Cellular Responses of Astrangia poculata (Ellis and Solander, 1786) and Its Symbiont to Experimental Heat Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coral Collection and Husbandry
2.2. Experimental Design
2.3. Maximum Quantum Yield (Fv/Fm)
2.4. Symbiotic Density via Photo Quantification
2.5. ROS Concentrations via Imaging Flow Cytometry (IFCM)
2.6. Statistical Analyses
3. Results
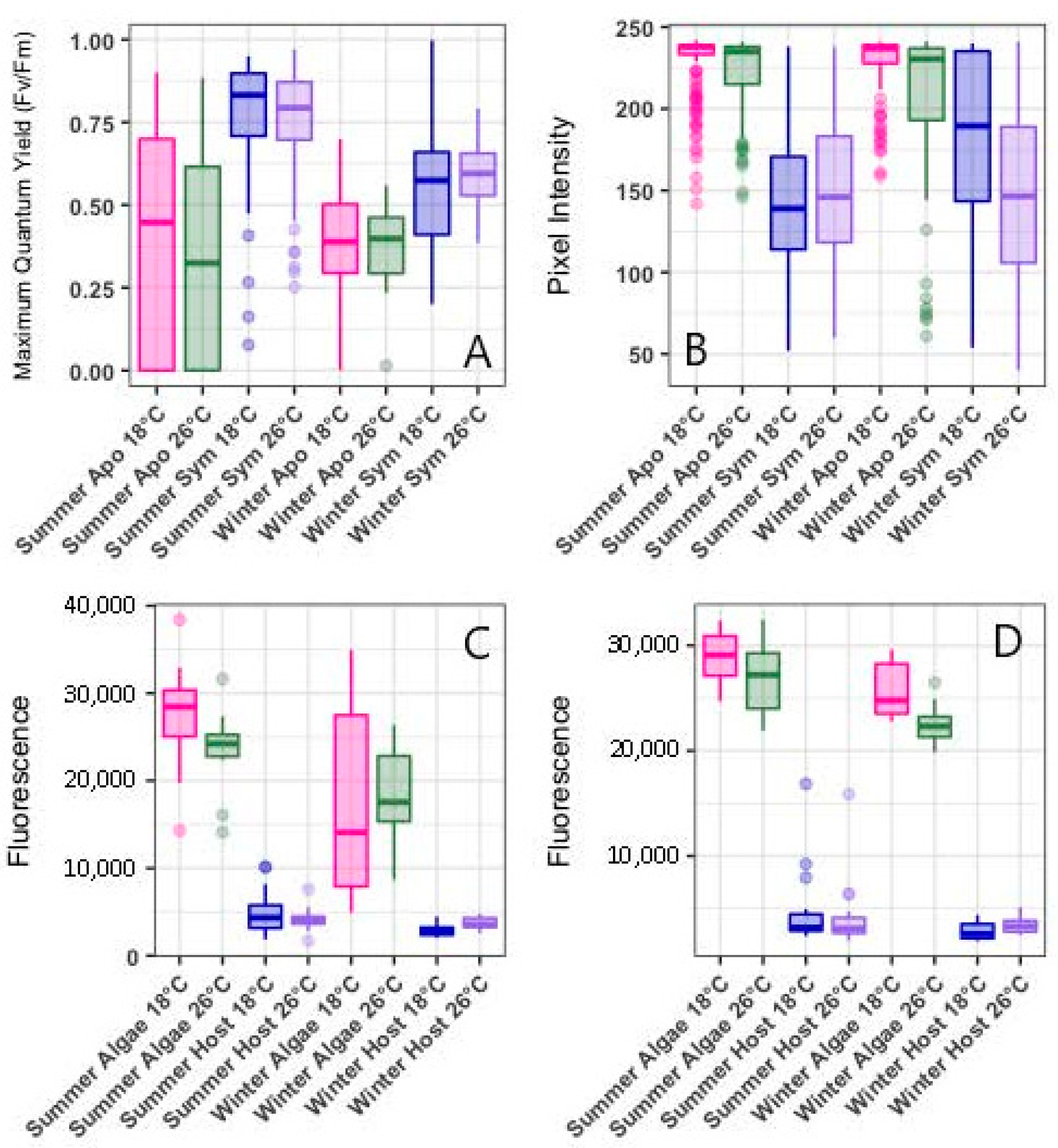
3.1. Photosynthetic Health
3.2. Symbiotic Density (Associated with Pixel Intensity)
3.3. Imaging Flow Cytometry (IFCM)
4. Discussion
4.1. Photosynthetic Health
4.2. Symbiotic Density (Pixel Intensity)
4.3. ROS Concentrations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacDowell, N.; Fennell, P.S.; Shah, N.; Maitland, G.C. The role of CO2 capture and utilization in mitigating climate change. Nat. Clim. Change 2017, 7, 243–249. [Google Scholar] [CrossRef]
- Giannakis, E.; Serghides, D.; Dimitriou, S.; Zittis, G. Land transport CO2 emissions and climate change: Evidence from Cyprus. Int. J. Sustain. Energy 2020, 39, 634–647. [Google Scholar] [CrossRef]
- Forster, P.M.; Smith, C.J.; Walsh, T.; Lamb, W.F.; Lamboll, R.; Hauser, M.; Ribes, A.; Rosen, D.; Gillett, N.; Palmer, M.D.; et al. Indicators of global climate change 2022: Annual update of large-scale indicators of the state of the climate systems and human influence. Earth Syst. Sci. Data 2023, 15, 2295–2327. [Google Scholar] [CrossRef]
- Carpenter, K.E.; Abrar, M.; Aeby, G.; Aronson, R.B.; Banks, S.; Bruckner, A.; Chiriboga, A.; Cortés, J.; Delbeek, J.C.; Devantier, L.; et al. One-third of reef-building corals face elevated extinction risk from climate change and local impacts. Science 2008, 321, 560–563. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Bruno, J.F. The impact of climate change on the world’s marine ecosystems. Science 2010, 328, 1523–1528. [Google Scholar] [CrossRef]
- Cheng, L.; Abraham, J.; Hausfather, Z.; Trenberth, K.E. How fast are the oceans warming? Science 2019, 363, 28–129. [Google Scholar] [CrossRef]
- Global Climate Highlights. C3S/ECMWF, ERA5 data, Global Climate Highlights 2024. Available online: https://climate.copernicus.eu/global-climate-highlights-2024 (accessed on 16 January 2025).
