Electron Beam Irradiation-Induced Degradation of Sulfadiazine in Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. SDZ Degradation
2.3. Analytical Method
3. Results and Discussions
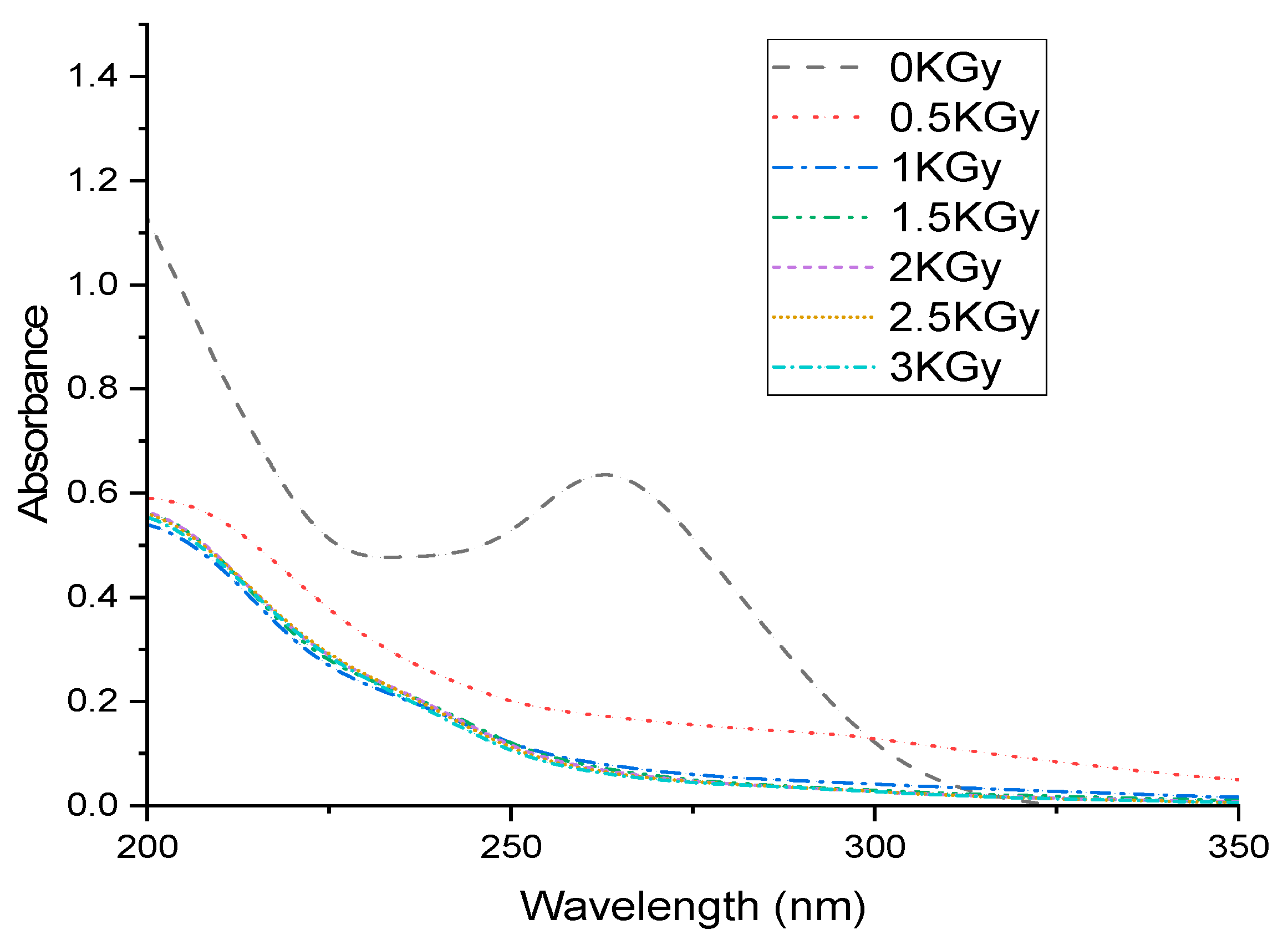
3.1. Dose Influence on SDZ Degradation
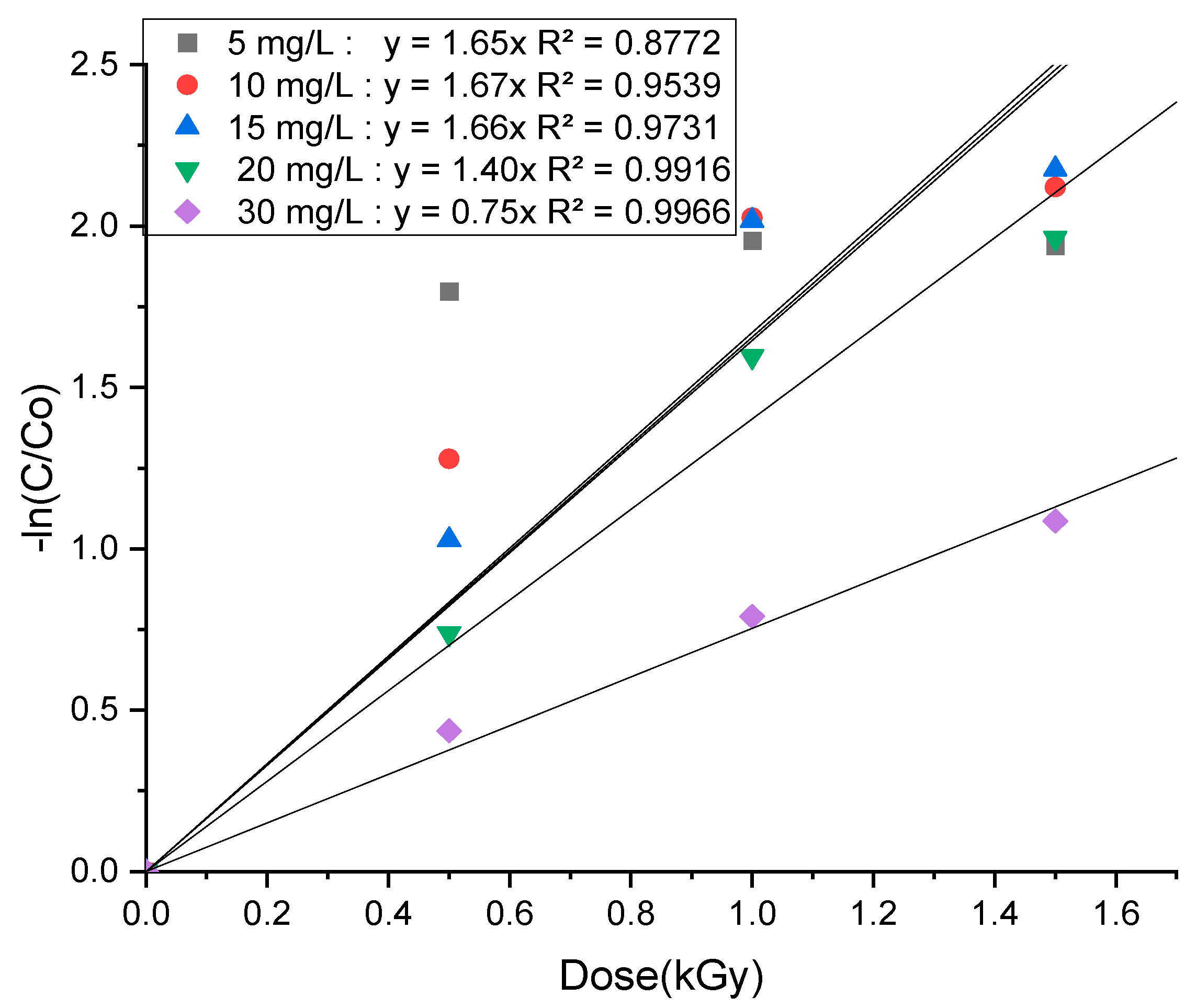
3.2. Effect of SDZ Initial Concentration Under EBI
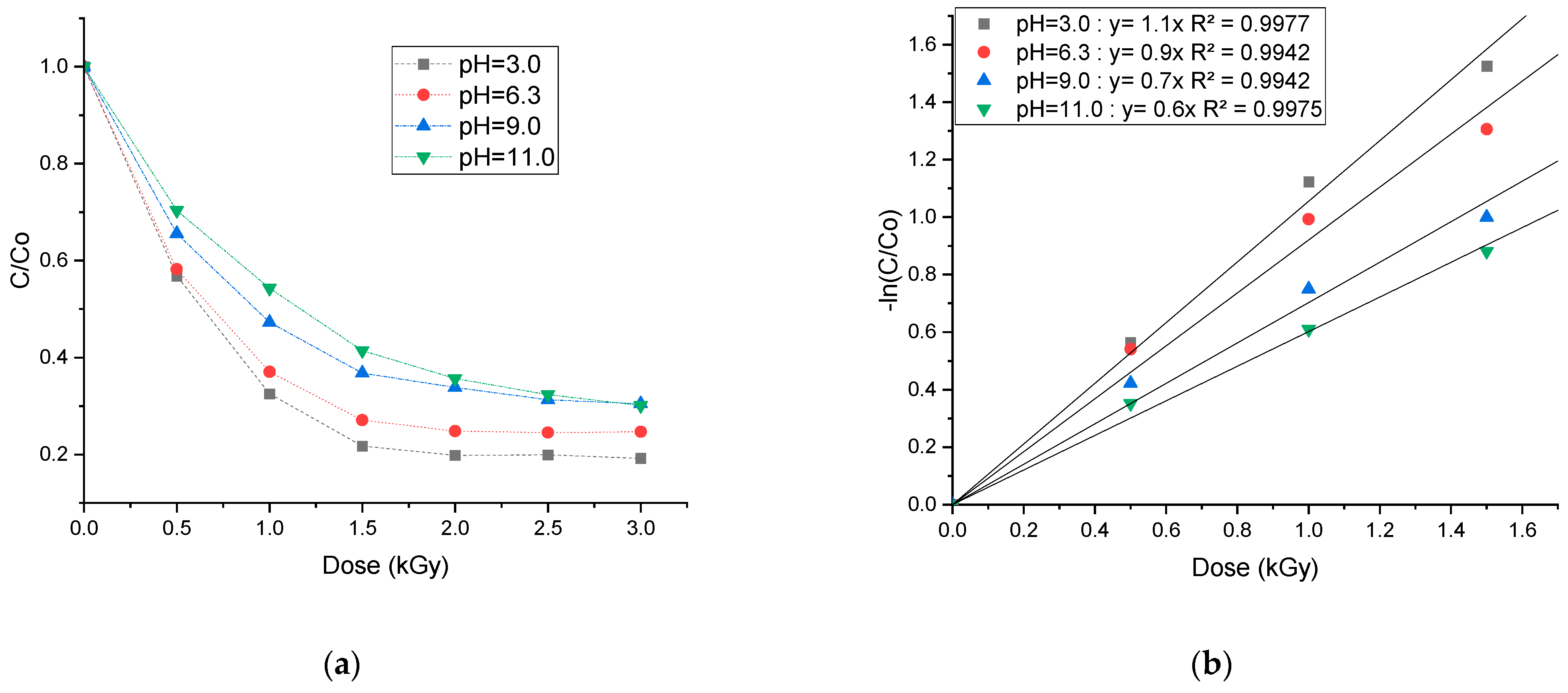
3.3. Effect of Initial pH
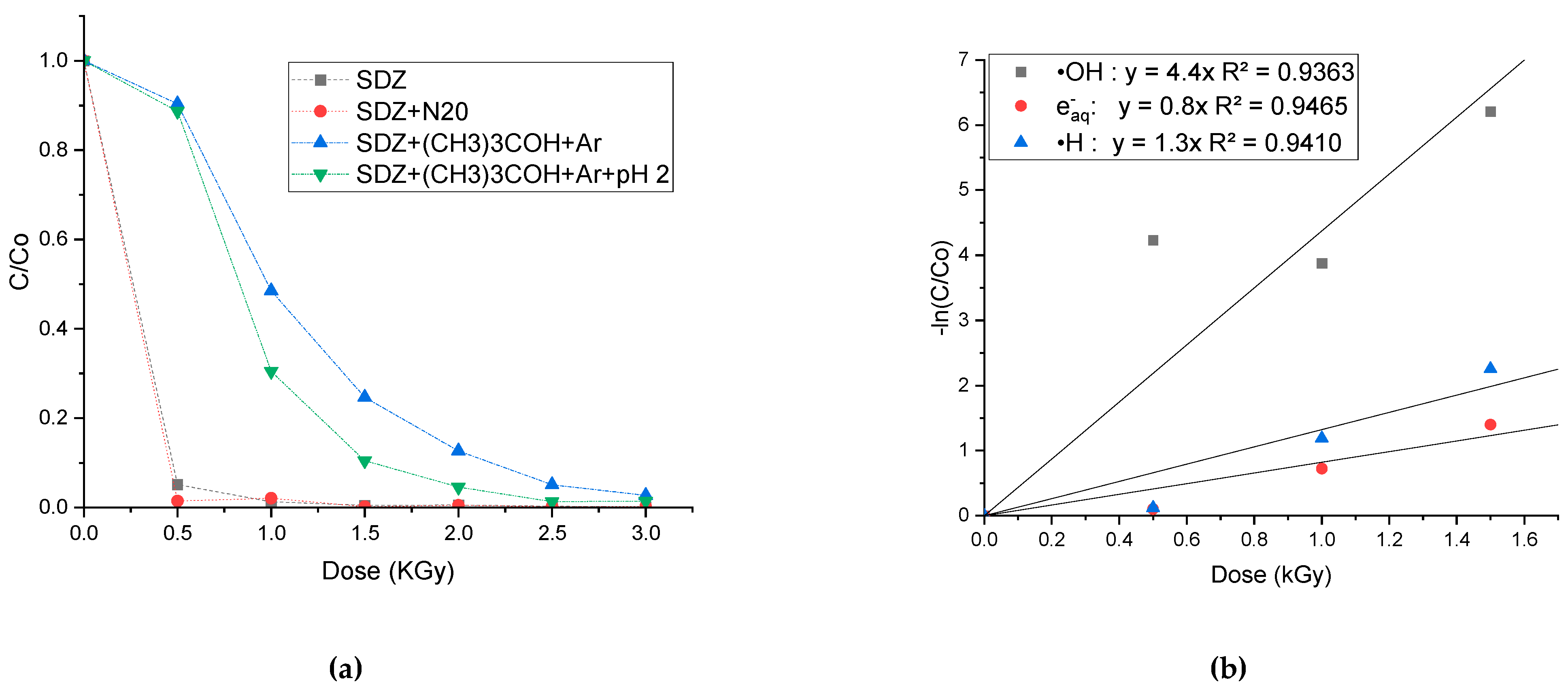
3.4. Effect of Reactive Species on SDZ Degradation
3.5. LC-MS Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Connor, E.E. Sulfonamide Antibiotics. Prim. Care Update OB/GYNS 1998, 5, 32–35. [Google Scholar] [CrossRef]
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and Personal Care Products in the Environment: Agents of Subtle Change? Env. Health Perspect. 1999, 107, 907–938. [Google Scholar] [CrossRef] [PubMed]
- Rong, S.; Sun, Y. Wetted-wall Corona Discharge Induced Degradation of Sulfadiazine Antibiotics in Aqueous Solution. J. Chem. Tech. Biotech. 2014, 89, 1351–1359. [Google Scholar] [CrossRef]
- García-Galán, M.J.; Díaz-Cruz, M.S.; Barceló, D. Occurrence of Sulfonamide Residues along the Ebro River basinRemoval in Wastewater Treatment Plants and Environmental Impact Assessment. Environ. Int. 2011, 37, 462–473. [Google Scholar] [CrossRef]
