Insight into Adsorption Kinetics, Equilibrium, Thermodynamics, and Modeling of Ciprofloxacin onto Iron Ore Tailings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Methods
2.3. Adsorption Experiments
2.4. Adsorption Analysis
2.4.1. Kinetic Characterization of CIP Removal
2.4.2. Isotherms of CIP Removal
2.4.3. Thermodynamic Characterization of CIP Removal
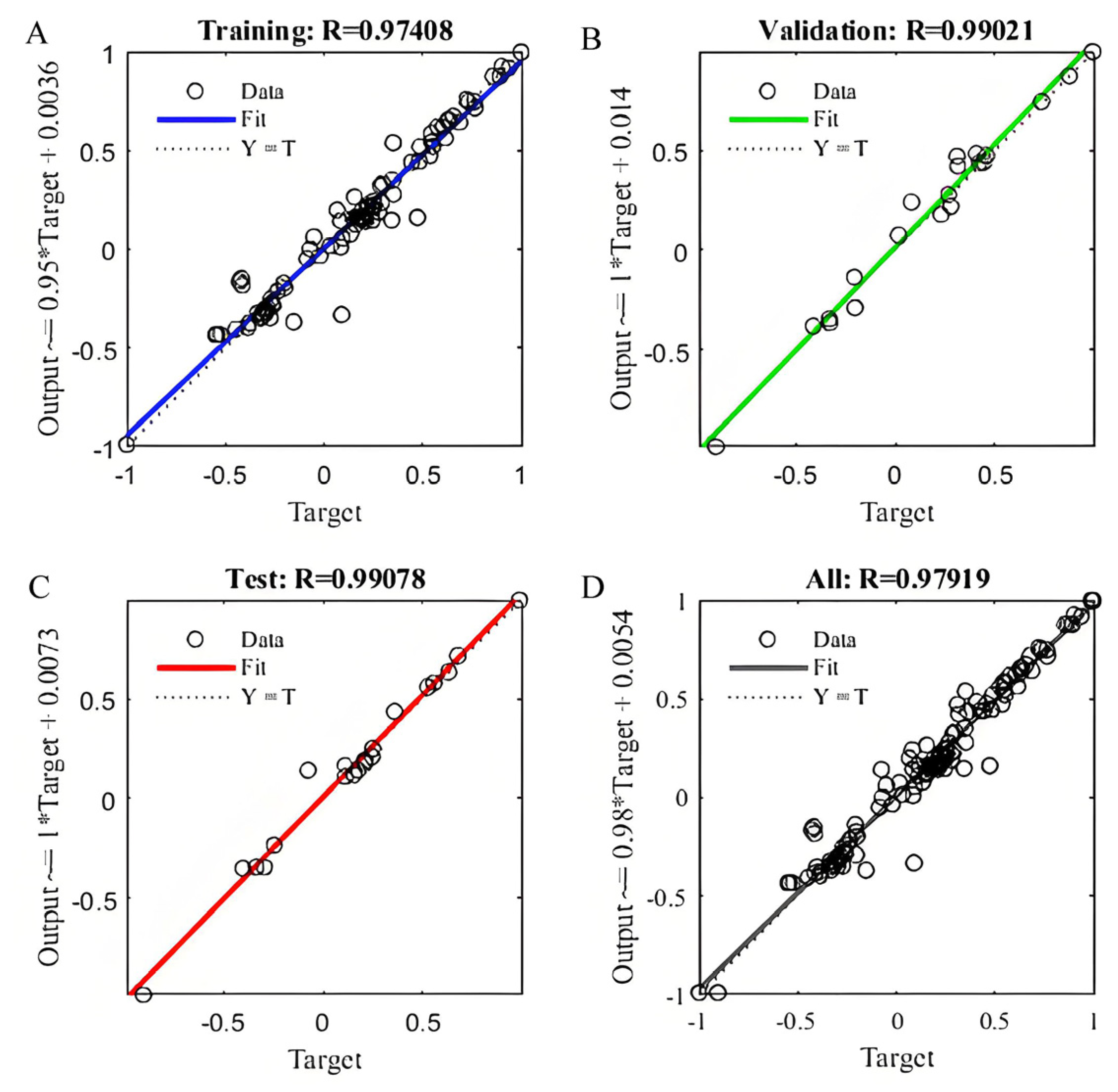
2.5. BP-ANN Model and Statistical Analysis
3. Result and Discussion
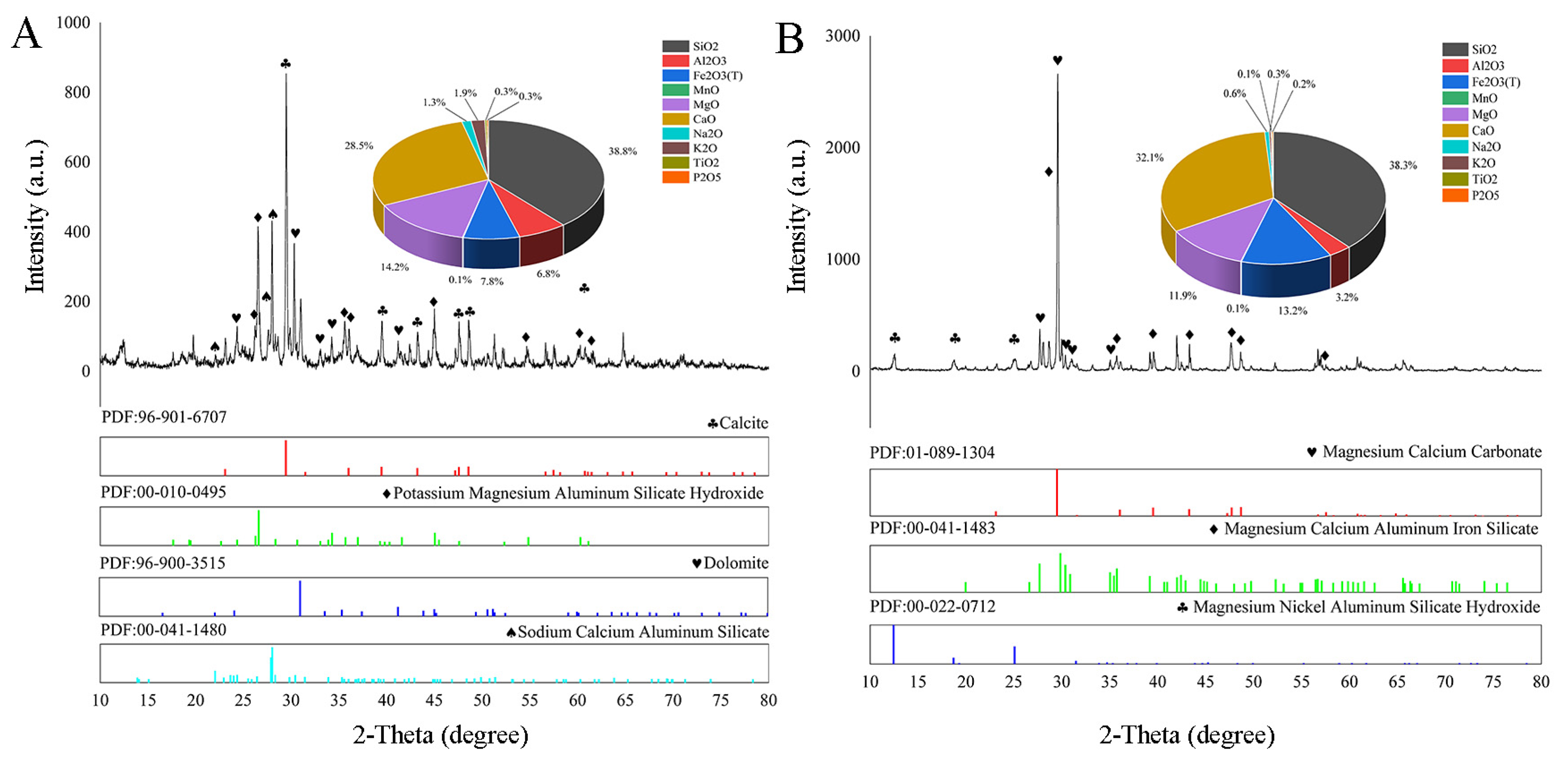
3.1. The Characterization of IOT
3.2. Batch Experiment Results
3.2.1. Effect of pH
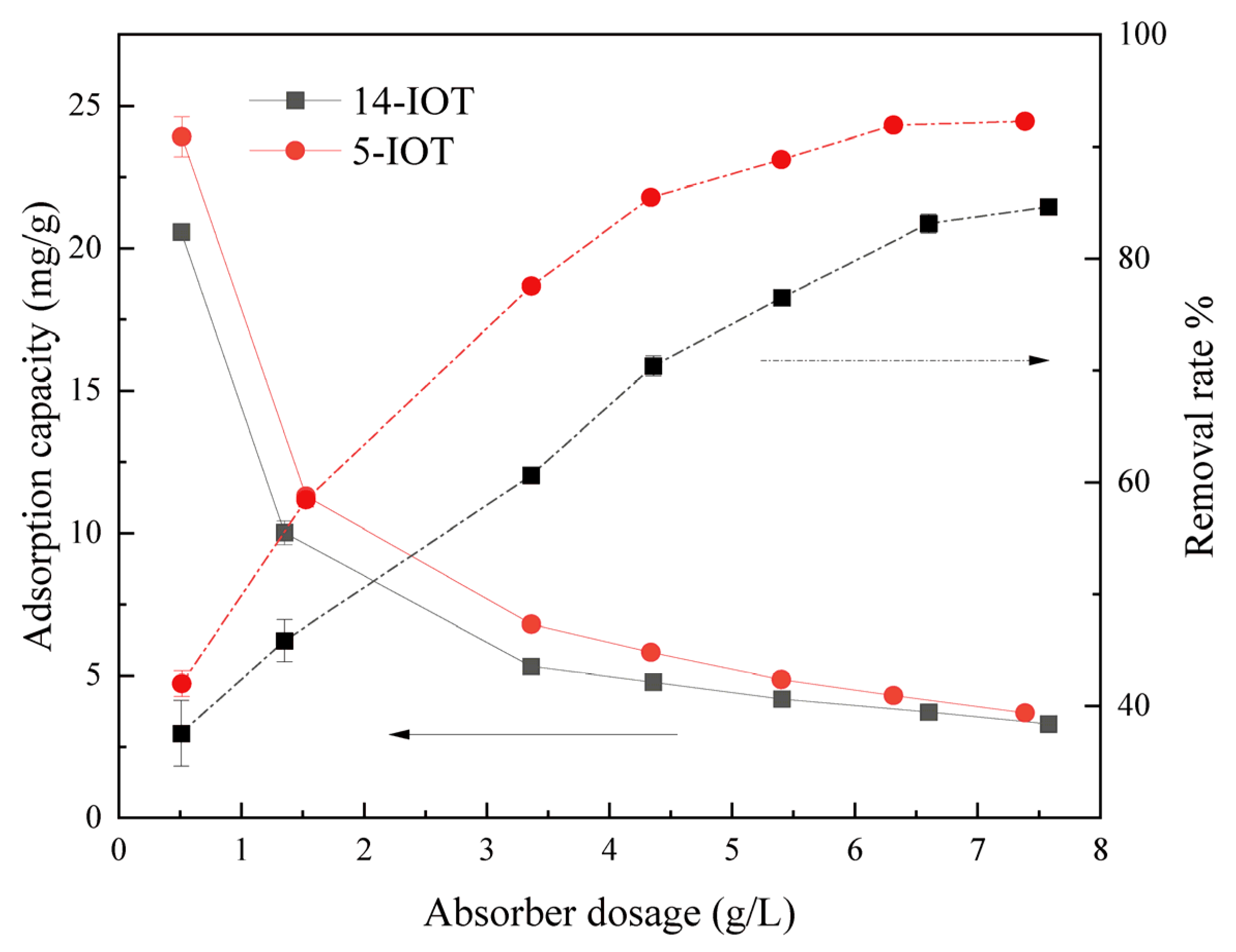
3.2.2. Effect of Sorbent Dosage
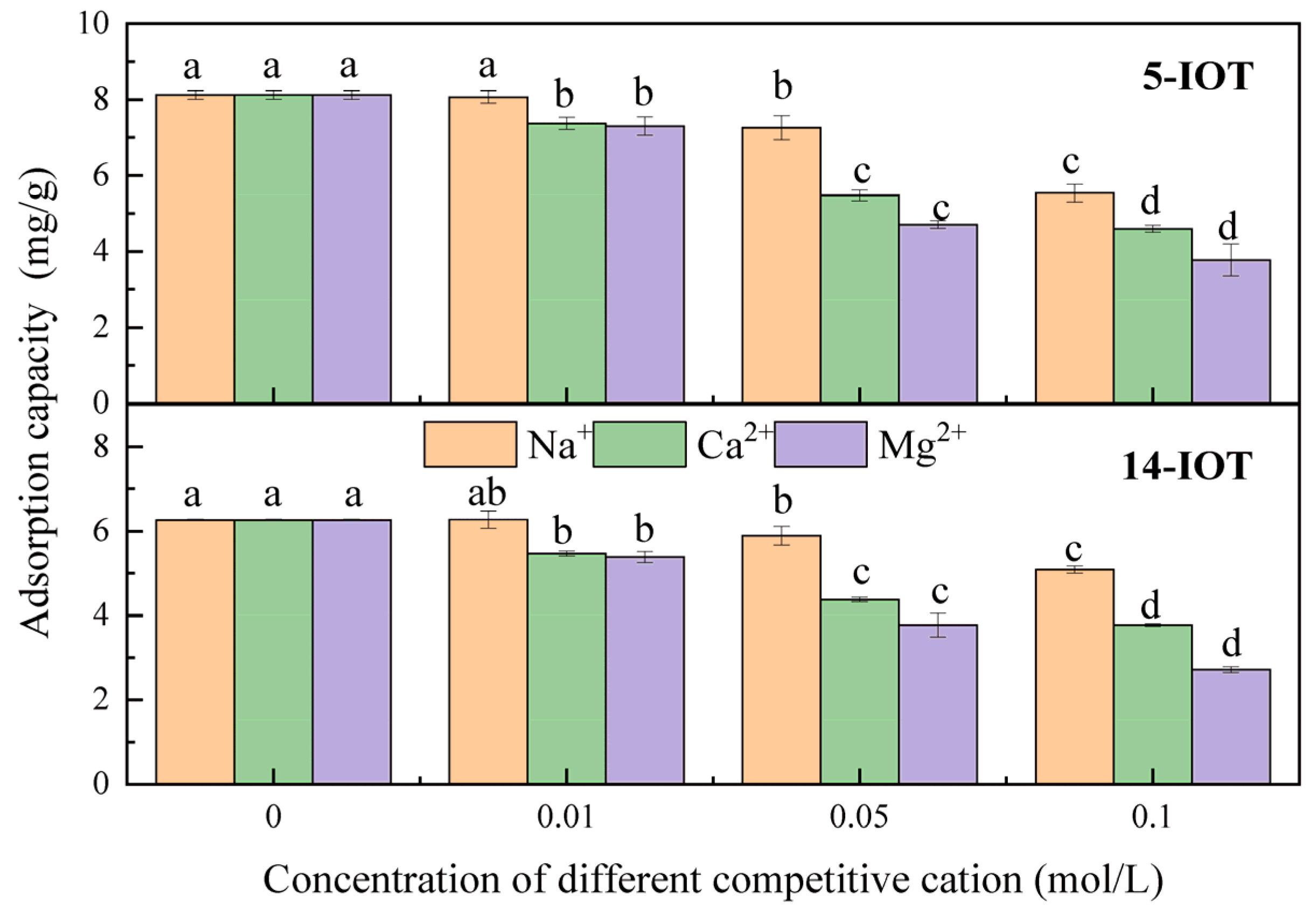
3.2.3. Effect of Competitive Cation
3.2.4. Effect of Contact Time
3.3. Adsorption Kinetic
3.4. Adsorption Isotherms
3.5. Adsorption Thermodynamics
3.6. The Potential Mechanism of CIP Removal by IOTs
3.7. Leaching Toxicity After Adsorption
3.8. Modeling by IOT with Back-Propagation Artificial Neural Network (ANN)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Shi, W.; You, M.; Zhang, R.; Kuang, Y.; Dang, C.; Sun, W.; Zhou, Y.; Wang, W.; Ni, J. Antibiotics in water and sediments of Danjiangkou Reservoir, China: Spatiotemporal distribution and indicator screening. Environ. Pollut. 2019, 246, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Xiao, S.-K.; Pan, C.-G.; Yin, C.; Wang, Y.-H.; Yu, K.-F. Occurrence, source apportionment and risk assessment of antibiotics in water and sediment from the subtropical Beibu Gulf, South China. Sci. Total Environ. 2022, 806, 150439. [Google Scholar] [CrossRef] [PubMed]
- Desbiolles, F.; Malleret, L.; Tiliacos, C.; Wong-Wah-Chung, P.; Laffont-Schwob, I. Occurrence and ecotoxicological assessment of pharmaceuticals: Is there a risk for the Mediterranean aquatic environment? Sci. Total Environ. 2018, 639, 1334–1348. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.Q.; Zehra, A.; Naqvi, B.S.; Shah, S.; Bushra, R. Resistance pattern of ciprofloxacin against different pathogens. Oman Med. J. 2010, 25, 294–298. [Google Scholar] [CrossRef]
- Shariati, A.; Arshadi, M.; Khosrojerdi, M.A.; Abedinzadeh, M.; Ganjalishahi, M.; Maleki, A.; Heidary, M.; Khoshnood, S. The resistance mechanisms of bacteria against ciprofloxacin and new approaches for enhancing the efficacy of this antibiotic. Front. Public Health 2022, 10, 1025633. [Google Scholar] [CrossRef]
- Meng, F.; Sun, S.; Geng, J.; Ma, L.; Jiang, J.; Li, B.; Yabo, S.D.; Lu, L.; Fu, D.; Shen, J.; et al. Occurrence, distribution, and risk assessment of quinolone antibiotics in municipal sewage sludges throughout China. J. Hazard. Mater. 2023, 453, 131322. [Google Scholar] [CrossRef]
- Li, Y.; Ding, J.; Zhang, L.; Liu, X.; Wang, G. Occurrence and ranking of pharmaceuticals in the major rivers of China. Sci. Total Environ. 2019, 696, 133991. [Google Scholar] [CrossRef]