- Hoegh-Guldberg, O. Coral reef ecosystems and anthropogenic climate change. Reg. Environ. Change 2011, 11 (Suppl. S1), 215–227. [Google Scholar] [CrossRef]
- Strychar, K.B.; Hauff-Salas, B.; Haslun, J.A.; DeBoer, J.; Cryer, K.; Keith, S.; Wooten, S. Stress resistance and adaptation of the aquatic invasive species Tubastrea coccinea (Lesson, 1829) to climate change and ocean acidification. Water 2021, 13, 3645. [Google Scholar] [CrossRef]
- Muscatine, L.; Porter, J.W. Reef corals: Mutualistic symbioses adapted to nutrient-poor environments. Bioscience 1977, 27, 454–460. [Google Scholar] [CrossRef]
- Douglas, A.E. Coral bleaching—How and why? Mar. Pollut. Bull. 2003, 46, 385–392. [Google Scholar] [CrossRef]
- Pandolfi, J.M.; Connolly, S.R.; Marshall, D.J.; Cohen, A.L. Projecting coral reef futures under global warming and ocean acidification. Science 2011, 333, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.E. Coral bleaching: Causes and consequences. Coral Reefs 1997, 16, S129–S138. [Google Scholar] [CrossRef]
- Berkelmans, R.; Van Oppen, M.J.H. The role of zooxanthellae in the thermal tolerance of corals: A “nugget of hope” for coral reefs in an era of climate change. Proc. R. Soc. B 2006, 273, 2305–2312. [Google Scholar] [CrossRef]
- Camp, E.F.; Schoepf, V.; Suggett, D.J. How can “Super Corals” facilitate global coral reef survival under rapid environmental and climatic change? Glob. Chang. Biol. 2018, 24, 2755–2757. [Google Scholar] [CrossRef]
- Strychar, K.B.; Sammarco, P.W. Exaptation in corals to high seawater temperatures: Low concentrations of apoptotic and necrotic cells in host coral tissue under bleaching conditions. J. Exp. Mar. Biol. Ecol. 2009, 369, 31–42. [Google Scholar] [CrossRef]
- Sammarco, P.W.; Strychar, K.B. Ecological and evolutionary considerations regarding corals in a rapidly changing environment. In The Cnidaria, Past, Present, and Future. The world of Medusa and Her Sisters; Chapter 34; Dubinsky, Z., Goffredo, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Antonelli, P.; Rutz, S.; Sammarco, P.W.; Strychar, K.B. Evolution of symbiosis in hermatypic corals: A model of the past, present, and future. Nonlinear Anal. Real World Appl. 2016, 32, 389–402. [Google Scholar] [CrossRef]
- Buerger, P.; Alvarez-Roa, C.; Coppin, C.W.; Pearce, S.L.; Chakravarti, L.J.; Oakeshott, J.G.; Edwards, O.R.; van Oppen, M.J.H. Heat-evolved microalgal symbionts increase coral bleaching tolerance. Sci. Adv. 2020, 6, eaba2498. [Google Scholar] [CrossRef]
- Quigley, K.M.; Ramsby, B.; Laffy, P.; Harris, J.; Mocellin, V.J.L.; Bay, L.K. Symbioses are restructured by repeated mass coral bleaching. Sci. Adv. 2022, 8, eabq8349. [Google Scholar] [CrossRef]
- Schoepf, V.; Sanderson, H.; Larcombe, E. Coral heat tolerance under variable temperatures: Effects of 707 different variability regimes and past environmental history vs. current exposure. Limnol. Oceanogr. 2022, 67, 404–418. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, X.; Kvitt, H.; Sheng, H.; Sun, D.; Niu, G.; Tchernov, D.; Shi, T. Lineage-specific symbionts mediate differential coral responses to thermal stress. Microbiome 2023, 11, 211. [Google Scholar] [CrossRef]
- Warner, M.E.; Fitt, W.K.; Schmidt, G.W. Damage to photosystem II in symbiotic dinoflagellates: A determinant of coral bleaching. Proc. Natl. Acad. Sci. USA 1999, 96, 8007–8012. [Google Scholar] [CrossRef] [PubMed]
- Strychar, K.B.; Sammarco, P.W. Effects of heat stress on phytopigments of zooxanthellae (Symbiodinium spp.) symbiotic with the corals Acropora hyacinthus, Porites solida and Favites complanata. Int. J. Biol. 2012, 4, 3. [Google Scholar] [CrossRef]
- Cziesielski, M.J.; Schmidt-Roach, S.; Aranda, M. The past, present, and future of coral heat stress studies. Ecol. Evol. 2019, 9, 10055–10066. [Google Scholar] [CrossRef] [PubMed]
- Camp, E.F.; Kahlke, T.; Nitschke, M.R.; Varkey, D.; Fisher, N.L.; Fujise, L.; Goyen, S.; Hughes, D.J.; Lawson, C.A.; Ros, M.; et al. Revealing changes in the microbiome of Symbiodiniaceae under thermal stress. Environ. Microbiol. 2020, 22, 1294–1309. [Google Scholar] [CrossRef] [PubMed]
- Hazraty-Kari, S.; Morita, M.; Tavakoli-Kolour, P.; Nakamura, T.; Harii, S. Reactions of juvenile coral to three years of consecutive thermal stress. Sci. Total Environ. 2023, 863, 161227. [Google Scholar] [CrossRef]
