An Overview on Microplastics Hazards to the Marine Ecosystem and Humans’ Health
Abstract
:1. Introduction
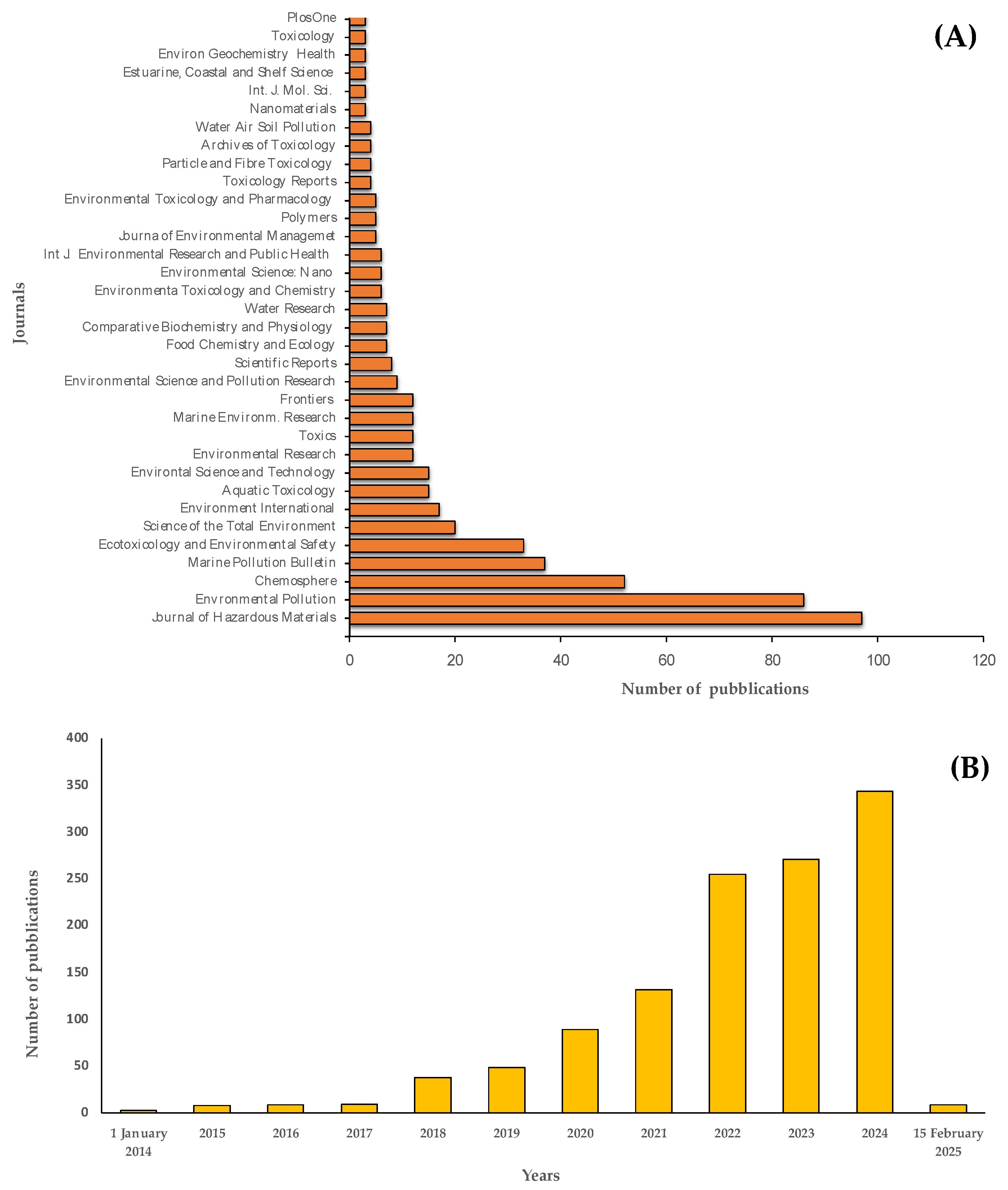
2. Literature Review Search Methodology
3. Plastics Polymer: Types and Physical–Chemical Characteristics
3.1. Plastic Types
3.2. Microplastics: Size, Shape, and Density
3.3. Addittives
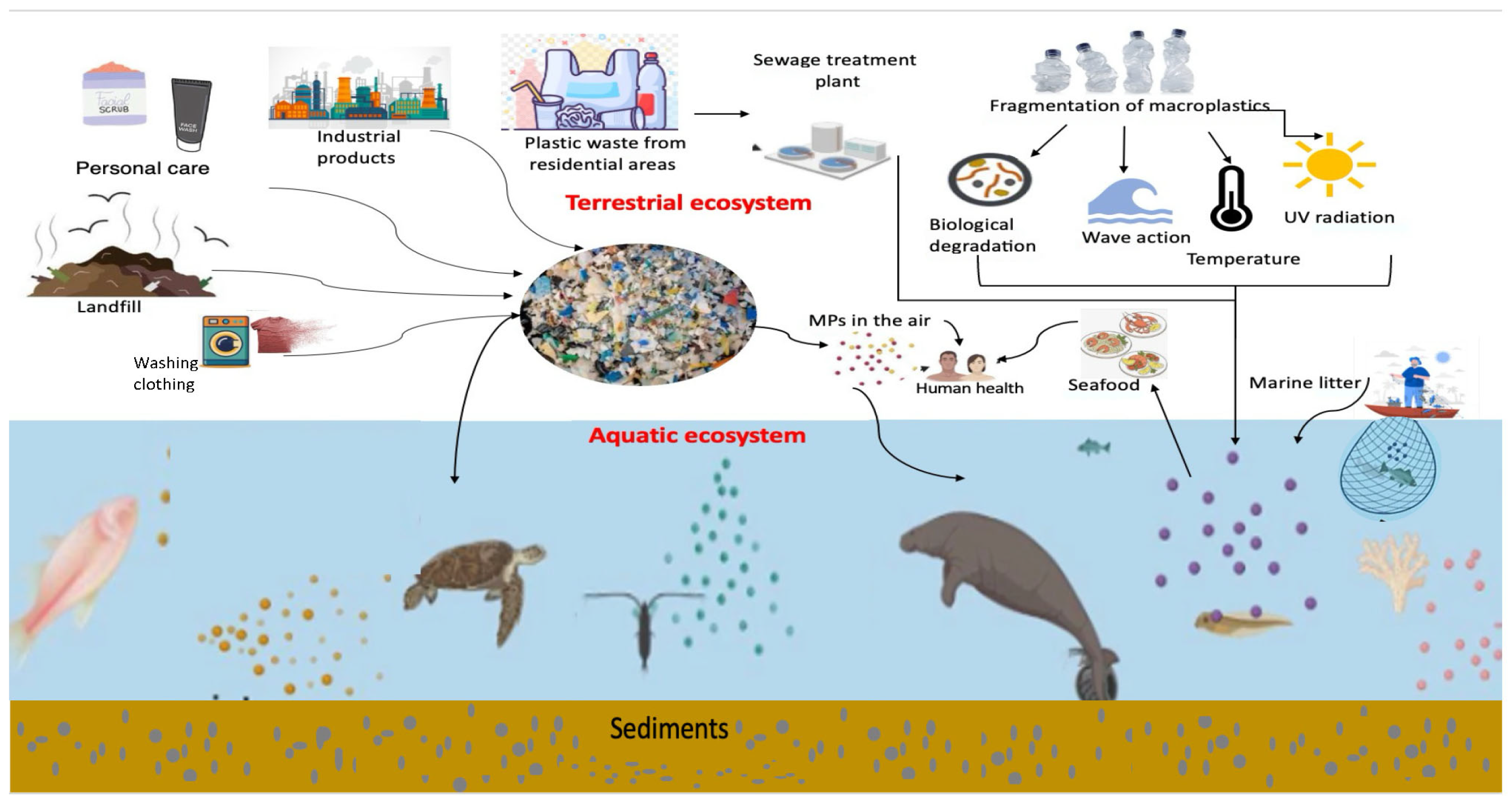
4. Sources and Transport of MPs in Marine Environment
5. Effects of Microplastics on Marine Organisms
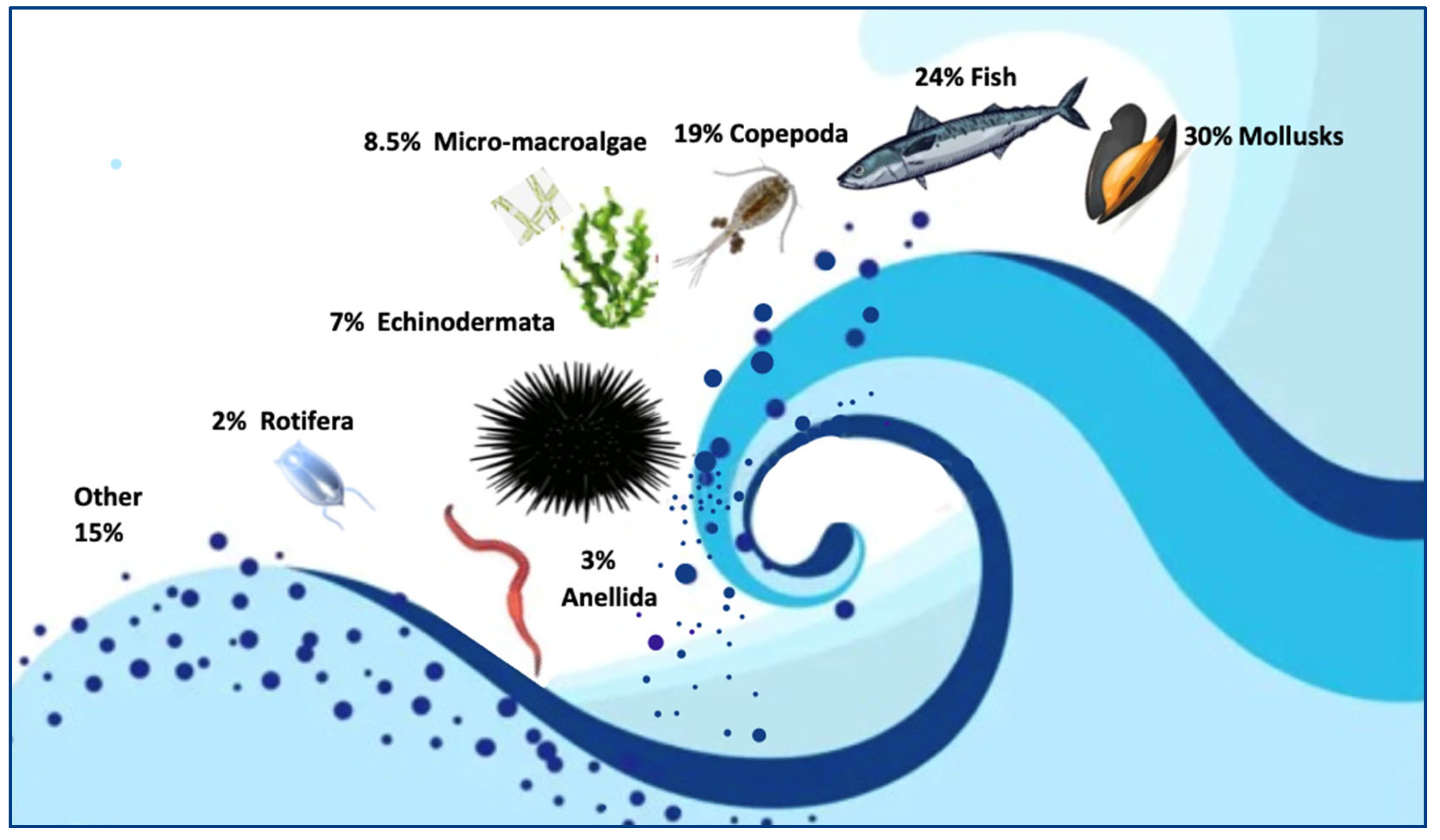
5.1. MPs Uptake by Marine Organisms
5.2. Toxic Effect of Microplastics on Marine Organisms
5.2.1. Phytoplankton
5.2.2. Zooplankton
5.2.3. Other Marine Invertebrates
5.2.4. Fish
5.3. Occurrence of Microplastics in Commercial Marine Species
6. Impact of Plastic on Human Health
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- OECD. Policies to Reduce Microplastics Pollution in Water. 2024. Available online: https://www.oecd.org/en/publications/policies-to-reduce-microplastics-pollution-in-water_7ec7e5ef-en.html (accessed on 16 February 2025).