- Ngeno, E.C.; Shikuku Victor, O.; Orata Francis Baraza Lilechi, D.; Kimosop Selly, J. Caffeine and Ciprofloxacin Adsorption from Water onto Clinoptilolite: Linear Isotherms Kinetics, Thermodynamic and Mechanistic Studies. S. Afr. J. Chem. 2019, 72, 136–142. [Google Scholar] [CrossRef]
- Wei, Z.; Li, W.; Zhao, D.; Seo, Y.; Spinney, R.; Dionysiou, D.D.; Wang, Y.; Zeng, W.; Xiao, R. Electrophilicity index as a critical indicator for the biodegradation of the pharmaceuticals in aerobic activated sludge processes. Water Res. 2019, 160, 10–17. [Google Scholar] [CrossRef]
- Li, W.; Li, S.; Tang, Y.; Yang, X.; Zhang, W.; Zhang, X.; Chai, H.; Huang, Y. Highly efficient activation of peroxymonosulfate by cobalt sulfide hollow nanospheres for fast ciprofloxacin degradation. J. Hazard. Mater. 2020, 389, 121856. [Google Scholar] [CrossRef]
- Nure, J.F.; Nkambule, T.T.I. The recent advances in adsorption and membrane separation and their hybrid technologies for micropollutants removal from wastewater. J. Ind. Eng. Chem. 2023, 126, 92–114. [Google Scholar] [CrossRef]
- Jin, Q.; Liu, W.; Dong, Y.; Lu, Y.; Yang, C.; Lin, H. Single atom catalysts for degradation of antibiotics from aqueous environments by advanced oxidation processes: A review. J. Clean. Prod. 2023, 423, 138688. [Google Scholar] [CrossRef]
- Bueno, I.; He, H.; Kinsley, A.C.; Ziemann, S.J.; Degn, L.R.; Nault, A.J.; Beaudoin, A.L.; Singer, R.S.; Wammer, K.H.; Arnold, W.A. Biodegradation, photolysis, and sorption of antibiotics in aquatic environments: A scoping review. Sci. Total Environ. 2023, 897, 165301. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, M.J.; Ansari, A.J.; Hai, F.I. Antibiotic sorption onto microplastics in water: A critical review of the factors, mechanisms and implications. Water Res. 2023, 233, 119790. [Google Scholar] [CrossRef]
- Li, S.; Zhang, X.; Huang, Y. Zeolitic imidazolate framework-8 derived nanoporous carbon as an effective and recyclable adsorbent for removal of ciprofloxacin antibiotics from water. J. Hazard. Mater. 2017, 321, 711–719. [Google Scholar] [CrossRef]
- Hacıosmanoğlu, G.G.; Mejías, C.; Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Antibiotic adsorption by natural and modified clay minerals as designer adsorbents for wastewater treatment: A comprehensive review. J. Environ. Manag. 2022, 317, 115397. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, Q.; Huang, B.; Cheng, H.; Wang, L.; He, Q. Removal of ciprofloxacin as an emerging pollutant: A novel application for bauxite residue reuse. J. Clean. Prod. 2020, 253, 120049. [Google Scholar] [CrossRef]
- Ren, J.; Cuan, Y.; Ma, E.; Wang, Z.; Xie, G.; Wang, H. Effect of fly ash micromotors on expression of antibiotic resistance genes in straw composting. J. Environ. Chem. Eng. 2024, 12, 112736. [Google Scholar] [CrossRef]
