Abstract
The presence of fungal spores in water poses a significant risk to public health, requiring effective inactivation strategies. Ultraviolet (UV) radiation is a widely used approach, traditionally employing mercury vapor lamps. However, these lamps have efficiency limitations and contain hazardous materials. As an alternative, ultraviolet light-emitting diodes (UV-LEDs) have emerged as a safer and more sustainable option. Despite their advantages, research on their efficacy against fungal spores remains limited. This study investigates the inactivation and post-exposure response of Aspergillus niger and Penicillium sp. spores using a collimated UV-LED system. The impact of two different wavelengths (265 nm and 280 nm) and post-treatment conditions (light and darkness for 24 h) on fungal viability was analyzed. Kinetic modeling was applied to assess the resistance of the spores and their capacity for photoreactivation. The results demonstrate that both the UV wavelength and the environmental conditions after exposure significantly influence disinfection outcomes. Penicillium sp. exhibited greater susceptibility to UV radiation but also higher photoreactivation potential, while A. niger showed stronger resistance and lower recovery capacity. The UV dose required for 99% inactivation, considering photoreactivation effects, was 323.7 ± 90.0 mJ cm−2 and 321.9 ± 43.8 mJ cm−2 for A. niger, whereas for Penicillium sp., it was 167.7 ± 13.0 mJ cm−2 and 146.5 ± 29.2 mJ cm−2 at 265 nm and 280 nm, respectively. These findings emphasize the necessity of tailoring UV-LED disinfection strategies based on the specific characteristics of the target organisms and post-treatment environmental factors.
1. Introduction
To ensure safe drinking water, it is important to disinfect it and prevent contamination from harmful microorganisms like bacteria, viruses, and fungi [1,2]. Filamentous fungi, usually known as molds, are eukaryotic organisms that belong to the kingdom Fungi. They have a multicellular structure comprising branched filaments called hyphae, which interconnect to form a complex network called mycelium [3]. This provides them the ability to grow and expand quickly. They can also produce spores (sexually and asexually), which are specialized reproductive structures that can be dispersed over long distances by air or water. This ability to spread makes them significant agents in microbiological contamination, posing risks to human health and the environment [1].
In water systems, molds can penetrate drinking water systems through different sources, such as contaminated irrigation water, rainwater, sediments, and organic matter in pipes where they can grow and reproduce to create colonies, increasing the risk of microbiological contamination of drinking water [1]. Reviews of a wide selection of studies on fungi in drinking water highlight that the most commonly isolated genera are Aspergillus, Penicillium, Cladosporium, Fusarium, and Trichoderma [4,5]. Multiple studies have identified these genera and show a high occurrence in drinking water [6,7]. In addition, certain genera such as Aspergillus, Penicillium, Cladosporium, and Alternaria have the potential to cause allergic diseases, raising additional concerns for human health [8]; these genera can also produce T&O (Taste and Odor) compounds (unpleasant tastes and odors) and mycotoxin compounds, indicating the possibility of chemical contamination associated with their presence in drinking water [1,7,9].
Conventional drinking water treatment methods fail to eliminate fungi. Common disinfection strategies, such as the use of chlorine and ozone, are ineffective in eliminating them [1,6,10]. Studies have shown that inactivation of fungal spores requires considerably higher Ct (concentration × time) values than bacteria and viruses [11]. One promising alternative for microbial control is UV radiation, which is capable of penetrating biofilms and cell walls, leading to DNA damage that inhibits fungal proliferation. In recent years, ultraviolet (UV) light exposure within the 200–320 nm range has gained recognition as a reliable technique for disinfection [12,13]. This is largely attributed to its adaptability, effectiveness, and minimal risk of producing hazardous by-products, making it a safer option than conventional chemical disinfection methods [14].
The most common sources of UV radiation include low-pressure mercury lamps (LP-UV), which emit light at 254 nm, and medium-pressure lamps (MP-UV), which generate radiation over a wide range of wavelengths. More recently, the UV-LED technology has made it possible to operate at multiple wavelengths, offering a more versatile and efficient alternative. Various studies have highlighted its superior effectiveness, making it a promising alternative for water disinfection [15,16,17,18]. In terms of technological comparison, LP-UV, although historically efficient (30–40% conversion efficiency, 40–60 W consumption, 8000–12,000 h lifetime), raises concerns due to the use of mercury and the need for frequent replacement [15,19]. In contrast, several studies point out that UV-LED emitters, with a lower conversion efficiency (5–15%) and 20–30% higher initial cost, offer the absence of mercury, instantaneous ignition, flexibility in wavelength selection, and more than 20,000 h of lifetime before requiring replacement [15,19]. On the one hand, it is argued that this lower efficiency forces UV-LEDs to consume between 50–100 W to achieve comparable irradiances, creating scalability challenges; on the other hand, the higher durability and no need to handle hazardous waste favor operational savings and reduce environmental impact [20]. Thus, the higher initial investment of UV-LEDs is mitigated by their medium- and long-term benefits, especially as production costs decrease and reactor designs are optimized to maximize their irradiance. In addition, the efficiency and lifetime of UV-LEDs are wavelength dependent [21]. It has been reported that 265 nm LEDs have a lower energy efficiency and shorter lifetime than 280 nm LEDs [15]. If the performance at 280 nm is similar or higher, their implementation in water treatment systems could be justified to prolong equipment durability and reduce maintenance costs, making the technology more competitive with conventional mercury lamps [12]. Another key aspect is the specific resistance of microorganisms. Different fungi have structural variations in their cell wall and protective pigments, such as melanin in A. niger and carotenoids in Penicillium sp., which influence their susceptibility to UV radiation [22]. If a wavelength is particularly effective against a specific type of fungus, treatments could be designed to match the contamination profile of each system [23].
The efficacy of ultraviolet (UV) disinfection can be compromised by photoreactivation, a process in which certain microorganisms repair damage to their DNA after exposure to light, regaining their viability and infective potential [24]. This phenomenon is especially relevant in water quality management, where storage and distribution conditions can significantly influence the success of UV treatment. Photoreactivation in drinking water systems represents a major risk, particularly when treated water is stored in uncovered tanks or open distribution systems with exposure to sunlight or artificial light [8]. In these scenarios, microorganisms that were not completely inactivated may regain viability, compromising the security of supply and resulting in public health risks. In the case of treated wastewater, the relevance of photoreactivation depends largely on the reused water’s purpose and subsequent exposure to light sources. If UV-disinfected wastewater is intended for reuse in agricultural irrigation or aquifer recharge, exposure to sunlight may favor the reactivation of microorganisms, reducing the effectiveness of the treatment [25]. Since photoreactivation varies according to the water matrix and storage or re-use conditions, its analysis is crucial to optimize UV-LED disinfection treatments. Understanding these effects allows treatment strategies to adjust and ensure safe microbiological quality in different water systems. Like other microorganisms, fungal spores can reactivate following UV treatment, which becomes a significant challenge when studying treatment efficacy; thus, the importance of analyzing photoreactivation as a function of the species studied and the wavelength used [26], represents a challenge in UV disinfection [27]. If radiation at 280 nm has a lower risk of allowing spore recovery compared to 265 nm, its strategic use could be crucial to improve long-term treatment effectiveness [28]. A suitable indicator should have high UV resistance to ensure inactivation of the most resistant organisms but, at the same time, a high potential for photoreactivation in case of exposure to ambient light, which would allow assessing the need for complementary mitigation strategies [29].
