Investigation on Lanthanum Modified Kaolinite for Control of Cyanobacterial Growth and Microcystin Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Treatment of M. aeruginosa by HKL-LH
2.3. Analytical Methods
2.3.1. Kinetic Model
2.3.2. Growth Assessment of M. aeruginosa
2.3.3. Fv/Fm and Cell Integrity Analysis
2.3.4. Determination of Cyanobacterial Cell Morphology
2.3.5. MC-LR Analysis
2.3.6. Measurement of Phosphate and AOMs
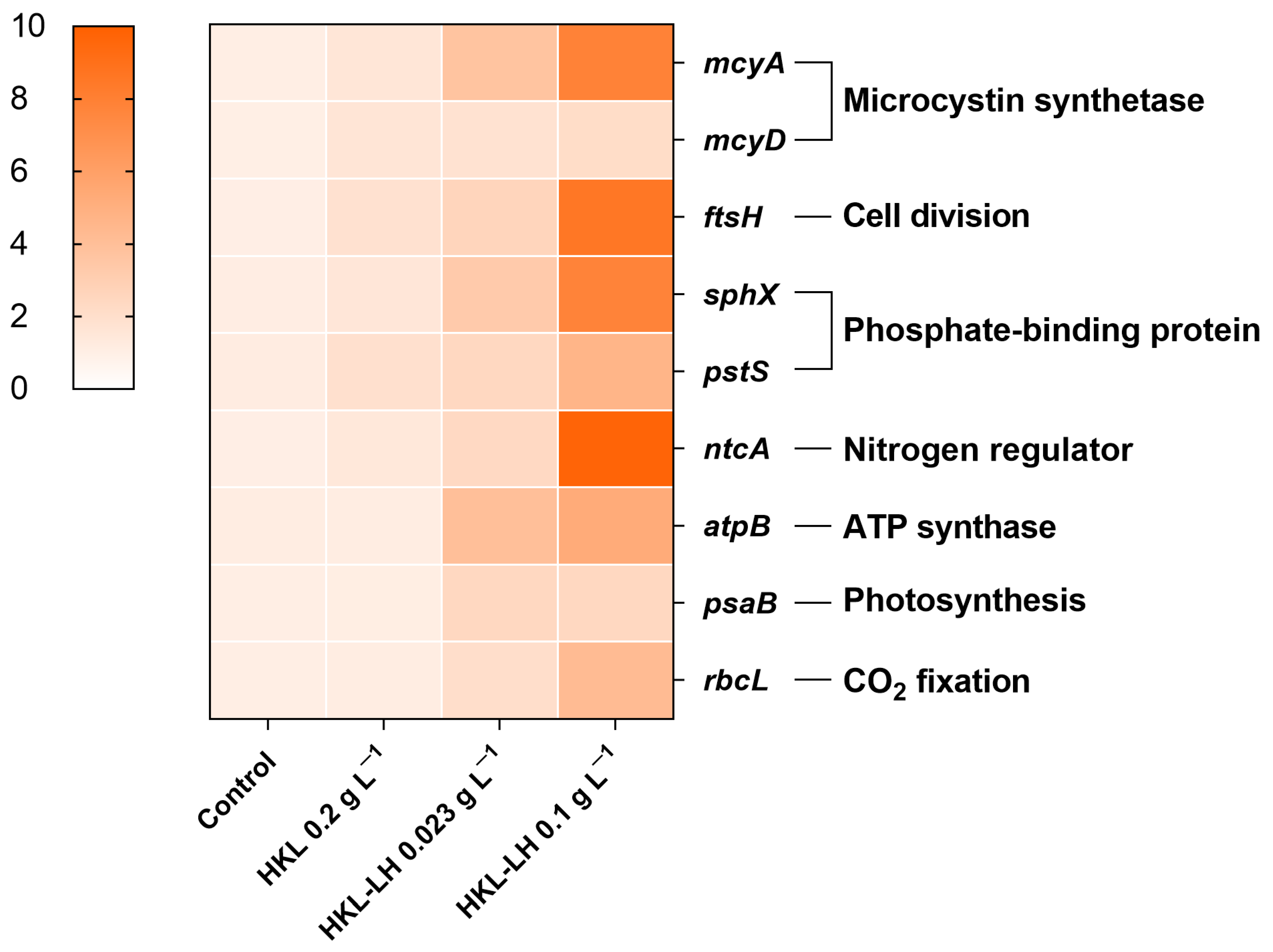
2.3.7. Gene Expression Test
2.4. Statistical Analysis
3. Results and Discussion
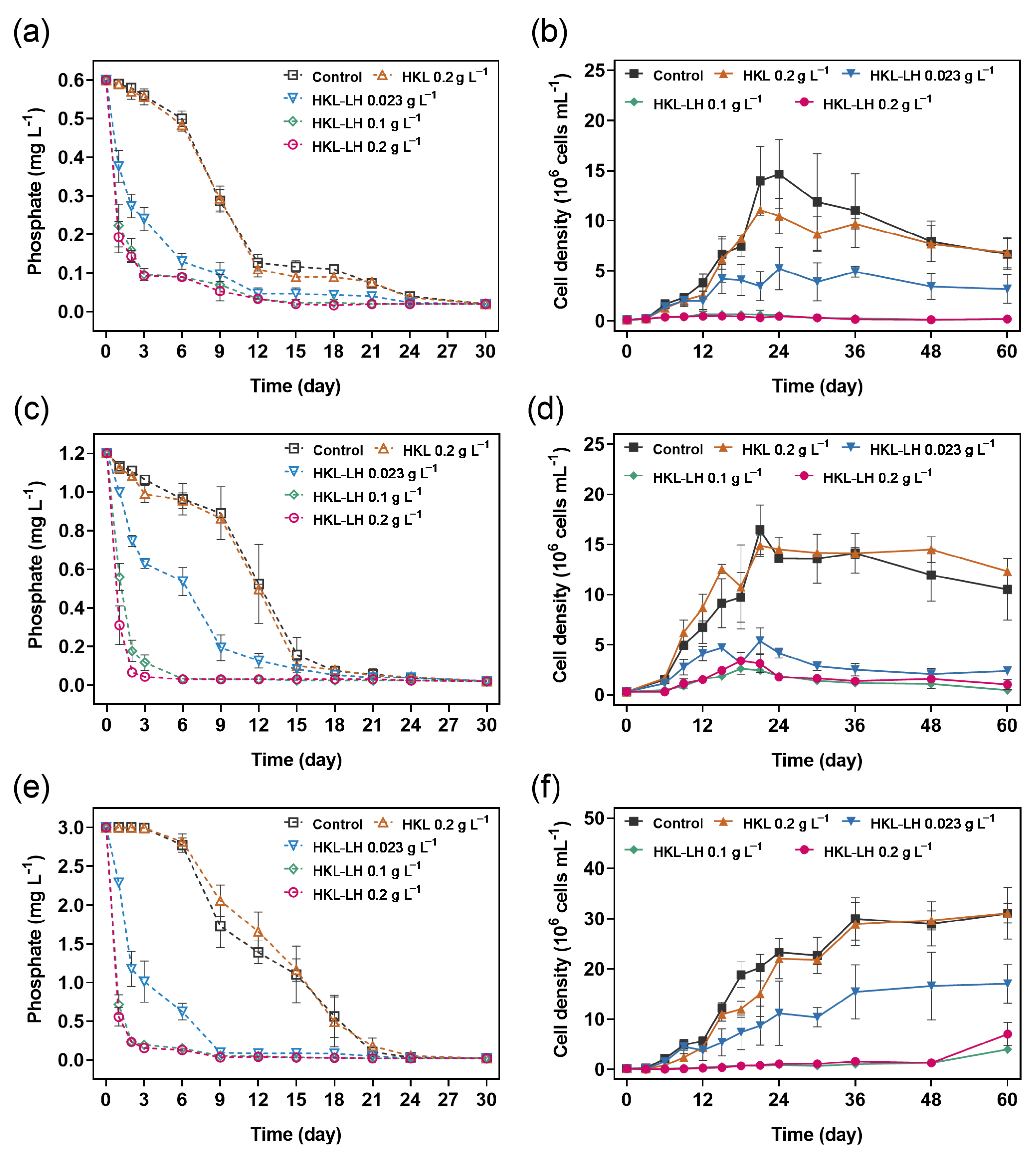
3.1. Phosphate Removal by HKL-LH in M. aeruginosa Samples
3.2. The Impact of HKL-LH on the Growth of M. aeruginosa
3.3. Impacts of HKL-LH on the Viability of M. aeruginosa
3.4. Changes in MCs and AOMs by HKL-LH Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Paerl, H.W.; Huisman, J. Climate. Blooms like it hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Facey, J.A.; Michie, L.E.; King, J.J.; Hitchcock, J.N.; Apte, S.C.; Mitrovic, S.M. Severe cyanobacterial blooms in an Australian lake; causes and factors controlling succession patterns. Harmful. Algae 2022, 117, 102284. [Google Scholar] [CrossRef] [PubMed]
- Cook, K.V.; Li, C.; Cai, H.; Krumholz, L.R.; Hambright, K.D.; Paerl, H.W.; Steffen, M.M.; Wilson, A.E.; Burford, M.A.; Grossart, H.-P.; et al. The global Microcystis interactome. Limnol. Oceanogr. 2020, 65, S194–S207. [Google Scholar] [CrossRef]
- Codd, G.A.; Morrison, L.F.; Metcalf, J.S. Cyanobacterial toxins: Risk management for health protection. Toxicol. Appl. Pharmacol. 2005, 203, 264–272. [Google Scholar] [CrossRef]
- Gonzalez-Torres, A.; Pivokonsky, M.; Henderson, R.K. The impact of cell morphology and algal organic matter on algal floc properties. Water Res. 2019, 163, 114887. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Z.; Rietveld, L.C.; Gao, N.; Hu, J.; Yin, D.; Yu, S. Comparison of the effects of extracellular and intracellular organic matter extracted from Microcystis aeruginosa on ultrafiltration membrane fouling: Dynamics and mechanisms. Environ. Sci. Technol. 2014, 48, 14549–14557. [Google Scholar] [CrossRef]
- Tomlinson, A.; Drikas, M.; Brookes, J.D. The role of phytoplankton as pre-cursors for disinfection by-product formation upon chlorination. Water Res. 2016, 102, 229–240. [Google Scholar] [CrossRef]
- Ihsanullah, I.; Khan, M.T.; Hossain, M.F.; Bilal, M.; Ali Shah, I. Eco-Friendly Solutions to Emerging Contaminants: Unveiling the Potential of Bioremediation in Tackling Microplastic Pollution in Water. Adv. Sustain. Syst. 2024, 8, 2400172. [Google Scholar] [CrossRef]