- Fridovich, I. The biology of oxygen radicals. Science 1978, 201, 875–880. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A. Singlet oxygen production in photosynthesis. J. Exp. Bot. 2005, 56, 337–346. [Google Scholar] [CrossRef]
- Slavov, C.; Schrameyer, V.; Reus, M.; Ralph, P.J.; Hill, R.; Büchel, C.; Larkum, A.W.; Holzwarth, A.R. “Super-quenching” state protects Symbiodinium from thermal stress - Implications for coral bleaching. Biochim. Biophys. Acta. 2016, 1857, 840–847. [Google Scholar] [CrossRef]
- Hillyer, K.E.; Tumanov, S.; Villas-Boas, S.; Davy, S.K. Metabolite profiling of symbiont and host during thermal stress and bleaching in a model cnidarian-dinoflagellate symbiosis. J. Exp. Biol. 2016, 219, 516–527. [Google Scholar] [CrossRef]
- Wietheger, A.; Starzak, D.E.; Gould, K.S.; Davy, S.K. Differential ROS generation in response to stress in Symbiodinium spp. Biol. Bull. 2018, 234, 11–21. [Google Scholar] [CrossRef]
- Harman, T.E.; Hauff-Salas, B.; Haslun, J.A.; Cervino, J.M.; Strychar, K.B. De-creased photosynthetic efficiency in response to site translocation and elevated temperature is mitigated with LPS exposure in Porites astreoides symbionts. Water 2022, 14, 366. [Google Scholar] [CrossRef]
- Hayat, M.A. Introduction to Autophagy: Cancer, Other Pathologies, Inflammation, Immunity, Infection, and Aging. In Autophagy: Cancer, Other Pathologies, Inflammation, Immunity, Infection, and Aging; Hayat, M.A., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 7, pp. 1–53. [Google Scholar] [CrossRef]
- Kristiansen, K.A.; Jensen, P.E.; Moller, I.M.; Schulz, A. Monitoring reactive oxy-gen species formation and localisation in living cells by use of the fluorescent probe CM-H(2)DCFDA and confocal laser microscopy. Physiol. Plant 2009, 136, 369–383. [Google Scholar] [CrossRef] [PubMed]
- McGinty, E.S.; Pieczonka, J.; Mydlarz, L.D. Variations in reactive oxygen release and antioxidant activity in multiple Symbiodinium types in response to elevated temperature. Microb. Ecol. 2012, 64, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Lesser, M. Oxidative stress causes coral bleaching during exposure to elevated temperatures. Coral Reefs. 1997, 16, 187–192. [Google Scholar] [CrossRef]
- Tolleter, D.; Seneca, F.O.; DeNofrio, J.C.; Krediet, C.J.; Palumbi, S.R.; Pringle, J.R.; Grossman, A.R. Coral bleaching independent of photosynthetic activity. Curr. Biol. 2013, 23, 1782–1786. [Google Scholar] [CrossRef]
- Gardner, S.G.; Raina, J.-B.; Ralph, P.J.; Petrou, K. Reactive oxygen species (ROS) and dimethylated sulphur compounds in coral explants under acute thermal stress. J. Exp. Biol. 2017, 220, 1787–1791. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A.; Fufezan, C.; Trebst, A. Singlet oxygen production in photosystem II and related protection mechanism. Photosynth. Res. 2008, 98, 551–564. [Google Scholar] [CrossRef]
- Toledo-Hernandez, C.; Ruiz-diaz, C.P. The immune responses of the coral. Invertebr. Surviv. J. 2014, 11, 319–328, ISSN 1824-307X. [Google Scholar]
- Roberty, S.; Furla, P.; Plumier, J.C. Differential antioxidant response between two Symbiodinium species from contrasting environments. Plant Cell. Environ. 2016, 39, 2713–2724. [Google Scholar] [CrossRef]
- Gottlieb, E.; Armour, S.; Harris, M.; Thompson, C.B. Mitochondrial membrane potential regulates matrix configuration and cytochrome c release during apoptosis. Cell Death Differ. 2003, 10, 709–717. [Google Scholar] [CrossRef]
- Tchernov, D.; Gorbunov, M.Y.; de Vargas, C.; Narayan Yadav, S.; Milligan, A.J.; Häggblom, M.; Falkowski, P.G. Membrane lipids of symbiotic algae are diagnostic of sensitivity to thermal bleaching in corals. Proc. Natl. Acad. Sci. USA 2004, 101, 13531–13535. [Google Scholar] [CrossRef] [PubMed]
- Downs, C.A.; Fauth, J.E.; Halas, J.C.; Dustan, P.; Bemiss, J.; Woodley, C.M. Oxidative stress and seasonal coral bleaching. Free Radic. Biol. Med. 2002, 33, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Garzón-Ferreira, J.; Gil-Agudelo, D.L.; Barrios, L.M.; Zea, S. Stony coral diseases observed in southwestern Caribbean reefs. Bull. Mar. Sci. 2001, 460, 65–69. [Google Scholar] [CrossRef]
- Morgans, C.A.; Hung, J.Y.; Bourne, D.G.; Quigley, K.M. Symbiodiniaceae probiotics for use in bleaching recovery. Restor. Ecol. 2019, 28, 282–288. [Google Scholar] [CrossRef]
- NOAA. Extreme Ocean Temperatures are Affecting Florida’s Coral Reef. 2023. Available online: https://www.nesdis.noaa.gov/news/extreme-ocean-temperatures-are-affecting-floridas-coral-reef (accessed on 16 January 2025).
