Tailoring the Structural and Morphological Properties of Biogenic Nano-Chlorapatite to Enhance the Capture Efficiency Towards Cr(VI)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Biogenic nClAP and CMC-nClAP
2.2. Characterization
2.3. Batch Adsorption Experiments
2.4. Adsorption Models
2.5. Approximate Site Energy Distribution Analysis
3. Results and Discussion
3.1. Characteristics of Biogenic nClAP and CMC-nClAP
3.1.1. XRD Analysis
3.1.2. FTIR Analysis
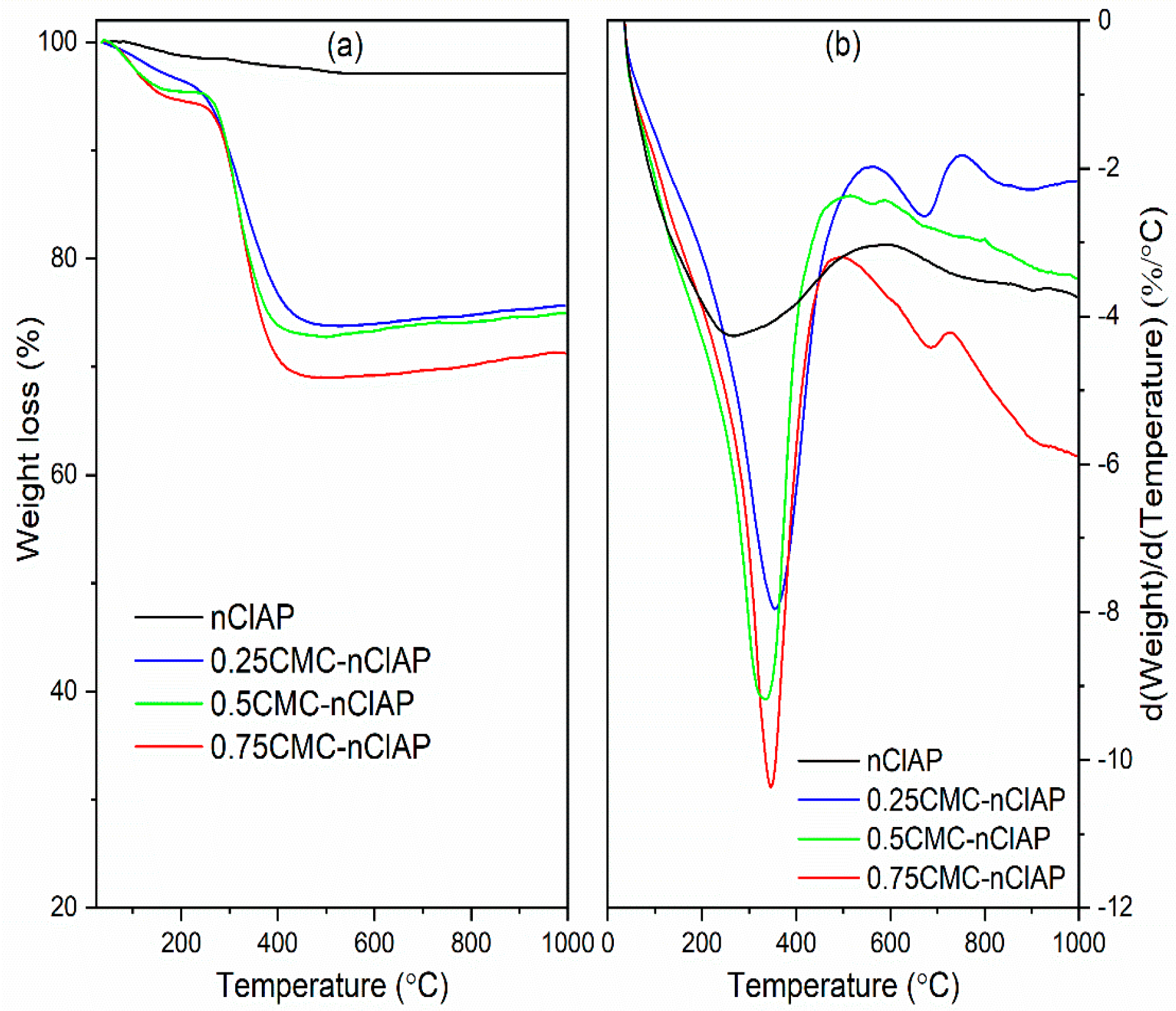
3.1.3. TG Analysis
3.1.4. TEM Analysis
3.2. Affecting Factors for Cr(VI) Adsorption by CMC-nClAP
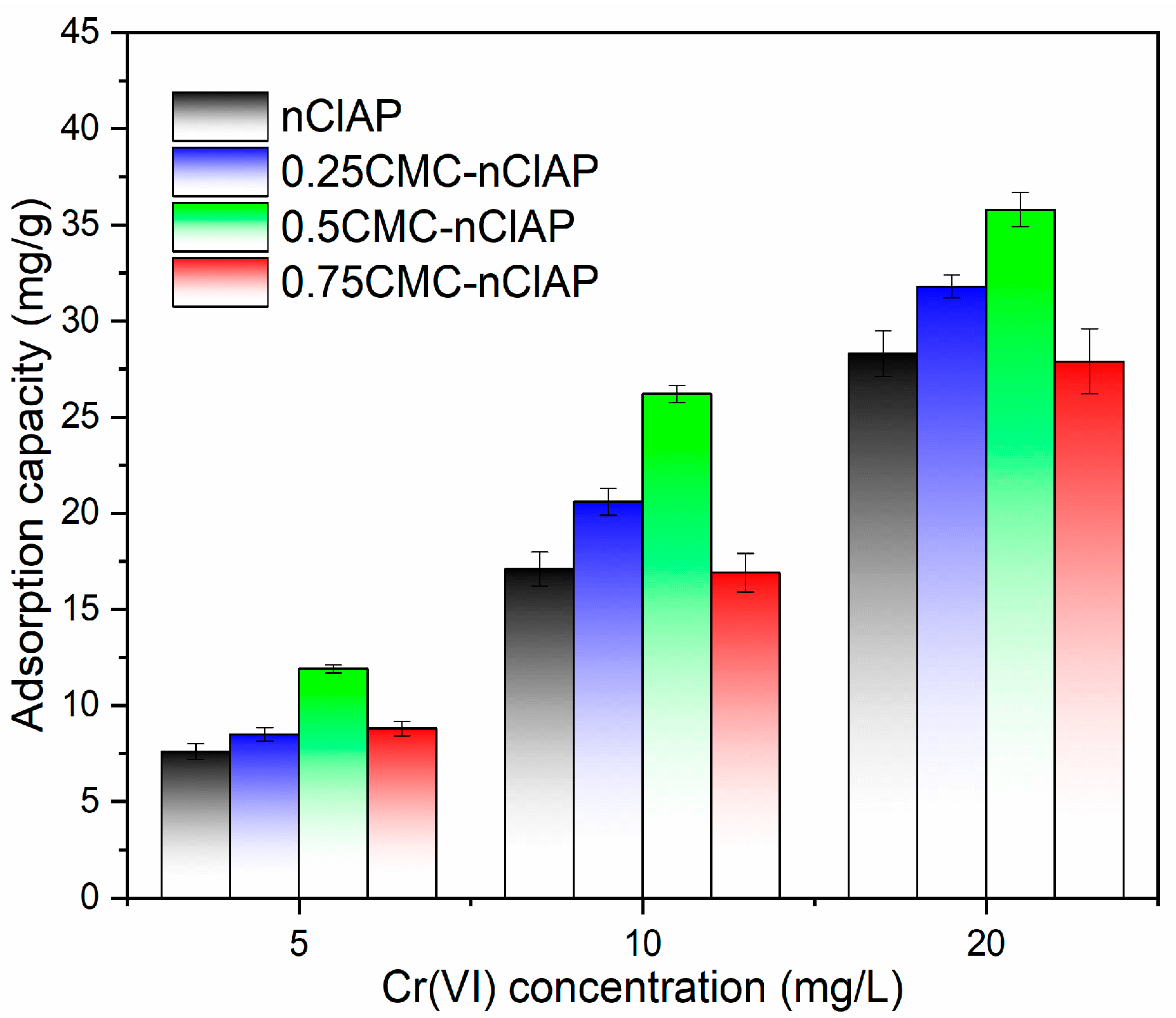
3.2.1. Effect of CMC Content on the Adsorption Ability of nClAP
3.2.2. Effect of pH
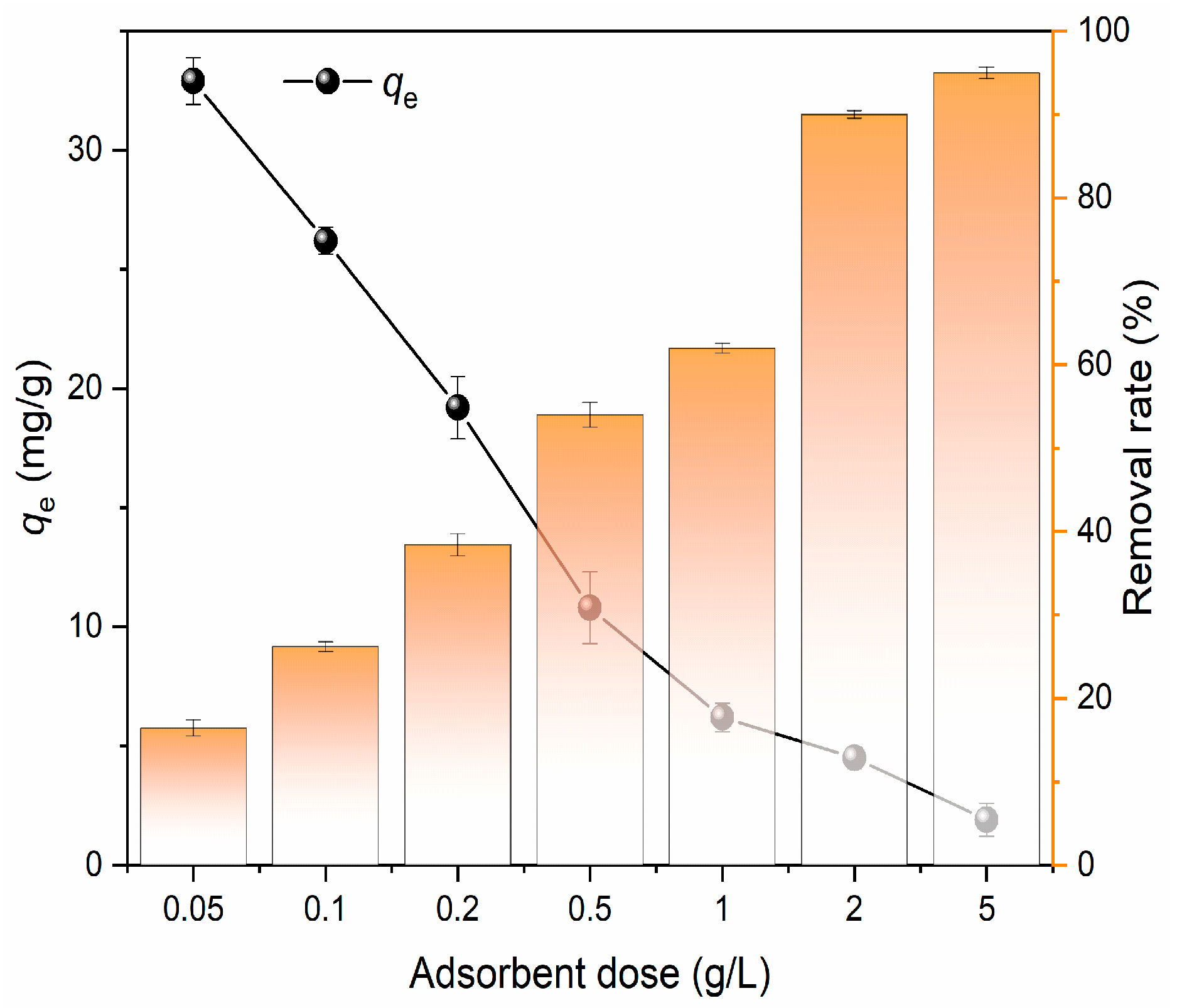
3.2.3. Effect of Adsorbent Dosage
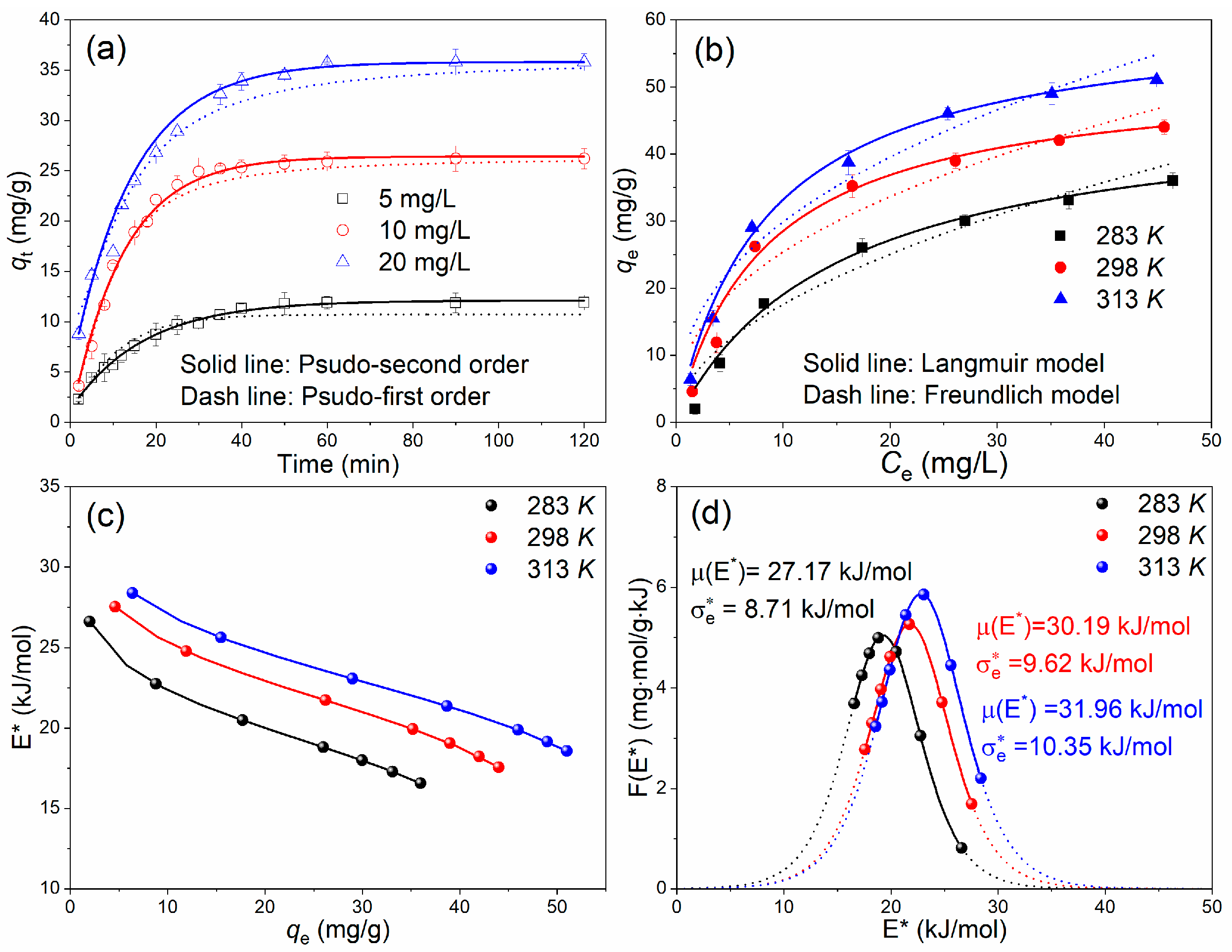
3.2.4. Adsorption Kinetics and Isotherms
3.3. Langmuir-Based Analysis of Adsorption Site Energy Distribution
3.4. Adsorption Thermodynamics
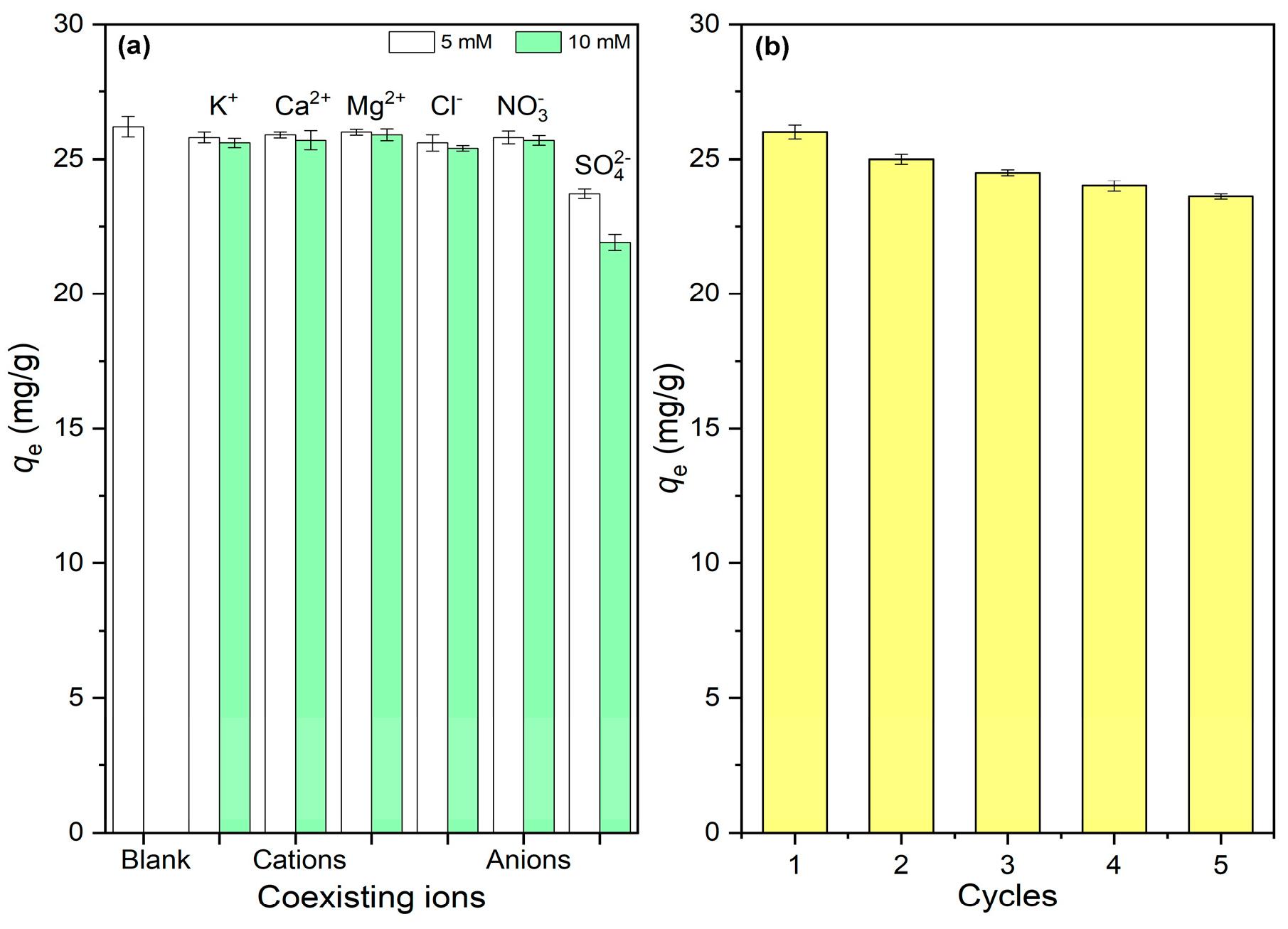
3.5. Effects of Coexisting Ions
3.6. Reusability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ren, G.; Wang, X.; Huang, P.; Zhong, B.; Zhang, Z.; Yang, L.; Yang, X. Chromium (VI) adsorption from wastewater using porous magnetite nanoparticles prepared from titanium residue by a novel solid-phase reduction method. Sci. Total Environ. 2017, 607, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Chen, X.; Liu, L.; Wang, S.; Yu, X.; Jia, Z.; Zhou, X. Cr (VI)-bioremediation mechanism of a novel strain Bacillus paramycoides Cr6 with the powerful ability to remove Cr (VI) from contaminated water. J. Hazard. Mater. 2023, 455, 131519. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, P.; Li, Y.; Tian, H.; Zhang, Y.; Zheng, X.; Liu, R.; Zhao, M.; Huang, X. Electro-peroxone enables efficient Cr removal and recovery from Cr (III) complexes and inhibits intermediate Cr (VI) generation in wastewater: Performance and mechanism. Water Res. 2022, 218, 118502. [Google Scholar] [CrossRef] [PubMed]
- Fenti, A.; Chianese, S.; Iovino, P.; Musmarra, D.; Salvestrini, S. Cr (VI) sorption from aqueous solution: A review. Appl. Sci. 2020, 10, 6477. [Google Scholar] [CrossRef]
- Ren, Y.; Han, Y.; Lei, X.; Lu, C.; Liu, J.; Zhang, G.; Zhang, B.; Zhang, Q. A magnetic ion exchange resin with high efficiency of removing Cr (VI). Colloids Surf. A 2020, 604, 125279. [Google Scholar] [CrossRef]
