Determination of the Occurrence of Trihalomethanes in the Drinking Water Supply of the City of Cuenca, Ecuador
Abstract
1. Introduction
2. Materials and Methods
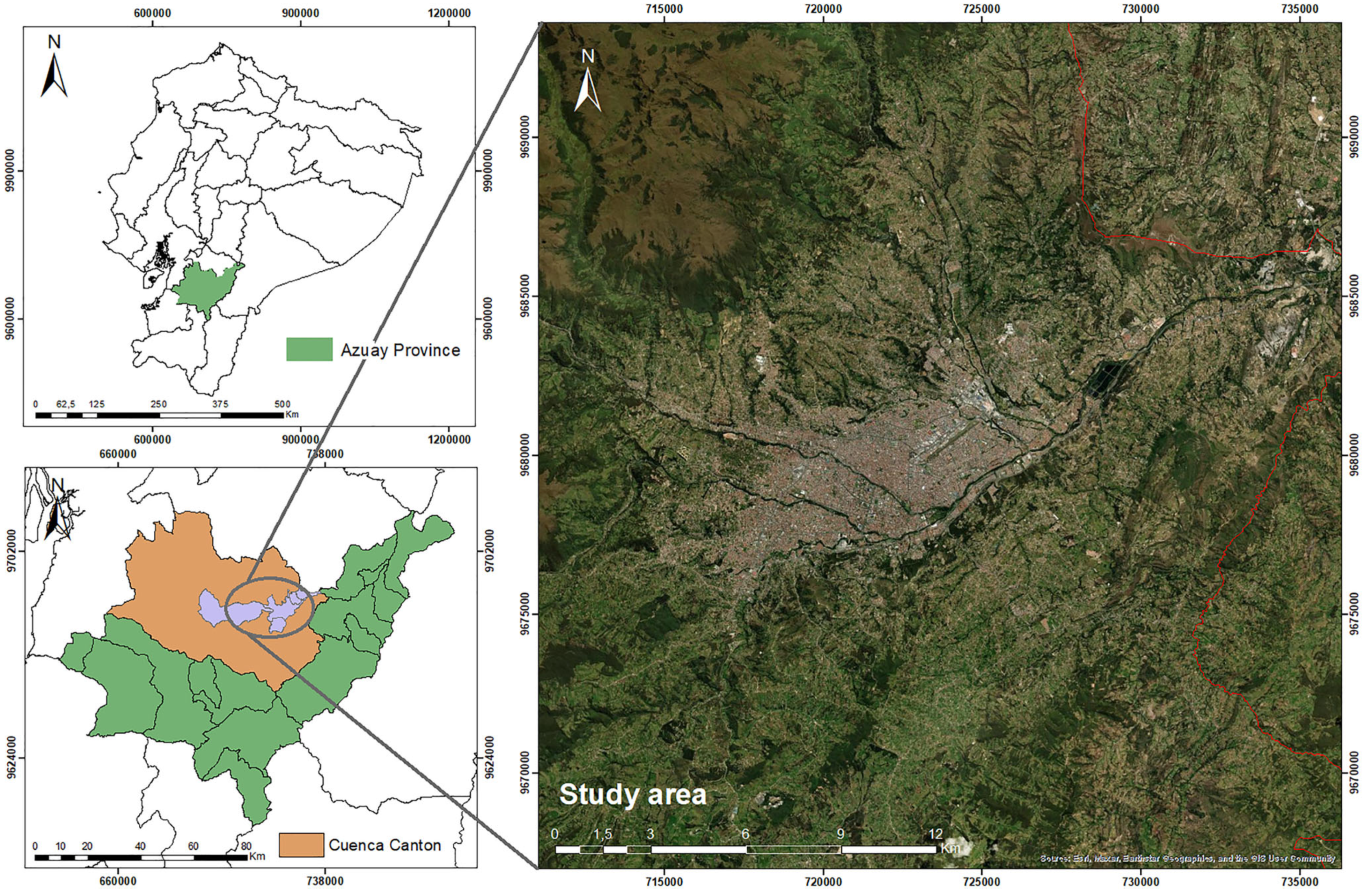
2.1. Description of the Study Area
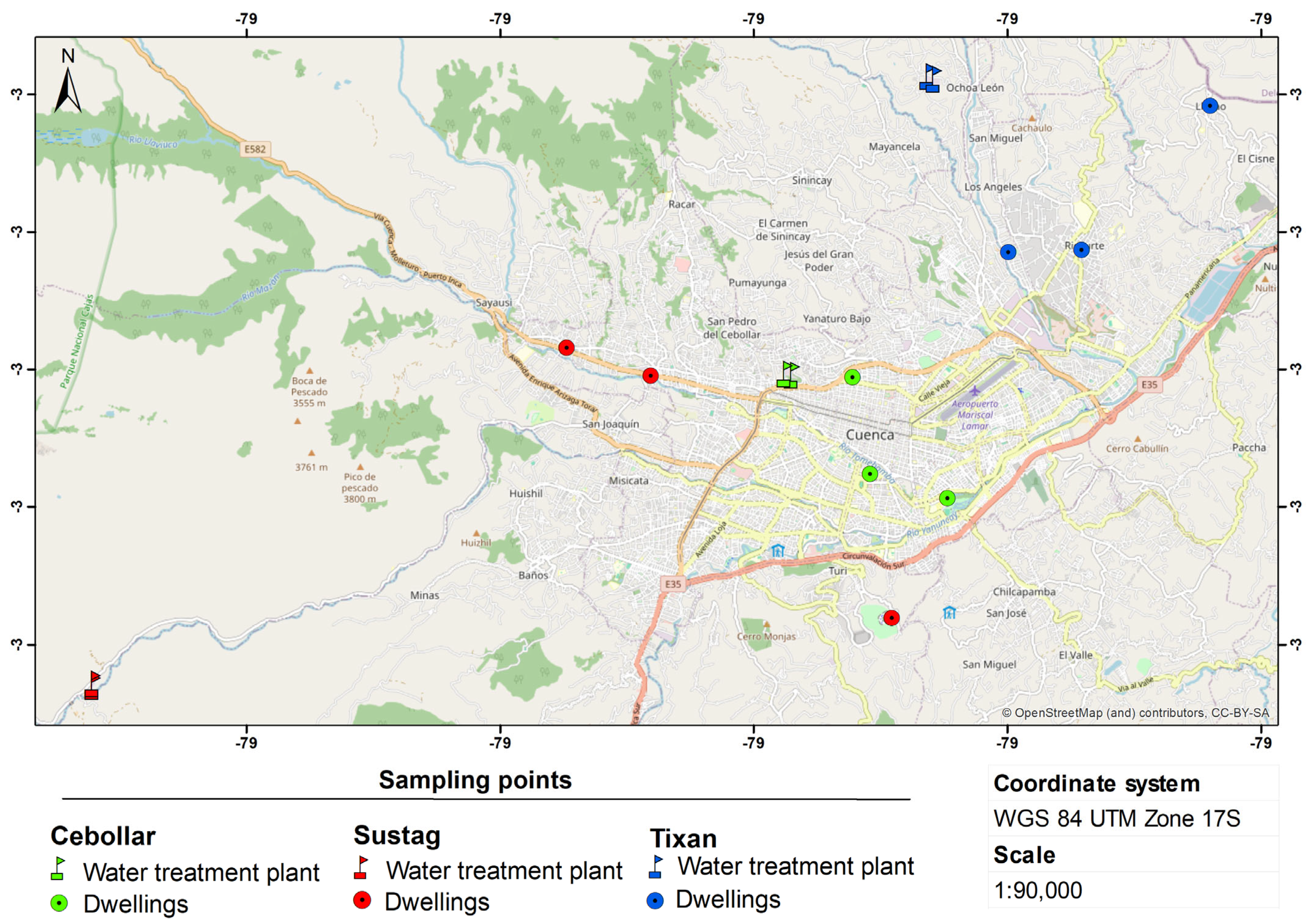
2.2. Sampling
2.3. Determination of CHCl3
2.4. Statistical Analysis
3. Results
3.1. Physicochemical Characteristics of Water Quality
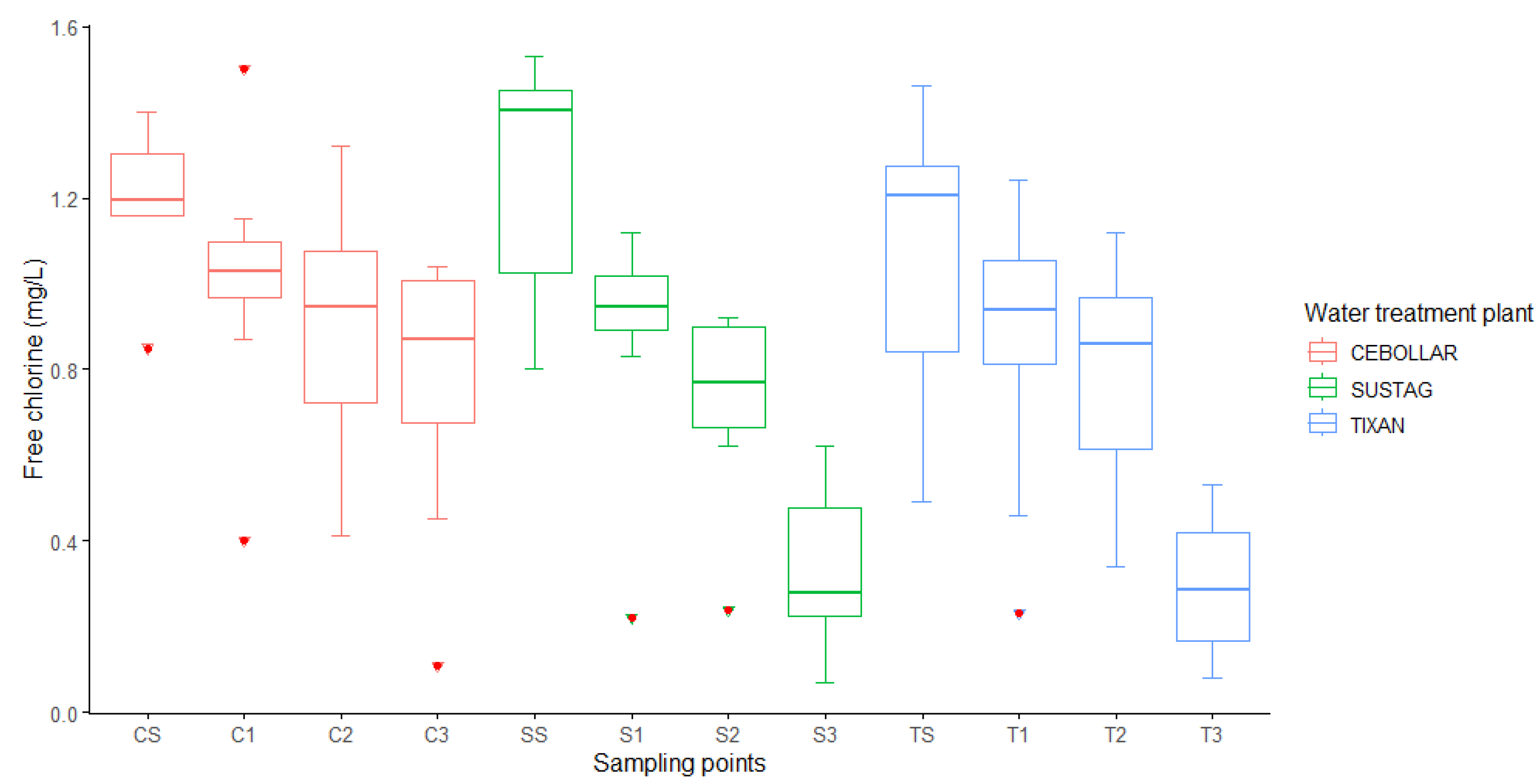
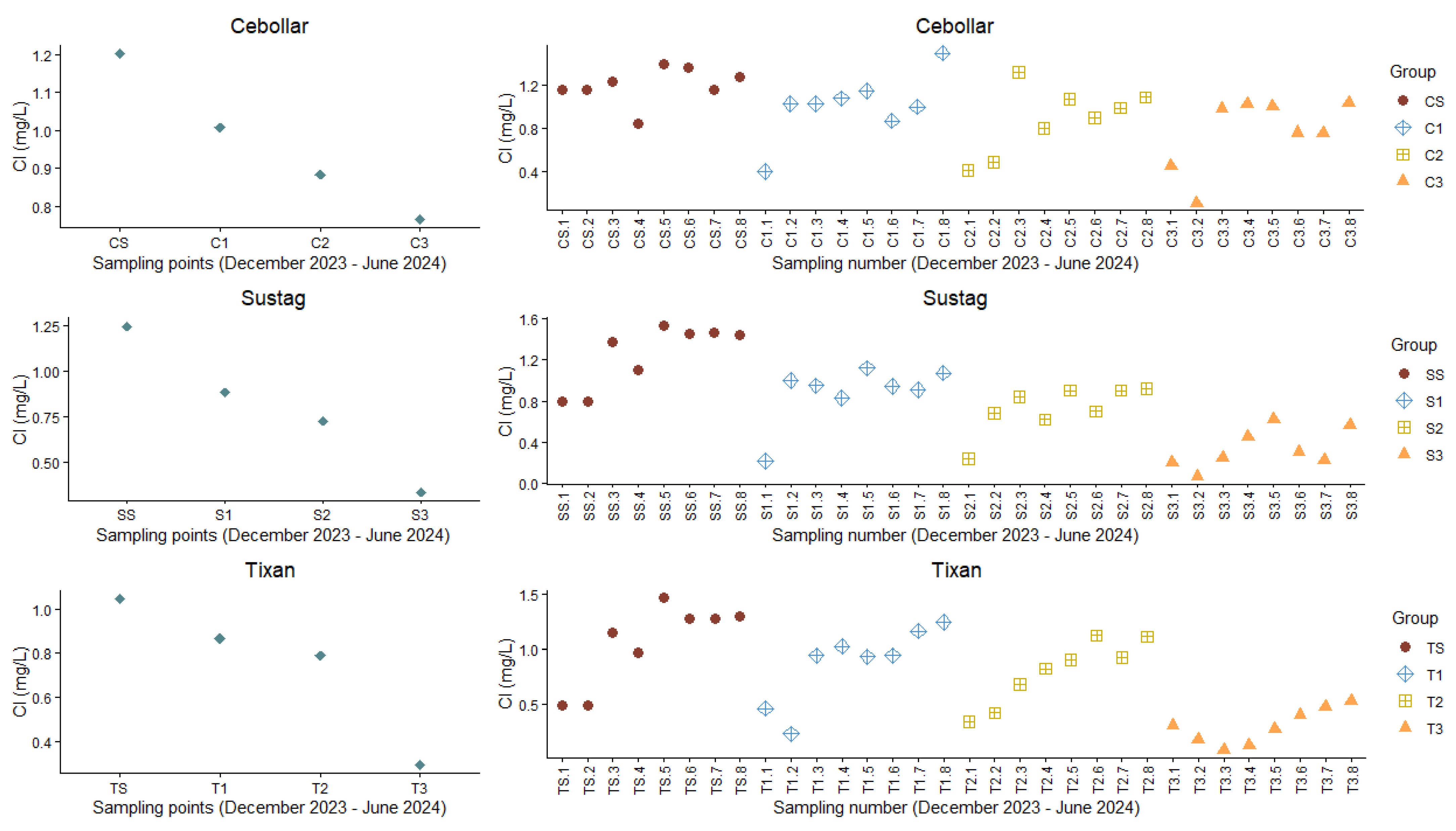
3.2. Free Chlorine Levels
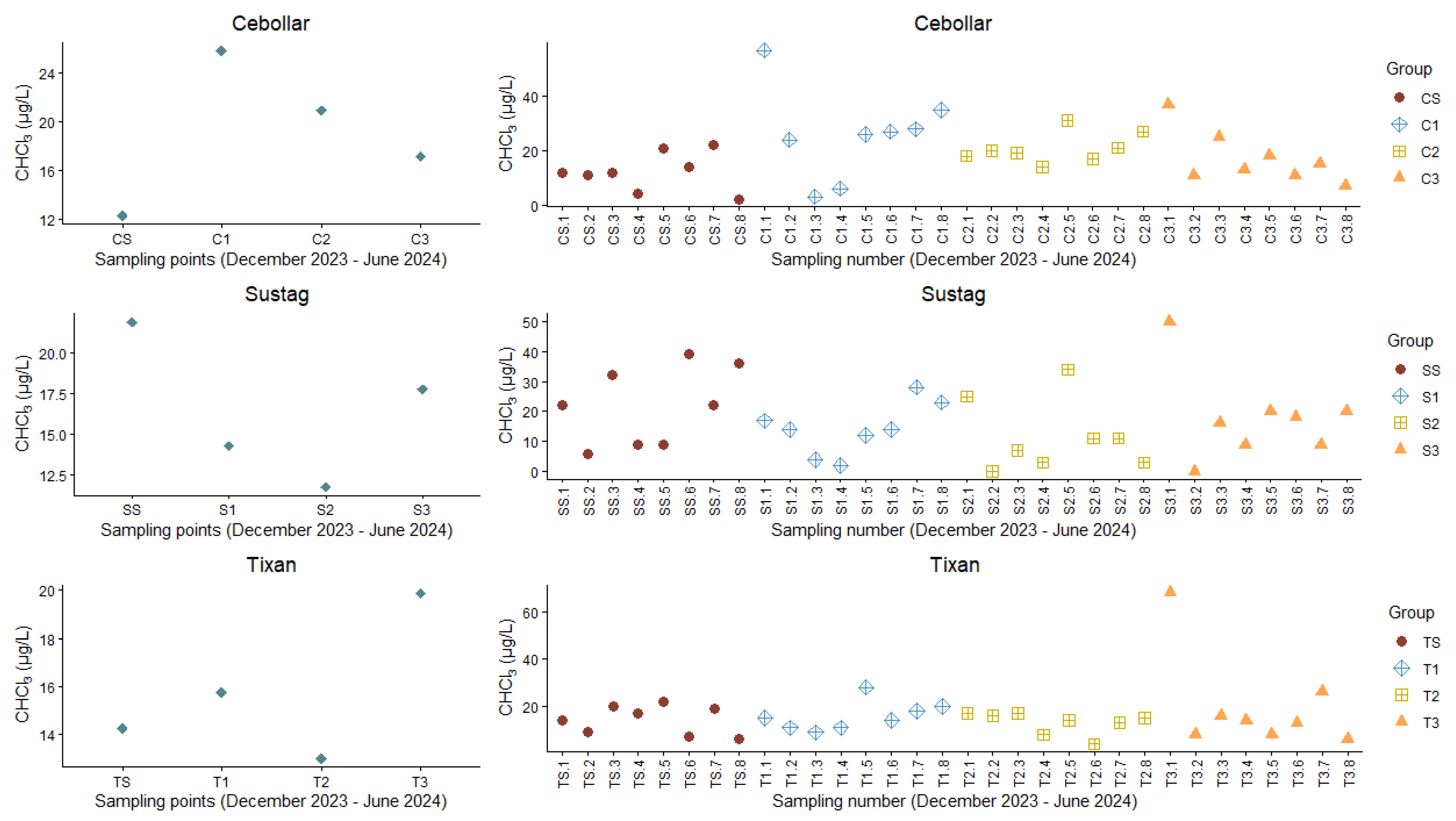
3.3. CHCl3 Concentrations
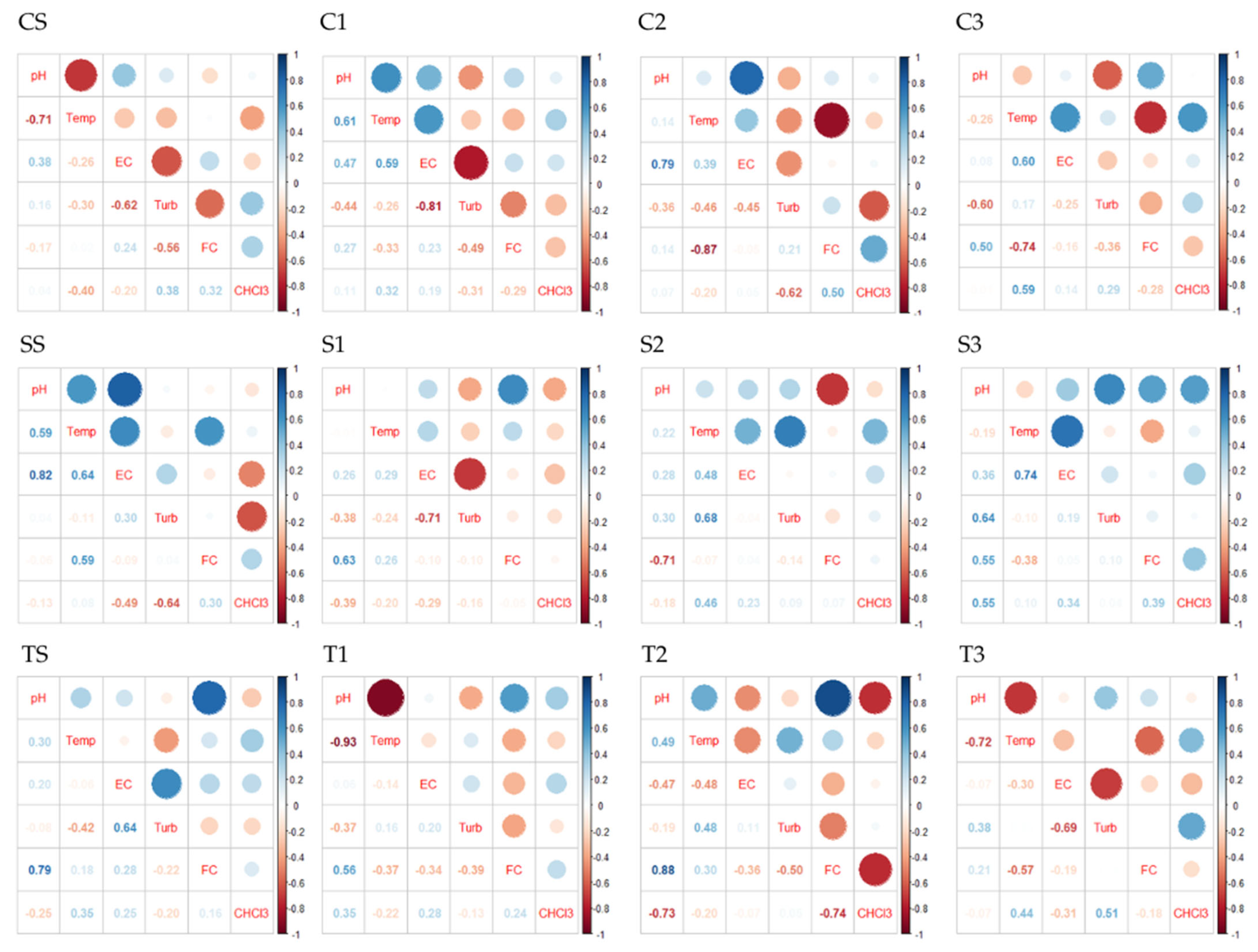
3.4. Spearman’s Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duque-Sarango, P.; Romero-Martínez, L.; Pinos-Vélez, V.; Sánchez-Cordero, E.; Samaniego, E. Comparative Study of UV Radiation Resistance and Reactivation Characteristics of E. coli ATCC 8739 and Native Strains: Implications for Water Disinfection. Sustainability 2023, 15, 9559. [Google Scholar] [CrossRef]
- Duque-Sarango, P.; Delgado-Armijos, N.; Romero-Martínez, L.; Pinos-Vélez, V. Assessing the potential of ultraviolet irradiation for inactivating waterborne fungal spores: Kinetics and photoreactivation studies. Front. Environ. Sci. 2023, 11, 1212807. [Google Scholar] [CrossRef]
- Cheswick, R.; Moore, G.; Nocker, A.; Hassard, F.; Jefferson, B.; Jarvis, P. Chlorine disinfection of drinking water assessed by flow cytometry: New insights. Environ. Technol. Innov. 2020, 19, 101032. [Google Scholar] [CrossRef]
- Srivastav, A.L.; Patel, N.; Chaudhary, V.K. Disinfection by-products in drinking water: Occurrence, toxicity and abatement. Environ. Pollut. 2020, 267, 115474. [Google Scholar] [CrossRef]