This study aimed to evaluate the effects of UV radiation on the inactivation and potential reactivation of A. niger and Penicillium sp. spores using a UV-LED reactor operating at wavelengths of 265 and 280 nm. Additionally, the spores’ ability to recover after 24 h of incubation under both light and dark conditions was analyzed and quantified. To achieve this, kinetic models were applied to assess and compare the UV resistance of the strains and their capacity for damage repair following exposure.
2. Materials and Methods
2.1. Preparation and Cultivation of Fungal Strains
For this study, Aspergillus niger and Penicillium sp. were utilized as model fungi, sourced from the mycological collection at the University of Azuay, Ecuador. These species were chosen due to their frequent detection in aquatic environments and their role as indicators of fungal contamination in water systems.
The fungal strains underwent a multi-step cultivation process to ensure their viability for experimentation. Initially, they were incubated for 14 days at 27 °C in a 30 mL potato dextrose agar (PDA) medium to promote growth. Following this stage, the cultures were transferred to Dichloran Rose Bengal Chloramphenicol Agar (DRBC) and maintained under the same temperature conditions for an additional two weeks to facilitate spore maturation. Upon reaching maturity, the spores were carefully extracted, washed three times with sterile phosphate-buffered saline (PBS) at pH 7.3, and subjected to centrifugation at 8000 rpm for 10 min per cycle. A Neubauer chamber was used to determine spore concentration, which was adjusted to a range of 107–108 spores per milliliter using a phosphate-buffered solution. The prepared stock suspension was kept at 4 °C until further experimentation. After the experiments were concluded, the concentration of spores of fungal strains was determined using the surface spread method combined with plate counting [30], with results expressed as spores per milliliter (spores mL−1).
Once the experiments were completed, the surface spreading and plate count technique [30] was applied to quantify the fungal concentration expressed in spores per milliliter (spores mL−1). To achieve this, serial dilutions were performed on a tenfold scale for each sample, ensuring a final concentration from 20 to 300 spores per plate. This strategy was implemented to limit colony growth to a manageable number and to avoid overlaps, which could lead to inaccuracies in counting. Subsequently, a 100 μL aliquot from each dilution was spread onto 90 mm Petri dishes containing DRBC agar, followed by incubation at 27 °C for a period of three days.
2.2. UV Exposure System and Radiation Measurement
In this study, ultraviolet exposure was administered using an LED-based collimated beam reactor, manufactured by AquiSense Technologies (Erlanger, KY, USA). The system included a UVinaire™ module, incorporating three LEDs per wavelength, along with a digital interface for controlling operational settings, a diffusion unit to ensure uniform light distribution, and a 12 V, 90 W adapter for AC–DC conversion. The experiment focused on two wavelengths, 265 nm and 280 nm. To accurately measure radiation intensity at the 6 cm working distance—matching the height used during the tests—a high-precision ILT 950 UV spectroradiometer (International Light Technologies, Peabody, MA, USA) was employed.
2.3. UV Dose Estimation and Correction Methodology
To facilitate the comparison of different inactivation experiments, the applied UV dose (mJ cm−2) was determined by integrating radiation intensity with exposure duration. Since various factors influence light distribution in liquid media, corrections were applied based on established methodologies by Bolton and Linden (2003) [31]. These included a reflection adjustment (0.975), a dish-induced light dispersion coefficient (0.911), and an angular divergence factor (0.966). Additionally, the reduction in UV intensity due to sample absorbance was analyzed separately for each fungal species. Absorbance levels at 265 nm and 280 nm were measured using a Jenway 6315 UV/Visible spectrophotometer to quantify this effect. Following these assessments, exposure times were calibrated to achieve UV doses of 40, 80, 120, 160, 200, 240, and 300 mJ cm−2.
2.4. Experimental Procedure
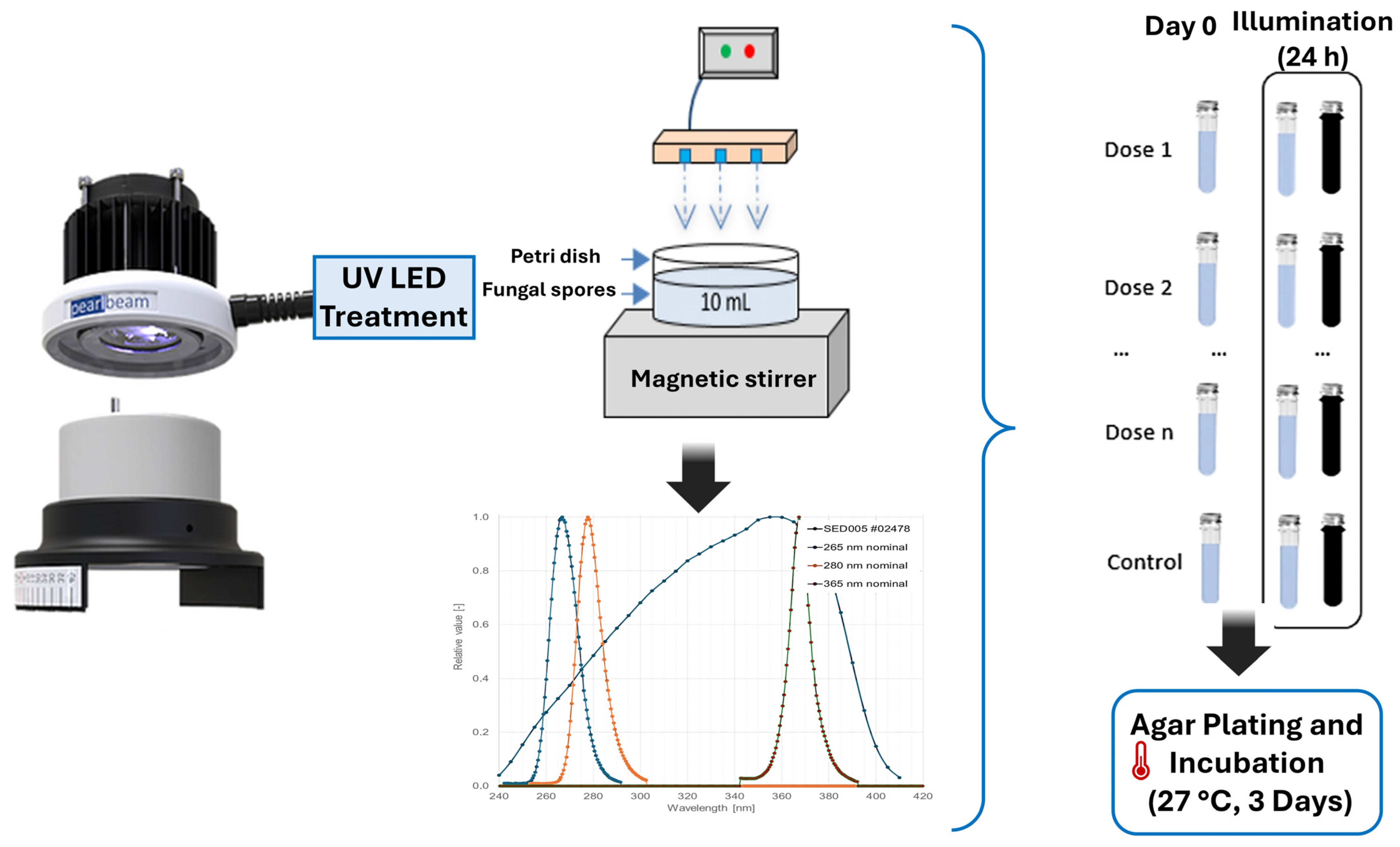
Assays for both fungal spores were carried out in triplicate for each wavelength investigated (265 nm and 280 nm) and under each post-treatment condition (photoreactivation and reactivation in the dark). Each experiment used 10 mL aliquots of 9 mL of autoclaved distilled water and 1 mL of the fungal spore stock solution. This mixture was placed in 5 cm diameter Petri dishes. The Petri dishes were placed in the collimated beam UV-LED system, ensuring that the dish’s center coincided with the equipment’s central irradiation point.