- He, P.Y.; Zhang, Y.J.; Chen, H.; Han, Z.C.; Liu, L.C. Low-cost and facile synthesis of geopolymer-zeolite composite membrane for chromium (VI) separation from aqueous solution. J. Hazard. Mater. 2020, 392, 122359. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, J.; Liu, Y. Removal of chromium(VI) from aqueous solutions by electrochemical reduction-precipitation. Int. J. Electrochem. Sci. 2017, 12, 11387–11396. [Google Scholar] [CrossRef]
- Li, Y.; Huang, T.; Liu, X.; Chen, Z.; Yang, H.; Wang, X. Sorption-catalytic reduction/extraction of hexavalent Cr (VI) and U (VI) by porous frameworks materials. Sep. Purif. Technol. 2023, 314, 123615. [Google Scholar] [CrossRef]
- Lal, S.; Singhal, A.; Kumari, P. Exploring carbonaceous nanomaterials for arsenic and chromium removal from wastewater. J. Water Proc. Eng. 2020, 36, 101276. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, H.; Xu, Y.; Yuan, M.; Jing, X.; Huang, J.; Li, Q.; Sun, D. Ultra-efficient removal of chromium from aqueous medium by biogenic iron based nanoparticles. Sep. Purif. Technol. 2017, 174, 466–473. [Google Scholar] [CrossRef]
- Tran, H.N.; Nguyen, D.T.; Le, G.T.; Tomul, F.; Lima, E.C.; Woo, S.H.; Sarmah, A.K.; Nguyen, H.Q.; Nguyen, P.T.; Nguyen, D.D.; et al. Adsorption mechanism of hexavalent chromium onto layered double hydroxides-based adsorbents: A systematic in-depth review. J. Hazard. Mater. 2019, 373, 258–270. [Google Scholar] [CrossRef]
- Hsu, C.S.; Haag, S.L.; Bernards, M.T.; Li, Q. Effects of chloride substitution on physical, mechanical, and biological properties of hydroxyapatite. Ceram. Int. 2021, 47, 13207–13215. [Google Scholar] [CrossRef]
- Han, X.; Zhang, Y.; Zheng, C.; Yu, X.; Li, S.; Wei, W. Enhanced Cr (VI) removal from water using a green synthesized nanocrystalline chlorapatite: Physicochemical interpretations and fixed-bed column mathematical model study. Chemosphere 2021, 264, 128421. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, D. Synthesis and characterization of a new class of stabilized apatite nanoparticles and applying the particles to in situ Pb immobilization in a fire-range soil. Chemosphere 2013, 91, 594–601. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, C.; Zeng, G.; Huang, D.; Hu, L.; Huang, C.; Wu, H.; Wang, L. Synthesis and evaluation of a new class of stabilized nano-chlorapatite for Pb immobilization in sediment. J. Hazard. Mater. 2016, 320, 278–288. [Google Scholar] [CrossRef]