- Patel, A.K.; Katiyar, R.; Chen, C.-W.; Singhania, R.R.; Awasthi, M.K.; Bhatia, S.; Bhaskar, T.; Dong, C.-D. Antibiotic bioremediation by new generation biochar: Recent updates. Bioresour. Technol. 2022, 358, 127384. [Google Scholar] [CrossRef]
- Lu, C.; Yang, H.; Wang, J.; Tan, Q.; Fu, L. Utilization of iron tailings to prepare high-surface area mesoporous silica materials. Sci. Total Environ. 2020, 736, 139483. [Google Scholar] [CrossRef]
- Saravanan, M.; Sudalai, S.; Dharaneesh, A.B.; Prahaaladhan, V.; Srinivasan, G.; Arumugam, A. An extensive review on mesoporous silica from inexpensive resources: Properties, synthesis, and application toward modern technologies. J. Sol.-Gel Sci. Technol. 2023, 105, 1–29. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Y.; Southam, G.; Robertson, L.; Chiu, T.H.; Cross, A.T.; Dixon, K.W.; Stevens, J.C.; Zhong, H.; Chan, T.-S.; et al. Geochemical and mineralogical constraints in iron ore tailings limit soil formation for direct phytostabilization. Sci. Total Environ. 2019, 651, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Hou, H.; Long, J.; Guo, X.; Xu, H. Underestimated environmental benefits of tailings resource utilization: Evidence from a life cycle perspective. Environ. Impact Assess. Rev. 2022, 96, 106832. [Google Scholar] [CrossRef]
- Xu, D.-M.; Zhan, C.-L.; Liu, H.-X.; Lin, H.-Z. A critical review on environmental implications, recycling strategies, and ecological remediation for mine tailings. Environ. Sci. Pollut. Res. 2019, 26, 35657–35669. [Google Scholar] [CrossRef]
- Shi, T.; Jia, S.; Chen, Y.; Wen, Y.; Du, C.; Guo, H.; Wang, Z. Adsorption of Pb(II), Cr(III), Cu(II), Cd(II) and Ni(II) onto a vanadium mine tailing from aqueous solution. J. Hazard. Mater. 2009, 169, 838–846. [Google Scholar] [CrossRef]
- Chakraborty, R.; Asthana, A.; Singh, A.K.; Jain, B.; Susan, A.B.H. Adsorption of heavy metal ions by various low-cost adsorbents: A review. Int. J. Environ. Anal. Chem. 2022, 102, 342–379. [Google Scholar] [CrossRef]
- Humelnicu, D.; Zinicovscaia, I.; Humelnicu, I.; Ignat, M.; Yushin, N.; Grozdov, D. Study on the SBA-15 Silica and ETS-10 Titanosilicate as Efficient Adsorbents for Cu(II) Removal from Aqueous Solution. Water 2022, 14, 857. [Google Scholar] [CrossRef]
- Ahmed, S.B.; Mahmoud, N.M.R.; Manda, A.A.; Refaat, H.M. Study of the optimization and mechanism for the remediation process of Malachite green dye via hybrid-based Magnetite-date’s stones. Alex. Eng. J. 2022, 61, 9879–9889. [Google Scholar] [CrossRef]
- Wu, S.; Sun, T.; Kou, J. A novel and clean utilization of converter sludge by co-reduction roasting with high-phosphorus iron ore to produce powdery reduced iron. J. Clean. Prod. 2022, 363, 132362. [Google Scholar] [CrossRef]
- Ghaedi, A.M.; Vafaei, A. Applications of artificial neural networks for adsorption removal of dyes from aqueous solution: A review. Adv. Colloid Interface Sci. 2017, 245, 20–39. [Google Scholar] [CrossRef]