To ensure homogeneous exposure to UV radiation, samples were continually stirred using a magnetic rod throughout the process. When the required irradiation period for the designated dose was completed, 1 mL of the treated sample was immediately withdrawn to quantify viable spore concentration at the initial measurement point (0 days). The remaining portion was reserved for further analysis of potential reactivation under distinct environmental settings. For this step, the sample was divided into two separate containers: one was wrapped in aluminum foil to maintain 1-day dark conditions, while the other remained exposed to light. Both sets of samples were incubated for 24 h in a controlled growth chamber (Binder Series KBWF) maintained at 20 °C and continuously agitated at 30 rpm. After this incubation period, the culturable spore concentration was quantified following the methodology detailed in Section 2.1, with sterile conditions verified by blank control plates. The container exposed to light received an illumination intensity of 36 µEinstein m−2 s−1, a value selected to simulate moderate indoor or partially covered storage conditions—environments that typically register well under 100 µEinstein m−2 s−1 [22,32] and can approximate the 20–80 µEinstein m−2 s−1 range observed in some growth chambers [33]. This setup reflects realistic low-light scenarios rather than direct solar irradiation and aligns with plausible water storage and distribution conditions Figure 1.
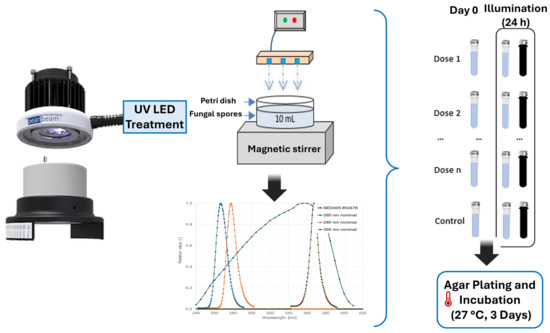
Figure 1.
Layout of the laboratory system and experimental process.
2.5. Evaluation of Inactivation Kinetics and Statistical Analysis
The survival rate (S) of each sample was determined by comparing the concentration of culturable spores of fungal strains in the irradiated sample to that in a non-irradiated control. This ratio was used to generate inactivation curves by plotting Log(S) (is defined as the logarithm in base 10 of the survival rate (S), where S is the ratio of the concentration of viable spores in the irradiated sample to the concentration of viable spores in the non-irradiated control) as a function of UV dose. To analyze nonlinear survival trends and extract inactivation kinetic parameters, the GInaFiT tool in MS Excel (version 2.1) was employed [34]. Due to the presence of tailing in the survival curves, the log-linear plus tail model was applied, as described in Equation (1) [35]:
where the terms are defined as follows:
- S: Survival fraction at a specific UV dose;
- Sres: Residual survival fraction;
- k: Inactivation rate constant;
- D: UV dose.
The required UV dose (D2) to achieve a 2-log reduction, corresponding to a 99% decrease in viable spores of fungal strains, was derived using inactivation kinetic parameters obtained through model fitting.
To assess statistical significance, an ANCOVA analysis was conducted in Statgraphics Centurion (version 16.1.03), where Log(S) was the dependent variable and UV dose was treated as a covariate. The independent factors included the following:
- Microbial species (A. niger or Penicillium sp.);
- Wavelength (265 nm and 280 nm);
- Post-treatment incubation period (0 days, 1 day with light exposure, or 1 day in darkness).
Additionally, the extent of photoreactivation was quantified using the photoreactivation percentage (PPR), calculated with Equation (2) [25]:
where the terms are defined as follows:
- Np: Concentration of organisms in the photoreactivated sample;
- N: Bacterial concentration immediately after UV exposure;
- N0: Initial bacterial concentration before UV treatment.
This equation was used to determine the fraction of spores of fungal strains that regained viability following UV disinfection.
3. Results and Discussion
3.1. Characteristics of Fungal Reactivation
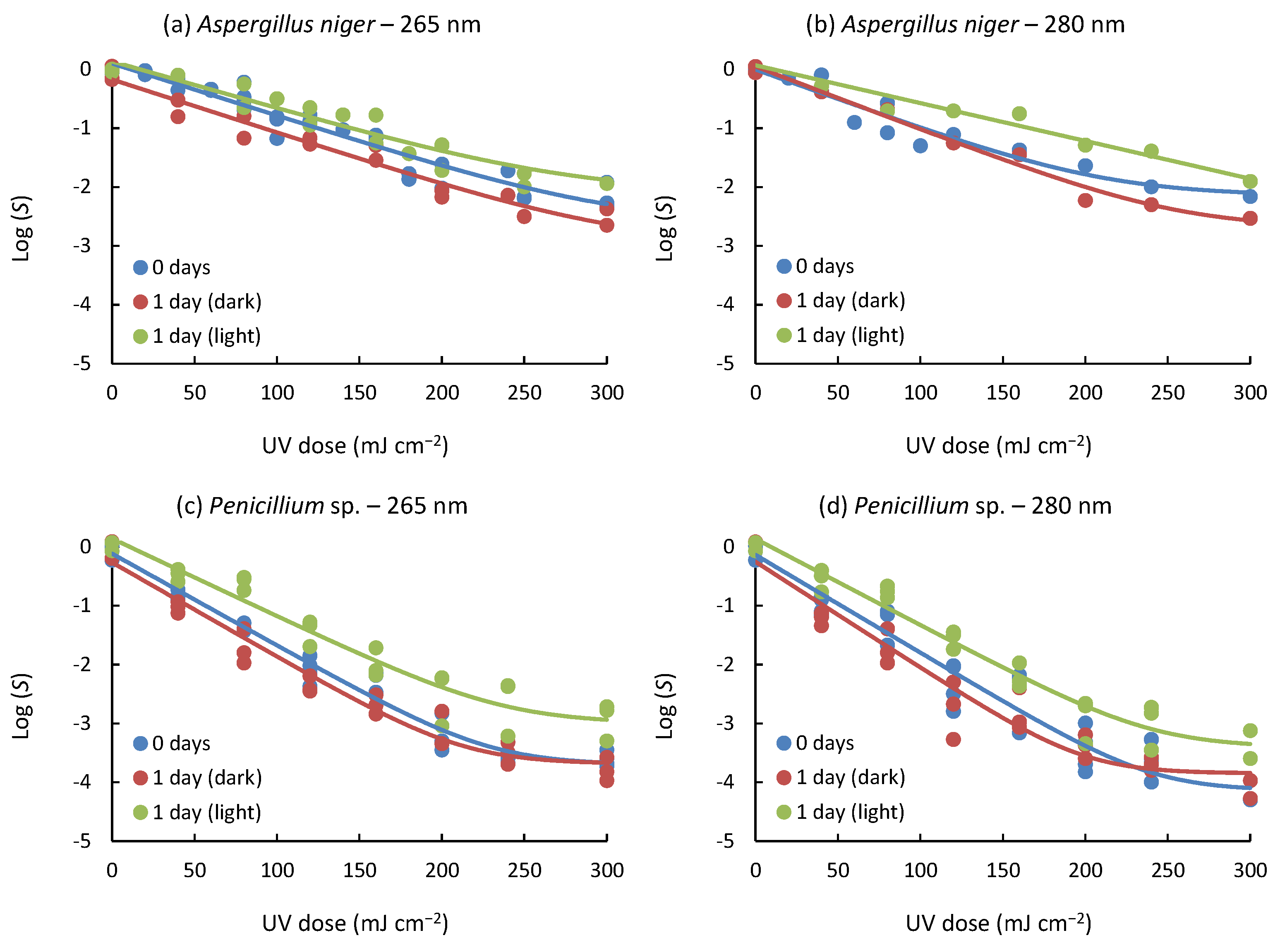
The inactivation curves for spores of fungal strains evaluated under different conditions, such as strain type, wavelength, and post-treatment, are presented in Figure 2. Samples exposed to UV light immediately after irradiation undergo photorepair processes, while samples subjected to post-treatment in the dark avoid these processes due to the absence of light. In all cases, tailing phenomena were observed at high UV doses, regardless of incubation time and applied wavelength emission up to 300 mJ cm−2 (Equation (2)), with a high level of correlation R2 (0.927–0.986). On the other hand, shoulders were not observed in all experiments.