- Li, Z.; Gong, Y.; Zhao, D.; Dang, Z.; Lin, Z. Enhanced removal of zinc and cadmium from water using carboxymethyl cellulose-bridged chlorapatite nanoparticles. Chemosphere 2021, 263, 128038. [Google Scholar] [CrossRef]
- Keochaiyom, B.; Wan, J.; Zeng, G.; Huang, D.; Xue, W.; Hu, L.; Huang, C.; Zhang, C.; Cheng, M. Synthesis and application of magnetic chlorapatite nanoparticles for zinc (II), cadmium (II) and lead (II) removal from water solutions. J. Colloid Interf. Sci. 2017, 505, 824–835. [Google Scholar] [CrossRef]
- Zhang, R.; Li, D.; Sun, J.; Cui, Y.; Sun, Y. In situ synthesis of FeS/Carbon fibers for the effective removal of Cr (VI) in aqueous solution. Front. Environ. Sci. Eng. 2020, 14, 68. [Google Scholar] [CrossRef]
- Shan, B.; Hao, R.; Zhang, J.; Ye, Y.; Li, J.; Xu, H.; Lu, A. Exploring the mechanism of enhanced Cr (VI) removal by Lysinibacillus cavernae microcapsules loaded with synthetic nano-hydroxyapatite. Environ. Sci. Pollut. Res. 2023, 30, 106571–106584. [Google Scholar] [CrossRef]
- Zhang, M.; Wei, W.; Chen, Y.; Han, X. Effects of Cr (VI) oxyanion, humic acid and solution chemistry on the aggregation and colloidal stability of green synthesized chlorapatite nanoparticles. Chemosphere 2023, 342, 140147. [Google Scholar] [CrossRef]
- Hoch, L.B.; Mack, E.J.; Hydutsky, B.W.; Hershman, J.M.; Skluzacek, J.M.; Mallouk, T.E. Carbothermal synthesis of carbon-supported nanoscale zero-valent iron particles for the remediation of hexavalent chromium. Environ. Sci. Technol. 2008, 42, 2600–2605. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Zeng, G.; Huang, D.; Hu, L.; Xu, P.; Huang, C.; Deng, R.; Xue, W.; Lai, C.; Zhou, C.; et al. Rhamnolipid stabilized nano-chlorapatite: Synthesis and enhancement effect on Pb-and Cd-immobilization in polluted sediment. J. Hazard. Mater. 2018, 343, 332–339. [Google Scholar] [CrossRef]
- Deng, R.; Huang, D.; Xue, W.; Lei, L.; Chen, S.; Zhou, C.; Liu, X.; Wen, X.; Li, B. Eco-friendly remediation for lead-contaminated riverine sediment by sodium lignin sulfonate stabilized nano-chlorapatite. Chem. Eng. J. 2020, 397, 125396. [Google Scholar] [CrossRef]
- He, F.; Zhao, D.; Liu, J.; Roberts, C.B. Stabilization of Fe-Pd nanoparticles with sodium carboxymethyl cellulose for enhanced transport and dechlorination of trichloroethylene in soil and groundwater. Ind. Eng. Chem. Res. 2007, 46, 29–34. [Google Scholar] [CrossRef]