- Romero-Martínez, L.; Duque-Sarango, P.; González-Martín, C.; Moreno-Andrés, J.; Acevedo-Merino, A.; Nebot, E. Inactivation efficacy and reactivation of fecal bacteria with a flow-through LED ultraviolet reactor: Intraspecific response prevails over interspecific differences. J. Water Process Eng. 2023, 52, 103497. [Google Scholar] [CrossRef]
- WHO. “Drinking-Water”, World Health Organization. Available online: https://lc.cx/wNxWH5 (accessed on 29 September 2024).
- Chawla, H.; Chandra, A.; Ingole, P.P.; Garg, S. Recent advancements in enhancement of photocatalytic activity using bismuth-based metal oxides Bi2MO6 (M = W, Mo, Cr) for environmental remediation and clean energy production. J. Ind. Eng. Chem. 2021, 95, 1–15. [Google Scholar] [CrossRef]
- Tsitsifli, S.; Kanakoudis, V. Disinfection Impacts to Drinking Water Safety—A Review. In EWaS3 2018; MDPI: Basel Switzerland, 2018; p. 603. [Google Scholar] [CrossRef]
- Dubey, S.; Gusain, D.; Sharma, Y.C.; Bux, F. The occurrence of various types of disinfectant by-products (trihalomethanes, haloacetic acids, haloacetonitrile) in drinking water. In Disinfection By-Products in Drinking Water; Elsevier: Amsterdam, The Netherlands, 2020; pp. 371–391. [Google Scholar] [CrossRef]
- Villanueva, C.M.; Evlampidou, I.; Ibrahim, F.; Donat-Vargas, C.; Valentin, A.; Tugulea, A.M.; Echigo, S.; Jovanovic, D.; Lebedev, A.T.; Lemus-Pérez, M.; et al. Global assessment of chemical quality of drinking water: The case of trihalomethanes. Water Res. 2023, 230, 119568. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.; Bridgeman, J.; West, J.R. Predicting chlorine decay and THM formation in water supply systems. Rev. Environ. Sci. Biotechnol. 2011, 10, 79–99. [Google Scholar] [CrossRef]