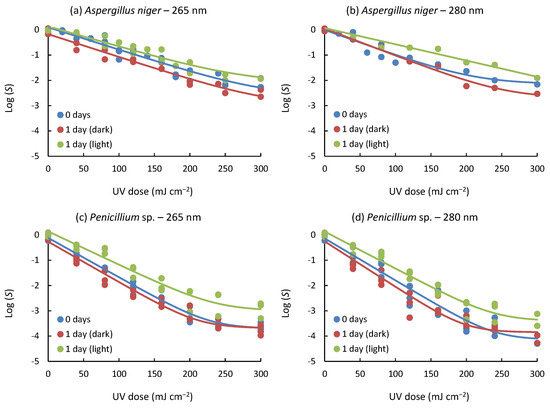
Figure 2.
Inactivation curves representing the treatment of spores of fungal strains under varying post-treatment conditions: immediate assessment without incubation (0 days), incubation for 24 h in darkness (1 day (dark)), and incubation for 24 h under light exposure (1 day (light)). Experimental data points are depicted by symbols, while the fitted log-linear + tail model is represented by continuous lines. Blue lines represent 0 days; red lines represent 1 day (dark); green lines represent 1 day (light).
The study of inactivation curves has a valuable application when determining the optimal treatment conditions. In this case, the log-linear + tail model is helpful because it shows that beyond a UV dose, no further inactivation of the fungal spores studied would be achieved, i.e., it will not be necessary to prolong the treatment beyond the indicated doses, since no further inactivation would be obtained. Previous studies [19,36], together with the experiments conducted in the study presented herein, support the idea that the tailing and shouldering phenomena observed in the response curves can be attributed to spore aggregation [37]. Furthermore, the possibility is raised that factors such as hydraulic conditions and inactivation levels close to the detection limit may also influence these phenomena [38,39,40].
To evaluate the relationship between UV radiation dose and microbial survival, an analysis of covariance (ANCOVA) was conducted, incorporating an interaction term. In this statistical assessment, Log(S) was designated as the dependent variable, while UV dose was introduced as a covariate. Additionally, three independent variables were examined: the spores of fungal strains subjected to the treatment, the wavelength of irradiation applied, and the post-treatment incubation environment. The analysis revealed that both UV dose and the independent factors (wavelength, type of spores of fungal, and post-treatment condition) played a significant role in determining the inactivation response, as indicated by variations in Log(S) values (p < 0.001).
The effectiveness of UV LED irradiation at 265 nm and 280 nm was compared by analyzing the logarithmic count reduction in spores of fungal strains. The findings indicate that 280 nm achieved a slightly higher inactivation rate than 265 nm, though the difference remained marginal for both tested species. Specifically, for A. niger, a UV dose of 254.6 ± 9.9 mJ cm−2 was necessary to reach a 2−log10 reduction at 265 nm, while a slightly increased dose of 289.9 ± 42.8 mJ cm−2 was required at 280 nm. Meanwhile, for Penicillium sp., the required UV dose showed minimal variation between the two wavelengths, with values of 108.7 ± 4.2 mJ cm−2 at 265 nm and 107.2 ± 7 mJ cm−2 at 280 nm.
Comparing the resistance of the two organisms studied, the treatment effect on Penicillium sp. was significantly higher than on A. niger. Both the wavelength used and post-treatment (procedures performed after treatment) significantly influenced the treatment efficacy for Penicillium sp. This means that both wavelength and post-treatment actions played an essential role in the inactivation of Penicillium sp. The p-value < 0.001 indicates that the influence is highly significant. In the case of A. niger, it was observed that wavelength had no significant impact on treatment efficacy. However, post-treatment did have a significant effect on the inactivation of A. niger. The p-value < 0.001 indicates that post-treatment is a relevant factor in treatment efficacy for A. niger.
These findings suggest that the impact of UV light treatment varies according to the fungal species studied. In the case of Penicillium sp., both wavelength and post-treatment are essential factors, while in A. niger, post-treatment seems to play a more relevant role in treatment efficacy. The resistance to UV radiation observed in this study is in agreement with previous research [41], where A. niger showed a higher tolerance compared to Penicillium sp. and a high photoreactivation capacity [19], suggesting that fungal resistance to ultraviolet radiation is not strictly dependent on the type of UV light source. On the one hand, differences in enzymatic activity (e.g., fotolyases), internal cellular structures, and protective pigments have been widely hypothesized to explain this resistance [8,22]; A. niger, for example, would have evolved highly effective defenses by producing melanin, which can absorb and dissipate UV energy, protecting DNA and reducing the need for repair [27,42]. On the other hand, although carotenoids present in Penicillium sp. have been proposed to provide some protection [22,43], the evidence is insufficient to fully explain their reduced UV resistance and increased susceptibility to photoreactivation, as the role of the enzymatic pathways involved has not been fully characterized. Furthermore, while several studies have pointed out that hydrophobicity and spore aggregation contribute to the efficiency of UV inactivation [44], there are still gaps regarding the influence of water chemistry and the interaction between pigment and DNA repair mechanisms. Thus, further research is needed to determine the extent of these factors on fungal resistance and assess the extent to which melanin production, carotenoids, and other cellular components influence tolerance and photoreactivation of spores.
3.2. Kinetic Parameters in Inactivation
The kinetics of UV inactivation in fungal spores showed a linear shape with tail. This suggests that some spores may resist UV radiation and survive despite rapid inactivation initially, resulting in a gradual tail in the survival curve. Parameters of inactivation kinetics allowed quantification of the efficiency of UV inactivation in fungal spores and the importance of photoreactivation processes. These parameters provide information on the relative resistance of spores and the ability of certain strains to recover or reactivate.