- Aguilera, A.; Almanza, V.; Haakonsson, S.; Palacio, H.; Benitez Rodas, G.A.; Barros, M.U.G.; Capelo-Neto, J.; Urrutia, R.; Aubriot, L.; Bonilla, S. Cyanobacterial bloom monitoring and assessment in Latin America. Harmful. Algae 2023, 125, 102429. [Google Scholar] [CrossRef]
- Ho, J.C.; Michalak, A.M.; Pahlevan, N. Widespread global increase in intense lake phytoplankton blooms since the 1980s. Nature 2019, 574, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Huo, D.; Gan, N.; Geng, R.; Cao, Q.; Song, L.; Yu, G.; Li, R. Cyanobacterial blooms in China: Diversity, distribution, and cyanotoxins. Harmful. Algae 2021, 109, 102106. [Google Scholar] [CrossRef]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Ecology. Controlling eutrophication: Nitrogen and phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.Q.; Xie, P.; Tang, H.J. Enhancement of dissolved phosphorus release from sediment to lake water by Microcystis blooms--an enclosure experiment in a hyper-eutrophic, subtropical Chinese lake. Environ. Pollut. 2003, 122, 391–399. [Google Scholar] [CrossRef]
- Correll, D.L. The Role of Phosphorus in the Eutrophication of Receiving Waters: A Review. J. Environ. Qual. 1998, 27, 261–266. [Google Scholar] [CrossRef]
- Nollet, L.M.L.; Gelder, L.S.P.D. (Eds.) Handbook of Water Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Bolan, N.S. Removal and Recovery of Phosphate from Water Using Sorption. Crit. Rev. Environ. Sci. Technol. 2014, 44, 847–907. [Google Scholar] [CrossRef]
- Wang, Z.; Xia, D.; Cui, S.; Yu, W.; Wang, B.; Liu, H. A high-capacity nanocellulose aerogel uniformly immobilized with a high loading of nano-La(OH)3 for phosphate removal. Chem. Eng. J. 2022, 433, 134439. [Google Scholar] [CrossRef]
- D’Haese, P.C.; Douglas, G.; Verhulst, A.; Neven, E.; Behets, G.J.; Vervaet, B.A.; Finsterle, K.; Lürling, M.; Spears, B. Human health risk associated with the management of phosphorus in freshwaters using lanthanum and aluminium. Chemosphere 2019, 220, 286–299. [Google Scholar] [CrossRef]
- Ma, C.; Hu, W.; Pei, H.; Xu, H.; Pei, R. Enhancing integrated removal of Microcystis aeruginosa and adsorption of microcystins using chitosan-aluminum chloride combined coagulants: Effect of chemical dosing orders and coagulation mechanisms. Colloids Surf. A Physicochem. Eng. Asp. 2016, 490, 258–267. [Google Scholar] [CrossRef]