- Zhu, F.; Wang, S.; Liu, Y.; Wu, M.; Wang, H.; Xu, G. Antibiotics in the Surface Water of Shanghai, China: Screening, Distribution, and Indicator Selecting. Environ. Sci. Pollut. Res. 2021, 28, 9836–9848. [Google Scholar] [CrossRef]
- Boxall, A.B.A.; Blackwell, P.; Cavallo, R.; Kay, P.; Tolls, J. The Sorption and Transport of a Sulphonamide Antibiotic in Soil Systems. Toxicol. Lett. 2002, 131, 19–28. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, J.-L. Removal of Sulfonamide Antibiotics from Wastewater by Gamma Irradiation in Presence of Iron Ions. Nucl. Sci. Tech. 2016, 27, 104. [Google Scholar] [CrossRef]
- Zaviska, F.; Drogui, P.; Mercier, G.; Blais, J.-F. Procédés d’oxydation avancée dans le traitement des eaux et des effluents industriels: Application à la dégradation des polluants réfractaires. Rseau 2009, 22, 535–564. [Google Scholar] [CrossRef]
- Deogaonkar, S.C.; Wakode, P.; Rawat, K.P. Electron Beam Irradiation Post Treatment for Degradation of Non Biodegradable Contaminants in Textile Wastewater. Radiat. Phys. Chem. 2019, 165, 108377. [Google Scholar] [CrossRef]
