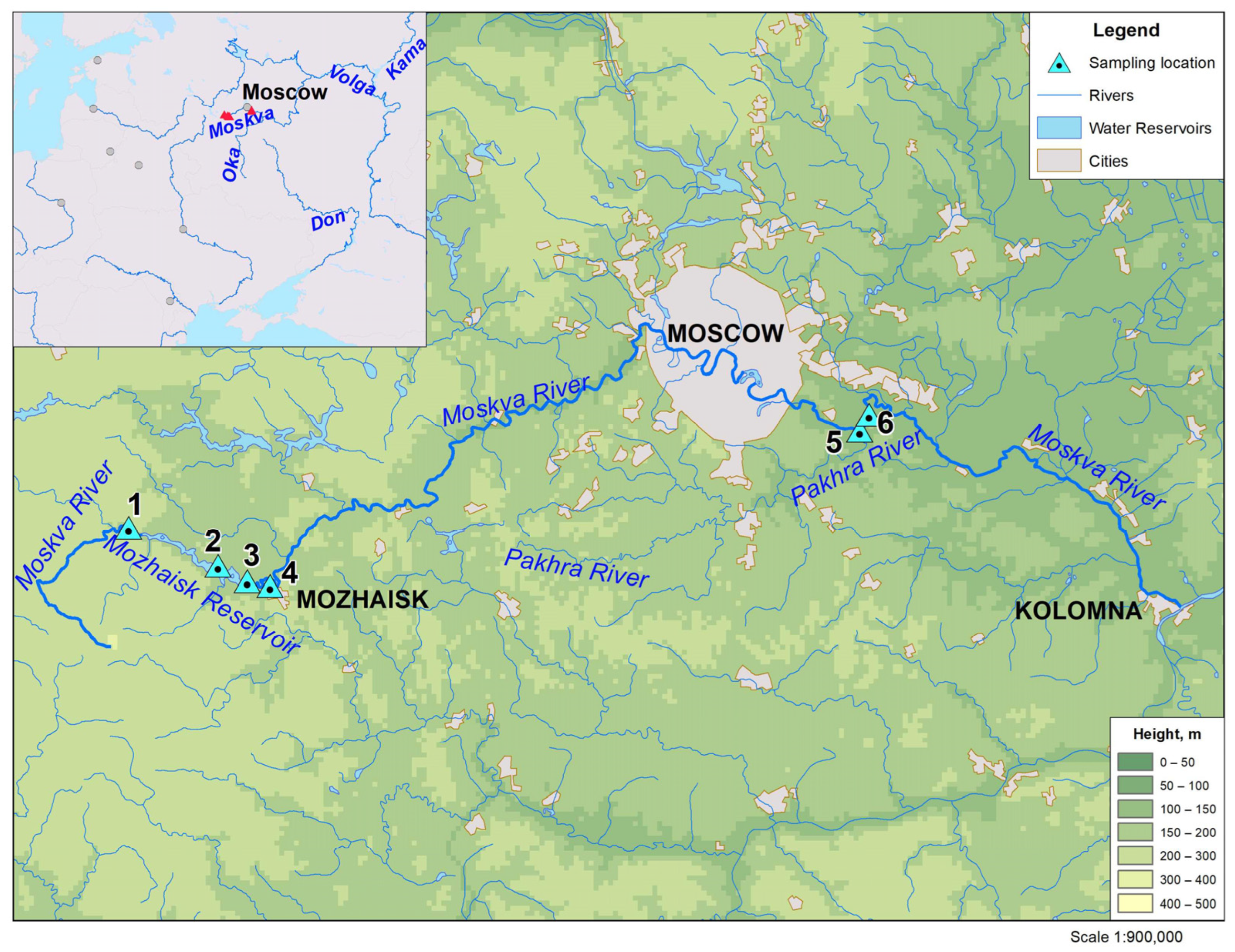
| Sample No. | Sampling Site | River/Reservoir Width, m |
|---|---|---|
| 1 | Moskva River at Barsuki Village | 30 |
| 2 | Mozhaisk Reservoir, Krasnovidovo Village | 1500 |
| 3 | Mozhaisk Reservoir, Blaznovo Village, near the dam | 1500 |
| 4 | Moskva River at Isavitsy Village | 40 |
| 5 | Moskva River, upstream of the Pakhra River inflow, Nizhnee Myachkovo Village | 150 |
| 6 | Moskva River, downstream of the Pakhra River inflow, Telman Settlement | 150 |
| No. | W % | OM % | The Concentration of Particles (Diameter, mm), % Dry Weight | Ground Type | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ˃2 | 2–1 | 1–0.5 | 0.5–0.25 | 0.25–0.1 | 0.1–0.05 | 0.05–0.01 | 0.01–0.005 | 0.005–0.001 | ˂0.001 | ||||
| 1 | 1.78 | 3.4 | 0.08 | 0.34 | 2.92 | 6.33 | 17.18 | 29.67 | 27.46 | 4.88 | 5.65 | 5.49 | clay sand |
| 2 | 4.93 | 5.2 | n.a. | n.a. | 0.27 | 1.22 | 1.3 | 17.28 | 40.91 | 11.34 | 15.25 | 12.43 | medium clay loam |
| 3 | 4.71 | 6.4 | 20.67 | n.a. | 0.12 | 0.86 | 0.17 | 8.48 | 25.02 | 11.46 | 17.39 | 15.83 | heavy clay loam |
| 4 | 0.72 | 5 | 22.62 | 10.34 | 35.21 | 18.62 | 7.46 | 0.88 | 3.14 | 0.44 | 0.41 | 0.88 | loose sand |
| 5 | 0.66 | 0.8 | 8.59 | 6.22 | 16.3 | 19.84 | 39.77 | 6.01 | 1.06 | 0.18 | 0.51 | 1.52 | loose sand |
| 6 | 0.38 | 0.4 | 0.57 | 0.5 | 4.84 | 39.11 | 59.11 | 0.98 | 0.08 | 0.08 | 0.51 | 1.22 | loose sand |
| Proportion of Compounds in the Total Content, % | |||||||
|---|---|---|---|---|---|---|---|
| Loosely Bound (LB) | Strongly Bound (SB) | LB/SB, % | |||||
| Sampling Site | Total Concentration, mg/kg | Exchange Forms | Bound with Carbonates | Associated with Hydromorphic Hydroxides and Fe–Mn | Associated with Organic Matter | Strongly Bound with Silicates | |
| Manganese | |||||||
| 1 | 444 | 3 | 44 | 20 | 4 | 29 | 47/53 |
| 2 | 1115 | 3 | 47 | 28 | 3 | 19 | 50/50 |
| 3 | 1832 | 14 | 44 | 24 | 4.5 | 13.5 | 58/42 |
| 4 | 702 | 4 | 63 | 20 | 4 | 9 | 67/33 |
| 5 | 267 | 7 | 38 | 31 | 5 | 19 | 45/55 |