- Reitzel, K.; Andersen, F.Ø.; Egemose, S.; Jensen, H.S. Phosphate adsorption by lanthanum modified bentonite clay in fresh and brackish water. Water Res. 2013, 47, 2787–2796. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Ren, C.; Li, W. Introducing hydrate aluminum into porous thermally-treated calcium-rich attapulgite to enhance its phosphorus sorption capacity for sediment internal loading management. Chem. Eng. J. 2018, 348, 704–712. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Omer, A.M.; El-Aqapa, H.G.; Gaber, N.M.; Attia, N.F.; El-Subruiti, G.M.; Mohy-Eldin, M.S.; Abd El-Monaem, E.M. Chitosan based adsorbents for the removal of phosphate and nitrate: A critical review. Carbohydr. Polym. 2021, 274, 118671. [Google Scholar] [CrossRef] [PubMed]
- Lürling, M.; Noyma, N.P.; de Magalhães, L.; Miranda, M.; Mucci, M.; van Oosterhout, F.; Huszar, V.L.M.; Marinho, M.M. Critical assessment of chitosan as coagulant to remove cyanobacteria. Harmful. Algae 2017, 66, 1–12. [Google Scholar] [CrossRef]
- Awad, M.E.; López-Galindo, A.; Setti, M.; El-Rahmany, M.M.; Iborra, C.V. Kaolinite in pharmaceutics and biomedicine. Int. J. Pharm. 2017, 533, 34–48. [Google Scholar] [CrossRef]
- Detellier, C. Functional Kaolinite. Chem. Rec. 2018, 18, 868–877. [Google Scholar] [CrossRef]
- Johnson, E.B.G.; Arshad, S.E. Hydrothermally synthesized zeolites based on kaolinite: A review. Appl. Clay Sci. 2014, 97–98, 215–221. [Google Scholar] [CrossRef]
- Dill, H.G. Kaolin: Soil, rock and ore: From the mineral to the magmatic, sedimentary and metamorphic environments. Earth Sci. Rev. 2016, 161, 16–129. [Google Scholar] [CrossRef]
- García, K.I.; Quezada, G.R.; Arumí, J.L.; Toledo, P.G. Phosphate aggregation, diffusion, and adsorption on kaolinite in saline solutions by molecular dynamics simulation. Appl. Clay Sci. 2023, 233, 106844. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Z.; Lu, S.; Wu, D.; Zhang, Z.; Kong, H. Removal and recovery of phosphate from water by lanthanum hydroxide materials. Chem. Eng. J. 2014, 254, 163–170. [Google Scholar] [CrossRef]
- Yang, Z.; Hou, J.; Pan, Z.; Wu, M.; Zhang, M.; Yin, X.; Wu, J.; Miao, L.; Liu, Q. A novel La(OH)3 decorated co-graft tannin and polyethyleneimine co-coating magnetic adsorbent for effective and selective phosphate removal from natural water and real wastewater. J. Clean. Prod. 2022, 369, 133345. [Google Scholar] [CrossRef]
- Zheng, S.; Fan, J.; Lu, X. Heated kaolinite-La(III) hydroxide complex for effective removal of phosphate from eutrophic water. Appl. Clay Sci. 2023, 231, 106729. [Google Scholar] [CrossRef]