- Ponomarev, A.V.; Ershov, B.G. The Green Method in Water Management: Electron Beam Treatment. Environ. Sci. Technol. 2020, 54, 5331–5344. [Google Scholar] [CrossRef]
- Trojanowicz, M. Removal of Persistent Organic Pollutants (POPs) from Waters and Wastewaters by the Use of Ionizing Radiation. Sci. Total Environ. 2020, 718, 134425. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S.; Chen, C.; Hu, J.; He, S.; Zhou, Y.; Zhu, H.; Wang, X.; Hu, D.; Lin, J. Treatment of Hospital Wastewater by Electron Beam Technology: Removal of COD, Pathogenic Bacteria and Viruses. Chemosphere 2022, 308, 136265. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhuan, R.; Wang, J. Assessment of Degradation Characteristic and Mineralization Efficiency of Norfloxacin by Ionizing Radiation Combined with Fenton-like Oxidation. J. Hazard. Mater. 2021, 404, 124172. [Google Scholar] [CrossRef] [PubMed]
- Folcik, A.M.; Ruggles, S.A.; Pillai, S.D. Applicability of Electron Beam Technology for the Degradation of Microcystin-LR in Surface Waters. ACS Omega 2023, 8, 12664–12670. [Google Scholar] [CrossRef]
- Lee, C.-S.; Londhe, K.; Grdanovska, S.; Cooper, C.A.; Venkatesan, A.K. Emerging Investigator Series: Low Doses of Electron Beam Irradiation Effectively Degrade 1,4-Dioxane in Water within a Few Seconds. Environ. Sci. Water Res. Technol. 2023, 9, 2226–2237. [Google Scholar] [CrossRef]