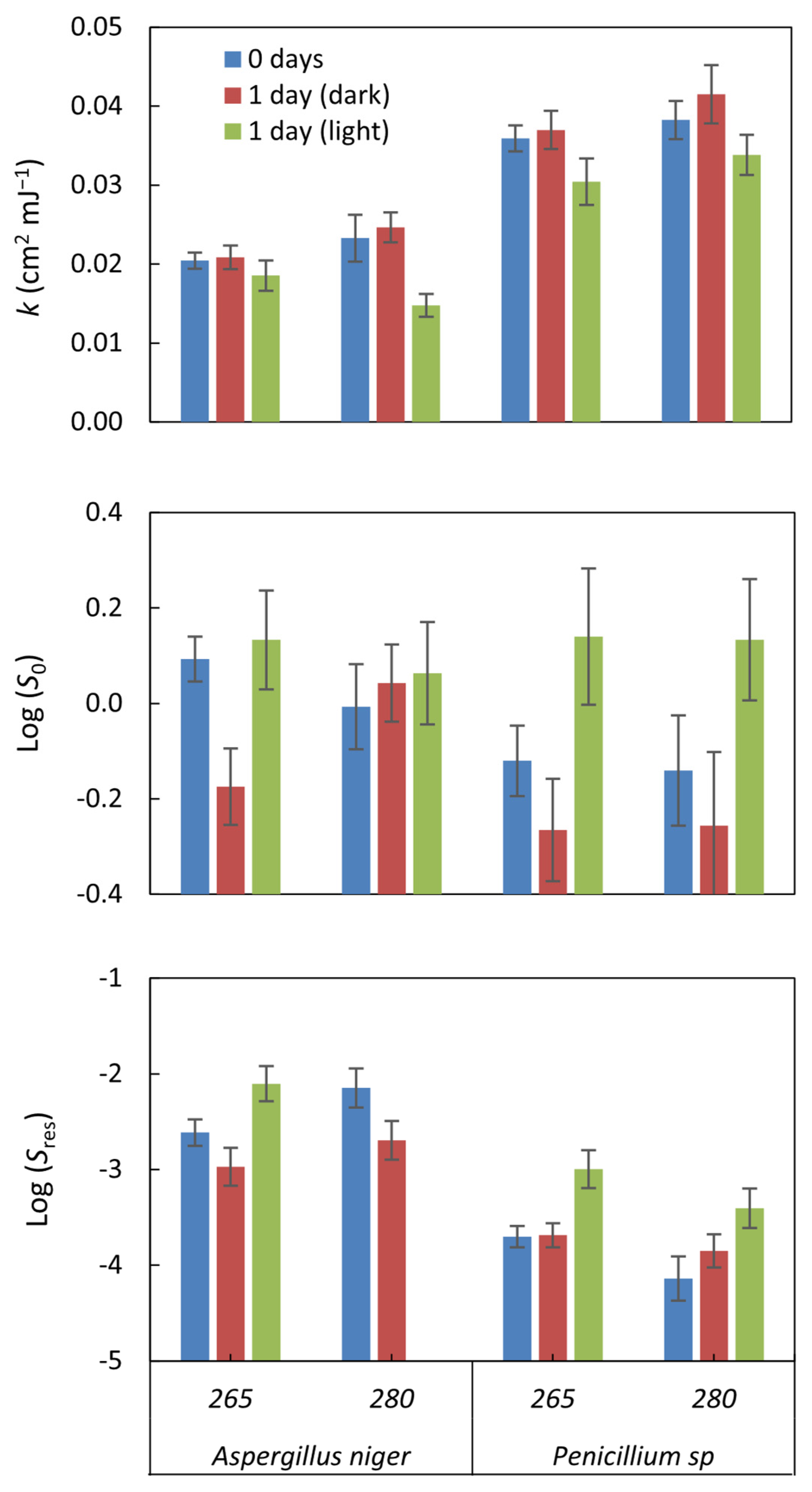
Log(S0) shows the survival of organisms without UV radiation; for incubated samples, Log(S0) measures the effect of incubation on the survival of the strains tested. Incubation alone does not generally impact survival, as the values are close to 0 in all cases. Furthermore, the values were not significant (p-value > 0.05) in all cases (Figure 3). On the other hand, UV radiation achieved several logarithmic reductions in the viability of the organisms. These results indicate that the impact of incubation alone on organism survival was very low compared to the efficacy of UV treatment in reducing organism viability.
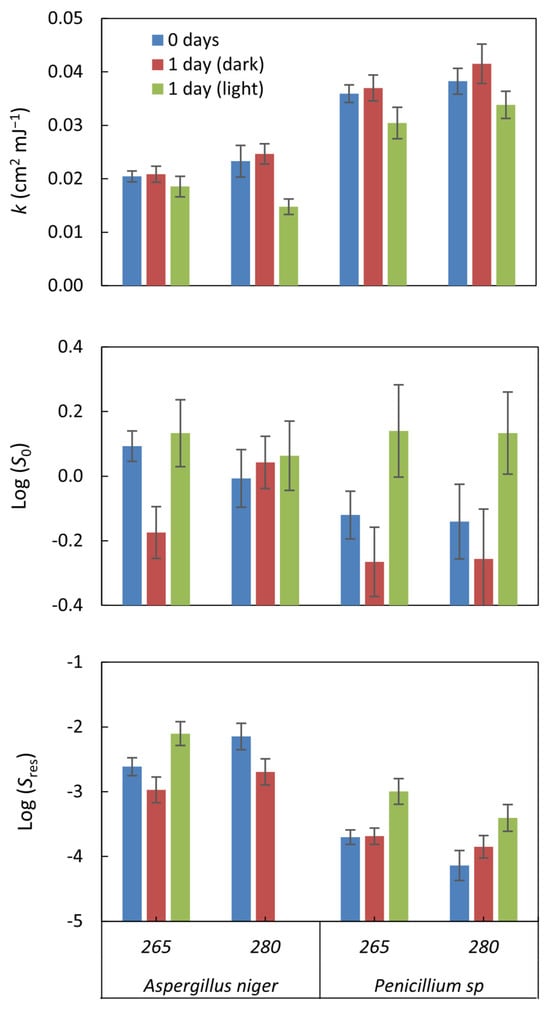
Figure 3.
The inactivation kinetics parameters were estimated by applying the log-linear + tail model to the inactivation curves. The parameter k represents the rate at which inactivation occurs within the linear region of the curve under UV exposure. Log(S0) denotes the survival fraction before any UV dose is applied (0 mJ cm−2), whereas Log(Sres) indicates the fraction of microorganisms that remained viable after treatment. The error bars, computed using GInaFiT, illustrate the standard error.
The Log (Sres) value indicates the residual survival of the organisms after applying the UV treatment, i.e., the fraction of organisms that managed to survive the treatment. In our case, values between −2.1 ± 0.19 and −4.14 ± 0.23 log10 were observed, indicating a significant reduction in the viability of the organisms due to the UV treatment. The lower the Log (Sres) value, the greater the treatment’s efficacy in reducing organisms’ survival. In this case, for A. niger, it reached a reduction of −2.97 ± 0.2 log10, while for Penicillium sp., it was higher, with values up to −4.14 ± 0.23 log10.