- OECD. Plastic Pollution Is Growing Relentlessly as Waste Management and Recycling Fall Short, Say OECD. 2022. Available online: https://www.oecd.org/en/about/news/press-releases/2022/02/plastic-pollution-is-growing-relentlessly-as-waste-management-and-recycling-fall-short.html (accessed on 16 February 2025).
- End Plastic Pollution: Towards an International Legally Binding Instrument (Draft Resolution). UNEP. 2022. Available online: https://wedocs.unep.org/20.500.11822/3852 (accessed on 16 February 2025).
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [PubMed]
- Woodall, L.C.; Sanchez-Vidal, A.; Canals, M.; Paterson, G.L.; Coppock, R.; Sleight, V.; Calafat, A.; Rogers, A.D.; Narayanaswamy, B.E.; Thompson, R.C. The deep sea is a major sink for microplastic debris. R. Soc. Open Sci. 2014, 1, 140317. [Google Scholar] [CrossRef] [PubMed]
- Obbard, R.W.; Sadri, S.; Wong, Y.Q.; Khitun, A.A.; Baker, I.; Thompson, R.C. Global warming releases microplastic legacy frozen in Arctic Sea ice. Earth’s Future 2014, 2, 315–320. [Google Scholar]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar]
- Andrady, A.L. Persistence of plastic litter in the oceans. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 57–72. [Google Scholar]
- Watkins, E. The socio-economic impacts of marine litter, including the costs of policy inaction and action. In Handbook on the Economics and Management of Sustainable Oceans; Nunes, P., Markandya, A., Eds.; Edward Elgar Publishing: Cheltenham, UK, 2017. [Google Scholar]
- Beaumont, N.J.; Aanesen, M.; Austen, M.C.; Börger, T.; Clark, J.R.; Cole, M.; Hooper, T.; Lindeque, P.; Pascoe, C.; Wyles, K.J. Global ecological, social and economic impacts of marine plastic. Mar. Pollut. Bull. 2019, 142, 189–195. [Google Scholar]
- Kühn, S.; Rebolledo, E.L.B.; Van Franeker, J.A. Deleterious effects of litter on marine life. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 75–116. [Google Scholar]
- Schuyler, Q.; Hardesty, B.D.; Lawson, T.J.; Opie, K.; Wilcox, C. Economic incentives reduce plastic inputs to the ocean. Mar. Policy 2018, 96, 250–255. [Google Scholar]
- ASTM D4000-20; Standard Classification System for Specifying Plastic Materials. ASTM. 2023. Available online: https://www.astm.org/d4000-20.html (accessed on 12 December 2024).
- ASTM D7611; Standard Practice for Coding Plastic Manufactured Articles for Resin Identification. ASTM. 2021. Available online: https://www.astm.org/ (accessed on 12 December 2024).
- Nizamuddin, S.; Baloch, A.J.; Chen, C.; Arif, M.; Mubarak, N.M. Bio-based plastics, biodegradable plastics, and compostable plastics: Biodegradation mechanism, biodegradability standards and environmental stratagem. Int. Biodeterior. Biodegrad. 2024, 195, 105887. [Google Scholar]
- Plastics Europe. Plastics—The Facts An Analysis of European Plastics Production, Demand and Waste Data. Plastics Europe. 2019. Available online: https://www.plasticseurope.org/en/resources/publications/1804-plastics-facts-2019 (accessed on 17 May 2024).
- Guzzetti, E.; Sureda, A.; Tejada, S.; Faggio, C. Microplastic in marine organism: Environmental and toxicological effects. Environ. Toxicol. Pharmacol. 2018, 64, 164–171. [Google Scholar]
- Nihei, Y.; Ota, H.; Tanaka, M.; Kataoka, T.; Kashiwada, J. Comparison of concentration, shape, and polymer composition between microplastics and mesoplastics in Japanese river waters. Water Res. 2024, 249, 120979. [Google Scholar]
- Oluwoye, I.; Machuca, L.L.; Higgins, S.; Suh, S.; Galloway, T.S.; Halley, P.; Tanaka, S.; Iannuzzi, M. Degradation and lifetime prediction of plastics in subsea and offshore infrastructures. Sci. Total Environ. 2023, 904, 166719. [Google Scholar]
- Huang, Y.Z.; Wang, T.; Sun, N.; Duan, Z.; Wigmosta, M.; Maurer, B. Quantifying the influence of size, shape, and density of microplastics on their transport modes: A modeling approach. Mar. Pollut. Bull. 2024, 203, 116461. [Google Scholar] [PubMed]
- UNEP. Guidance on Best Available Techniques and Best Environmental Practices for the Recycling and Disposal of Articles Containing Polybrominated Diphenyl Ethers (PBDEs) Listed Under the Stockholm. Convention on Persistent Organic Pollutants; UNEP/POPS/COP.7/INF/22 2015; UNEP: Geneva, Switzerland, 2015. [Google Scholar]
- UNEP. Guidance for the Inventory, Identification and Substitution of Hexabromocyclododecane (HBCD); UNEP: Osaka, Japan, 2015. [Google Scholar]
- Crema, A.; Dinelli, E.; Fabbri, E.; Galletti, P.; Greggio, N.; Lastella, V.; Parodi, A.; Pasteris, A.; Pedrizzi, M.; Samorì, C. Additives in bioplastics: Chemical characterization, migration in water and effects on photosynthetic organisms. Sci. Total Environ. 2024, 955, 177205. [Google Scholar] [PubMed]
- Krivohlavek, A.; Mikulec, N.; Budec, M.; Barusic, L.; Bosnir, J.; Sikic, S.; Jakasa, I.; Begovic, T.; Janda, R.; Vitale, K. Migration of BPA from Food Packaging and Household Products on the Croatian Market. Int. J. Environ. Res. Public. Health 2023, 20, 2877. [Google Scholar] [PubMed]
- Nasello, S.; Beiguel, E.; Fitó-Friedrichs, G.; Irala, C.; Berenstein, G.; Basack, S.; Montserrat, J.M. Thermal paper as a potential source of Bisphenol A for humans and the environment: Migration and ecotoxicological impact. Environ. Sci. Pollut. Res. Int. 2022, 29, 53382–53394. [Google Scholar]
- Liu, B.; Yan, Y.; Xie, J.; Sun, J.; Lehmler, H.-J.; Trasande, L.; Wallace, R.B.; Bao, W. Bisphenol S, bisphenol F, bisphenol a exposure and body composition in US adults. Chemosphere 2024, 346, 140537. [Google Scholar]
- Naderi, M.; Wong, M.Y.; Gholami, F. Developmental exposure of zebrafish (Danio rerio) to bisphenol-S impairs subsequent reproduction potential and hormonal balance in adults. Aquat. Toxicol. 2014, 148, 195–203. [Google Scholar] [CrossRef]
- Sokołowski, A.; Kończak, M.; Oleszczuk, P.; Gao, Y.; Czech, B. Environmental and Food Contamination by Phthalic Acid Esters (PAEs): Overview. Water Air Soil. Pollut. 2024, 235, 313. [Google Scholar] [CrossRef]
- Janssen, M.P.M.; Spijker, J.; Lijzen, J.P.A.; Wesselink, L.G. Plastics That Contain Hazardous Substances: Recycle or Incinerate? Letter report; Dutch National Institute for Public Health and the Environment, RIVM: Bilthoven, The Netherlands, 2016. [Google Scholar]
- Lahimer, M.C.; Ayed, N.; Horriche, J.; Belgaied, S. Characterization of plastic packaging additives: Food contact, stability and toxicity. Arab. J. Chem. 2017, 10, S1938–S1954. [Google Scholar]
- Munier, B.; Bendell, L.I. Macro and micro plastics sorb and desorb metals and act as a point source of trace metals to coastal ecosystems. PLoS ONE 2018, 13, e0191759. [Google Scholar]
- Wang, J.; Peng, J.; Tan, Z.; Gao, Y.; Zhan, Z.; Chen, Q.; Cai, L. Microplastics in the surface sediments from the Beijiang River littoral zone: Composition, abundance, surface textures and interaction with heavy metals. Chemosphere 2017, 171, 248–258. [Google Scholar]
- Turner, A.; Holmes, L.; Thompson, R.C.; Fisher, A. Metals and marine microplastics: Adsorption from the environment versus addition during manufacture, exemplified with lead. Water Res. 2020, 173, 115577. [Google Scholar] [PubMed]
- Massos, A.; Turner, A. Cadmium, lead and bromine in beached microplastics. Environ. Pollut. 2017, 227, 139–145. [Google Scholar] [PubMed]
- Turner, A.; Filella, M. The role of titanium dioxide on the behaviour and fate of plastics in the aquatic environment. Sci. Total Environ. 2023, 869, 161727. [Google Scholar] [PubMed]
- Guo, H.; Zheng, X.; Ru, S.; Luo, X.; Mai, B. The leaching of additive-derived flame retardants (FRs) from plastics in avian digestive fluids: The significant risk of highly lipophilic FRs. J. Environ. Sci. 2019, 85, 200–207. [Google Scholar]
- Löhr, A.; Savelli, H.; Beunen, R.; Kalz, M.; Ragas, A.; Belleghem, F.V. Solutions for global marine litter pollution. Curr. Opin. Environ. Sustain. 2017, 28, 90–99. [Google Scholar]
- UNEP. Picking up Litter: Pointless Exercise or Powerful Tool in the Battle to Beat Plastic Pollution? 2023. Available online: https://www.unep.org/news-and-stories/story/picking-litter-pointless-exercise-or-powerful-tool-battle-beat-plastic (accessed on 15 August 2024).