- IPCC. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; pp. 35–115. [Google Scholar] [CrossRef]
- Miller, M.W. Growth of a temperate coral: Effects of temperature, light, depth, and heterotrophy. Mar. Ecol. Prog. Ser. 1995, 122, 217–226. [Google Scholar] [CrossRef]
- Maier, C.; Schubert, A.; Berzunza Sànchez, M.M.; Weinbauer, M.G.; Watremez, P.; Gattuso, J.-P. End of the Century pCO2 Levels Do Not Impact Calcification in Medi-terranean Cold-Water Corals. PLoS ONE 2013, 8, e62655. [Google Scholar] [CrossRef]
- Aichelman, H.E.; Townsend, J.E.; Courtney, T.A.; Baumann, J.H.; Davies, S.W.; Castillo, K.D. Heterotrophy mitigates the response of the temperate coral Oculina arbuscula to temperature stress. Ecol. Evol. 2016, 6, 6758–6769. [Google Scholar] [CrossRef]
- Fitt, W.K.; McFarland, F.K.; Warner, M.E.; Chilcoat, G.C. Seasonal patterns of tissue biomass and densities of symbiotic dinoflagellates in reef corals and relation to coral bleaching. Limnol. Oceanogr. 2000, 75, 677–685. [Google Scholar] [CrossRef]
- Levy, O.; Achituv, Y.; Yacobi, Y.Z.; Dubinsky, Z.; Stambler, N. Diel ‘tuning’ of coral metabolism: Physiological responses to light cues. J. Exp. Biol. 2006, 209 Pt 2, 273–283. [Google Scholar] [CrossRef]
- McCauley, M.; Banaszak, A.T.; Goulet, T.L. Species traits dictate seasonal-dependent responses of octocoral–algal symbioses to elevated temperature and ultraviolet radiation. Coral Reefs 2018, 37, 901–917. [Google Scholar] [CrossRef]
- van de Water, J.A.J.M.; Voolstra, C.R.; Rottier, C.; Cocito, S.; Peirano, A.; Allemand, D.; Ferrier-Pagès, D. Seasonal stability in the microbiomes of temperate gorgonians and the red coral Corallium rubrum across the Mediterranean Sea. Microb. Ecol. 2018, 75, 274–288. [Google Scholar] [CrossRef] [PubMed]
- McCauley, M.; Jackson, C.R.; Goulet, T.L. Microbiomes of Caribbean octocorals vary over time but are resistant to environmental change. Front. Microbiol. 2020, 11, 538614. [Google Scholar] [CrossRef] [PubMed]
- Rodolfo-Metalpa, R.; Martin, S.; Ferrier-Pagès, C.; Gattuso, J.-P. Response of the temperate coral Cladocora caespitosa to mid- and long-term exposure to pCO2 and temperature levels projected for the year 2100 AD. Biogeosciences 2010, 7, 289–300. [Google Scholar] [CrossRef]
- Cummings, C. The Biology of Astrangia danae. Ph.D. Dissertation, University of Rhode Island, Kingston, RI, USA, 1983. [Google Scholar]
- Sharp, K.H.; Pratte, Z.A.; Kerwin, A.H.; Rotjan, R.D.; Stewart, F.J. Season, but not symbiont state, drives microbiome structure in the temperate coral Astrangia poculata. Microbiome 2017, 5, 120. [Google Scholar] [CrossRef]
- Changsut, I.V.; Borbee, E.M.; Womack, H.R.; Shickle, A.; Sharp, K.H.; Fuess, L.E. Photosymbiont density is correlated with constitutive and induced immunity in the facultatively symbiotic coral, Astrangia poculata. Integr. Comp. Biol. 2024, 64, 1278–1290. [Google Scholar] [CrossRef]
- Harman, T.E.; Barshis, D.J.; Hauff Salas, B.; Hamsher, S.E.; Strychar, K.B. Indications of symbiotic state influencing melanin-synthesis immune response in the facultative coral Astrangia poculata. Dis. Aquat. Organ. 2022, 151, 63–74. [Google Scholar] [CrossRef]
- LaJeunesse, T.C.; Parkinson, J.E.; Gabrielson, P.W.; Jeong, H.J.; Reimer, J.D.; Voolstra, C.R.; Santos, S.R. Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Curr. Biol. 2018, 28, 2570–2580.e6. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M.; AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway (Taxonomic Information Republished from Al-gaeBase with Permission of M.D. Guiry). Breviolum Psygmophilum (LaJeunesse, J.E.Parkinson & J.D.Reimer) J.E.Parkinson & LaJeunesse, 2018. 2024. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=1643622 (accessed on 9 April 2024).
- Szmant-Froelich, A.; Yevich, P.; Pilson, M.E.Q. Gametogenesis and early development of the temperate coral Astrangia danae (Anthozoa: Scleractinia). Biol. Bull. 1980, 158, 257–269. [Google Scholar] [CrossRef]
- Jacques, T.G.; Marshall, N.; Pilson ME, Q. Experimental ecology of the temperate scleractinian coral Astrangia danae-II. Effect of temperature, light intensity and symbiosis with zooxanthellae on metabolic rate and calcification. Mar. Biol. 1983, 76, 135–148. [Google Scholar] [CrossRef]
- Dimond, J.; Carrington, E. Temporal variation in the symbiosis and growth of the temperate scleractinian coral Astrangia poculata. Mar. Ecol. Prog. Ser. 2007, 348, 161–172. [Google Scholar] [CrossRef]
- Aichelman, H.E.; Zimmerman, R.C.; Barshis, D.J. Adaptive signatures in thermal performance of the temperate coral Astrangia poculata. J. Exp. Biol. 2019, 222 Pt 5, jeb189225. [Google Scholar] [CrossRef]
- Dimond, J.L.; Kerwin, A.H.; Rotjan, R.; Sharp, K.; Stewart, F.J.; Thornhill, D.J. A simple temperature-based model predicts the upper latitudinal limit of the temperate coral Astrangia poculata. Coral Reefs 2013, 32, 401–409. [Google Scholar] [CrossRef]
- Thornhill, D.J.; Kemp, D.W.; Bruns, B.U.; Fitt, W.K.; Schmidt, G.W. Correspondence between cold tolerance and temperate biogeography in a western Atlantic Symbiodinium (Dinophyta) lineage. J. Phycol. 2008, 44, 1126–1135. [Google Scholar] [CrossRef]
- Jaap, W.C. Stony coral (Milleporidae and Scleractinia) communities in the eastern Gulf of Mexico: A synopsis with insights from the Hourglass collections. Bull. Mar. Sci. 2015, 91, 207–253. [Google Scholar] [CrossRef]
- IPCC. IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Pörtner, H.-O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Alegría, A., Nicolai, M., Okem, A., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA,, 2019; p. 755. [Google Scholar] [CrossRef]