- Hu, G.; Mian, H.R.; Mohammadiun, S.; Rodriguez, M.J.; Hewage, K.; Sadiq, R. Appraisal of machine learning techniques for predicting emerging disinfection byproducts in small water distribution networks. J. Hazard. Mater. 2023, 446, 130633. [Google Scholar] [CrossRef] [PubMed]
- Sathasivan, A.; Kastl, G.; Korotta-Gamage, S.; Gunasekera, V. Trihalomethane species model for drinking water supply systems. Water Res. 2020, 184, 116189. [Google Scholar] [CrossRef]
- Hua, P.; Gao, Q.; Wang, Z.; Jiang, S.; de Oliveira, K.R.F.; Macedo, D.O. Modeling and elucidation the effects of iron deposits on chlorine decay and trihalomethane formation in drinking water distribution system. Water Res. 2021, 207, 117804. [Google Scholar] [CrossRef] [PubMed]
- Clayton, G.E.; Thorn, R.M.S.; Reynolds, D.M. Comparison of Trihalomethane Formation Using Chlorine-Based Disinfectants Within a Model System; Applications Within Point-of-Use Drinking Water Treatment. Front. Environ. Sci. 2019, 7, 35. [Google Scholar] [CrossRef]
- Padhi, R.K.; Subramanian, S.; Mohanty, A.K.; Satpathy, K.K. Comparative assessment of chlorine reactivity and trihalomethanes formation potential of three different water sources. J. Water Process Eng. 2019, 29, 100769. [Google Scholar] [CrossRef]
- Kumari, M.; Gupta, S.K. Occurrence and Exposure to Trihalomethanes in Drinking Water: A Systematic Review and Meta-analysis. Expo. Health 2022, 14, 915–939. [Google Scholar] [CrossRef]