- Peters, C.A.; Thomas, P.A.; Rieper, K.B.; Bratton, S.P. Foraging preferences influence microplastic ingestion by six marine fish species from the Texas Gulf Coast. Mar. Pollut. Bull. 2017, 124, 82–88. [Google Scholar]
- Almroth, B.M.C.; Astrom, L.; Roslund, S.; Petersson, H.; Johansson, M.; Persson, N.-K. Quantifying shedding of synthetic fibers from textiles; a source of microplastics released into the environment. Environ. Sci. Pollut. Res. 2017, 25, 1191–1199. [Google Scholar]
- Sundt, P.; Schulze, P.-E.; Syversen, F. Sources of Microplastics-Pollution to the Marine Environment; Mepex Consult AS: Asker, Norway, 2014; p. 108. [Google Scholar]
- Essel, R.; Engel, L.; Carus, M.; Ahrens, R.H. Sources of Microplastics Relevant to Marine Protection in Germany Texte 64/2015; Report No. (UFA-FB) 002147/E; Umweltbundesamt: Dessau-Roßlau, Germany, 2015. [Google Scholar]
- Ashrafy, A.; Liza, A.A.; Islam, M.N.; Billah, M.M.; Arafat, S.T.; Rahman, M.M.; Rahman, S.M. Microplastics Pollution: A Brief Review of Its Source and Abundance in Different Aquatic Ecosystems. J. Hazard. Mater. Adv. 2023, 9, 100215. [Google Scholar]
- Karbalaei, S.; Karbalaei, S.; Golieskardi, A.; Hamzah, H.B.; Abdulwahid, S.; Hanachi, P.; Walker, T.R.; Karami, A. Abundance and characteristics of microplastics in commercial marine fish from Malaysia. Mar. Pollut. Bull. 2019, 148, 5–15. [Google Scholar]
- Ahmed, S.F.; Islam, N.; Tasannum, N.; Mehjabin, A.; Momtahin, A.; Chowdhury, A.A.; Almomani, F.; Mofijur, M. Microplastic removal and management strategies for wastewater treatment plants. Chemosphere 2024, 347, 140648. [Google Scholar]
- Lares, M.; Ncibi, M.; Sillanpaa, M.; Sillanpaa, M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018, 133, 236–246. [Google Scholar] [PubMed]
- Talvitie, J.; Mikola, A.; Setal, O.; Heinonen, M.; Koistinen, A. How well is microlitter purified from wastewater? A detailed study on the stepwise removal of microlitter in a tertiary level wastewater treatment plant. Water Res. 2016, 109, 164–172. [Google Scholar] [PubMed]
- Parolini, M.; Stucchi, M.; Ambrosini, R.; Romano, A. A global perspective on microplastic bioaccumulation in marine organisms. Ecol. Indic. 2023, 149, 110179. [Google Scholar]
- Setala, O.; Norkko, J.; Lehtiniemi, M. Feeding type affects microplastic ingestion in coastal invertebrate community. Mar. Pollut. Bull. 2016, 102, 95–101. [Google Scholar]
- Avio, C.G.; Gorbi, S.; Milan, M.; Benedetti, M.; Fattorini, D.; d’Errico, G.; Pauletto, M.; Bargelloni, L.; Regoli, F. Pollutants bioavailability and toxicological risk from microplastics to mussels. Environ. Pollut. 2015, 198, 211–222. [Google Scholar]
- Green, D.S.; Boots, B.; Sigwart, J.; Jiang, S.; Rocha, C. Effects of conventional and biodegradable microplastics on a marine ecosystem engineer (Arenicola marina) and sediment nutrient cycling. Environ. Pollut. 2016, 208, 426–434. [Google Scholar]
- Cole, M.; Galloway, T.S. Ingestion of Nanoplastics and Microplastics by Pacific Oyster Larvae. Environ. Sci. Technol. 2015, 49, 14625–14632. [Google Scholar]
- Rochman, C.M.; Tahir, A.; Williams, S.L.; Baxa, D.V.; Lam, R.; Miller, J.T.; Teh, F.; Werorilangi, S.; Teh, S.J. Anthropogenic debris in seafood: Plastic debris and fibers from textiles in fish and bivalves sold for human consumption. Sci. Rep. 2015, 5, 14340. [Google Scholar]
- Da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T.A.P. Microplastics-Occurence, Fate and Behaviour in the Environment. In Comprehensive Analytical Chemistry; Rocha-Santos, T.A.P., Duarte, A.C., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2017; Volume 75, pp. 1–24. [Google Scholar]
- Porter, A.; Godbold, J.A.; Lewis, C.N.; Savage, G.; Solan, M.; Galloway, T.S. Microplastic burden in marine benthic invertebrates depends on species traits and feeding ecology within biogeographical provinces. Nat. Commun. 2023, 14, 8023. [Google Scholar]
- Naji, A.; Nuri, M.; Vethaak, A.D. Microplastics contamination in molluscs from the northern part of the Persian Gulf. Environ. Pollut. 2018, 235, 113–120. [Google Scholar]
- de Sá, L.C.; Luís, L.G.; Guilhermino, L. Effects of microplastics on juveniles of the common goby (Pomatoschistus microps): Confusion with prey, reduction of the predatory performance and efficiency, and possible influence of developmental conditions. Environ. Pollut. 2015, 196, 359–362. [Google Scholar]
- Luis, L.G.; Ferreira, P.; Fonte, E.; Oliveira, M.; Guilhermino, L. Does the presence of microplastics influence the acute toxicity of chromium (VI) to early juveniles of the common goby (Pomatoschistus microps)? A study with juveniles from two wild estuarine populations. Aquat. Toxicol. 2015, 164, 163–174. [Google Scholar] [PubMed]
- Abd-Elkader, A.; Hamed, E.; Sayed, A.E.; Mahdy, A.; Shabaka, S. Microplastics in marine invertebrate from the Red Sea Coast Egypt: Abundance, composition, and risks. Mar. Pollut. Bull. 2023, 197, 115760. [Google Scholar] [PubMed]
- Walkinshaw, C.; Lindeque, P.K.; Thompson, R.; Tolhurst, T.; Cole, M. Microplastics and seafood: Lower trophic organisms at highest risk of contamination. Ecotoxicol. Environ. Saf. 2020, 190, 110066. [Google Scholar]
- Anderson, G.; Shenkar, N. Potential effects of biodegradable single-use items in the sea: Polylactic acid (PLA) and solitary ascidians. Environ. Pollut. 2021, 268 Pt. A, 115364. [Google Scholar]
- Vandermeersch, G.; Van Cauwenberghe, L.; Janssen, C.R.; Marques, A.; Granby, K.; Fait, G.; Kotterman, M.J.J.; Diogene, J.; Bekaert, K.; Robbens, J.; et al. A critical view on microplastic quantification in aquatic organisms. Environ. Res. 2015, 143, 46–53. [Google Scholar]
- Alfaro-Núñez, A.; Astorga, D.; Cáceres-Farías, L.; Bastidas, L.; Villegas, C.S.; Macay, K.; Christensen, J.H. Microplastic pollution in seawater and marine organisms across the Tropical Eastern Pacific and Galápagos. Sci. Rep. 2021, 11, 6424. [Google Scholar]
- Mason, V.G.; Skov, M.W.; Hiddink, J.G.; Walton, M. Microplastics alter multiple biological processes of marine benthic fauna. Sci. Total Environ. 2022, 845, 157362. [Google Scholar]
- Mizraji, R.; Ahrendt, C.; Perez-Venegas, D.; Vargas, J.; Pulgar, J.; Aldana, M.F.; Ojeda, P.; Duarte, C.; Galbán-Malagón, C. Is the feeding type related with the content of microplastics in intertidal fish gut? Mar. Pollut. Bull. 2017, 116, 498–500. [Google Scholar]
- Garcia, T.D.; Cardozo, A.L.P.; Quirino, B.A.; Yofukuji, K.Y.; Ganassin, M.J.M.; dos Santos, N.C.L.; Fugi, R. Ingestion of Microplastic by Fish of Different Feeding Habits in Urbanized and Non-urbanized Streams in Southern Brazil. Water Air Soil. Pollut. 2020, 231, 434. [Google Scholar]
- Yang, Z.; De Loid, G.M.; Zarbl, H.; Baw, J.; Demokritou, P. Micro- and nanoplastics (MNPs) and their potential toxicological outcomes: State of science, knowledge gaps and research needs. NanoImpact 2023, 32, 100481. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-C.; Fang, C.; Zheng, R.-H.; Chen, M.-L.; Kim, D.-H.; Lee, Y.-H.; Bailey, C.; Wang, K.-J.; Lee, J.-S.; Bo, J. Environmentally relevant concentrations of microplastics modulated the immune response and swimming activity, and impaired the development of marine medaka Oryzia melastigma larvae. Ecotoxicol. Environ. Saf. 2022, 241, 113843. [Google Scholar] [CrossRef] [PubMed]
- Tumwesigye, E.; Nnadozie, C.F.; Akamagwuna, F.C.; Noundou, X.S.; Nyakairu, G.W.; Odume, O.N. Microplastics as vectors of chemical contaminants and biological agents in freshwater ecosystems: Current knowledge status and future perspectives. Environ. Pollut. 2023, 330, 121829. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, C.; Morgana, S.; Bramini, M.; Rotini, A.; Manfra, L.; Migliore, L.; Piazza, V.; Garaventa, F.; Faimali, M. Ecotoxicological effects of polystyrene microbeads in a battery of marine organisms belonging to different trophic levels. Mar. Environ. Res. 2018, 141, 313–321. [Google Scholar] [CrossRef]