- Lürling, M.; Faassen, E.J. Controlling toxic cyanobacteria: Effects of dredging and phosphorus-binding clay on cyanobacteria and microcystins. Water Res. 2012, 46, 1447–1459. [Google Scholar] [CrossRef]
- Peschek, G.A.; Obinger, C.; Renger, G. (Eds.) Bioenergetic Processes of Cyanobacteria; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Briddon, C.L.; Szekeres, E.; Hegedüs, A.; Nicoară, M.; Chiriac, C.; Stockenreiter, M.; Drugă, B. The combined impact of low temperatures and shifting phosphorus availability on the competitive ability of cyanobacteria. Sci. Rep. 2022, 12, 16409. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Otten, T.G. Harmful cyanobacterial blooms: Causes, consequences, and controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Bacelo, H.; Pintor, A.M.A.; Santos, S.C.R.; Boaventura, R.A.R.; Botelho, C.M.S. Performance and prospects of different adsorbents for phosphorus uptake and recovery from water. Chem. Eng. J. 2020, 381, 122566. [Google Scholar] [CrossRef]
- Sun, F.; Pei, H.-Y.; Hu, W.-R.; Ma, C.-X. The lysis of Microcystis aeruginosa in AlCl3 coagulation and sedimentation processes. Chem. Eng. J. 2012, 193–194, 196–202. [Google Scholar] [CrossRef]
- Du, H.; Chen, Z.; Mao, G.; Chen, L.; Crittenden, J.; Li, R.Y.M.; Chai, L. Evaluation of eutrophication in freshwater lakes: A new non-equilibrium statistical approach. Ecol. Indic 2019, 102, 686–692. [Google Scholar] [CrossRef]
- de Farias Silva, C.E.; de Oliveira Cerqueira, R.B.; de Lima Neto, C.F.; de Andrade, F.P.; de Oliveira Carvalho, F.; Tonholo, J. Developing a kinetic model to describe wastewater treatment by microalgae based on simultaneous carbon, nitrogen and phosphorous removal. J. Environ. Chem. Eng. 2020, 8, 103792. [Google Scholar] [CrossRef]
- Fan, J.; Ho, L.; Hobson, P.; Brookes, J. Evaluating the effectiveness of copper sulphate, chlorine, potassium permanganate, hydrogen peroxide and ozone on cyanobacterial cell integrity. Water Res. 2013, 47, 5153–5164. [Google Scholar] [CrossRef]
- Macedo, R.S.; Lombardi, A.T.; Omachi, C.Y.; Rörig, L.R. Effects of the herbicide bentazon on growth and photosystem II maximum quantum yield of the marine diatom Skeletonema costatum. Toxicol. Vitr. 2008, 22, 716–722. [Google Scholar] [CrossRef]
- Wu, H.; Wei, G.; Tan, X.; Li, L.; Li, M. Species-dependent variation in sensitivity of Microcystis species to copper sulfate: Implication in algal toxicity of copper and controls of blooms. Sci. Rep. 2017, 7, 40393. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.; Hurry, V.; Clarke, A.K.; Gustafsson, P.; Öquist, G. Chlorophyll Fluorescence Analysis of Cyanobacterial Photosynthesis and Acclimation. Microbiol. Mol. Biol. Rev. 1998, 62, 667–683. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Yu, X.; Fang, J.; Fan, J. Influences of the micropollutant erythromycin on cyanobacteria treatment with potassium permanganate. Water Res. 2020, 177, 115786. [Google Scholar] [CrossRef]