- Ahmad, Z.U.; Yao, L.; Lian, Q.; Islam, F.; Zappi, M.E.; Gang, D.D. The use of artificial neural network (ANN) for modeling adsorption of sunset yellow onto neodymium modified ordered mesoporous carbon. Chemosphere 2020, 256, 127081. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Bar, N.; Das, S.K. Sustainable bioremadiation of Cd(II) in fixed bed column using green adsorbents: Application of Kinetic models and GA-ANN technique. Environ. Technol. Innov. 2019, 13, 130–145. [Google Scholar] [CrossRef]
- Wong, Y.J.; Arumugasamy, S.K.; Chung, C.H.; Selvarajoo, A.; Sethu, V. Comparative study of artificial neural network (ANN), adaptive neuro-fuzzy inference system (ANFIS) and multiple linear regression (MLR) for modeling of Cu(II) adsorption from aqueous solution using biochar derived from rambutan (Nephelium lappaceum) peel. Environ. Monit. Assess. 2020, 192, 439. [Google Scholar] [CrossRef] [PubMed]
- Karlaftis, M.G.; Vlahogianni, E.I. Statistical methods versus neural networks in transportation research: Differences, similarities and some insights. Transp. Res. Part C Emerg. Technol. 2011, 19, 387–399. [Google Scholar] [CrossRef]
- Arnepalli, D.N.; Shanthakumar, S.; Hanumantha Rao, B.; Singh, D.N. Comparison of Methods for Determining Specific-surface Area of Fine-grained Soils. Geotech. Geol. Eng. 2008, 26, 121–132. [Google Scholar] [CrossRef]
- Li, S.; Wu, J.; Huo, Y.; Zhao, X.; Xue, L. Profiling multiple heavy metal contamination and bacterial communities surrounding an iron tailing pond in Northwest China. Sci. Total Environ. 2021, 752, 141827. [Google Scholar] [CrossRef]
- Lu, D.; Xu, S.; Qiu, W.; Sun, Y.; Liu, X.; Yang, J.; Ma, J. Adsorption and desorption behaviors of antibiotic ciprofloxacin on functionalized spherical MCM-41 for water treatment. J. Clean. Prod. 2020, 264, 121644. [Google Scholar] [CrossRef]
- Ofomaja, A.E.; Ho, Y.-S. Effect of temperatures and pH on methyl violet biosorption by Mansonia wood sawdust. Bioresour. Technol. 2008, 99, 5411–5417. [Google Scholar] [CrossRef]
- Kannamba, B.; Reddy, K.L.; AppaRao, B.V. Removal of Cu(II) from aqueous solutions using chemically modified chitosan. J. Hazard. Mater. 2010, 175, 939–948. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, X.; Kang, Y.; Shu, Y.; Sun, Q.; Li, L. Application of Mn/MCM-41 as an adsorbent to remove methyl blue from aqueous solution. J. Colloid Interface Sci. 2014, 429, 25–33. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, Y.; Zhou, Y.; Ma, X.; Wang, X.; Tong, W.; Luan, X.; Chu, P.K. Preparation and effectiveness of slow-release silicon fertilizer by sintering with iron ore tailings. Environ. Prog. Sustain. Energy 2018, 37, 1011–1019. [Google Scholar] [CrossRef]