- González-Fernández, C.; Toullec, J.; Lambert, C.; Le Goïc, N.; Seoane, M.; Moriceau, B.; Huvet, A.; Berchel, M.; Vincent, D.; Courcot, L.; et al. Do transparent exopolymeric particles (TEP) affect the toxicity of nanoplastics on Chaetoceros neogracile? Environ. Pollut. 2019, 250, 873–882. [Google Scholar] [CrossRef]
- Mojiri, A.; Vishkaei, M.N.; Zhou, J.L.; Trzcinski, A.P.; Lou, Z.; Kasmuri, N.; Rezania, S.; Gholami, A.; Vakili, M.; Kazeroon, R.A. Impact of polystyrene microplastics on the growth and photosynthetic efficiency of diatom Chaetoceros neogracile. Mar. Environ. Res. 2024, 194, 106343. [Google Scholar] [CrossRef]
- Sjollema, S.B.; Redondo-Hasselerharm, P.; Leslie, H.A.; Kraak, M.H.; Vethaak, A.D. Do plastic particles affect microalgal photosynthesis and growth? Aquat. Toxicol. 2016, 170, 259–261. [Google Scholar]
- Huang, J.; Wang, H.; Xue, X.; Zhang, R. Impacts of microplastic and seawater acidification on unicellular red algae: Growth response, photosynthesis, antioxidant enzymes, and extracellular polymer substances. Aquat. Toxicol. 2024, 272, 106960. [Google Scholar] [CrossRef]
- Long, M.; Moriceau, B.; Gallinari, M.; Lambert, C.; Huvet, A.; Raffray, J.; Soudant, P. Interactions between microplastics and phytoplankton aggregates: Impact on their respective fates. Mar. Chem. 2015, 175, 39–46. [Google Scholar] [CrossRef]
- Di Giannantonio, M.; Gambardella, C.; Miroglio, R.; Costa, E.; Sbrana, F.; Smerieri, M.; Carraro, G.; Utzeri, R.; Faimali, M.; Garaventa, F. Ecotoxicity of Polyvinylidene Difluoride (PVDF) and Polylactic Acid (PLA) Microplastics in Marine Zooplankton. Toxics 2022, 10, 479. [Google Scholar] [CrossRef]
- Seong, T.; Yamamoto, S.; Nakatani, H.; Yagi, M.; Kyozuka, Y.; Satuito, G.; Kim, H.J. Effects of microplastics on reproductive characteristics and mechanisms of the marine rotifer Brachionus plicatilis. Sci. Rep. 2024, 14, 18350. [Google Scholar]
- Beiras, R.; Bellas, J.; Cachot, J.; Cormier, B.; Cousin, X.; Engwall, M.; Gambardella, C.; Garaventa, F.; Keiter, S.; Le Bihanic, F.; et al. Ingestion and contact with polyethylene microplastics does not cause acute toxicity on marine zooplankton. J. Hazard. Mater. 2018, 360, 452–460. [Google Scholar] [PubMed]
- Gonçalves, J.M.; Benedetti, M.; d’Errico, G.; Regoli, F.; Bebianno, M.J. Polystyrene nanoplastics in the marine mussel Mytilus galloprovincialis. Environ. Pollut. 2023, 333, 122104. [Google Scholar] [PubMed]
- Pittura, L.; Avio, C.G.; Giuliani, M.E.; d’Errico, G.; Keiter, S.H.; Cormier, B.; Gorbi, S.; and Regoli, F. Microplastics as vehicles of environmental PAHs to marine organisms: Combined chemical and physical hazards to the Mediterranean Mussels, Mytilus galloprovincialis. Front. Mar. Sci. 2018, 5, 103. [Google Scholar]
- Yu, D.; Liu, S.; Yu, Y.; Wang, Y.; Li, L.; Peijnenburg, W.J.G.M.; Yuan, Y.; Peng, X. Transcriptomic analysis reveals interactive effects of polyvinyl chloride microplastics and cadmium on Mytilus galloprovincialis: Insights into non-coding RNA responses and environmental implications. Aquat. Toxicol. 2024, 275, 107062. [Google Scholar]
- Fernández, B.; Vidal-Liñán, L.; Bellas, J.; Campillo, J.A.; Chaves-Pozo, E.; Albentosa, M. The particle effect: Comparative toxicity of chlorpyrifos in combination with microplastics and phytoplankton particles in mussel. Aquat. Toxicol. 2024, 275, 107053. [Google Scholar]
- Aramendia, J.; García-Velasco, N.; Amigo, J.M.; Izagirre, U.; Seifert, A.; Soto, M.; Castro, K. Evidence of internalized microplastics in mussel tissues detected by volumetric Raman imaging. Sci. Total Environ. 2024, 914, 169960. [Google Scholar]
- Di, Y.; Li, L.; Xu, J.; Liu, A.; Zhao, R.; Li, S.; Li, Y.; Ding, J.; Chen, S.; Qu, M. MAPK signaling pathway enhances tolerance of Mytilus galloprovincialis to co-exposure of sulfamethoxazole and polyethylene microplastics. Environ. Pollut. 2024, 362, 125007. [Google Scholar]
- García-Pimentel, M.M.; Mezzelani, M.; Valdés, N.J.; Giuliani, M.E.; Gorbi, S.; Regoli, F.; León, V.M.; Campillo, J.A. Integrative oxidative stress biomarkers in gills and digestive gland of the combined exposure to citalopram and bezafibrate with polyethylene microplastics on mussels Mytilus galloprovincialis. Environ. Pollut. 2025, 366, 125508. [Google Scholar]
- Li, L.L.; Amara, R.; Souissi, S.; Dehaut, A.; Duflos, G.; Monchy, S. Impacts of microplastics exposure on mussel (Mytilus edulis) gut microbiota. Sci. Total Environ. 2020, 745, 141018. [Google Scholar]
- Zhong, Z.; Huang, W.; Yin, Y.; Wang, S.; Chen, L.; Chen, Z.; Wang, J.; Li, L.; Khalid, M.; Hu, M.; et al. Tris(1-chloro-2-propyl) phosphate enhances the adverse effects of biodegradable polylactic acid microplastics on the mussel Mytilus coruscus. Environ. Pollut. 2024, 359, 124741. [Google Scholar] [PubMed]
- Wang, S.; Ma, Y.; Khan, F.U.; Dupont, S.; Huang, W.; Tu, Z.; Shang, Y.; Wang, Y.; Hu, M. Size-dependent effects of plastic particles on antioxidant and immune responses of the thick-shelled mussel Mytilus coruscus. Sci. Total Environ. 2024, 914, 169961. [Google Scholar] [PubMed]
- Tallec, K.; Huvet, A.; Di Poi, C.; Gonzalez-Fernandez, C.; Lambert, C.; Petton, B.; Le Goic, N.; Berchel, M.; Soudant, P.; Paul-Pont, I. Nanoplastics impaired oyster free living stages, gametes and embryos. Environ. Pollut. 2018, 242, 1226–1235. [Google Scholar] [PubMed]
- Bringer, A.; Thomas, H.; Dubillot, E.; Le Floch, S.; Receveur, J.; Cachot, J.; Tran, D. Subchronic exposure to high-density polyethylene microplastics alone or in combination with chlortoluron significantly affected valve activity and daily growth of the Pacific oyster, Crassostrea gigas. Aquat. Toxicol. 2021, 237, 105880. [Google Scholar]
- Parizadeh, L.; Saint-Picq, C.; Barbier, P.; Bringer, A.; Huet, V.; Dubillot, E.; Thomas, H. Groundbreaking study: Combined effect of marine heatwaves and polyethylene microplastics on Pacific oysters, Crassostrea gigas. Environ. Pollut. 2025, 364 Pt 2, 125164. [Google Scholar]
- Green, D.S. Effects of microplastics on European flat oysters, Ostrea edulis and their associated benthic communities. Environ. Pollut. 2016, 216, 95e103. [Google Scholar]
- Ribeiro, F.; Garcia, A.R.; Pereira, B.P.; Fonseca, M.; Mestre, N.C.; Fonseca, T.G.; Ilharco, L.; Bebianno, M.J. Microplastics effects in Scrobicularia plana. Mar. Pollut. Bull. 2017, 122, 379–391. [Google Scholar]
- Santana, M.F.M.; Moreira, F.T.; Pereira, C.D.S.; Abessa, D.M.S.; Turra, A. Continuous exposure to microplastics does not cause physiological effects in the cultivated mussel Perna perna. Arch. Environ. Contam. Toxicol. 2018, 74, 594–604. [Google Scholar]
- Li, J.; You, L.; Gin, K.Y.-H.; He, Y. Impact of microplastics pollution on ciprofloxacin bioaccumulation in the edible mussel (Perna viridis): Implications for human gut health risks. Environ. Technol. Innov. 2024, 36, 103860. [Google Scholar]
- Lu, F.; Guo, C.; Mkuye, R.; Chen, W.; Yang, X.; Zhou, Z.; He, Y.; Yang, C.; Deng, Y. Effects of polyvinyl chloride microplastic on pearl oyster (Pinctada fucata martensii). Reg. Stud. Mar. Sci. 2024, 69, 103313. [Google Scholar]
- Xu, X.-Y.; Lee, W.T.; Chan, A.K.Y.; Lo, H.S.; Shin, P.K.S.; Cheung, S. Microplastic ingestion reduces energy intake in the clam Atactodea striata. Mar. Pollut. Bull. 2017, 124, 798–802. [Google Scholar] [PubMed]
- Tang, Y.; Rong, J.; Guan, X.; Zha, S.; Shi, W.; Han, Y.; Du, X.; Wu, F.; Huang, W.; Liu, G. Immunotoxicity of microplastics and two persistent organic pollutants alone or in combination to a bivalve species. Environ. Pollut. 2020, 258, 113845. [Google Scholar] [PubMed]
- Sun, Y.; Zhao, X.; Sui, Q.; Sun, X.; Zhu, L.; Booth, A.M.; Chen, B.; Qu, K.; Xia, B. Polystyrene nanoplastics affected the nutritional quality of Chlamys farreri through disturbing the function of gills and physiological metabolism: Comparison with microplastics. Sci. Total Environ. 2024, 910, 168457. [Google Scholar] [PubMed]
- Parolini, M.; De Felice, B.; Gazzotti, S.; Annunziata, L.; Sugni, M.; Bacchetta, R.; Ortenzi, M.A. Oxidative stress-related effects induced by micronized polyethylene terephthalate microparticles in the Manila clam. J. Toxicol. Environ. Health A. 2020, 83, 168–179. [Google Scholar]