- Ashokan, A.; Rajendran, V.; Kumar, T.S.; Jayaraman, G. Eggshell derived hydroxyapatite microspheres for chromatographic applications by a novel dissolution-precipitation method. Ceram. Int. 2021, 47, 18575–18583. [Google Scholar] [CrossRef]
- Carter, M.C.; Kilduff, J.E.; Walter, J.; Weber, J. Site energy distribution analysis of preloaded adsorbents. Environ. Sci. Technol. 1995, 29, 1773–1780. [Google Scholar] [CrossRef]
- Cerofolini, G.F. Localized adsorption on heterogeneous surfaces. Thin Solid Films 1974, 23, 129–152. [Google Scholar]
- Jiang, H.; Li, Q.Y.; Sun, J.X.; Huang, Y.Y.; Zhang, P.; Mao, Y.F.; Qu, Y.F.; Liu, X.L. Studies on competitive adsorption characteristics of bisphenol A and 17α-ethinylestradiol on thermoplastic polyurethane by site energy distribution theory. Environ. Geochem. Health 2023, 45, 5181–5194. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, Y.; Zheng, Z.; Mu, B. Synthesis of an absorption material based on oil shale semi-coke: Discussion to adsorption mechanism and corresponding site energy distribution analysis. Colloids Surf. A 2022, 637, 128251. [Google Scholar] [CrossRef]
- Chen, H.; Xiao, R.; Huang, D.; Deng, R.; Li, R.; Chen, Y.; Zhou, W. Three kinds of apatite adsorbents prepared by co-precipitation for Pb (II) and Cd (II) removal from wastewater: Performance, competitive effects and mechanisms. J. Mol. Liq. 2024, 400, 124478. [Google Scholar] [CrossRef]
- Venkateswarlu, K.; Sandhyarani, M.; Nellaippan, T.A.; Rameshbabu, N. Estimation of crystallite size, lattice strain and dislocation density of nanocrystalline carbonate substituted hydroxyapatite by X-ray peak variance analysis. Procedia Mater. Sci. 2014, 5, 212–221. [Google Scholar] [CrossRef]
- Grządka, E.; Matusiak, J.; Bastrzyk, A.; Polowczyk, I. CMC as a stabiliser of metal oxide suspensions. Cellulose 2020, 27, 2225–2236. [Google Scholar] [CrossRef]
- Okitsu, S.; Yokota, T.; Aizawa, M. Effect of ball-milling treatment on sinterability of hydroxyapatite ceramics including bone minerals. Phosphorus Res. Bull. 2022, 38, 25–31. [Google Scholar] [CrossRef]
- Jerdioui, S.; Bouammalia, H.; Mejdoubi, E.; Touzani, R.; Azzaoui, K.; Hammouti, B.; Sabbahi, R.; Nandiyanto, A.B.D.; Elansari, L.L. Physico-chemical characteristics of Ca/P ratio on the composition and structure of oxygenated apatite. Commun. Sci. Technol. 2024, 9, 100–106. [Google Scholar] [CrossRef]