- Shahrokhi-Shahraki, R.; Benally, C.; El-Din, M.G.; Park, J. High efficiency removal of heavy metals using tire-derived activated carbon vs commercial activated carbon: Insights into the adsorption mechanisms. Chemosphere 2021, 264, 128455. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, Z.; Zhu, H.; Liu, Q.; Liu, J. Dissolution-precipitation hydration mechanism of steel slag based on ion exchange of a layered alkali-activator. Constr. Build. Mater. 2024, 411, 134795. [Google Scholar] [CrossRef]
- Chen, T.; Wen, X.-C.; Zhang, L.-J.; Tu, S.-C.; Zhang, J.-H.; Sun, R.-N.; Yan, B. The geochemical and mineralogical controls on the release characteristics of potentially toxic elements from lead/zinc (Pb/Zn) mine tailings. Environ. Pollut. 2022, 315, 120328. [Google Scholar] [CrossRef]
- Geng, H.; Wang, F.; Yan, C.; Tian, Z.; Chen, H.; Zhou, B.; Yuan, R.; Yao, J. Leaching behavior of metals from iron tailings under varying pH and low-molecular-weight organic acids. J. Hazard. Mater. 2020, 383, 121136. [Google Scholar] [CrossRef]
- Chen, T.; Li, H.; Wang, H.; Zou, X.; Liu, H.; Chen, D.; Zhou, Y. Removal of Pb(II) from Aqueous Solutions by Periclase/Calcite Nanocomposites. Water Air Soil Pollut. 2019, 230, 299. [Google Scholar] [CrossRef]
- Thirunavukkarasu, A.; Nithya, R. Adsorption of acid orange 7 using green synthesized CaO/CeO2 composite: An insight into kinetics, equilibrium, thermodynamics, mass transfer and statistical models. J. Taiwan Inst. Chem. Eng. 2020, 111, 44–62. [Google Scholar] [CrossRef]
- Chen, G.; Yang, L.; Chen, J.; Miki, T.; Li, S.; Bai, H.; Nagasaka, T. Competitive mechanism and influencing factors for the simultaneous removal of Cr(III) and Zn(II) in acidic aqueous solutions using steel slag: Batch and column experiments. J. Clean. Prod. 2019, 230, 69–79. [Google Scholar] [CrossRef]
- Igwegbe, C.A.; Oba, S.N.; Aniagor, C.O.; Adeniyi, A.G.; Ighalo, J.O. Adsorption of ciprofloxacin from water: A comprehensive review. J. Ind. Eng. Chem. 2021, 93, 57–77. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, C.; Liu, F.; Yuan, Y.; Wu, H.; Li, A. Effects of ionic strength on removal of toxic pollutants from aqueous media with multifarious adsorbents: A review. Sci. Total Environ. 2019, 646, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Pan, X.; Zhao, X.; Guo, P.; Zhao, Z. Recovery and preparation of high-grade silica from iron ore tailings by S-HGMS coupling with acid leaching technology: Description of separation mechanism and leaching kinetics. Powder Technol. 2023, 424, 118523. [Google Scholar] [CrossRef]
- Ma, Y.; Li, M.; Li, P.; Yang, L.; Wu, L.; Gao, F.; Qi, X.; Zhang, Z. Hydrothermal synthesis of magnetic sludge biochar for tetracycline and ciprofloxacin adsorptive removal. Bioresour. Technol. 2021, 319, 124199. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, J. Adsorption study for removal of Congo red anionic dye using organo-attapulgite. Adsorption 2009, 15, 381–389. [Google Scholar] [CrossRef]