- Zheng, J.; Li, C.; Zheng, X. Toxic effects of polystyrene microplastics on the intestine of Amphioctopus fangsiao (Mollusca: Cephalopoda): From physiological responses to underlying molecular mechanisms. Chemosphere 2022, 308, 136362. [Google Scholar]
- Pinto, E.P.; Paredes, E.; Santos-Echeandía, J.; Campillo, J.A.; León, V.M.; Bellas, J. Comparative assessment of microplastics and microalgae as vectors of mercury and chlorpyrifos in the copepod Acartia tonsa. Sci. Total Environ. 2024, 945, 173791. [Google Scholar]
- Parlapiano, I.; Prato, E.M.; Libralato, G.; Biandolino, F. Impacts of some recyclable plastic on marine key species. In Proceedings of the 2023 IEEE International Workshop on Metrology for the Sea; Learning to Measure Sea Health Parameters (MetroSea), La Valletta, Malta, 4–6 October 2023; IEEE: New York, NY, USA, 2023; pp. 221–225. [Google Scholar]
- Choi, J.S.; Hong, S.H.; Park, J. Evaluation of microplastic toxicity in accordance with different sizes and exposure times in the marine copepod Tigriopus Japonicus. Mar. Env. Res. 2019, 153, 1048382020. [Google Scholar]
- Lee, K.W.; Shim, W.J.; Kwon, O.Y.; Kang, J.H. Size-dependent effects of micro polystyrene particles in the marine copepod Tigriopus japonicus. Environ. Sci. Technol. 2013, 47, 11278–11283. [Google Scholar]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Galloway, T.S. The impact of polystyrene microplastics on feeding, function and fecundity in the marine copepod Calanus helgolandicus. Environ. Sci. Technol. 2015, 49, 1130–1137. [Google Scholar]
- Jeong, C.B.; Kang, H.M.; Lee, M.C.; Kim, D.H.; Han, J.; Hwang, D.S.; Souissi, S.; Lee, S.-J.; Shin Kyung, H.H.; Park, G.; et al. Adverse effects of microplastics and oxidative stress-induced MAPK/Nrf2 pathway-mediated defense mechanisms in the marine copepod Paracyclopina nana. Sci. Rep. 2017, 7, 41323. [Google Scholar]
- Oliviero, M.; Tato, T.; Schiavo, S.; Fernández, V.; Manzo, S.; Beiras, R. Leachates of micronized plastic toys provoke embryotoxic effects upon sea urchin Paracentrotus lividus. Environ. Pollut. 2019, 247, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Uribe-Echeverría, T.; Beiras, R. Acute toxicity of bioplastic leachates to Paracentrotus lividus sea urchin larvae. Mar. Environ. Res. 2022, 176, 105605. [Google Scholar] [CrossRef] [PubMed]
- Beiras, R.; Tato, T. Microplastics do not increase toxicity of a hydrophobic organic chemical to marine plankton. Mar. Pollut. Bull. 2019, 138, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Messinetti, S.; Mercurio, S.; Parolini, M.; Sugni, M.; Pennati, R. Effects of polystyrene microplastics on early stages of two marine invertebrates with different feeding strategies. Environ. Pollut. 2018, 237, 1080–1087. [Google Scholar] [CrossRef]
- Martiínez-Gomez, C.; Leon, V.M.; Calles, S.; Gomariz-Olcina, M.; Vethaak, A.D. The adverse effects of virgin microplastics on the fertilization and larval development of sea urchins. Mar. Environ. Res. 2017, 130, 69–76. [Google Scholar] [CrossRef]
- Viel, T.; Cocca, M.; Manfra, L.; Caramiello, D.; Libralato, G.; Zupo, V.; Costantini, M. Effects of biodegradable-based microplastics in Paracentrotus lividus Lmk embryos: Morphological and gene expression analysis. Environ. Pollut. 2023, 334, 122129. [Google Scholar] [CrossRef]
- Viel, T.; Cocca, M.; Esposito, R.; Amato, A.; Russo, T.; Di Cosmo, A.; Polese, G.; Manfra, L.; Libralato, G.; Zupo, V.; et al. Effect of biodegradable polymers upon grazing activity of the sea urchin Paracentrotus lividus (Lmk) revealed by morphological, histological and molecular analyses. Sci. Total Environ. 2024, 929, 172586. [Google Scholar] [CrossRef]
- Kaposi, K.L.; Mos, B.; Kelaher, B.P.; Dworjanyn, S.A. Ingestion of microplastic has limited impact on a marine larva. Environ. Sci. Technol. 2014, 48, 1638–1645. [Google Scholar] [CrossRef]
- dos Santos, J.B.; Choueri, R.B.; dos Santos, F.E.M.; Santos, L.A.d.O.; da Silva, L.F.; Nobre, C.R.; Cardoso, M.A.; de Britto Mari, R.; Simões, F.R.; Delvalls, T.A.; et al. Are Microfibers a Threat to Marine Invertebrates? A Sea Urchin Toxicity Assessment. Toxics 2024, 12, 753. [Google Scholar] [CrossRef]
- Trifuoggi, M.; Pagano, G.; Oral, R.; Pavičić-Hamer, D.; Burić, P.; Kovačić, I.; Siciliano, A.; Toscanesi, M.; Thomas, P.J.; Paduano, L.; et al. Microplastic-induced damage in early embryonal development of sea urchin Sphaerechinus granularis. Environ. Res. 2019, 179 Pt A, 108815. [Google Scholar] [CrossRef]
- Maldeniya, M.U.S.; Ma, B.; Liu, Y.; Yin, J.; Pan, W.; Wen, S.; Luo, P. Potential harmful impacts of micro- and nanoplastics on the health of a tropical sea cucumber, Holothuria leucospilota, evidenced by changes of gut microflora, histology, immune and oxidative indexes. Sci. Total Environ. 2024, 954, 176487. [Google Scholar] [CrossRef] [PubMed]
- Cocci, P.; Stecconi, T.; Minicucci, M.; Gabrielli, S.; Mosconi, G.; Stramenga, A.; Tavoloni, T.; Piersanti, A.; Bracchetti, L.; Palermo, F.A. Levels and oxidative toxicity of microplastics and perfluoroalkyl substances (PFASs) in different tissues of sea cucumber (Holothuria tubulosa). Sci. Total Environ. 2025, 962, 178472. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, F.; Liu, S.; Cheng, X.; Xu, J.; Liu, X.; Zhang, L. Response and adaptation mechanisms of Apostichopus japonicus to single and combined anthropogenic stresses of polystyrene microplastics or cadmium. Mar. Pollut. Bull. 2024, 204, 116519. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, C.; Morgana, S.; Ferrando, S.; Bramini, M.; Piazza, V.; Costa, E.; Garaventa, F.; Faimali, M. Effects of polystyrene microbeads in marine planktonic crustaceans. Ecotoxicol. Environ. Saf. 2017, 145, 250–257. [Google Scholar] [CrossRef]
- Kim, L.; Kim, S.A.; Kim, T.H.; Kim, J.; An, Y.J. Synthetic and natural microfibers induce gut damage in the brine shrimp Artemia franciscana. Aquat. Toxicol. 2021, 232, 105748. [Google Scholar] [CrossRef]
- Manfra, L.; Albarano, L.; Rotini, A.; Biandolino, F.; Prato, E.; Carraturo, F.; Chiaretti, G.; Faraponova, O.; Salamone, M.; Sebbio, C.; et al. Can biodegradable plastics mitigate plastamination? Feedbacks from marine organisms. J. Hazard. Mater. 2025, 487, 137179. [Google Scholar] [CrossRef]
- Bergami, E.; Bocci, E.; Vannuccini, M.L.; Monopoli, M.; Salvati, A.; Dawson, K.A.; Corsi, I. Nano-sized polystyrene affects feeding, behavior and physiology of brine shrimp Artemia franciscana larvae. Ecotoxicol. Environ. Saf. 2016, 123, 18–25. [Google Scholar] [CrossRef]
- Bergami, E.; Pugnalini, S.; Vannuccini, M.L.; Manfra, L.; Faleri, C.; Savorelli, F.; Dawson, K.A.; Corsi, I. Long-term toxicity of surface-charged polystyrene nanoplastics to marine planktonic species Dunaliella tertiolecta and Artemia franciscana. Aquat. Toxicol. 2017, 89, 159–169. [Google Scholar] [CrossRef]
- Kim, L.; Kim, H.; Song, Y.; An, Y.J. Chronic effects of irregular and fibril microplastics on Artemia franciscana in a benthic environment: Size and shape-dependent toxicity. Mar. Pollut. Bull. 2024, 209 Pt B, 117270. [Google Scholar] [CrossRef]
- Jeyavani, J.; Sibiya, A.; Bhavaniramya, S.; Mahboob, S.; Al-Ghanim, K.A.; Nisa, Z.U.; Riaz, M.N.; Nicoletti, M.; Govindarajan, M.; Vaseeharan, B. Toxicity evaluation of polypropylene microplastic on marine microcrustacean Artemia salina: An analysis of implications and vulnerability. Chemosphere 2022, 296, 133990. [Google Scholar] [CrossRef]
- Athulya, P.A.; Sunil, Z.; Manzo, S.; Chandrasekaran, N. Prepared microplastics interaction with Artemia salina under low pH conditions representing ocean acidification; a simulated environmental exposure. J. Environ. Manag. 2023, 348, 119367. [Google Scholar] [PubMed]
- Suman, T.Y.; Jia, P.P.; Li, W.G.; Junaid, M.; Xin, G.Y.; Wang, Y.; Pei, D.S. Acute and chronic effects of polystyrene microplastics on brine shrimp: First evidence highlighting the molecular mechanism through transcriptome analysis. J. Hazard. Mater. 2020, 400, 123220. [Google Scholar] [PubMed]