- Nicholson, B.C.; Rositano, J.; Burch, M.D. Destruction of cyanobacterial peptide hepatotoxins by chlorine and chloramine. Water Res. 1994, 28, 1297–1303. [Google Scholar] [CrossRef]
- Knochen, M.; Rodríguez-Silva, J.C.; Silva-Silva, J. Exploitation of reaction mechanisms for sensitivity enhancement in the determination of phosphorus by sequential injection analysis. Talanta 2020, 209, 120589. [Google Scholar] [CrossRef]
- Li, L.; Gao, N.; Deng, Y.; Yao, J.; Zhang, K. Characterization of intracellular & extracellular algae organic matters (AOM) of Microcystic aeruginosa and formation of AOM-associated disinfection byproducts and odor & taste compounds. Water Res. 2012, 46, 1233–1240. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zhao, H.; Teng, Z.; Wang, Y.; Li, M.; Hoffmann, M.R. Phosphate removal and recovery by lanthanum-based adsorbents: A review for current advances. Chemosphere 2022, 303, 134987. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Yu, W.; Huang, H.; Han, J.-C.; Huang, Y.; Wu, X.; Young, B.; Wang, G. Lanthanum-based adsorbents for phosphate reutilization: Interference factors, adsorbent regeneration, and research gaps. Sustain. Horiz. 2022, 1, 100011. [Google Scholar] [CrossRef]
- de Lucena-Silva, D.; Molozzi, J.; Severiano, J.D.S.; Becker, V.; de Lucena Barbosa, J.E. Removal efficiency of phosphorus, cyanobacteria and cyanotoxins by the “flock & sink” mitigation technique in semi-arid eutrophic waters. Water Res. 2019, 159, 262–273. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Yang, Q.; Wang, D.; Xu, Q.; Yao, F.; Chen, F.; Tao, Z.; Huang, X. Hydrated lanthanum oxide-modified diatomite as highly efficient adsorbent for low-concentration phosphate removal from secondary effluents. J. Environ. Manag. 2019, 231, 370–379. [Google Scholar] [CrossRef]
- Correll, D.L. Phosphorus: A rate limiting nutrient in surface waters. Poult. Sci. 1999, 78, 674–682. [Google Scholar] [CrossRef]
- Ren, L.; Li, Y.; Wang, K.; Ding, K.; Sha, M.; Cao, Y.; Kong, F.; Wang, S. Recovery of phosphorus from eutrophic water using nano zero-valent iron-modified biochar and its utilization. Chemosphere 2021, 284, 131391. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Chen, Z.; Yuen Koh, K.; Cui, F.; Paul Chen, J. Development and application of lanthanum peroxide loaded sepiolite nanocomposites for simultaneous removal of phosphate and inhibition of cyanobacteria growth. J. Colloid Interface Sci. 2022, 624, 691–703. [Google Scholar] [CrossRef]