- Mohammad, A.; Inamuddin; Amin, A. Surfactant assisted preparation and characterization of carboxymethyl cellulose Sn (IV) phosphate composite nano-rod like cation exchanger: A thermodynamic study of pyridine adsorption. J. Therm. Anal. Calorim. 2012, 107, 127–134. [Google Scholar] [CrossRef]
- He, F.; Zhao, D. Manipulating the size and dispersibility of zerovalent iron nanoparticles by use of carboxymethyl cellulose stabilizers. Environ. Sci. Technol. 2007, 41, 6216–6221. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Effects of molecular weight and concentration of carboxymethyl cellulose on morphology of hydroxyapatite nanoparticles as prepared with one-step wet chemical method. Front. Environ. Sci. Eng. 2015, 9, 804–812. [Google Scholar] [CrossRef]
- Wang, G.; Chang, Q.; Zhang, M.; Han, X. Effect of pH on the removal of Cr (III) and Cr (VI) from aqueous solution by modified polyethyleneimine. React. Funct. Polym. 2013, 73, 1439–1446. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, K.; Zhao, L. Sono-assisted synthesis of nanostructured polyaniline for adsorption of aqueous Cr (VI): Effect of protonic acids. Chem. Eng. J. 2014, 239, 123–131. [Google Scholar] [CrossRef]
- Hu, Y.; Zhan, G.; Peng, X.; Liu, X.; Ai, Z.; Jia, F.; Cao, S.; Quan, F.; Shen, W.; Zhang, L. Enhanced Cr (VI) removal of zero-valent iron with high proton conductive FeC2O4·2H2O shell. Chem. Eng. J. 2020, 389, 124414. [Google Scholar] [CrossRef]
- Campisi, S.; Evangelisti, C.; Postole, G.; Gervasini, A. Combination of interfacial reduction of hexavalent chromium and trivalent chromium immobilization on tin-functionalized hydroxyapatite materials. Appl. Surf. Sci. 2021, 539, 148227. [Google Scholar] [CrossRef]
- Valsami-Jones, E.; Ragnarsdottir, K.V.; Putnis, A.; Bosbach, D.; Kemp, A.J.; Cressey, G. The dissolution of apatite in the presence of aqueous metal cations at pH 2-7. Chem. Geol. 1998, 151, 215–233. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, Y.; Cha, L. Removal of methyl orange dye using activated biochar derived from pomelo peel wastes: Performance, isotherm, and kinetic studies. J. Dispers. Sci. Technol. 2020, 41, 125–136. [Google Scholar] [CrossRef]
- Aliyu, M.; Abdullah, A.H.; bin Mohamed Tahir, M.I. Adsorption tetracycline from aqueous solution using a novel polymeric adsorbent derived from the rubber waste. J. Taiwan Inst. Chem. Eng. 2022, 136, 104333. [Google Scholar] [CrossRef]
- Shams Khorramabadi, G.; Darvishi Cheshmeh Soltani, R.; Rezaee, A.; Khataee, A.R.; Jonidi Jafari, A. Utilisation of immobilised activated sludge for the biosorption of chromium (VI). Can. J. Chem. Eng. 2012, 90, 1539–1546. [Google Scholar] [CrossRef]