- Pramanik, D.D.; Lei, S.; Kay, P.; Goycoolea, F.M. Investigating on the toxicity and bio-magnification potential of synthetic glitters on Artemia salina. Mar. Pollut. Bull. 2023, 190, 114828. [Google Scholar] [PubMed]
- Saha, G.; Chandrasekaran, N. Isolation and characterization of microplastics from skin care products; interactions with albumin proteins and in-vivo toxicity studies on Artemia salina. Environ. Toxicol. Pharmacol. 2023, 99, 104112. [Google Scholar]
- Pramanik, D.D.; Sharma, A.; Das, D.K.; Pramanik, A.; Kay, P.; Goycoolea, F.M. Toxicological impacts of plastic microfibers from face masks on Artemia salina: An environmental assessment using Box-Behnken design. Mar. Environ. Res. 2024, 202, 106810. [Google Scholar]
- Abessa, D.M.S.; Gonçalves, A.R.N.; Carvalho, M.U.; Spanghero, N.; Soares do Nasicmento, N.S.; Fornari, M.; Perina, F.C.; Cruz, A.C.F. Not all that glitters is gold: Glitter causes acute toxicity to nauplii of Artemia sp. Marit. Technol. Res. 2024, 6, 270722. [Google Scholar]
- Amato, A.; Esposito, R.; Viel, T.; Glaviano, F.; Cocca, M.; Manfra, L.; Libralato, G.; Somma, E.; Lorenti, M.; Costantini, M.; et al. Effects of biodegradable microplastics on the crustacean isopod Idotea balthica basteri Audouin, 1826. Environ Pollut. 2024, 361, 124897. [Google Scholar]
- Liang, J.; Abdullah, A.L.B.; Li, Y.; Wang, H.; Xiong, S.; Han, M. Long-term PS micro/nano-plastic exposure: Particle size effects on hepatopancreas injury in Parasesarma pictum. Sci. Total Environ. 2024, 954, 176530. [Google Scholar]
- Amato, A.; Esposito, R.; Pinto, B.; Viel, T.; Glaviano, F.; Cocca, M.; Manfra, L.; Libralato, G.; Aflalo, E.D.; Sagi, A.; et al. First evidence of molecular response of the shrimp Hippolyte inermis to biodegradable microplastics. J. Hazard. Mater. 2024, 487, 137069. [Google Scholar]
- Li, Y.; Ye, Y.; Rihan, N.; Zhu, B.; Jiang, Q.; Liu, X.; Zhao, Y.; Che, X. Polystyrene nanoplastics exposure alters muscle amino acid composition and nutritional quality of Pacific whiteleg shrimp (Litopenaeus vannamei). Sci. Total Environ. 2024, 912, 168904. [Google Scholar]
- Espinosa, C.; Esteban, M.Á; Cuesta, A. Dietary administration of PVC and PE microplastics produces histological damage, oxidative stress and immunoregulation in European sea bass (Dicentrarchus labrax L.). Fish. Shellfish. Immunol. 2019, 95, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, C.; Cuesta, A.; Esteban, M.Á. Effects of dietary polyvinylchloride microparticles on general health, immune status and expression of several genes related to stress in gilthead seabream (Sparus aurata L.). Fish. Shellfish. Immunol. 2017, 68, 251–259. [Google Scholar] [CrossRef]
- Fonte, E.; Ferreira, P.; Guilhermino, L. Temperature rise and MPs interact with the toxicity of the antibiotic cefalexin to juveniles of the common goby (Pomatoschistus microps): Post-exposure predatory behaviour, acetylcholinesterase activity and lipid peroxidation. Aquat. Toxicol. 2016, 180, 173–185. [Google Scholar] [CrossRef]
- Oliveira, M.; Ribeiro, A.; Hylland, K.; Guilhermino, L. Single and combined effects of microplastics and pyrene on juveniles (0+ group) of the common goby Pomatoschistus microps (Teleostei, Gobiidae). Ecol. Indic. 2013, 34, 641–647. [Google Scholar] [CrossRef]
- Byeon, E.; Jeong, H.; Lee, Y.-J.; Cho, Y.; Lee, K.-W.; Lee, E.; Jeong, C.-B.; Lee, J.-S.; Kang, H.-M. Effects of microplastics and phenanthrene on gut microbiome and metabolome alterations in the marine medaka Oryzias melastigma. J. Hazard. Mater. 2024, 461, 132620. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Yin, X.; Zhang, Y.; Diao, X. Chronic exposure to low concentrations of microplastics causing gut tissue damage but non-significant changes in the microbiota of marine medaka larvae (Oryzias melastigma). Mar. Environ. Res. 2024, 195, 106381. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, M.; Feng, Z.; Zhu, L.; Sui, Q.; Sun, X.; Xia, B. Combined toxicity of polyvinyl chloride microplastics and copper to marine jacopever (Sebastes schlegelii). Mar. Environ. Res. 2024, 199, 106598. [Google Scholar] [CrossRef]
- Bakhasha, J.; Saxena, V.; Arya, N.; Kumar, P.; Srivastava, A.; Yadav, K.K.; Trivedi, A. Copper-loaded microplastics unleash endoplasmic reticulum stress-driven liver apoptosis in fish Channa punctatus Emerging Contaminants. Emerg. Contam. 2025, 11, 100422. [Google Scholar] [CrossRef]
- Choi, J.S.; Jung, Y.J.; Hong, N.H.; Hong, S.H.; Park, J.W. Toxicological effects of irregularly shaped and spherical microplastics in a marine teleost, the sheepshead minnow (Cyprinodon variegatus). Mar. Pollut. Bull. 2018, 129, 231–240. [Google Scholar] [CrossRef]
- Saiz, E.; Griffell, K.; Isari, S.; Calbet, A. Ecophysiological response of marine copepods to dietary elemental imbalances. Mar. Environ. Res. 2023, 186, 105940. [Google Scholar] [CrossRef]
- Romeo, T.; Pietro, B.; Pedà, C.; Consoli, P.; Andaloro, F.; Fossi, M.C. First evidence of presence of plastic debris in stomach of large pelagic fish in the Mediterranean Sea. Mar. Pollut. Bull. 2015, 95, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.L.; McHugh, M.; Thompson, R.C. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar. Pollut. Bull. 2013, 67, 94–99. [Google Scholar] [CrossRef] [PubMed]
- McCormick, M.I.; Chivers, D.P.; Ferrari, M.C.O.; Blandford, M.I.; Nanninga, G.B.; Richardson, C.; Fakan, E.P.; Vamvounis, G.; Gulizia, A.M.; Allan, B.J. Microplastic exposure interacts with habitat degradation to affect behaviour and survival of juvenile fish in the field. Procoseeding, R. Soc. B 2020, 287, 20201947. [Google Scholar]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar]
- De Witte, B.; Devriese, L.; Bekaert, K.; Hoffman, S.; Vandermeersch, G.; Cooreman, K.; Robbens, J. Quality assessment of the blue mussel (Mytilus edulis): Comparison between commercial and wild types. Mar. Pollut. Bull. 2014, 85, 146–155. [Google Scholar] [CrossRef]
- Gedik, K.; Eryasar, A.R. Microplastic pollution profile of Mediterranean mussels (Mytilus galloprovincialis) collected along the Turkish coasts. Chemosphere 2020, 260, 127570. [Google Scholar] [CrossRef]
- Digka, N.; Tsangaris, C.; Torre, M.; Anastasopoulou, A.; Zeri, C. Microplastics in mussels and fish from the Northern Ionian Sea. Mar. Pollut. Bull. 2018, 135, 30–40. [Google Scholar] [CrossRef]
- Gomiero, A.; Strafella, P.; Øysæd, K.B.; Fabi, G. First occurrence and composition assessment of microplastics in native mussels collected from coastal and offshore areas of the northern and central Adriatic Sea. Environ. Sci. Pollut. Res. 2019, 26, 24407–24416. [Google Scholar]
- Renzi, M.; Guerranti, C.; Blaskovic, A. Microplastic contents from maricultured and natural mussels. Mar. Pollut. Bull. 2018, 131, 248–251. [Google Scholar]
- Wakkaf, T.; El Zrelli, R.; Kedzierski, M.; Balti, R.; Shaiek, M.; Mansour, L.; Tlig-Zouari, S.; Bruzaud, S.; Rabaoui, L. Microplastics in edible mussels from a southern Mediterranean lagoon: Preliminary results on seawater-mussel transfer and implications for environmental protection and seafood safety. Mar. Pollut. Bull. 2020, 158, 111355. [Google Scholar]
- Sparks, C. Microplastics in mussels along the coast of Cape Town, South Africa. Bull. Environ. Contam. Toxicol. 2020, 104, 423–431. [Google Scholar] [PubMed]
- Digka, N.; Patsiou, D.; Hatzonikolakis, Y.; Raitsos, D.E.; Skia, G.; Koutsoubas, D.; Dimitriadis, C.; Tsangaris, C. Microplastic ingestion in mussels from the East Mediterranean Sea: Exploring its impacts in nature and controlled conditions. Sci. Total Environ. 2024, 946, 174268. [Google Scholar] [PubMed]
- Phuong, N.N.; Zalouk-Vergnoux, A.; Kamari, A.; Mouneyrac, C.; Amiard, F.; Poirier, L.; Lagarde, F. Quantification and characterization of microplastics in blue mussels (Mytilus edulis): Protocol setup and preliminary data on the contamination of the French Atlantic coast. Environ. Sci. Pollut. Res. Int. 2018, 25, 6135–6144. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Green, C.; Reynolds, A.; Shi, H.; Rotchell, J.M. Microplastics in mussels sampled from coastal waters and supermarkets in the United Kingdom. Environ. Pollut. 2018, 241, 35–44. [Google Scholar]