- Borhani Yazdi, N.; Rezvani Ghalhari, M.; Parach, A.; Ehrampoush, M.H.; Ghadiri, K.; Ghorbanian, M.; Zare Hassanabadi, M.H.; Abouee Mehrizi, E. Degradation of Piroxicam and Celecoxib from Aqueous Solution by High-Energy Electron Beam as a Sustainable Method. Heliyon 2024, 10, e39839. [Google Scholar] [CrossRef]
- Zhu, F.; Pan, J.; Zou, Q.; Wu, M.; Wang, H.; Xu, G. Electron Beam Irradiation of Typical Sulfonamide Antibiotics in the Aquatic Environment: Kinetics, Removal Mechanisms, Degradation Products and Toxicity Assessment. Chemosphere 2021, 274, 129713. [Google Scholar] [CrossRef]
- Wang, D.; Huang, Y.; Xiao, S.; Xie, Q.; Bianbadrolma; Wang, H. Enhancement of Sulfadiazine Degradation by TiO2 Addition in a Simulated Sunlight Induced Periodate Activation System: Influence Parameter, Degradation Process and Solution Toxicity Analysis. J. Water Process Eng. 2024, 68, 106511. [Google Scholar] [CrossRef]
- ISO-ASTM-51401-2013; Practice for Use of a Dichromate Dosimetry System. ASTM International: West Conshohocken, PA, USA, 2013.
- Wang, S.; Wang, J. Radiation-Induced Degradation of Sulfamethoxazole in the Presence of Various Inorganic Anions. Chem. Eng. J. 2018, 351, 688–696. [Google Scholar] [CrossRef]