- Billah, R.E.K.; Shekhawat, A.; Mansouri, S.; Majdoubi, H.; Agunaou, M.; Soufiane, A.; Jugade, R. Adsorptive removal of Cr (VI) by chitosan-SiO2-TiO2 nanocomposite. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100695. [Google Scholar]
- Choe, J.; Ji, J.; Yu, J.; Jang, K.; Yun, J.; Choe, S.; Rim, Y.; Jo, C. Adsorption of Cr(VI) in aqueous solution by polypyrrole nanotube and polypyrrole nanoparticle; Kinetics, isotherm equilibrium, and thermodynamics. Inorg. Chem. Commun. 2022, 145, 109981. [Google Scholar] [CrossRef]
- Tang, Q.; Wu, H.; Zhou, M.; Yang, D. Preparation of a novel high-performance lignin-based anionic adsorption resin for efficient removal of Cr (VI) in aqueous solutions. Ind. Crops Prod. 2023, 199, 116720. [Google Scholar] [CrossRef]
- Mobarak, M.; Qaysi, S.; Ahmed, M.S.; Salama, Y.F.; Abbass, A.M.; Abd Elrahman, M.; Abdel-Gawwad, H.A.; Seliem, M.K. Insights into the adsorption performance and mechanism of Cr (VI) onto porous nanocomposite prepared from gossans and modified coal interface: Steric, energetic, and thermodynamic parameters interpretations. Chin. J. Chem. Eng. 2023, 61, 118–128. [Google Scholar] [CrossRef]
- Mutabazi, E.; Qiu, X.; Song, Y.; Li, C.; Jia, X.; Hakizimana, I.; Niu, J.; Nuramkhaan, M.; Zhao, Y. Cr (VI) adsorption on activated carbon, sludge derived biochar, and peanut shells derived biochar: Performance, mechanisms during the reuse process and site energy distribution analysis. J. Water Proc. Eng. 2024, 57, 104679. [Google Scholar] [CrossRef]
- Liao, P.; Li, B.; Xie, L.; Bai, X.; Qiao, H.; Li, Q.; Yang, B.; Liu, C. Immobilization of Cr (VI) on engineered silicate nanoparticles: Microscopic mechanisms and site energy distribution. J. Hazard. Mater. 2020, 383, 121145. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Zhao, C.; Song, Y.; Qiu, X.; Li, C.; Zhao, Y. Competitive adsorption of sulfamethoxazole and bisphenol A on magnetic biochar: Mechanism and site energy distribution. Environ. Pollut. 2023, 329, 121662. [Google Scholar] [CrossRef]
- Hussain, N.; Khan, H.; Hussain, S.; Arshad, M.; Umar, M.; Wahab, F. Unleashing the dye adsorption potential of polyaminoimide homopolymer: DFT, statistical physics, site energy and pore size distribution analyses. J. Environ. Chem. Eng. 2023, 11, 111383. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, C.; Yang, Y.; Li, Z.; Qiu, X.; Gao, J.; Ji, M. Adsorption of sulfamethoxazole on polypyrrole decorated volcanics over a wide pH range: Mechanisms and site energy distribution consideration. Sep. Purif. Technol. 2022, 283, 120165. [Google Scholar] [CrossRef]