- Tovar, S.S.T.; Susa, M.R. Cancer risk assessment from exposure to trihalomethanes in showers by inhalation. Environ. Res. 2021, 196, 110401. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.A.; Wright, J.M.; Evans, A.; Rivera-Núñez, Z.; Meyer, A.; Narotsky, M.G. Disinfection by-product exposures and the risk of musculoskeletal birth defects. Environ. Epidemiol. 2020, 4, e081. [Google Scholar] [CrossRef] [PubMed]
- Medgyesi, D.N.; Mohan, S.; Bangia, K.; Spielfogel, E.S.; Fisher, J.A.; Jones, R.R.; Ward, M.H.; Lacey, J.V., Jr.; Sanchez, T.R. Trihalomethanes from residential community water supplies and chronic kidney disease risk among women in California. In Proceedings of the ISEE 2024: 36th Annual Conference of the International Society of Environmental Epidemiology, Santiago, Chile, 25–28 August 2024. [Google Scholar] [CrossRef]
- An, S.L.; Xiong, S.M.; Shen, X.B.; Ni, Y.Q.; Chen, W.; He, C.D.; Zhou, Y.Z. The associations between exposure to trihalomethanes during pregnancy and adverse birth outcomes: A systematic review and meta-analysis. Chemosphere 2022, 293, 133524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Deng, Y.-L.; Liu, C.; Lu, W.-Q.; Zeng, Q. Impacts of disinfection byproduct exposures on male reproductive health: Current evidence, possible mechanisms and future needs. Chemosphere 2023, 331, 138808. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.L.; Luo, Q.; Liu, C.; Zeng, J.Y.; Lu, T.T.; Shi, T.; Cui, F.P.; Yuan, X.Q.; Miao, Y.; Zhang, M.; et al. Urinary biomarkers of exposure to drinking water disinfection byproducts and ovarian reserve: A cross-sectional study in China. J. Hazard. Mater. 2022, 421, 126683. [Google Scholar] [CrossRef]