The Inactivation rate constant (k) represents the rate at which fungal inactivation occurs. It was observed that the average inactivation rate constants (k) of A. niger were lower than those of Penicillium sp., consistent with the log-log count reduction analysis results, indicating that A. niger is more resistant to radiation. Specifically, the results revealed that the inactivation rate of Penicillium sp. was approximately 1.7 times higher than that of A. niger.
The k values obtained in this study for A. niger at 265 nm (k265: 0.02 ± 0.001 cm2 mJ−1) and at 280 nm (k280: 0.023 ± 0.003 cm2 mJ−1), as well as for Penicillium sp. at 265 nm (k265: 0. 036 ± 0.002 cm2 mJ−1) and at 280 nm (k280: 0.038 ± 0.002 cm2 mJ−1), were lower compared to the values reported in a previous study by Wan, Q. et al., 2020 [19]. In that study, k265 (0.072–0.111 cm2 mJ−1) and k280 (0.076–0.114 cm2 mJ−1) were found for A. niger and a Penicillium species, respectively. This difference suggests that the particular resistance of the species being experimented with could be responsible for this disparity in k values, since a comparison with previous studies with the same isolated species provided similar values: 0.022 cm2 mJ−1 for A. niger and 0.038 cm2 mJ−1 for Penicillium sp. [41]; this also suggests that the species’ resistance influences the type of UV radiation applied.
Comparing the kinetic coefficients obtained with results in bacteria, in the case of E. coli, ranges of k values between 4.4 and 0.91 cm2 mJ−1 are significantly higher than those found here with fungal spores [20,45,46]. Although it is known that bacteria exhibit greater sensitivity to UV radiation than other microorganisms that may be present in wastewater, fungal spores demonstrate that this can be attributed to various factors, including the composition of the fungal cell wall, mainly composed of chitin. This wall serves as a barrier that physically and chemically blocks UV radiation, preventing it from penetrating the cell [6,42,47], enhancing the resistance of fungal spores against the harmful effects of UV radiation. Unlike bacteria, fungi have larger and more complex cells, allowing them to have a variety of organelles and cellular structures that counteract the negative effects of UV radiation and maintain cellular integrity [48]. Fungi can also repair DNA damage and produce their own antioxidants to help repair and neutralize damage caused by reactive oxygen species generated by UV radiation [22]. All these defense mechanisms contribute to the higher resistance of fungal spores to ultraviolet radiation.
Furthermore, fungi have developed various adaptations, including producing protective pigments and forming spores with a protective layer known as sporopollenin. This coating acts as an additional barrier, safeguarding genetic material and other cellular components and preventing direct damage caused by UV radiation [29].
3.3. D2 and Comparison of Resistance to Other Microorganisms
An analysis of the photoreactivation and UV resistance characteristics of each organism studied was performed, evaluating the dose of UV radiation required to reduce two logarithmic units of the initial concentration (D2). This was determined using the inactivation kinetics parameters obtained by fitting the log-linear + tailed model (Table 1). The results showed that A. niger exhibited higher resistance to treatment at both UV-LED wavelengths evaluated compared to Penicillium sp. This difference in resistance was reflected in the dose required, where Penicillium sp. required a significantly lower dose compared to A. niger (approximately at a ratio of 1:2.5).

Table 1.
Required UV doses to obtain a 2-log reduction (D2) for the two spores of fungal strains, considering different wavelengths and post-treatment conditions.
Regarding the post-treatments, our results show that samples incubated in the dark for 24 h exhibited a slight overall decrease in D2 values, which indicates that the absence of light limits photoreactivation. In contrast, samples that were not incubated demonstrated minimal differences. Notably, the most significant improvement was observed for A. niger at both 265 nm and 280 nm, where the D2 decreased by 15% compared to day 0. This reduction suggests that, under dark conditions, the inhibition of light-dependent DNA repair mechanisms (e.g., photolyase activity) prevents the recovery of sub-lethally damaged spores, thereby enhancing the efficacy of the UV treatment.
Conversely, a notable increase in D2 was detected in samples subjected to light following UV exposure, attributed to photoreactivation. The most significant increase in the required dose was observed in Penicillium sp. treated at 265 nm, where the required dose increased up to 54% compared to day 0. It was followed by an increase in the required dose for the same species at 280 nm. On the other hand, A. niger was less affected by photoreactivation, showing an increase in D2 between 11% and 27% at 280 nm and 265 nm, respectively. These results support the idea that the UV dose required to achieve and maintain a −2 log10 reduction varies according to the fungal strain and wavelength used. With the recent discussion, post-treatment conditions also play a role. This highlights the importance of considering not only the characteristics of the fungi and the type of UV radiation but also the environmental conditions and photoreactivation or recovery processes that may affect treatment efficacy.
3.4. Effects of Photoreactivation
The analysis of inactivation curves demonstrated that spores of fungal strains exhibited variable levels of resistance to UV radiation. Additionally, differences in post-treatment illumination conditions influenced the extent of microbial reduction achieved at different UV exposure levels (p < 0.001). The results indicated that UV-LED treatment in the range of 265 and 280 nm did not inhibit the ability of the spores to repair part of the damage caused, especially when they were exposed to light after treatment. In response to post-treatments, a significant but slight increase in treatment efficacy was observed when organisms were kept in darkness for 24 h (p < 0.001). In contrast, in all cases, a marked and statistically significant decrease in treatment efficacy was observed when organisms were exposed to light for 24 h (p < 0.001). These results highlight the importance of photorepair in the spores of fungal strains (Figure 2).
On the other hand, incubation for 24 h under dark conditions did not produce significant changes in k values compared to samples without incubation (Figure 2). However, incubation with light reduced the k value in all cases, indicating a decrease in the inactivation rate after exposure to light (up to 37% for the case of A. niger at 280 nm). For Penicillium sp., experimental results showed a reduction in k in equal proportion for both wavelengths (Figure 2). These results highlight the differences in the inactivation rate between the spores of fungal strains studied and highlight the effect of incubation with light in reducing the inactivation rate.