- He, J.; Guo, J.; Zhou, Q.; Fang, F. Adsorption characteristics of nitrite on natural filter medium: Kinetic, equilibrium, and site energy distribution studies. Ecotoxicol. Environ. Saf. 2019, 169, 435–441. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, B.; Zhang, H.; Ma, J.; Mu, B.; Zhang, W. A novel Biochar modified by Chitosan-Fe/S for tetracycline adsorption and studies on site energy distribution. Bioresour. Technol. 2019, 294, 122152. [Google Scholar] [CrossRef]
- Shen, X.; Guo, X.; Zhang, M.; Tao, S.; Wang, X. Sorption mechanisms of organic compounds by carbonaceous materials: Site energy distribution consideration. Environ. Sci. Technol. 2015, 49, 4894–4902. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Gu, M.; Tao, M.; Xian, X. Adsorption of methane on shale: Statistical physics model and site energy distribution studies. Energy Fuels 2019, 34, 304–318. [Google Scholar] [CrossRef]
- Yan, B.; Niu, C.H.; Wang, J. Kinetics, electron-donor-acceptor interactions, and site energy distribution analyses of norfloxacin adsorption on pretreated barley straw. Chem. Eng. J. 2017, 330, 1211–1221. [Google Scholar] [CrossRef]
- Jamshidi, P.; Shemirani, F. Adsorption and desorption of Pb2+ on magnetic Mn2O3 as highly efficient adsorbent: Isotherm, kinetic and thermodynamic studies. Colloids Surf. A 2019, 571, 151–159. [Google Scholar] [CrossRef]
- Zeng, H.; Zeng, H.; Zhang, H.; Shahab, A.; Zhang, K.; Lu, Y.; Nabi, I.; Naseem, F.; Ullah, H. Efficient adsorption of Cr (VI) from aqueous environments by phosphoric acid activated eucalyptus biochar. J. Clean. Prod. 2021, 286, 124964. [Google Scholar] [CrossRef]
- Singh, S.; Anil, A.G.; Khasnabis, S.; Kumar, V.; Nath, B.; Adiga, V.; Naik, T.S.K.; Subramanian, S.; Kumar, V.; Singh, J.; et al. Sustainable removal of Cr (VI) using graphene oxide-zinc oxide nanohybrid: Adsorption kinetics, isotherms and thermodynamics. Environ. Res. 2022, 203, 111891. [Google Scholar] [CrossRef] [PubMed]
- Nie, D.; Ma, R.; Zhang, Y.; Wang, W.; Nie, G.; Liu, G.; Liu, W.; Zou, D. Efficient removal of Cr (VI) from wastewater by composite adsorptive membrane modified with polyethyleneimine (PEI). Sep. Purif. Technol. 2024, 346, 127410. [Google Scholar] [CrossRef]