- Bojanowska-Czajka, A.; Kciuk, G.; Gumiela, M.; Borowiecka, S.; Nałęcz-Jawecki, G.; Koc, A.; Garcia-Reyes, J.F.; Ozbay, D.S.; Trojanowicz, M. Analytical, Toxicological and Kinetic Investigation of Decomposition of the Drug Diclofenac in Waters and Wastes Using Gamma Radiation. Env. Sci. Pollut. Res. 2015, 22, 20255–20270. [Google Scholar] [CrossRef]
- Kwon, M.; Yoon, Y.; Cho, E.; Jung, Y.; Lee, B.-C.; Paeng, K.-J.; Kang, J.-W. Removal of Iopromide and Degradation Characteristics in Electron Beam Irradiation Process. J. Hazard. Mater. 2012, 227–228, 126–134. [Google Scholar] [CrossRef]
- Paul, J.; Rawat, K.P.; Sarma, K.S.S.; Sabharwal, S. Decoloration and Degradation of Reactive Red-120 Dye by Electron Beam Irradiation in Aqueous Solution. Appl. Radiat. Isot. 2011, 69, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Wu, M.; Deng, F.; Xu, G.; Liu, N.; Li, X.; Tang, L. Electron Beam Irradiation Induced Degradation of Antidepressant Drug Fluoxetine in Water Matrices. Chemosphere 2018, 190, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Zhuang, S.; Wang, J. Degradation Kinetics and Mechanism of Penicillin G in Aqueous Matrices by Ionizing Radiation. Radiat. Phys. Chem. 2018, 145, 34–38. [Google Scholar] [CrossRef]