- Dimos, B.A.; Mahmud, S.A.; Fuess, L.E.; Mydlarz, L.D.; Pellegrino, M.W. Uncovering a mitochondrial unfolded protein response in corals and its role in adapting to a changing world. Proc. Biol. Sci. 2019, 286, 20190470. [Google Scholar] [CrossRef]
- Wuitchik, D.M.; Almanzar, A.; Benson, B.E.; Brennan, S.; Chavez, J.D.; Liesegang, M.B.; Reavis, J.L.; Reyes, C.L.; Schniedewind, M.K.; Trumble, I.F.; et al. Characterizing environmental stress responses of aposymbiotic Astrangia poculata to divergent thermal challenges. Mol. Ecol. 2021, 30, 5064–5079. [Google Scholar] [CrossRef]
- Pfab, F.; Detmer, A.R.; Moeller, H.V.; Nisbet, R.M.; Putnam, H.M.; Cunning, R. Heat stress and bleaching in corals: A bioenergetic model. Coral Reefs 2024, 43, 1627–1645. [Google Scholar] [CrossRef]
- Palacio-Castro, A.M.; Smith, T.B.; Brandtneris, V.; Snyder, G.A.; van Hooidonk, R.; Maté, J.L.; Manzello, D.; Glynn, P.W.; Fong, P.; Baker, A.C. Increased dominance of heat-tolerant symbionts creates resilient coral reefs in near-term ocean warming. Proc. Natl. Acad. Sci. USA 2023, 120, e2202388120. [Google Scholar] [CrossRef]
- Martinez, S.; Grover, R.; Ferrier-Pagès, C. Unveiling the importance of heterotrophy for coral symbiosis under heat stress. mBio 2024, 15, e01966-24. [Google Scholar] [CrossRef]
- Downs, C.A.; Woodley, C.M.; Richmond, R.H.; Lanning, L.L.; Owen, R. Shifting the paradigm of coral-reef “health” assessment. Mar. Poll. Bull. 2005, 51, 486–494. [Google Scholar] [CrossRef]
- Tang, X.; Yang, Q.; Zhang, Y.; Wang, H.; Ling, J.; Sun, H.; Dong, J.; Zhang, Y. Validating the use of ROS-scavenging bacteria as probiotics to increase coral resilience to thermal stress. J. Ocean. Limnol. 2024, 42, 1242–1260. [Google Scholar] [CrossRef]
- Wuitchik, D.M.; Aichelman, H.E.; Atherton, K.F.; Brown, C.M.; Chen, X.; DiRoberts, L.; Pelose, G.E.; Tramonte, C.A.; Davies, S.W. Photosymbiosis reduces the environmental stress response under a heat challenge in a facultatively symbiotic coral. bioRxiv 2023. [Google Scholar] [CrossRef]
- Fuess, L.E.; Palacio-Castro, A.M.; Butler, C.C.; Baker, A.C.; Mydlarz, L.D. Increased algal symbiont density reduces host immunity in a threatened Caribbean coral species, Orbicella faveolata. Front. Ecol. Evol. 2020, 8, 572942. [Google Scholar] [CrossRef]
- Jury, C. The Great Temperature Debate, Part III. 2015. Available online: https://reefs.com/magazine/the-great-temperature-debate-part-iii/ (accessed on 16 January 2025).
- Putnam, H.M.; Ritson-Williams, R.; Cruz, J.A.; Davidson, J.M.; Gates, R.D. Environmentally-induced parental or developmental conditioning influences coral offspring ecological performance. Sci. Rep. 2020, 10, 13664. [Google Scholar] [CrossRef]
- McLachlan, R.H.; Price, J.T.; Solomon, S.L.; Grottoli, A.G. Thirty years of coral heat-stress experiments: A review of methods. Coral Reefs 2020, 39, 885–902. [Google Scholar] [CrossRef]
- Fuess, L.E.; Pinzón, C.J.H.; Weil, E.; Grinshpon, R.D.; Mydlarz, L.D. Life or death: Disease-tolerant coral species activate autophagy following immune challenge. Proc. Biol. Sci. 2017, 284, 20170771. [Google Scholar] [CrossRef]
- Winters, G.; Holzman, R.; Blekhan, A.; Beer, S.; Loya, Y. Photographic assessment of coral chlorophyll contents: Implications for ecophysiological studies and coral monitoring. J. Exp. Mar. Biol. Ecol. 2009, 380, 25–35. [Google Scholar] [CrossRef]
- Burmester, E.M. Insights into Coral Recovery Based on Symbiont State and Environmental Conditions in the Temperate, Facultatively Symbiotic Coral Astrangia poculata. Boston University. 2017. Available online: https://open.bu.edu/server/api/core/bitstreams/aa1f63ce-7b6d-465e-a886-dc29c35e2930/content (accessed on 16 January 2025).
- Grottoli, A.G.; Toonen, R.J.; van Woesik, R.; Vega Thurber, R.; Warner, M.E.; McLachlan, R.H.; Price, J.T.; Bahr, K.D.; Baums, I.B.; Castillo, K.D.; et al. Increasing comparability among coral bleaching experiments. Ecol. Appl. 2020, 31, e02262. [Google Scholar] [CrossRef]
- Ralph, P.J.; Hill, R.; Doblin, M.A.; Davy, S.K. Theory and application of pulse amplitude modulated chlorophyll fluorometry in coral health assessment. In Diseases of Coral; Woodley, C.M., Downs, C.A., Bruckner, A.W., Porter, J.W., Galloway, S.B., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 506–523. [Google Scholar] [CrossRef]
- Schreiber, U.; Gademann, R.; Ralph, P.J.; Larkum, A.W.D. Assessment of photosynthetic performance of prochloron in Lissoclinum patella in hospite by chlorophyll fluorescence measurements. Plant Cell Physio. 1997, 38, 945–951. [Google Scholar] [CrossRef]
- Jones, R.J.; Kildea, T.; Hoegh-Guldberg, O. PAM Chlorophyll Fluorometry: A New in situ Technique for Stress Assessment in Scleractinian Corals, used to Examine the Effects of Cyanide from Cyanide Fishing. Mar. Pollut. Bull. 1999, 38, 864–874. [Google Scholar] [CrossRef]
- Warner, M.E.; Lesser, M.P.; Ralph, P.J. Chlorophyll fluorescence in reef building corals. In Chlorophyll a Fluorescence in Aquatic Sciences: Methods and Applications. Developments in Applied Phycology; Suggett, D., Prášil, O., Borowitzka, M., Eds.; Springer: Dordrecht, The Netherlands, 2010; Volume 4. [Google Scholar] [CrossRef]
- Wangpraseurt, D.; Lichtenberg, M.; Jacques, S.L.; Larkum, A.W.D.; Kühl, M. Optical properties of corals distort variable chlorophyll fluorescence measurements. Plant Physiol. 2019, 179, 1608–1619. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Komenda, J.; Sobotka, R.; Nixon, P.J. The biogenesis and maintenance of PSII: Recent advances and current challenges. Plant Cell 2024, 36, 3997–4013. [Google Scholar] [CrossRef]
- Ralph, P. Pulse Amplitude Modulation Fluorometry and the Stress Biology of Reef-Building Corals. 2005. Available online: https://gefcoral.org/portals/25/theme%201%20pam%20fluorescence%20and%20the%20stress%20biology%20of%20reef%20building%20corals.pdf (accessed on 16 January 2025).