- Lagergren, S. About theory of so-called adsorption of soluble substances. K. Sven. Vetenskapsakad. Andl. 1898, 24, 1–39. Available online: https://sid.ir/paper/563615/en (accessed on 2 March 2025).
- Ho, Y.S.; McKay, G. Kinetics of pollutant sorption by biosorbents: Review. Sep. Purif. Methods 2000, 29, 189–232. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Udber die adsorption in Loesungen. Z. Physik. Chem. 1907, 57, 385–470. [Google Scholar] [CrossRef]





| Cr(VI) Concentration | qe,exp (mg/g) | PFO Model | PSO Model | ||||
|---|---|---|---|---|---|---|---|
| k1 | qe,cal | R2 | k2 | qe,cal | R2 | ||
| (1/h) | (mg/g) | (g/(mg·h)) | (mg/g) | ||||
| 5 mg/L | 11.8 | 0.099 | 10.7 | 0.919 | 0.008 | 12.2 | 0.967 |
| 10 mg/L | 26.2 | 0.082 | 24.4 | 0.946 | 0.003 | 25.4 | 0.969 |
| 20 mg/L | 35.8 | 0.077 | 33.3 | 0.934 | 0.002 | 36.5 | 0.978 |
| Temperature | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| KL | qmax | R2 | KF | 1/n | R2 | |
| (L/mg) | (mg/g) | [(mg/g)·(L/mg)1/n] | ||||
| 283 K | 0.067 | 47.4 | 0.985 | 5.386 | 0.513 | 0.933 |
| 298 K | 0.119 | 52.1 | 0.989 | 9.950 | 0.407 | 0.915 |
| 313 K | 0.151 | 61.1 | 0.991 | 11.745 | 0.405 | 0.932 |
| Temperature (K) | R2 | ΔG0 (KJ/mol) | ΔH0 (KJ/mol) | ΔS0 (J/(mol·K)) |
|---|---|---|---|---|
| 283 | 0.9557 | −9.89 | 19.89 | 105.62 |
| 298 | −11.84 | |||
| 313 | −13.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Z.; Huan, X.; Li, Y.; Zhi, J.; Wei, W. Tailoring the Structural and Morphological Properties of Biogenic Nano-Chlorapatite to Enhance the Capture Efficiency Towards Cr(VI). Water 2025, 17, 762. https://doi.org/10.3390/w17050762
Tian Z, Huan X, Li Y, Zhi J, Wei W. Tailoring the Structural and Morphological Properties of Biogenic Nano-Chlorapatite to Enhance the Capture Efficiency Towards Cr(VI). Water. 2025; 17(5):762. https://doi.org/10.3390/w17050762
Chicago/Turabian StyleTian, Zhuangzhuang, Xinyu Huan, Yuanyi Li, Jiaqi Zhi, and Wei Wei. 2025. "Tailoring the Structural and Morphological Properties of Biogenic Nano-Chlorapatite to Enhance the Capture Efficiency Towards Cr(VI)" Water 17, no. 5: 762. https://doi.org/10.3390/w17050762
APA StyleTian, Z., Huan, X., Li, Y., Zhi, J., & Wei, W. (2025). Tailoring the Structural and Morphological Properties of Biogenic Nano-Chlorapatite to Enhance the Capture Efficiency Towards Cr(VI). Water, 17(5), 762. https://doi.org/10.3390/w17050762