| 6 | 173 | 6 | 27 | 23 | 3 | 41 | 33/67 |
| Iron | |||||||
| 1 | 13528 | 0.1 | 0.8 | 17 | 1.5 | 80.6 | 0.9/99.1 |
| 2 | 36481 | 0 | 0.7 | 15 | 0.8 | 83.5 | 0.7/99.3 |
| 3 | 45043 | 0 | 0.8 | 14 | 1 | 84.2 | 0.8/99.2 |
| 4 | 9380 | 0.2 | 2 | 35 | 1.5 | 61.3 | 2.2/97.8 |
| 5 | 8142 | 0.2 | 1.2 | 25 | 1 | 72.6 | 1.4/98.6 |
| 6 | 4351 | 0.4 | 0.9 | 24 | 0.7 | 74 | 1.3/98.7 |
| Cobalt | |||||||
| 1 | 6.92 | 8.7 | 13.4 | 29.6 | 10.7 | 37.6 | 22/78 |
| 2 | 12.54 | 4 | 6 | 28 | 8 | 54 | 10/90 |
| 3 | 15.8 | 4 | 9 | 26 | 8 | 53 | 13/87 |
| 4 | 3.51 | 14 | 9.7 | 33 | 11 | 32.3 | 24/76 |
| 5 | 2.98 | 20 | 7.4 | 33 | 6 | 33.6 | 27/73 |
| 6 | 1.97 | 25 | 5.6 | 29 | 4.6 | 35.8 | 31/69 |
| Nickel | |||||||
| 1 | 18.3 | 3.8 | 11 | 29.5 | 12 | 43.7 | 15/85 |
| 2 | 37.5 | 1.9 | 5.3 | 19.2 | 10.4 | 63.2 | 7/93 |
| 3 | 44.8 | 2 | 6.3 | 15 | 9.3 | 67.4 | 8/92 |
| 4 | 11.8 | 6 | 15.5 | 37 | 13 | 28.5 | 22/78 |
| 5 | 11.1 | 6.3 | 12.6 | 42 | 11.7 | 27.4 | 19/81 |
| 6 | 8.5 | 8.2 | 11.8 | 47 | 14 | 19 | 20/80 |
| Copper | |||||||
| 1 | 22.9 | 1.3 | 8.7 | 4.3 | 8.7 | 77 | 10/90 |
| 2 | 37.5 | 1 | 8 | 2.7 | 8 | 80.3 | 9/91 |
| 3 | 45.2 | 0.9 | 6.6 | 2.2 | 6.6 | 83.7 | 8/92 |
| 4 | 19.8 | 1.5 | 15 | 5 | 10 | 68.5 | 17/83 |
| 5 | 22.5 | 1.8 | 13.3 | 22 | 9 | 53.7 | 15/85 |
| 6 | 26.8 | 1.5 | 11.2 | 18.7 | 3.7 | 64.9 | 13/87 |
| Zinc | |||||||
| 1 | 62.6 | 1.6 | 9.7 | 37 | 7 | 44.7 | 11/89 |
| 2 | 114 | 0.9 | 4.6 | 24.6 | 4 | 65.9 | 5/95 |
| 3 | 125.8 | 0.8 | 0.5 | 21 | 4 | 73.7 | 1/99 |
| 4 | 30.9 | 3.2 | 12 | 35.6 | 10 | 39.2 | 15/85 |
| 5 | 79.8 | 1.3 | 21 | 55 | 7.7 | 15 | 22/78 |
| 6 | 49.1 | 2 | 18 | 55 | 7 | 18 | 20/80 |
| Cadmium | |||||||
| 1 | 0.29 | 6.9 | 31 | 52 | 3.4 | 6.7 | 38/62 |
| 2 | 0.46 | 8.7 | 24 | 54 | 4.3 | 9 | 33/67 |
| 3 | 0.47 | 8.5 | 21 | 51 | 8.5 | 11 | 30/70 |
| 4 | 0.14 | 14 | 36 | 28 | 7 | 15 | 50/50 |
| 5 | 0.23 | 22 | 35 | 35 | 4 | 4 | 57/43 |
| 6 | 0.16 | 25 | 25 | 37.5 | 6.3 | 6.2 | 50/50 |
| Lead | |||||||
| 1 | 17.5 | 1 | 9 | 23 | 5.7 | 61.3 | 10/90 |
| 2 | 29.94 | 0.1 | 4.7 | 24 | 5 | 66.2 | 5/95 |