- Belgio, E.; Trsková, E.; Kotabová, E.; Ewe, D.; Prášil, O.; Kaňa, R. High light acclimation of Chromera velia points to photoprotective NPQ. Photosynth. Res. 2018, 135, 263–274. [Google Scholar] [CrossRef]
- Jones, R.J.; Hoegh-Guldberg, O.; Larkum, A.W.D.; Schreiber, U. Temperature-induced bleaching of corals begins with impairment of the CO2 fixation mechanism in zooxanthellae. Plant Cell Environ. 2002, 21, 1219–1230. [Google Scholar] [CrossRef]
- Demmig, B.; Björkman, O. Comparison of the effect of excessive light on chlorophyll fluorescence (77K) and photon yield of O2 evolution in leaves of higher plants. Planta 1987, 171, 171–184. [Google Scholar] [CrossRef]
- Chan, A.N.; González-Guerrero, L.A.; Iglesias-Prieto, R.; Burmester, E.M.; Rotjan, R.D.; Finnerty, J.R. An algal symbiont (Breviolum psygmophilum) responds more strongly to chronic high temperatures than its facultatively symbiotic coral host (Astrangia poculata). bioRxiv 2021. [Google Scholar] [CrossRef]
- Camp, E.F.; Kahlke, T.; Signal, B.; Oakley, C.A.; Lutz, A.; Davy, S.K.; Suggett, D.J.; Leggat, W.P. Proteome metabolome and transcriptome data for three Symbiodiniaceae under ambient and heat stress conditions. Sci. Data 2022, 9, 153. [Google Scholar] [CrossRef]
- Burmester, E.M.; Finnerty, J.R.; Kaufman, L.; Rotjan, R.D. Temperature and symbiosis affect lesion recovery in experimentallywounded, facultatively symbiotic temperate corals. Mar. Ecol. Prog. Ser. 2017, 570, 87–99. [Google Scholar] [CrossRef]
- Ferris, Z.; Ribeiro, E.; Nagata, T.; van Woesik, R. ReScape: Transforming coral-reefscape images for quantitative analysis. Sci. Rep. 2024, 14, 8915. [Google Scholar] [CrossRef]
- Chow, M.H.; Tsang, R.H.L.; Lam, E.K.Y.; Ang, P., Jr. Quantifying the degree of coral bleaching using digital photographic technique. J. Exp. Mar. Biol. Ecol. 2016, 479, 60–68. [Google Scholar] [CrossRef]
- DeFilippo, L.; Burmester, E.M.; Kaufman, L.; Rotjan, R.D. Patterns of surface lesion recovery in the northern star coral, Astrangia poculata. J. Exp. Mar. Bio. Ecol. 2016, 481, 15–24. [Google Scholar] [CrossRef]
- Li, S.; Roger, L.M.; Kumar, L.; Lewinski, N.A.; Klein-Seetharaman, J.; Gagnon, A.; Putnam, H.M.; Yang, J. Digital image processing to detect subtle motion in stony coral. Sci. Rep. 2021, 11, 7722. [Google Scholar] [CrossRef]
- Sunoj, S.; Hammed, A.; Igathinathane, C.; Eshkhabilov, S.; Simsek, H. Identification, quantification, and growth profiling of eight different microalgae species using image analysis. Algal Res. 2021, 60, 102487. [Google Scholar] [CrossRef]
- Salgueiro, J.L.; Pérez, L.; Sanchez, Á.; Cancela, Á.; Míguez, C. Microalgal biomass quantification from the non-invasive technique of image processing through red–green–blue (RGB) analysis. J. Appl. Phycol. 2022, 34, 871–881. [Google Scholar] [CrossRef]
- Sarrafzadeh, M.H.; La, H.J.; Seo, S.H.; Asgharnejad, H.; Oh, H.M. Evaluation of various techniques for microalgal biomass quantification. J. Biotechnol. 2015, 216, 90–97. [Google Scholar] [CrossRef]
- Jing, S.; Hu, C. Response of South Florida Estuaries to the 2023 Heatwave, 25 November 2023, PREPRINT (Version 1). Research Square, 2023. [Google Scholar] [CrossRef]
- Neely, K.L.; Nowicki, R.J.; Dobler, M.A.; Chaparro, A.A.; Miller, S.M.; Toth, K.A. Too hot to handle? The impact of the 2023 marine heatwave on Florida Keys coral. bioRxiv 2024. [Google Scholar] [CrossRef]
- Amario, M.; Villela, L.B.; Jardim-Messender, D.; Silva-Lima, A.W.; Rosado, P.M.; Leão de Moura, R.; Sachetto-Martins, G.; Chaloub, R.M.; Salomon, P.S. Physiological response of Symbiodiniaceae to thermal stress: Reactive oxygen species, photosynthesis, and relative cell size. PLoS ONE 2023, 18, e0284717. [Google Scholar] [CrossRef]
- Kihika, J.K.; Wood, S.A.; Rhodes, L.; Smith, K.F.; Butler, J.; Ryan, K.G. Assessment of the recovery and photosynthetic efficiency of Breviolum psygmophilum and Effrenium voratum (Symbiodiniaceae) following cryopreservation. PeerJ 2023, 11, e14885. [Google Scholar] [CrossRef]
- Chen, R.-W.; Li, Z.; Huang, J.; Liu, X.; Zhu, W.; Li, Y.; Wang, A.; Li, X. The community stability of Symbiodiniaceae and bacteria of different morphological corals and linkages to coral susceptibility to anthropogenic disturb-ance. Coral Reefs 2024, 43, 467–481. [Google Scholar] [CrossRef]
- Rädecker, N.; Pogoreutz, C.; Gegner, H.M.; Voolstra, C.R. Heat stress destabilizes symbiotic nutrient cycling in corals. Proc. Natl. Acad. Sci. USA 2021, 118, e2022653118. [Google Scholar] [CrossRef]
- Krämer, W.E.; Iglesias-Prieto, R.; Enríquez, S. Evaluation of the current understanding of the impact of climate change on coral physiology after three decades of experimental research. Commun. Biol. 2022, 5, 1418. [Google Scholar] [CrossRef]
- Turnham, K.E.; Aschaffenburg, M.D.; Pettay, D.T.; Paz-García, D.A.; Reyes-Bonilla, H.; Pinzón, J.; Timmins, E.; Smith, R.T.; McGinley, M.P.; Warner, M.E.; et al. High physiological function for corals with thermally tolerant, host-adapted symbionts. Proc. R. Soc. B 2023, 290, 20231021. [Google Scholar] [CrossRef]
- Aichelman, H.E.; Barshis, D.J. Adaptive divergence, neutral panmixia, and algal symbiont population structure in the temperate coral Astrangia poculata along the Mid-Atlantic United States. PeerJ. 2020, 8, e10201. [Google Scholar] [CrossRef]
- Bent, S.M.; Miller, C.A.; Sharp, K.H.; Hansel, C.M.; Apprill, A. Differential patterns of microbiota recovery in symbiotic and aposymbiotic corals following antibiotic disturbance. mSystems 2021, 6, e01086-20. [Google Scholar] [CrossRef]
- Dimond, J.; Carrington, E. Symbiosis regulation in a facultatively symbiotic temper-ate coral: Zooxanthellae division and expulsion. Coral Reefs 2008, 27, 601–604. [Google Scholar] [CrossRef]
- Berardelli, J.; Never Been So Worried about Florida’s Reefs’: Experts Fear Dangerous Marine Heatwave. Climate Classroom. 2023. Available online: https://www.wfla.com/weather/climate-classroom/record-florida-ocean-temperatures-may-be-death-knell-for-coral-reefs-expert-fears/ (accessed on 16 January 2025).