- Hu, Q.; Lan, R.; He, L.; Liu, H.; Pei, X. A critical review of adsorption isotherm models for aqueous contaminants: Curve characteristics, site energy distribution and common controversies. J. Environ. Manag. 2023, 329, 117104. [Google Scholar] [CrossRef]
- Ozkul, S.; Arbabzadeh, O.; Bisselink, R.J.M.; Kuipers, N.J.M.; Bruning, H.; Rijnaarts, H.H.M.; Dykstra, J.E. Selective adsorption in ion exchange membranes: The effect of solution ion composition on ion partitioning. Water Res. 2024, 254, 121382. [Google Scholar] [CrossRef]
- Saxena, M.; Sharma, N.; Saxena, R. Highly efficient and rapid removal of a toxic dye: Adsorption kinetics, isotherm, and mechanism studies on functionalized multiwalled carbon nanotubes. Surf. Interfaces 2020, 21, 100639. [Google Scholar] [CrossRef]
- Zhang, C.-L.; Qiao, G.-L.; Zhao, F.; Wang, Y. Thermodynamic and kinetic parameters of ciprofloxacin adsorption onto modified coal fly ash from aqueous solution. J. Mol. Liq. 2011, 163, 53–56. [Google Scholar] [CrossRef]
- MacKay, A.A.; Seremet, D.E. Probe Compounds to Quantify Cation Exchange and Complexation Interactions of Ciprofloxacin with Soils. Environ. Sci. Technol. 2008, 42, 8270–8276. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, C.-H. Adsorption and oxidation of fluoroquinolone antibacterial agents and structurally related amines with goethite. Chemosphere 2007, 66, 1502–1512. [Google Scholar] [CrossRef]
- Wu, Q.; Li, Z.; Hong, H.; Yin, K.; Tie, L. Adsorption and intercalation of ciprofloxacin on montmorillonite. Appl. Clay Sci. 2010, 50, 204–211. [Google Scholar] [CrossRef]
- Rasoulzadeh, H.; Mohseni-Bandpei, A.; Hosseini, M.; Safari, M. Mechanistic investigation of ciprofloxacin recovery by magnetite–imprinted chitosan nanocomposite: Isotherm, kinetic, thermodynamic and reusability studies. Int. J. Biol. Macromol. 2019, 133, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Zhou, Y.; Cheng, M.; Zhang, L.; Zhou, T.; Cen, Q.; Li, B.; Liu, Z. A review on modified red mud-based materials in removing organic dyes from wastewater:Application, mechanisms and perspectives. J. Mol. Liq. 2024, 407, 125171. [Google Scholar] [CrossRef]
- Zhang, P.; He, M.; Huo, S.; Li, F.; Li, K. Recent progress in metal-based composites toward adsorptive removal of phosphate: Mechanisms, behaviors, and prospects. Chem. Eng. J. 2022, 446, 137081. [Google Scholar] [CrossRef]
- Jin, H.; Li, L.; Luo, N.; Niu, H.; Han, J.; Xu, L.; Hao, Z.; Cao, D.; Cai, Y. Biochars-FexPCu hybrides deriving from solid waste and waste acids for elimination of refractory organic pollutants from pharmaceutical wastewater. Chem. Eng. J. 2023, 465, 142727. [Google Scholar] [CrossRef]
- Ateş, A.; Mert, Y.; Timko, M.T. Evaluation of characteristics of raw tea waste-derived adsorbents for removal of metals from aqueous medium. Biomass Convers. Biorefinery 2023, 13, 7811–7826. [Google Scholar] [CrossRef]
- Ministry of Health PRC. GB 5749-2006; Standards for drinking water quality. Standards Press of China: Beijing China, 2006.