| 3 | 32 | 0.3 | 5.6 | 24 | 5.6 | 64.5 | 6/94 |
| 4 | 13.9 | 0.8 | 6.5 | 29 | 5.8 | 57.9 | 7/93 |
| 5 | 20.61 | 0 | 8.7 | 40 | 6.8 | 44.5 | 9/91 |
| 6 | 63.23 | 0.5 | 28 | 55 | 5.2 | 11.3 | 29/71 |
| Element | The Share of the Total Content of Migratory Forms, % | |||||
|---|---|---|---|---|---|---|
| Bound with Carbonates | Bound with Hydroxides Fe–Mn | Bound with Organic Matter | ||||
| Mozhaisk Reservoir | Ivan’kovo Reservoir [38] | Mozhaisk Reservoir | Ivan’kovo Reservoir [38] | Mozhaisk Reservoir | Ivan’kovo Reservoir [38] | |
| Mn | 60–61 61 | 47–77 71 | 33–36 34 | 19–50 25 | 4–6 5 | 2–6 4 |
| Fe | 4–5 4.5 | 10–30 20 | 89–91 90 | 42–80 62 | 5–6 5.5 | 9–48 18 |
| Co | 14–21 17 | 10–35 22 | 60–67 64 | 35–77 49 | 19 | 13–51 29 |
| Ni | 15–21 18 | 9–22 15 | 49–55 52 | 28–43 39 | 30 | 37–63 46 |
| Cu | 43 | 2–34 12 | 14 | 2–18 6 | 43 | 54–96 82 |
| Zn | 2–14 8 | 23–47 38 | 74–82 78 | 31–52 46 | 12–16 14 | 10–46 16 |
| Cd | 26–29 27 | 28–88 52 | 63–66 65 | 8–56 38 | 5–11 8 | 3–34 10 |
| Pb | 14–16 15 | 16–37 29 | 68–71 70 | 22–59 44 | 15–16 15 | 15–61 27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grishantseva, E.S.; Georgiadi, A.G.; Groisman, P.Y. Speciation of Trace Metals in the Bottom Sediments of the Mozhaisk Reservoir and the Moskva River. Water 2025, 17, 367. https://doi.org/10.3390/w17030367
Grishantseva ES, Georgiadi AG, Groisman PY. Speciation of Trace Metals in the Bottom Sediments of the Mozhaisk Reservoir and the Moskva River. Water. 2025; 17(3):367. https://doi.org/10.3390/w17030367
Chicago/Turabian StyleGrishantseva, Elena S., Aleksandr G. Georgiadi, and Pavel Y. Groisman. 2025. "Speciation of Trace Metals in the Bottom Sediments of the Mozhaisk Reservoir and the Moskva River" Water 17, no. 3: 367. https://doi.org/10.3390/w17030367
APA StyleGrishantseva, E. S., Georgiadi, A. G., & Groisman, P. Y. (2025). Speciation of Trace Metals in the Bottom Sediments of the Mozhaisk Reservoir and the Moskva River. Water, 17(3), 367. https://doi.org/10.3390/w17030367