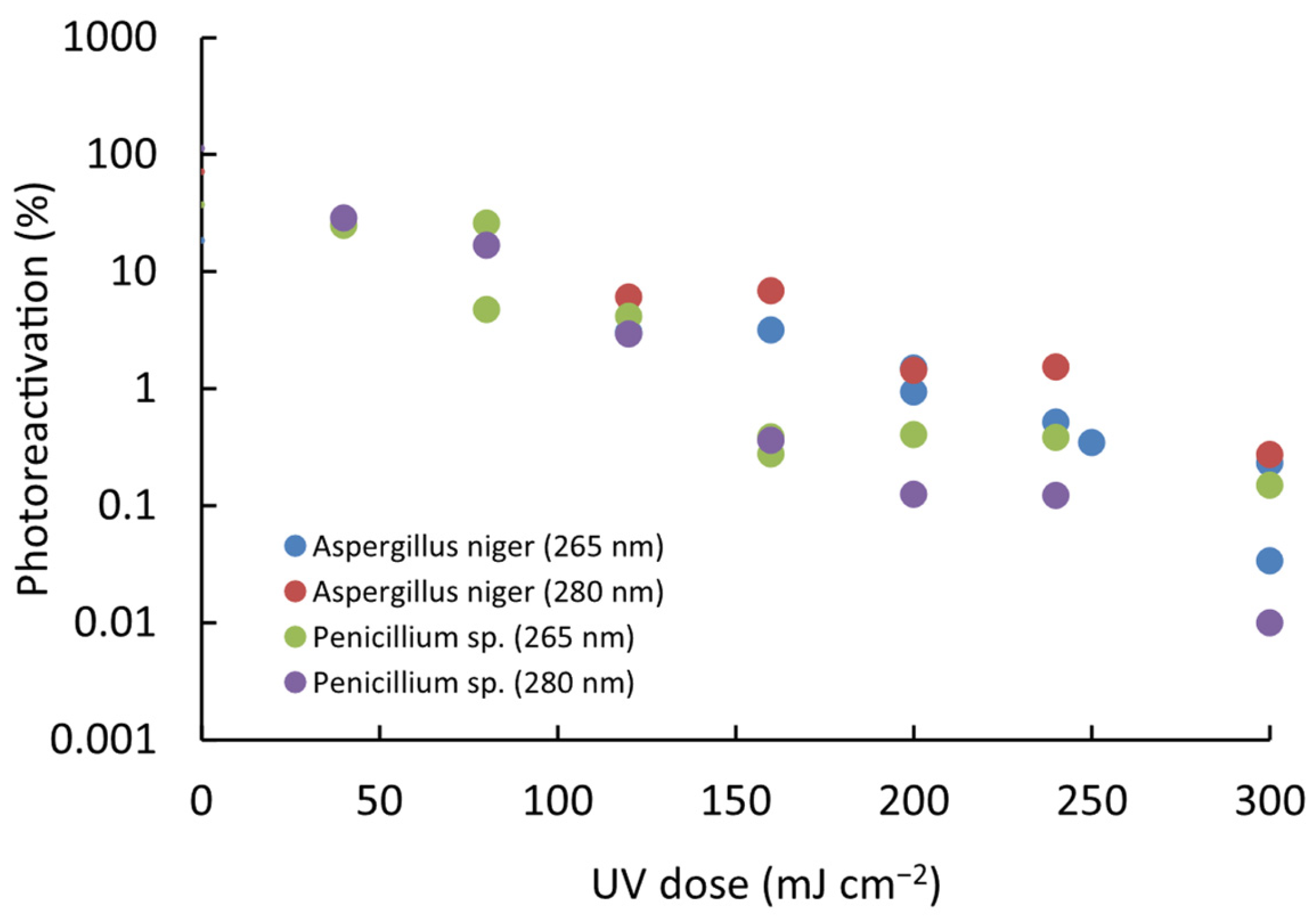
The photoreactivation percentage (PPR) was evaluated for both microorganisms and modeled as a function of the UV dose using a first-order kinetic equation (Log(PPR) = a + b − UV dose). The results indicate that photoreactivation follows a proportional pattern across all tested conditions. Regression analysis consistently revealed a significant linear relationship between Log(PPR) and the administered UV dose (p < 0.01). Furthermore, variations in photoreactivation levels were dependent on the intensity of UV exposure applied under these specific conditions (Figure 4).
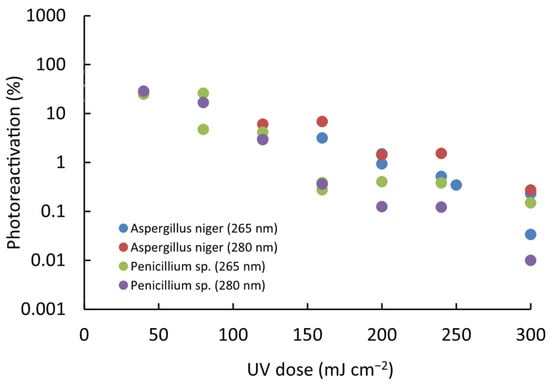
Figure 4.
Relationship between photoreactivation and UV LED radiation dose for the studied spores of fungal strains. The trend follows a first-order kinetic model (Log(PPR) = a + b − UV dose), demonstrating a proportional relationship across all tested conditions.
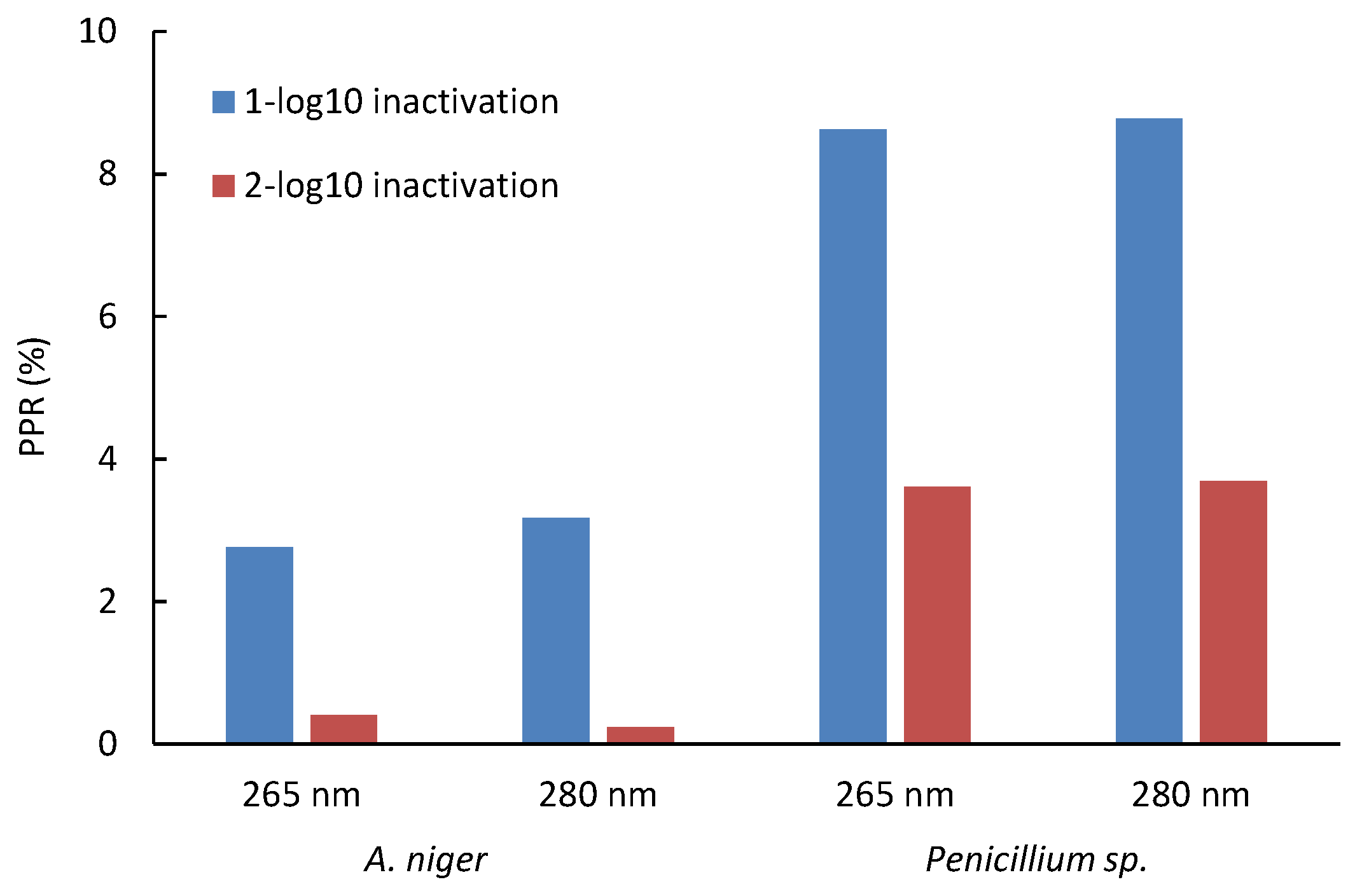
Specifically for A. niger, a PRR of up to 6.9% was found, while for Penicillium sp., a value of up to 28.6% was reached in all the trials. With the equations obtained in the correlation, the increase in the D1 and D2 doses due to the effect of photoreactivation was determined; it was found that for Penicillium sp., 8.6% and 8.8% of spores can be photoreactivated after 1-log reduction at 265 nm and 280 nm, respectively. In comparison, 3.6% and 3.7% photoreactivation percentages were found after 2-log reductions at 265 nm and 280 nm, respectively, for A. niger; these percentages are much lower for both wavelengths (Figure 5). Thus, A. niger is more challenging to inactivate but less likely to photoreactivate, whereas Penicillium sp. requires a lower UV dose for inactivation yet exhibits a higher percentage of photoreactivation.