- Kang, L.; Mucci, M.; Lürling, M. Compounds to mitigate cyanobacterial blooms affect growth and toxicity of Microcystis aeruginosa. Harmful. Algae 2022, 118, 102311. [Google Scholar] [CrossRef]
- Graham, J.L.; Loftin, K.A.; Ziegler, A.C.; Meyer, M.T. Guidelines for Design and Sampling for Cyanobacterial Toxin and Taste-and-Odor Studies in Lakes and Reservoirs; Scientific Investigations Report No. 2008–5038; USGS: Reston, VA, USA, 2008. [Google Scholar] [CrossRef]
- Sipka, G.B.; Magyar, M.; Mezzetti, A.; Akhtar, P.; Zhu, Q.; Xiao, Y.; Han, G.; Santabarbara, S.; Shen, J.-R.; Lambrev, P.H.; et al. Light-adapted charge-separated state of photosystem II: Structural and functional dynamics of the closed reaction center. Plant Cell 2021, 33, 1286–1302. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Lin, Q.; Liu, B.; Huang, S.; Yan, W.; Zhang, L.; Ge, F.; Zhang, Y.; Wu, Z. Effect of submerged plant coverage on phytoplankton community dynamics and photosynthetic activity in situ. J. Environ. Manag. 2022, 301, 113822. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zeng, B.; Li, R.; Song, L. Physiological regulation of Cylindrospermopsis raciborskii (Nostocales, Cyanobacteria) in response to inorganic phosphorus limitation. Harmful. Algae 2012, 15, 53–58. [Google Scholar] [CrossRef]
- Butusov, M.; Jernelöv, A. Phosphorus in the Organic Life: Cells, Tissues, Organisms. In Phosphorus: An Element That Could Have Been Called Lucifer; Butusov, M., Jernelöv, A., Eds.; Springer: New York, NY, USA, 2013; pp. 13–17. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, G.; Wang, Y.; Guo, C.; Zhou, J. Physiological and molecular responses of Prorocentrum donghaiense to dissolved inorganic phosphorus limitation. Mar. Pollut. Bull. 2018, 129, 562–572. [Google Scholar] [CrossRef]
- Song, Q.; Huang, S.; Xu, L.; Li, Q.; Luo, X.; Zheng, Z. Response of Magnetite/Lanthanum hydroxide composite on cyanobacterial bloom. Chemosphere 2021, 275, 130017. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, M.; Zhang, J.; Shi, W.; Mynett, A.E.; Yan, H.; Hu, L. Physiological effects of nitrate, ammonium, and urea on the growth and microcystins contamination of Microcystis aeruginosa: Implication for nitrogen mitigation. Water Res. 2019, 163, 114890. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, G.; Fastner, J.; Erhard, M.; Börner, T.; Dittmann, E. Microcystin biosynthesis in planktothrix: Genes, evolution, and manipulation. J. Bacteriol. 2003, 185, 564–572. [Google Scholar] [CrossRef] [PubMed]