- Evlampidou, I.; Font-Ribera, L.; Rojas-Rueda, D.; Gracia-Lavedan, E.; Costet, N.; Pearce, N.; Vineis, P.; Jaakkola, J.J.; Delloye, F.; Makris, K.C.; et al. Trihalomethanes in Drinking Water and Bladder Cancer Burden in the European Union. Environ. Health Perspect. 2020, 128, 017001. [Google Scholar] [CrossRef]
- Helte, E.; Säve-Söderbergh, M.; Larsson, S.C.; Martling, A.; Åkesson, A. Disinfection by-products in drinking water and risk of colorectal cancer: A population-based cohort study. JNCI J. Natl. Cancer Inst. 2023, 115, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Wang, H.; Yu, K.; Shen, W.; Hou, Y.; Ji, M.; Zhu, Y.; Tian, W.; Li, X. Determination of four trihalomethanes in ship ballast water by gas chromatography-negative chemical ionization-mass spectrometry. Chin. J. Chromatogr. 2022, 40, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Tafesse, N.; Porcelli, M.; Hirpessa, B.B.; Gasana, J.; Padhi, R.K.; Robele, S.; Ambelu, A. Trihalomethanes and physicochemical quality of drinking water in Addis Ababa, Ethiopia. Heliyon 2023, 9, e19446. [Google Scholar] [CrossRef]
- Sánchez-Duque, G.; Lozada-Castro, J.J.; Hara, E.L.Y.; Grassi, M.T.; Rosero-Moreano, M.; Ríos-Acevedo, J.J. Alternative Ecosorbent for the Determination of Trihalomethanes in Aqueous Samples in SPME Mode. Molecules 2022, 27, 8653. [Google Scholar] [CrossRef] [PubMed]
- Momeniha, F.; Oskouie, A.A.; Esrafili, A.; Janani, L.; Jafari, A.J. Trihalomethanes in exhaled air, indoor air and water: A parallel design trial in chlorine and ozone-chlorine disinfected swimming pools. Build. Environ. 2021, 205, 108316. [Google Scholar] [CrossRef]