- Zhang, Y.; Gao, W.; Ni, W.; Zhang, S.; Li, Y.; Wang, K.; Huang, X.; Fu, P.; Hu, W. Influence of calcium hydroxide addition on arsenic leaching and solidification/stabilisation behaviour of metallurgical-slag-based green mining fill. J. Hazard. Mater. 2020, 390, 122161. [Google Scholar] [CrossRef]
- Ghaedi, M.; Ghaedi, A.M.; Ansari, A.; Mohammadi, F.; Vafaei, A. Artificial neural network and particle swarm optimization for removal of methyl orange by gold nanoparticles loaded on activated carbon and Tamarisk. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 132, 639–654. [Google Scholar] [CrossRef]
- Dehghanian, N.; Ghaedi, M.; Ansari, A.; Ghaedi, A.; Vafaei, A.; Asif, M.; Agarwal, S.; Tyagi, I.; Gupta, V.K. A random forest approach for predicting the removal of Congo red from aqueous solutions by adsorption onto tin sulfide nanoparticles loaded on activated carbon. Desalination Water Treat. 2016, 57, 9272–9285. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Alam, M.S.B.; Hassan, M.; Rozbu, M.R.; Ishtiak, T.; Rafa, N.; Mofijur, M.; Shawkat Ali, A.B.M.; Gandomi, A.H. Deep learning modelling techniques: Current progress, applications, advantages, and challenges. Artif. Intell. Rev. 2023, 56, 13521–13617. [Google Scholar] [CrossRef]
- Balci, B.; Keskinkan, O.; Avci, M. Use of BDST and an ANN model for prediction of dye adsorption efficiency of Eucalyptus camaldulensis barks in fixed-bed system. Expert Syst. Appl. 2011, 38, 949–956. [Google Scholar] [CrossRef]
- Chowdhury, S.; Saha, P.D. Artificial neural network (ANN) modeling of adsorption of methylene blue by NaOH-modified rice husk in a fixed-bed column system. Environ. Sci. Pollut. Res. 2013, 20, 1050–1058. [Google Scholar] [CrossRef]

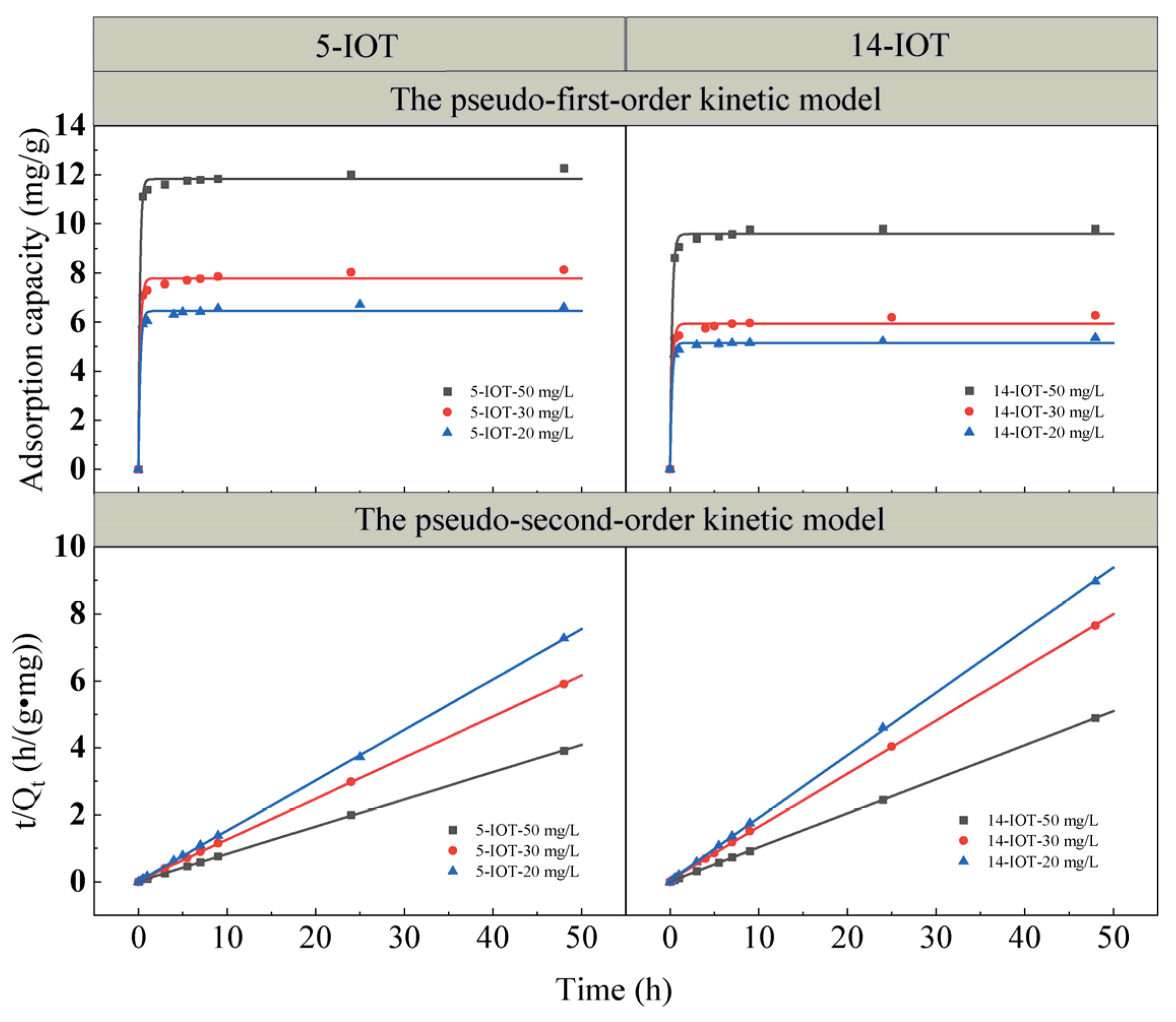

| Initial Concentration (mg/L) | The Pseudo-First-Order Kinetic Equation | The Pseudo-Second-Order Kinetic Equation | |||||
|---|---|---|---|---|---|---|---|
| Qa | Qe | k1 | R2 | Qe | K2 | R2 | |
| (mg/g) | (mg/g) | (1/h) | (mg/g) | (g/(mg·h)) | |||
| 5-IOT | |||||||
| 50 | 12.26 | 11.83 | 5.52 | 0.9965 | 12.27 | 0.54 | 0.9999 |
| 30 | 8.13 | 7.78 | 4.57 | 0.9920 | 8.15 | 0.50 | 0.9999 |
| 20 | 6.60 | 6.45 | 4.74 | 0.9932 | 6.64 | 1.38 | 0.9999 |
| 14-IOT | |||||||
| 50 | 9.80 | 9.58 | 4.40 | 0.9955 | 9.83 | 0.88 | 0.9999 |
| 30 | 6.27 | 5.93 | 4.24 | 0.9872 | 6.30 | 0.48 | 0.9998 |
| 20 | 5.35 | 5.13 | 4.67 | 0.9953 | 5.35 | 0.77 | 0.9998 |