- Zhuan, R.; Wang, J. Enhanced Degradation and Mineralization of Sulfamethoxazole by Integrating Gamma Radiation with Fenton-like Processes. Radiat. Phys. Chem. 2020, 166, 108457. [Google Scholar] [CrossRef]
- Kabasa, S.; Sun, Y.; Bułka, S.; Chmielewski, A.G. Chloroquine Degradation in Aqueous Solution under Electron Beam Irradiation. Nukleonika 2024, 69, 53–63. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, J.; Wang, J. Radiation-Induced Removal of Sulphadiazine Antibiotics from Wastewater. Environ. Technol. 2014, 35, 2028–2034. [Google Scholar] [CrossRef]
- Tominaga, F.K.; Dos Santos Batista, A.P.; Silva Costa Teixeira, A.C.; Borrely, S.I. Degradation of Diclofenac by Electron Beam Irradiaton: Toxicitiy Removal, by-Products Identification and Effect of Another Pharmaceutical Compound. J. Environ. Chem. Eng. 2018, 6, 4605–4611. [Google Scholar] [CrossRef]
- He, S.; Wang, J.; Ye, L.; Zhang, Y.; Yu, J. Removal of Diclofenac from Surface Water by Electron Beam Irradiation Combined with a Biological Aerated Filter. Radiat. Phys. Chem. 2014, 105, 104–108. [Google Scholar] [CrossRef]