- Hermabessiere, L.; Paul-Pont, I.; Cassone, A.L.; Himber, C.; Receveur, J.; Jezequel, R.; Soudant, P. Microplastic contamination and pollutant levels in mussels and cockles collected along the channel coasts. Environ. Pollut. 2019, 250, 807–819. [Google Scholar] [CrossRef]
- Catarino, A.I.; Macchia, V.; Sanderson, W.G.; Thompson, R.C.; Henry, T.B. Low levels of microplastics (MP) in wild mussels indicate that MP ingestion by humans is minimal compared to exposure via household fibers fallout during a meal. Environ. Pollut. 2018, 237, 675–684. [Google Scholar]
- Martinelli, J.C.; Phan, S.; Luscombe, C.K.; Padilla-Gamiño, J.L. Low incidence of microplastic contaminants in Pacific oysters (Crassostrea gigas Thunberg) from the Salish Sea, USA. Sci. Total Environ. 2020, 715, 136826. [Google Scholar]
- Teng, J.; Wang, Q.; Ran, W.; Wu, D.; Liu, Y.; Sun, S.; Zhao, J. Microplastic in cultured oysters from different coastal areas of China. Sci. Total Environ. 2019, 653, 1282–1292. [Google Scholar]
- Oliveira, S.; Krelling, A.P.; Turra, A. Contamination by microplastics in oysters shows a widespread but patchy occurrence in a subtropical estuarine system. Mar. Pollut. Bull. 2024, 203, 116380. [Google Scholar]
- Pazos, R.S.; Spaccesi, F.; Gómez, N. First record of microplastics in the mussel Limnoperna fortune. Reg. Stud. Mar. Sci. 2020, 38, 101360. [Google Scholar]
- Daniel, D.B.; Ashraf, P.M.; Thomas, S.N. Abundance, characteristics and seasonal variation of microplastics in Indian white shrimps (Fenneropenaeus indicus) from coastal waters off Cochin, Kerala, India. Sci. Total Environ. 2020, 737, 139839. [Google Scholar] [PubMed]
- Gurjar, U.R.; Xavier, M.; Nayak, B.B.; Ramteke, K.; Deshmukhe, G.; Jaiswar, A.K.; Shukla, S.P. Microplastics in shrimps: A study from the trawling grounds of north eastern part of Arabian Sea, Environ. Sci. Pollut. Res. 2021, 28, 48494–48504. [Google Scholar]
- Hossain, M.S.; Rahman, M.S.; Uddin, M.N.; Sharifuzzaman, S.M.; Chowdhury, S.R.; Sarker, S.; Chowdhury, M.S.N. Microplastic contamination in penaeid shrimp from the northern bay of bengal. Chemosphere 2020, 238, 124688. [Google Scholar] [CrossRef] [PubMed]
- Curren, E.; Leaw, C.P.; Lim, P.T.; Leong, S.C.Y. Evidence of marine microplastics in commercially harvested seafood. Front. Bioeng. Biotechnol. 2020, 8, 1–9. [Google Scholar]
- Akhbarizadeh, R.; Moore, F.; Keshavarzi, B. Investigating microplastics bioaccumulation and biomagnification in seafood from the Persian Gulf: A threat to human health? Food Addit. Contam. Part. A Chem. Anal. Control Expo. Risk Assess. 2019, 36, 1696–1708. [Google Scholar]
- Wu, F.; Wang, Y.; Leung, J.Y.S.; Huang, W.; Zeng, J.; Tang, Y.; Chen, J.; Shi, A.; Yu, X.; Xu, X.; et al. Accumulation of microplastics in typical commercial aquatic species: A case study at a productive aquaculture site in China. Sci. Total Environ. 2020, 708, 135432. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, Y.; Song, K.; Du, W.; Huang, W.; Gu, Z.; Feng, Z. Microplastics in different tissues of wild crabs at three important fishing grounds in China. Chemosphere 2021, 271, 129479. [Google Scholar]
- Devriese, L.I.; Van der Meulen, M.D.; Maes, T.; Bekaert, K.; Paul-Pont, I.; Frère, L.; Vethaak, A.D. Microplastic contamination in brown shrimp (Crangon crangon, Linnaeus 1758) from coastal waters of the Southern North Sea and Channel area. Mar. Pollut. Bull. 2015, 98, 179–187. [Google Scholar] [CrossRef]
- Amponsah, A.K.; Afrifa, E.K.A.P.; Essandoh, K. Plastic in the food chain: Investigating microplastic consumption by the blue-swimming crab (de Rochebrune, 1883) and shrimp (Pérez-Farfante, 1967) from an estuarine system in Ghana. Sci. Afr. 2024, 25, e02261. [Google Scholar]
- Waddell, E.N.; Lascelles, N.; Conkle, J.L. Microplastic contamination in Corpus Christi Bay blue crabs, Callinectes sapidus. Limnol. Oceanogr. Lett. 2020, 5, 92–102. [Google Scholar]
- Piarulli, S.; Scapinello, S.; Comandini, P.; Magnusson, K.; Wong, J.X.W.; Sciutto, G.; Prati, S.; Mazzeo, R.; Andy, M.; Airoldi, L. Microplastic in wild populations of the omnivorous crab Carcinus aestuarii: A review and a regional-scale test of extraction methods, including microfibres. Environ. Pollut. 2019, 251, 117–127. [Google Scholar] [PubMed]
- Renzi, M.; Specchiulli, A.; Blašković, A.; Manzo, C.; Mancinelli, G.; Cilenti, L. Marine litter in stomach content of small pelagic fishes from the Adriatic Sea: Sardines (Sardina pilchardus) and anchovies (Engraulis encrasicolus). Environ. Sci. Pollut. Res. 2019, 26, 2771–2781. [Google Scholar]
- Kazour, M.; Jemaa, S.; Issa, C.; Khalaf, G.; Amara, R. Microplastics pollution along the Lebanese coast (Eastern Mediterranean Basin): Occurrence in surface water, sediments and biota samples. Sci. Total Environ. 2019, 696, 133933. [Google Scholar] [PubMed]
- Capone, A.; Petrillo, M.; Misic, C. Ingestion and elimination of anthropogenic fibers and microplastic fragments by the European anchovy (Engraulis encrasicolus) of the NW Mediterranean Sea. Mar. Biol. 2020, 167, 166. [Google Scholar]
- Lefebvre, C.; Saraux, C.; Heitz, O.; Nowaczyk, A.; Bonnete, D. Microplastics FTIR characterization and distribution in the water column and digestive tracts of small pelagic fish in the Gulf of Lions. Mar. Pollut. Bull. 2019, 142, 510–519. [Google Scholar]
- Avio, C.G.; Gorbi, S.; Regoli, F. Experimental development of a new protocol for extraction and characterization of microplastics in fish tissues: First observations in commercial species from Adriatic Sea. Mar. Environ. Res. 2015, 111, 18e26. [Google Scholar]
- Güven, O.; Gökdag, K.; Jovanovic, B.; Kıdeys, A.E. Microplastic litter composition of the Turkish territorial waters of the Mediterranean Sea, and its occurrence in the gastrointestinal tract of fish. Environ. Pollut. 2017, 223, 286–294. [Google Scholar]
- Eryaşar, A.R.; Mutlu, T.; Karaoğlu, K.; Veske, E.; Gedik, K. Assessment of microplastic pollution in eleven commercial fish species in the Gulf of İzmir (Aegean Sea, eastern Mediterranean). Mar. Pollut. Bull. 2024, 208, 116932. [Google Scholar]
- Compa, M.; Ventero, A.; Iglesias, M.; Deudero, S. Ingestion of microplastics and natural fibres in Sardina pilchardus (Walbaum, 1792) and Engraulis encrasicolus (Linnaeus, 1758) along the Spanish Mediterranean coast. Mar. Pollut. Bull. 2018, 128, 89–96. [Google Scholar]
- Giani, D.; Baini, M.; Galli, M.; Casini, S.; Fossi, M.C. Microplastics occurrence in edible fish species (Mullus barbatus and Merluccius merluccius) collected in three different geographical sub-areas of the Mediterranean Sea. Mar. Pollut. Bull. 2019, 140, 129–137. [Google Scholar]
- Bellas, J.; Martínez-Armental, J.; Martínez-Cámara, A.; Besada, V.; Martínez-Gómez, C. Ingestion of microplastics by demersal fish from the Spanish Atlantic and Mediterranean coasts. Mar. Pollut. Bull. 2016, 109, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, L.; Coppa, S.; Camedda, A.; Cocca, M.; De Falco, F.; Vianello, A.; de Lucia, G.A. A novel approach based on multiple fish species and water column compartments in assessing vertical microlitter distribution and composition. Environ. Pollut. 2021, 272, 116419. [Google Scholar] [CrossRef] [PubMed]
- Avio, C.G.; Pittura, L.; d’Errico, G.; Abel, S.; Amorello, S.; Marino, G.; Regoli, F. Distribution and characterization of microplastic particles and textile microfibers in Adriatic food webs: General insights for biomonitoring strategies. Environ. Pollut. 2020, 258, 113766. [Google Scholar] [CrossRef] [PubMed]
- Cheung, L.T.; Lui, C.Y.; Fok, L. Microplastic contamination of wild and captive flathead grey mullet (Mugil cephalus). Int. J. Environ. Res. Public. Health 2018, 15, 597. [Google Scholar] [CrossRef]
- Jimenez-Cárdenas, V.; Luna-Acosta, A.; Gómez-Méndez, L.D. Differential presence of microplastics and mesoplastics in coral reef and mangrove fishes in Isla Grande, Colombia. Microplastics 2022, 1, 477–493. [Google Scholar] [CrossRef]