- NOAA. Rising Ocean Temps Raise New Concerns for Coral Reefs: Q&A with NOAA Coral Reef Watch Director, Dr. Derek Manzello. 2023. Available online: https://www.nesdis.noaa.gov/news/rising-ocean-temps-raise-new-concerns-coral-reefs (accessed on 26 July 2023).
- Brown, A.L.; Sharp, K.; Apprill, A. Reshuffling of the coral microbiome during dormancy. Appl. Environ. Microbiol. 2022, 88, e0139122. [Google Scholar] [CrossRef]
- Hoadley, K.D.; Lewis, A.M.; Wham, D.D.; Petay, D.T.; Grasso, C.; Smith, R.; Kemp, D.W.; LaJeunesse, T.C.; Warner, M.E. Host-symbiont combinations dictate the photo-physiological response of reef-building corals to thermal stress. Sci. Rep. 2019, 9, 9985. [Google Scholar] [CrossRef]
- Jurriaans, S.; Hoogenboom, M.O. Seasonal acclimation of thermal performance in two species of reef-building corals. Mar. Ecol. Prog. Ser. 2020, 635, 55–70. [Google Scholar] [CrossRef]
- Borell, E.M.; Bischof, K. Feeding sustains photosynthetic quantum yield of a scleractinian coral during thermal stress. Oecologia 2008, 157, 593–601. [Google Scholar] [CrossRef]
- Marubini, F.; Davies, P.S. Nitrate increases zooxanthellae population density and re-duces skeletogenesis in corals. Mar. Biol. 1996, 127, 319–328. [Google Scholar] [CrossRef]
- Shantz, A.A.; Burkepile, D.E. Context-dependent effects of nutrient loading on the coral-algal mutualism. Ecology 2014, 95, 1995–2005. [Google Scholar] [CrossRef]
- Gorbunov, M.Y.; Kolber, Z.S.; Lesser, M.P.; Falkowski, P.G. Photosynthesis and photoprotection in symbiotic corals. Limnol. Oceanogr. 2001, 46, 75–85. [Google Scholar] [CrossRef]
- Ralph, P.J.; Gademann, R. Rapid light curves: A powerful tool to assess photosynthetic activity. Aq. Bot. 2005, 82, 222–237. [Google Scholar] [CrossRef]
- Wall, M.; Doering, T.; Pohl, D.; Putchim, L.; Ratanawongwan, T.; Roik, A. Natural thermal stress-hardening of corals through cold temperature pulses in the Thai Andaman Sea. bioRxiv 2023. [Google Scholar] [CrossRef]
- Bowling, C.M. Improving the heat tolerance of vulnerable corals through their algal symbionts. OUR J. ODU Undergrad. Res. J. 2022, 9, 2. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; p. 1535. [Google Scholar] [CrossRef]
- Lesser, M.P. Exposure of symbiotic dinoflagellates to elevated temperatures and ultraviolet radiation causes oxidative stress and photosynthesis. Limnol. Oceanogr. 1996, 41, 271–283. [Google Scholar] [CrossRef]
- Szabó, M.; Larkum, A.W.D.; Vass, I. A Review: The role of reactive oxygen species in mass coral bleaching. In Photosynthesis in Algae: Biochemical and Physiological Mechanisms. Advances in Photosynthesis and Respiration; Larkum, A., Grossman, A., Raven, J., Eds.; Springer: Cham, Switzerland, 2020; Volume 45. [Google Scholar] [CrossRef]
- Stankiewicz, K.H.; Guiglielmoni, N.; Kitchen, S.A.; Flot, J.-F.; Barott, K.L.; Davies, S.W.; Finnerty, J.R.; Grace, S.P.; Kaufman, L.S.; Putnam, H.M.; et al. Genomic comparison of the temperate coral Astrangia poculata with tropical corals yields insights into winter quiescence, innate immunity, and sexual reproduction. bioRxiv. [CrossRef]
- Tate, A.T.; van Cleve, J. Bet-hedging in innate and adaptive immune systems. Evol. Med. Public Health 2022, 10, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Gianella, M.; Bradford, K.J.; Guzzon, F. Ecological, (epi)genetic and physiological aspects of bet-hedging in angiosperms. Plant Reprod. 2021, 34, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.; Deogharia, R.; Sil, S. Influence of the solar penetration depth and heat-fluxes on the sea surface temperature using an ocean mixed layer model. Reg. Stud. Mar. Sci. 2024, 2024, 74. [Google Scholar] [CrossRef]
- Venegas, R.M.; Acevedo, J.; Treml, E.A. Three decades of ocean warming impacts on marine ecosystems: A review and perspective. Deep Sea Res. 2024, 212, 1–19. [Google Scholar] [CrossRef]
- Fuller, A.; Dawson, T.; Helmuth, B.; Hetem, R.S.; Mitchell, D.; Maloney, S.K. Physiological mechanisms in coping with climate change. Physiol. Biochem. Zool. 2010, 83, 713–720. [Google Scholar] [CrossRef]
- Navas-Martín, M.Á.; Cuerdo-Vilches, T.; López-Bueno, J.A.; Díaz, J.; Linares, C.; Sánchez-Martínez, G. Human adaptation to heat in the context of climate change: A conceptual framework. Environ. Res. 2024, 252, 118803. [Google Scholar] [CrossRef]