- MacKeigan, P.W.; Zastepa, A.; Taranu, Z.E.; Westrick, J.A.; Liang, A.; Pick, F.R.; Beisner, B.E.; Gregory-Eaves, I. Microcystin concentrations and congener composition in relation to environmental variables across 440 north-temperate and boreal lakes. Sci. Total Environ. 2023, 884, 163811. [Google Scholar] [CrossRef]
- Xin, R.; Yu, X.; Fan, J. Physiological, biochemical and transcriptional responses of cyanobacteria to environmentally relevant concentrations of a typical antibiotic-roxithromycin. Sci. Total Environ. 2022, 814, 152703. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Li, Z.; Yan, F.; An, L.; Du, W.; Li, X. Evaluation of changes in M. aeruginosa growth and microcystin production under phosphorus starvation via transcriptomic surveys. Sci. Total Environ. 2023, 893, 164848. [Google Scholar] [CrossRef]
- Rantala, A.; Fewer, D.P.; Hisbergues, M.; Rouhiainen, L.; Vaitomaa, J.; Börner, T.; Sivonen, K. Phylogenetic evidence for the early evolution of microcystin synthesis. Proc. Natl. Acad. Sci. USA 2004, 101, 568–573. [Google Scholar] [CrossRef]
- Wang, X.; Qian, Y.; Chen, Y.; Liu, F.; An, D.; Yang, G.; Dai, R. Application of fluorescence spectra and molecular weight analysis in the identification of algal organic matter-based disinfection by-product precursors. Sci. Total Environ. 2023, 882, 163589. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Shao, Y.; Gao, N.; Deng, Y.; Li, L.; Deng, J.; Tan, C. Characterization of algal organic matters of Microcystis aeruginosa: Biodegradability, DBP formation and membrane fouling potential. Water Res. 2014, 52, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Harke, M.J.; Berry, D.L.; Ammerman, J.W.; Gobler, C.J. Molecular response of the bloom-forming cyanobacterium, Microcystis aeruginosa, to phosphorus limitation. Microb. Ecol. 2012, 63, 188–198. [Google Scholar] [CrossRef]
- Kaebernick, M.; Neilan, B.A.; Börner, T.; Dittmann, E. Light and the transcriptional response of the microcystin biosynthesis gene cluster. Appl. Environ. Microbiol. 2000, 66, 3387–3392. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Kang, X.; Chu, L.; Wang, Y.; Song, X.; Zhao, X.; Cao, X. Algicidal mechanism of Raoultella ornithinolytica against Microcystis aeruginosa: Antioxidant response, photosynthetic system damage and microcystin degradation. Environ. Pollut. 2021, 287, 117644. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, J.; Yu, Y.; Shi, L.; Kong, F. Changes in the physiology and gene expression of Microcystis aeruginosa under EGCG stress. Chemosphere 2014, 117, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Muro-Pastor, M.I.; Florencio, F.J. Regulation of ammonium assimilation in cyanobacteria. Plant Physiol. Biochem. 2003, 41, 595–603. [Google Scholar] [CrossRef]
- Pimentel, J.S.M.; Giani, A. Microcystin production and regulation under nutrient stress conditions in toxic microcystis strains. Appl. Environ. Microbiol. 2014, 80, 5836–5843. [Google Scholar] [CrossRef]
- Qian, H.; Yu, S.; Sun, Z.; Xie, X.; Liu, W.; Fu, Z. Effects of copper sulfate, hydrogen peroxide and N-phenyl-2-naphthylamine on oxidative stress and the expression of genes involved photosynthesis and microcystin disposition in Microcystis aeruginosa. Aquat. Toxicol. 2010, 99, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Rinta-Kanto, J.M.; Ouellette, A.J.A.; Boyer, G.L.; Twiss, M.R.; Bridgeman, T.B.; Wilhelm, S.W. Quantification of toxic Microcystis spp. during the 2003 and 2004 blooms in western Lake Erie using quantitative real-time PCR. Environ. Sci. Technol. 2005, 39, 4198–4205. [Google Scholar] [CrossRef]




| [HKL-LH] (g L−1) | Initial Phosphate Concentrations (mg L−1) | |||||
|---|---|---|---|---|---|---|
| 0.6 | 1.2 | 3.0 | ||||
| k (L mg−1 d−1) | R2 | k (L mg−1 d−1) | R2 | k (L mg−1 d−1) | R2 | |
| 0.023 | 0.86 | 0.9908 | 0.21 | 0.9853 | 0.22 | 0.9721 |
| 0.1 | 2.68 | 0.9904 | 2.38 | 0.9704 | 1.67 | 0.9823 |
| 0.2 | 2.91 | 0.9943 | 6.93 | 0.9670 | 2.00 | 0.9943 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, Y.; Zheng, S.; Lu, X.; Zhang, K.; Fan, J. Investigation on Lanthanum Modified Kaolinite for Control of Cyanobacterial Growth and Microcystin Production. Water 2025, 17, 428. https://doi.org/10.3390/w17030428
Miao Y, Zheng S, Lu X, Zhang K, Fan J. Investigation on Lanthanum Modified Kaolinite for Control of Cyanobacterial Growth and Microcystin Production. Water. 2025; 17(3):428. https://doi.org/10.3390/w17030428
Chicago/Turabian StyleMiao, Yige, Songhai Zheng, Xiancai Lu, Kejia Zhang, and Jiajia Fan. 2025. "Investigation on Lanthanum Modified Kaolinite for Control of Cyanobacterial Growth and Microcystin Production" Water 17, no. 3: 428. https://doi.org/10.3390/w17030428
APA StyleMiao, Y., Zheng, S., Lu, X., Zhang, K., & Fan, J. (2025). Investigation on Lanthanum Modified Kaolinite for Control of Cyanobacterial Growth and Microcystin Production. Water, 17(3), 428. https://doi.org/10.3390/w17030428