- Getoff, N. Radiation-induced degradation of water pollutants--state of the ARTt. Radiat. Phys. Chem. 1996, 47, 581–593. [Google Scholar] [CrossRef]
- Dorfman, L.M. Reactivity of the Hydroxyl Radical in Aqueous Solutions. Natl. Stand. Ref. Data Ser. 1973, 46, 1–56. [Google Scholar]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of Rate Constants for Reactions of Hydrated Electrons, Hydrogen Atoms and Hydroxyl Radicals (OH/⋅O− in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Hayati, F.; Isari, A.A.; Anvaripour, B.; Fattahi, M.; Kakavandi, B. Ultrasound-Assisted Photocatalytic Degradation of Sulfadiazine Using MgO@CNT Heterojunction Composite: Effective Factors, Pathway and Biodegradability Studies. Chem. Eng. J. 2020, 381, 122636. [Google Scholar] [CrossRef]
- Wang, S.; Hu, Y.; Wang, J. Biodegradation of Typical Pharmaceutical Compounds by a Novel Strain Acinetobacter sp. J. Environ. Manag. 2018, 217, 240–246. [Google Scholar] [CrossRef]

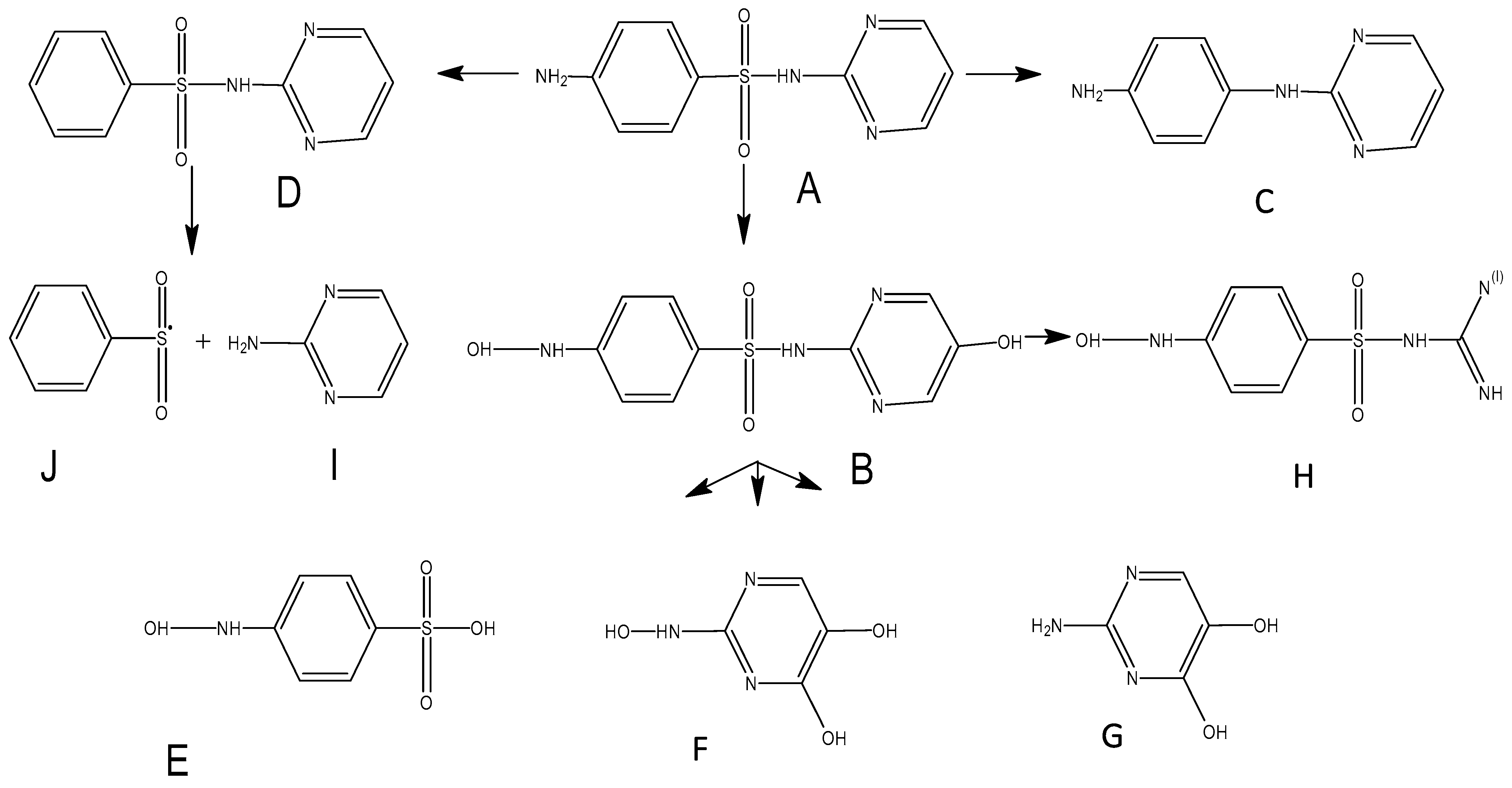
| Compounds | Retention Time | Main Fragments (m/z) | Molecular Structure |
|---|---|---|---|
| A 4-amino-N-pyrimidin-2yl-benzenesulfonamide (SDZ) | 7.17 | 251 |  |
| B | 15.74 | 283 |  |
| C | 1.003 | 187 | 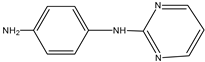 |