| T (K) | Freundlich Model | Langmuir Model | Dubin–Radushkevich Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | KF | R2 | Qmax | KL | RL | R2 | Qmax | Kd | E | R2 | |
| 5-IOT | |||||||||||
| 308.15 | 0.526 | 0.136 | 0.9986 | 15.610 | 0.178 | 0.208–0.922 | 0.9412 | 0.00032 | 0.003 | 12.127 | 0.9977 |
| 298.15 | 0.598 | 0.181 | 0.9533 | 16.077 | 0.215 | 0.209–0.916 | 0.8560 | 0.00047 | 0.004 | 11.180 | 0.9582 |
| 288.15 | 0.594 | 0.22 | 0.9812 | 16.639 | 0.160 | 0.198–0.900 | 0.9792 | 0.00047 | 0.004 | 10.660 | 0.9898 |
| 14-IOT | |||||||||||
| 308.15 | 0.356 | 0.058 | 0.9912 | 11.876 | 0.139 | 0.091–0.590 | 0.9425 | 0.00009 | 0.023 | 14.744 | 0.9771 |
| 298.15 | 0.387 | 0.081 | 0.9920 | 12.484 | 0.137 | 0.092–0.594 | 0.9425 | 0.00012 | 0.027 | 13.609 | 0.9816 |
| 288.15 | 0.405 | 0.114 | 0.9978 | 13.680 | 0.104 | 0.118–0.659 | 0.8721 | 0.00015 | 0.032 | 12.500 | 0.9716 |
| T (K) | Qmax (mg/g) | Reference | |
|---|---|---|---|
| 5-IOT | 288 | 16.64 (predicted) | This study |
| 14-IOT | 288 | 13.68 (predicted) | This study |
| Modified coal fly ash | 313 | 1.157 | [58] |
| Red mud | 293 | 6.13 | [17] |
| Kaolinite | 308 | 7.95 | [59] |
| Goethite | 295 | 19.88 | [60] |
| Montmorillonite | 310 | 397.6 | [61] |
| T (K) | ΔG (KJ/mol) | ΔH (KJ/mol) | ΔS (J/(mol·K)) |
|---|---|---|---|
| 5-IOT | |||
| 308.15 | −27.462 | −17.665 | 31.941 |
| 298.15 | −27.272 | ||
| 288.15 | −26.829 | ||
| 14-IOT | |||
| 308.15 | −25.253 | −25.041 | 0.714 |
| 298.15 | −25.268 | ||
| 288.15 | −25.240 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, N.; Xi, Y.; Zhang, J.; Wu, J.; Cheng, H.; He, Q. Insight into Adsorption Kinetics, Equilibrium, Thermodynamics, and Modeling of Ciprofloxacin onto Iron Ore Tailings. Water 2025, 17, 760. https://doi.org/10.3390/w17050760
Fang N, Xi Y, Zhang J, Wu J, Cheng H, He Q. Insight into Adsorption Kinetics, Equilibrium, Thermodynamics, and Modeling of Ciprofloxacin onto Iron Ore Tailings. Water. 2025; 17(5):760. https://doi.org/10.3390/w17050760
Chicago/Turabian StyleFang, Nan, Yanhua Xi, Jing Zhang, Jian Wu, Huicai Cheng, and Qiang He. 2025. "Insight into Adsorption Kinetics, Equilibrium, Thermodynamics, and Modeling of Ciprofloxacin onto Iron Ore Tailings" Water 17, no. 5: 760. https://doi.org/10.3390/w17050760
APA StyleFang, N., Xi, Y., Zhang, J., Wu, J., Cheng, H., & He, Q. (2025). Insight into Adsorption Kinetics, Equilibrium, Thermodynamics, and Modeling of Ciprofloxacin onto Iron Ore Tailings. Water, 17(5), 760. https://doi.org/10.3390/w17050760