- Jin, M.; Wen, Z.F.; Liu, Y.J.; Qian, M.; Zhou, Y.; Bian, Y.; Zhang, Y.; Feng, X.S. Trihalomethanes in water samples: Recent update on pretreatment and detection methods. Chemosphere 2023, 341, 140005. [Google Scholar] [CrossRef]
- Mahato, J.K.; Gupta, S.K. Exploring applicability of artificial intelligence and multivariate linear regression model for prediction of trihalomethanes in drinking water. Int. J. Environ. Sci. Technol. 2022, 19, 5275–5288. [Google Scholar] [CrossRef]
- Cheshmekhezr, S.; Alimoradi, S.; Torabian, A.; Pardakhti, A.; Babaei, L. Investigation of the formation and effects of upstream trihalomethanes in Tehran’s raw and drinking water using the spectrophotometric method. J. Water Process Eng. 2021, 44, 102426. [Google Scholar] [CrossRef]
- Shariati-Rad, M.; Fattahi, F. A simple equipment and colorimetric method for determination of chloroform in water. Anal. Chim. Acta 2020, 1100, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Rasheduzzaman, M.; O’Connell, B.; Krometis, L.-A.; Brown, T.; Cohen, A. Point-of-use chlorine residuals and disinfection byproduct occurrences in rural households served by public water utilities in Appalachian Virginia. J. Water Health 2024, 22, 1064–1074. [Google Scholar] [CrossRef]
- Ali, S.I.; Arnold, M.; Liesner, F.; Fesselet, J.-F. Characterization of Disinfection By-Products Levels at an Emergency Surface Water Treatment Plant in a Refugee Settlement in Northern Uganda. Water 2019, 11, 647. [Google Scholar] [CrossRef]
- EPA. “Current EPA Microbial and Disinfection Byproduct Regulations”, Agencia de Protección Ambiental de los Estados Unidos. Available online: https://archive.epa.gov/enviro/html/icr/web/html/regulations.html (accessed on 20 November 2024).
- WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum, 4th ed.; World Health Organization: Geneva, Switzerland, 2017; Available online: https://www.who.int/publications/i/item/9789241549950 (accessed on 20 November 2024).
- EU. Directive (Eu) 2020/2184 of the European Parliament and of The Council of 16 December 2020 on the Quality of Water Intended for Human Consumption (Recast). The European Parliament and the Council of the European Union. 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020L2184&from=EN (accessed on 20 November 2024).
- NTE INEN 1108. Agua Potable. Requisitos, 5th ed.; Norma Técnica Ecuatoriana: Quito, Ecuador, 2014; Available online: https://lc.cx/EEUYoT (accessed on 10 November 2024).
- Salazar-Flores, C.A.; Kurbatova, A.I.; Mikhaylichenko, K.Y.; Milutka, A.C. Health risk assessment of drinking water: Case study of city of Latacunga and the canton of Pedro Vicente Maldonado (Ecuador). Hyg. Sanit. 2022, 101, 344–356. [Google Scholar] [CrossRef]
- Cuenca, G.A. Plan de Desarrollo y Ordenamiento Territorial del Cantón Cuenca—Actualización 2022. Cuenca—Ecuador: Gobierno Autónomo Descentralizado del Cantón Cuenca. 2022. Available online: https://lc.cx/rBKHuI (accessed on 11 November 2024).
- Arcentales-Ríos, R.; Carrión-Méndez, A.; Cipriani-Ávila, I.; Acosta, S.; Capparelli, M.; Moulatlet, G.M.; Pinos-Vélez, V. Assessment of metals, emerging contaminants, and physicochemical characteristics in the drinking water and wastewater of Cuenca, Ecuador. J. Trace Elem. Miner. 2022, 2, 100030. [Google Scholar] [CrossRef]
- ETAPA. “Plantas de Potabilización”, Empresa Pública Municipal de Telecomunicaciones, Agua Potable, Saneamiento y Gestión Ambiental del Cantón Cuenca. Available online: https://www.etapa.net.ec/agua-potable-y-saneamiento/operacion-y-mantenimiento-agua-potable-y-saneamiento/plantas-de-potabilizacion-agua-potable/ (accessed on 11 November 2024).
- HACH. “THM PlusTM Method: Hach Method 10132—Total Trihalomethanes (THM) Reagent Set”, HACH. Available online: https://ca.hach.com/product.detail-print.version.jsa?id=14533963759 (accessed on 11 November 2024).
- HACH. “Trihalomethanes: Method 10132”, HACH COMPANY. 2017. Available online: https://lc.cx/Sv_ZL6 (accessed on 11 November 2024).
- Hung, Y.-C.; Waters, B.W.; Yemmireddy, V.K.; Huang, C.-H. pH effect on the formation of THM and HAA disinfection byproducts and potential control strategies for food processing. J. Integr. Agric. 2017, 16, 2914–2923. [Google Scholar] [CrossRef]
- Sriboonnak, S.; Induvesa, P.; Wattanachira, S.; Rakruam, P.; Siyasukh, A.; Pumas, C.; Wongrueng, A.; Khan, E. Trihalomethanes in Water Supply System and Water Distribution Networks. Int. J. Environ. Res. Public Health 2021, 18, 9066. [Google Scholar] [CrossRef]
- Valdivia-Garcia, M.; Weir, P.; Graham, D.W.; Werner, D. Predicted Impact of Climate Change on Trihalomethanes Formation in Drinking Water Treatment. Sci. Rep. 2019, 9, 9967. [Google Scholar] [CrossRef] [PubMed]
- Masoud, M.S.; Ismail, A.M.; El-Hoshy, M.M. Kinetics and thermodynamics of the formation of trihalomethanes. Appl. Water Sci. 2019, 9, 99. [Google Scholar] [CrossRef]
- Amarasooriya, A.A.G.D.; Weragoda, S.K.; Makehelwala, M.; Weerasooriya, R. Occurrence of trihalomethane in relation to treatment technologies and water quality under tropical conditions. H2Open J. 2018, 1, 69–83. [Google Scholar] [CrossRef]
- Kalantary, R.R.; Oshidari, Y.; Amoohadi, V.; Niknejad, H.; Zeraatkar, R.; Arani, M.H. Investigating the relationship between free chlorine concentration and heterotrophs in water of swimming pool in Iran. Heliyon 2024, 10, e36804. [Google Scholar] [CrossRef] [PubMed]