| D | 1.003 | 236 | 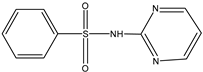 |
| E | 14.408 | 191 | 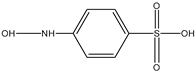 |
| F | 1.003 | 145 |  |
| G | 9.73 | 128 | 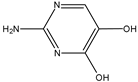 |
| H | 18.494 | 229 | 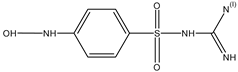 |
| I | 0.471 | 97 | 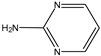 |
| J | 0.471 | 141 |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kengne, B.T.; Wang, S.; Sun, Y.; Wang, J.; Bulka, S. Electron Beam Irradiation-Induced Degradation of Sulfadiazine in Aqueous Solutions. Water 2025, 17, 1077. https://doi.org/10.3390/w17071077
Kengne BT, Wang S, Sun Y, Wang J, Bulka S. Electron Beam Irradiation-Induced Degradation of Sulfadiazine in Aqueous Solutions. Water. 2025; 17(7):1077. https://doi.org/10.3390/w17071077
Chicago/Turabian StyleKengne, Boris Tende, Shizong Wang, Yongxia Sun, Jianlong Wang, and Sylwester Bulka. 2025. "Electron Beam Irradiation-Induced Degradation of Sulfadiazine in Aqueous Solutions" Water 17, no. 7: 1077. https://doi.org/10.3390/w17071077
APA StyleKengne, B. T., Wang, S., Sun, Y., Wang, J., & Bulka, S. (2025). Electron Beam Irradiation-Induced Degradation of Sulfadiazine in Aqueous Solutions. Water, 17(7), 1077. https://doi.org/10.3390/w17071077