- Guilhermino, L.; Martins, A.; Lopes, C.; Raimundo, J.; Vieira, L.R.; Barboza, L.G.A.; Vale, C. Microplastics in fishes from an estuary (Minho River) ending into the NE Atlantic Ocean. Mar. Pollut. Bull. 2021, 173, 113008. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, S.; Pan, Z.; Sun, D.; Xie, S.; Zhou, A.; Wang, J.; Zou, J. Occurrence and distribution of microplastics in commercial fishes from estuarine areas of Guangdong, South China. Chemosphere 2020, 260, 127656. [Google Scholar]
- Lopes, C.; Raimundo, J.; Caetano, M.; Garrido, S. Microplastic ingestion and diet composition of planktivorous fish. LO Lett. 2020, 5, 103–112. [Google Scholar] [CrossRef]
- Neves, D.; Sobral, P.; Ferreira, J.L.; Pereira, T. Ingestion of microplastics by commercial fish off the Portuguese coast. Mar. Pollut. Bull. 2015, 101, 119–126. [Google Scholar]
- Sultana, S.; Anisuzzaman, M.; Hossain, M.K.; Rana, M.S.; Paray, B.A.; Arai, T.; Yu, J.; Hossain, M.B. Ecological risk assessment of microplastics and mesoplastics in six common fishes from the Bay of Bengal Coast. Mar. Pollut. Bull. 2024, 204, 116544. [Google Scholar]
- Jabeen, K.; Su, L.; Li, J.; Yang, D.; Tong, C.; Mu, J.; Shi, H. Microplastics and mesoplastics in fish from coastal and fresh waters of China. Environ. Pollut. 2017, 221, 141–149. [Google Scholar] [PubMed]
- Bessa, F.; Barría, P.; Neto, J.M.; Frias, J.P.G.L.; Otero, V.; Sobral, P.; Marques, J.C. Occurrence of microplastics in commercial fish from a natural estuarine environment. Mar. Pollut. Bull. 2018, 128, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.; Martyniuk, C.J.; Lee, J.-S.; Sayed El-Din, H.H. Distribution, abundance, and composition of microplastics in market fishes from the Red and Mediterranean seas in Egypt. J. Sea Res. 2023, 194, 102407. [Google Scholar]
- Ghosh, G.C.; Akter, S.M.; Islam, R.M.; Habib, A.; Chakraborty, T.K.; Zaman, S.; Wahid, M.A. MPs contamination in commercial marine fish from the Bay of Bengal. Reg. Stud. Mar. Sci. 2021, 44, 101728. [Google Scholar]
- Pellini, G.; Gomiero, A.; Fortibuoni, T.; Ferrà, C.; Grati, F.; Tassetti, A.N.; Polidori, P.; Fabi, G.; Scarcella, G. Characterization of microplastic litter in the gastrointestinal tract of Solea solea from the Adriatic Sea. Environ. Pollut. 2018, 234, 943–952. [Google Scholar]
- Tsangaris, C.; Digka, N.; Valente, T.; Aguilar, A.; Borrell, A.; de Lucia, G.A.; Gambaiani, D.; Garcia-Garin, O.; Kaberi, H.; Martin, J.; et al. Using Boops boops (osteichthyes) to assess microplastic ingestion in the Mediterranean Sea. Mar. Pollut. Bull. 2020, 158, 111397. [Google Scholar] [CrossRef]
- Sbrana, A.; Valente, T.; Scacco, U.; Bianchi, J.; Silvestri, C.; Palazzo, L.; de Lucia, G.A.; Valerani, C.; Ardizzone, G.; Matiddi, M. Spatial variability and influence of biological parameters on microplastic ingestion by Boops boops (L.) along the Italian coasts (Western Mediterranean Sea). Environ. Pollut. 2020, 263, 114429. [Google Scholar]
- Ineyathendral, T.R.; Govindarajulu, B.; Priyanka, R. Characterization and distribution of microplastics in the commercial fishes along the coast of Chennai. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100898. [Google Scholar] [CrossRef]
- Kılıç, E. Abundance and ecological risk of microplastics in commercial fish species from northeastern Mediterranean Sea. Environ. Pollut. 2024, 363, 125252. [Google Scholar]
- Karami, A.; Golieskardi, A.; Ho, Y.B.; Larat, V.; Salamatinia, B. Microplastics in eviscerated flesh and excised organs of dried fish. Sci. Rep. 2017, 7, 5473. [Google Scholar]
- World Health Organization. Dietary and Inhalation Exposure to Nano- and Microplastic Particles and Potential Implications for Human Health; World Health Organization: Geneva, Switzerland, 2022; Licence: CC BY- NC-SA 3.0; Available online: https://iris.who.int/bitstream/handle/10665/362049/9789240054608-eng.pdf?sequence= (accessed on 15 December 2024).
- Dris, R.; Gasperi, J.; Rocher, V.; Saad, M.; Renault, N.; Tassin, B. Microplastic contamination in an urban area: A case study in Greater Paris. Environ. Chem. 2015, 12, 592–599. [Google Scholar]
- Saha, S.C.; Saha, G. Effect of microplastics deposition on human lung airways: A review with computational benefits and challenges. Helyon 2024, 10, e24355. [Google Scholar]
- Vethaak, A.D.; Leslie, H.A. Plastic Debris Is a Human Health Issue. Environ. Sci. Technol. 2016, 50, 6825–6826. [Google Scholar] [PubMed]
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Kelly, F.J.; Tassin, B. Microplastics in air: Are we breathing it in? Curr. Opin. Environ. Sci. Health 2018, 1, 1–5. [Google Scholar]
- Yee, M.S.; Hii, L.W.; Looi, C.K.; Lim, W.M.; Wong, S.F.; Kok, Y.Y.; Tan, B.-K.; Wong, C.-Y.; Leong, C.-O. Impact of microplastics and nanoplastics on human health. Nanomaterials 2021, 11, 496. [Google Scholar] [CrossRef]
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in Seafood and the Implications for Human Health. Curr. Environ. Health Rep. 2018, 5, 375–386. [Google Scholar]
- Yang, D.; Shi, H.; Li, L.; Li, J.; Jabeen, K.; Kolandhasamy, P. Microplastic Pollution in Table Salts from China. Environ. Sci. Technol. 2015, 49, 13622–13627. [Google Scholar]
- Liebezeit, G.; Liebezeit, E. Non-pollen particulates in honey and sugar. Food Addit. Contam. 2023, 30 Pt A, 2136–2140. [Google Scholar]
- Liebezeit, G.; Liebezeit, E. Synthetic particles as contaminants in German beers. Food Addit. Contam. 2014, 31, 1574–1578. [Google Scholar]
- Damaj, S.; Trad, F.; Goevert, D.; Wilkesmann, J. Bridging the Gaps between Microplastics and Human Health. Microplastics 2024, 3, 46–66. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [PubMed]
- Schwabl, P.; Koppel, S.; Konigshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of various microplastics in human stool a prospective case series. Ann. Int. Med. 2019, 171, 453. [Google Scholar] [PubMed]
- Ho, Y.-W.; Lim, J.Y.; Yeoh, Y.K.; Chiou, J.-C.; Zhu, Y.; Lai, K.P.; Li, L.; Chan, P.K.S.; Fang, J.K.-H. Preliminary Findings of the High Quantity of Microplastics in Faeces of Hong Kong Residents. Toxics 2022, 10, 414. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Liu, Y.; Zhang, T.; Zhang, F.; Ren, H.; Zhang, Y. Analysis of microplastics in human feces reveals a correlation between fecal microplastics and inflammatory bowel disease status. Environ. Sci. Technol. 2022, 56, 414–421. [Google Scholar]
- Sun, A.; Wang, W.-X. Human Exposure to Microplastics and Its Associated Health Risks. Environ. Health 2023, 1, 139–149. [Google Scholar]
- Revel, M.; Chatel, A.; Mouneyrac, C. Micro(nano)plastics:A threat to human health? Curr. Opin. Environ. Sci. Health 2018, 1, 17–23. [Google Scholar]
- Burgos-Aceves, M.A.; Abo-Al-Ela, H.G.; Faggio, C. Physiological and metabolic approach of plastic additive effects: Immune cells responses. J. Hazard. Mater. 2021, 404, 124114. [Google Scholar]
- Sozener, Z.C.; Yücel, Ü.Ö.; Altiner, S.; Oztürk, B.O.; Cerci, P.; Türk, M.; Akin, B.G.; Akdis, M.; Yilmaz, I.; Ozdemir, C.; et al. The external exposome and allergies: From the perspective of the epithelial barrier hypothesis. Front. Allergy 2022, 3, 887672. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grattagliano, A.; Grattagliano, Z.; Manfra, L.; Libralato, G.; Biandolino, F.; Prato, E. An Overview on Microplastics Hazards to the Marine Ecosystem and Humans’ Health. Water 2025, 17, 916. https://doi.org/10.3390/w17070916
Grattagliano A, Grattagliano Z, Manfra L, Libralato G, Biandolino F, Prato E. An Overview on Microplastics Hazards to the Marine Ecosystem and Humans’ Health. Water. 2025; 17(7):916. https://doi.org/10.3390/w17070916
Chicago/Turabian StyleGrattagliano, Asia, Zaira Grattagliano, Loredana Manfra, Giovanni Libralato, Francesca Biandolino, and Ermelinda Prato. 2025. "An Overview on Microplastics Hazards to the Marine Ecosystem and Humans’ Health" Water 17, no. 7: 916. https://doi.org/10.3390/w17070916
APA StyleGrattagliano, A., Grattagliano, Z., Manfra, L., Libralato, G., Biandolino, F., & Prato, E. (2025). An Overview on Microplastics Hazards to the Marine Ecosystem and Humans’ Health. Water, 17(7), 916. https://doi.org/10.3390/w17070916