- Kwio-Tamale, J.C.; Onyutha, C. Influence of physical and water quality parameters on residual chlorine decay in water distribution network. Heliyon 2024, 10, e30892. [Google Scholar] [CrossRef] [PubMed]
- Saldaña-González, I.; Ávila, H. Evaluación de la concentración de compuestos trihalometanos en el agua potable distribuida en la Ciudad David, Panamá y sus riesgos a la salud humana. Rev. I+D Tecnológico 2022, 18, 2022. Available online: http://portal.amelica.org/ameli/journal/339/3393183007/ (accessed on 19 November 2024). [CrossRef]
- Sarmiento, A.; Rojas, M.; Medina, E.; Olivet, C.; Casanova, J. Investigación de trihalometanos en agua potable del Estado Carabobo, Venezuela. Gac. Sanit. 2003, 17, 137–143. [Google Scholar] [CrossRef] [PubMed]
| Treatment Plant | Code | pH | Temperature (°C) | CE (µS/cm) | Turbidity (NTU) | Free Cl (mg/L) |
|---|---|---|---|---|---|---|
| Cebollar | CS | 7.20 ± 0.31 | 15.01 ± 0.66 | 114.48 ± 17.88 | 0.71 ± 0.68 | 1.20 ± 0.17 |
| C1 | 7.34 ± 0.24 | 20.23 ± 2.17 | 123.25 ± 17.64 | 1.07 ± 0.58 | 1.01 ± 0.31 | |
| C2 | 7.26 ± 0.48 | 20.36 ± 1.93 | 115.50 ± 21.16 | 1.04 ± 0.32 | 0.88 ± 0.31 | |
| C3 | 7.33 ± 0.15 | 20.64 ± 1.70 | 123.08 ± 14.26 | 1.57 ± 1.35 | 0.77 ± 0.33 | |
| Sustag | SS | 7.06 ± 0.50 | 15.18 ± 2.11 | 77.14 ± 8.05 | 0.46 ± 0.24 | 1.24 ± 0.30 |
| S1 | 6.76 ± 0.65 | 22.61 ± 4.02 | 82.38 ± 13.26 | 0.53 ± 0.39 | 0.88 ± 0.28 | |
| S2 | 6.81 ± 0.65 | 21.50 ± 2.28 | 77.77 ± 9.68 | 0.53 ± 0.18 | 0.73 ± 0.23 | |
| S3 | 7.01 ± 0.32 | 19.98 ± 2.80 | 83.63 ± 11.77 | 0.62 ± 0.33 | 0.34 ± 0.19 | |
| Tixan | TS | 7.54 ± 0.49 | 15.15 ± 1.58 | 110.50 ± 11.29 | 0.54 ± 0.48 | 1.05 ± 0.37 |
| T1 | 7.44 ± 0.25 | 19.55 ± 2.73 | 103.75 ± 7.92 | 0.44 ± 0.25 | 0.87 ± 0.35 | |
| T2 | 7.46 ± 0.25 | 20.16 ± 1.87 | 106.13 ± 7.20 | 0.51 ± 0.26 | 0.79 ± 0.29 | |
| T3 | 7.70 ± 0.21 | 20.01 ± 2.52 | 118.13 ± 12.29 | 0.65 ± 0.41 | 0.29 ± 0.16 |
| PTA | Code | CHCl3 (µg/L) |
|---|---|---|
| Cebollar | CS | 12.25 ± 5.07 |
| C1 | 25.75 ± 6.82 | |
| C2 | 20.88 ± 5.54 | |
| C3 | 17.13 ± 3.69 | |
| Sustag | SS | 21.88 ± 3.98 |
| S1 | 14.25 ± 5.73 | |
| S2 | 11.75 ± 4.87 | |
| S3 | 17.75 ± 6.74 | |
| Tixan | TS | 14.25 ± 6.23 |
| T1 | 15.75 ± 6.18 | |
| T2 | 13.00 ± 5.66 | |
| T3 | 19.88 ± 6.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández, B.; Duque-Sarango, P.; Tonón, M.D.; Abril-González, M.; Pinos-Vélez, V.; García-Sánchez, C.R.; Rodríguez, M.J. Determination of the Occurrence of Trihalomethanes in the Drinking Water Supply of the City of Cuenca, Ecuador. Water 2025, 17, 591. https://doi.org/10.3390/w17040591
Hernández B, Duque-Sarango P, Tonón MD, Abril-González M, Pinos-Vélez V, García-Sánchez CR, Rodríguez MJ. Determination of the Occurrence of Trihalomethanes in the Drinking Water Supply of the City of Cuenca, Ecuador. Water. 2025; 17(4):591. https://doi.org/10.3390/w17040591
Chicago/Turabian StyleHernández, Bolívar, Paola Duque-Sarango, María Dolores Tonón, Mónica Abril-González, Verónica Pinos-Vélez, Cristian R. García-Sánchez, and Manuel J. Rodríguez. 2025. "Determination of the Occurrence of Trihalomethanes in the Drinking Water Supply of the City of Cuenca, Ecuador" Water 17, no. 4: 591. https://doi.org/10.3390/w17040591
APA StyleHernández, B., Duque-Sarango, P., Tonón, M. D., Abril-González, M., Pinos-Vélez, V., García-Sánchez, C. R., & Rodríguez, M. J. (2025). Determination of the Occurrence of Trihalomethanes in the Drinking Water Supply of the City of Cuenca, Ecuador. Water, 17(4), 591. https://doi.org/10.3390/w17040591