- van Woesik, R.; Shlesinger, T.; Grottoli, A.G.; Toonen, R.J.; Thurber, R.V.; Warner, M.E.; Hulver, A.M.; Chapron, L.; McLachlan, R.H.; Albright, R.; et al. Coral-bleaching responses to climate change across biological scales. Glob. Change Biol. 2022, 28, 4229–4250. [Google Scholar] [CrossRef]



| Maximum Quantum Yield | ||||||
|---|---|---|---|---|---|---|
| Condition 1 | Condition 2 | p-value | ||||
| Season | Symbiotic State | Temperature | Season | Symbiotic State | Temperature | |
| Summer | Aposymbiotic | 18 °C | Summer | Symbiotic | 18 °C | 2.47×10−9 |
| Summer | Aposymbiotic | 18 °C | Summer | Aposymbiotic | 26 °C | 1 |
| Summer | Aposymbiotic | 18 °C | Winter | Aposymbiotic | 18 °C | 1 |
| Summer | Symbiotic | 18 °C | Summer | Symbiotic | 26 °C | 1 |
| Summer | Symbiotic | 18 °C | Winter | Symbiotic | 18 °C | 1.32×10−6 |
| Summer | Aposymbiotic | 26 °C | Summer | Symbiotic | 26 °C | 2.13×10−10 |
| Summer | Aposymbiotic | 26 °C | Winter | Aposymbiotic | 26 °C | 1 |
| Summer | Symbiotic | 26 °C | Winter | Symbiotic | 26 °C | 4.56×10−6 |
| Winter | Aposymbiotic | 18 °C | Winter | Symbiotic | 18 °C | 1.18×10−1 |
| Winter | Aposymbiotic | 18 °C | Winter | Aposymbiotic | 26 °C | 1 |
| Winter | Symbiotic | 18 °C | Winter | Symbiotic | 26 °C | 1 |
| Winter | Aposymbiotic | 26 °C | Winter | Symbiotic | 26 °C | 2.07×10−7 |
| Photo Quantification | ||||||
| Condition 1 | Condition 2 | p-value | ||||
| Season | Symbiotic State | Temperature | Season | Symbiotic State | Temperature | |
| Summer | Aposymbiotic | 18 °C | Summer | Symbiotic | 18 °C | 1.61×10−54 |
| Summer | Aposymbiotic | 18 °C | Summer | Aposymbiotic | 26 °C | 1×10−3 |
| Summer | Aposymbiotic | 18 °C | Winter | Aposymbiotic | 18 °C | 1 |
| Summer | Symbiotic | 18 °C | Summer | Symbiotic | 26 °C | 1.45×10−49 |
| Summer | Symbiotic | 18 °C | Winter | Symbiotic | 18 °C | 7.98×10−7 |
| Summer | Aposymbiotic | 26 °C | Summer | Symbiotic | 26 °C | 8.32×10−42 |
| Summer | Aposymbiotic | 26 °C | Winter | Aposymbiotic | 26 °C | 1.36×10−1 |
| Summer | Symbiotic | 26 °C | Winter | Symbiotic | 26 °C | 1 |
| Winter | Aposymbiotic | 18 °C | Winter | Symbiotic | 18 °C | 4.98×10−12 |
| Winter | Aposymbiotic | 18 °C | Winter | Aposymbiotic | 26 °C | 4×10−3 |
| Winter | Symbiotic | 18 °C | Winter | Symbiotic | 26 °C | 3×10−3 |
| Winter | Aposymbiotic | 26 °C | Winter | Symbiotic | 26 °C | 2.50×10−6 |
| ROS Fluorescence (Algae) | ||||||
| Condition 1 | Condition 2 | p-value | ||||
| Season | Symbiotic State | Temperature | Season | Symbiotic State | Temperature | |
| Summer | Aposymbiotic | 18 °C | Summer | Aposymbiotic | 26 °C | 6.12×10−1 |
| Summer | Aposymbiotic | 18 °C | Winter | Aposymbiotic | 18 °C | 1.48×10−3 |
| Summer | Aposymbiotic | 26 °C | Winter | Aposymbiotic | 26 °C | 2.17×10−1 |
| Winter | Aposymbiotic | 18 °C | Winter | Aposymbiotic | 26 °C | 9.90×10−1 |
| Summer | Symbiotic | 18 °C | Summer | Symbiotic | 26 °C | 3.12×10−1 |
| Summer | Symbiotic | 18 °C | Winter | Symbiotic | 18 °C | 4.47×10−2 |
| Summer | Symbiotic | 26 °C | Winter | Symbiotic | 26 °C | 4.50×10−4 |
| Winter | Symbiotic | 18 °C | Winter | Symbiotic | 26 °C | 1.35×10−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harman, T.E.; Barshis, D.; Salas, B.H.; Strychar, K.B. Cellular Responses of Astrangia poculata (Ellis and Solander, 1786) and Its Symbiont to Experimental Heat Stress. Water 2025, 17, 411. https://doi.org/10.3390/w17030411
Harman TE, Barshis D, Salas BH, Strychar KB. Cellular Responses of Astrangia poculata (Ellis and Solander, 1786) and Its Symbiont to Experimental Heat Stress. Water. 2025; 17(3):411. https://doi.org/10.3390/w17030411
Chicago/Turabian StyleHarman, Tyler E., Daniel Barshis, Briana Hauff Salas, and Kevin B. Strychar. 2025. "Cellular Responses of Astrangia poculata (Ellis and Solander, 1786) and Its Symbiont to Experimental Heat Stress" Water 17, no. 3: 411. https://doi.org/10.3390/w17030411
APA StyleHarman, T. E., Barshis, D., Salas, B. H., & Strychar, K. B. (2025). Cellular Responses of Astrangia poculata (Ellis and Solander, 1786) and Its Symbiont to Experimental Heat Stress. Water, 17(3), 411. https://doi.org/10.3390/w17030411