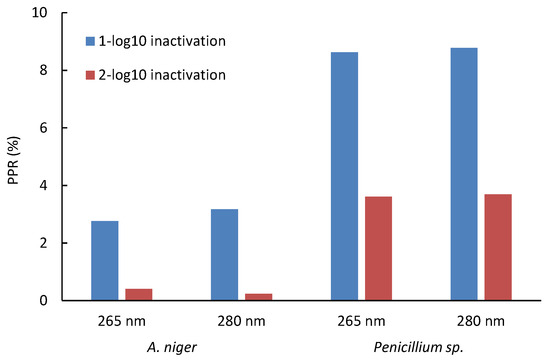
Figure 5.
Impact of UV-LED dose at 265 nm and 280 nm on photoreactivation of two spores of fungal strains. Initial concentration: (1–7) × 105 CFU mL−1.
The contrast observed between A. niger and Penicillium sp. spores was revealed as a highly relevant element in this study. This finding points to a critical point: by employing higher doses of UV radiation to inactivate these microorganisms, a marked reduction in the likelihood of photoreactivation is achieved.
3.5. Significance and Implications of Results
This study consistently demonstrates that UV-LED technologies can effectively inactivate fungal spores in water, even if the dose required varies depending on species, wavelength, and post-treatment conditions. On the one hand, the results confirm that A. niger and Penicillium sp. represent valuable models due to their high prevalence and documented resistance in drinking water samples, which justifies their selection; on the other hand, it is recognized that limiting the analysis to these two genera prevents full extrapolation of the findings to other fungi of interest, such as Cladosporium or Fusarium. Therefore, future research should cover a wider range of species to better understand variations in resistance and photoreactivation mechanisms, thus reinforcing the validity of the findings in different aquatic scenarios.
Furthermore, our results indicate that the efficacy of UV-LED inactivation depends not only on the dose applied but also on the storage conditions. On the one hand, it was observed that Penicillium sp. achieves a photoreactivation of up to 54% after 24 h under light, suggesting the need to increase the dose if the treated water is exposed to illumination; on the other hand, if storage is performed in the dark, the recovery of the fungi is limited, optimizing the effect of the radiation. This finding emphasizes the relevance of adapting post-treatment conditions according to the predominant fungal type and disinfection objectives. Finally, the importance of analyzing photoreactivation as a function of the species and wavelength used is underlined. A suitable UV efficacy indicator should show high resistance to ensure inactivation of more susceptible organisms and high photoreactivation power in case of exposure to light, which would allow the true robustness of the disinfection system to be assessed. This argues for choosing microbial models that reflect worst-case resistance and photoreactivation scenarios, ensuring that UV-LED disinfection protocols are robust and designed.
4. Conclusions
Resistance to UV-LED radiation varied between the investigated spores of fungal strains, and both the wavelength used and post-treatment actions significantly influenced the treatment efficacy. Concerning wavelength, it was observed that the inactivation efficacy of UV-LEDs emitted at 280 nm was considerably higher than those emitted at 265 nm for both strains studied, although with very low differences.
Penicillium sp. proved to be more susceptible to the treatment compared to A. niger, presenting, in addition, the highest photoreactivation value. The required dose increased up to 54% under subsequent photoreactivation conditions for 24 h. Therefore, both the wavelength used and the post-treatment actions significantly influenced the efficacy of the treatment for Penicillium sp. In the case of A. niger, the wavelength did not have a significant impact, but the post-treatment did.
The D2 value, representing the UV dose required to achieve 99% inactivation, while accounting for photoreactivation at 265 nm and 280 nm, was determined to be 323.7 ± 90.0 mJ cm−2 and 321.9 ± 43.8 mJ cm−2 for A. niger, respectively. In contrast, for Penicillium sp., the required doses were 167.7 ± 13 mJ cm−2 and 146.5 ± 29.2 mJ cm−2, respectively. These findings highlight the necessity of incorporating species-specific traits and post-treatment conditions into UV disinfection strategies, particularly when addressing less-studied microorganisms like fungal spores in drinking water treatment.
Author Contributions
Conceptualization, P.D.-S., V.P.-V. and L.R.-M.; Development of methodology, N.D.-A. and P.D.-S.; Experimental investigation, P.D.-S. and N.D.-A.; Resource acquisition, P.D.-S.; Manuscript drafting, P.D.-S.; Review and revision of the text, V.P.-V., L.R.-M. and D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Salesian Polytechnic University research funds under project number 010-005-2021-07-01.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We express our gratitude to Rodrigo Caroca for kindly providing the fungal strains utilized in this study. Additionally, we appreciate the support of the Life Sciences Laboratory at UPS for granting access to their facilities and for their invaluable assistance during the research process.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Al-Gabr, H.M.; Zheng, T.; Yu, X. Fungi Contamination of Drinking Water. Rev. Environ. Contam. Toxicol. 2014, 228, 121–139. [Google Scholar] [CrossRef] [PubMed]
- Doggett, M.S. Characterization of Fungal Biofilms within a Municipal Water Distribution System. Appl. Environ. Microbiol. 2000, 66, 1249–1251. [Google Scholar] [CrossRef] [PubMed]
- Afonso, T.B.; Simões, L.C.; Lima, N. Occurrence of Filamentous Fungi in Drinking Water: Their Role on Fungal-Bacterial Biofilm Formation. Res. Microbiol. 2021, 172, 103791. [Google Scholar] [CrossRef]
- Babič, M.N.; Gunde-Cimerman, N.; Vargha, M.; Tischner, Z.; Magyar, D.; Veríssimo, C.; Sabino, R.; Viegas, C.; Meyer, W.; Brandão, J. Fungal Contaminants in Drinking Water Regulation? A Tale of Ecology, Exposure, Purification and Clinical Relevance. Int. J. Environ. Res. Public Health 2017, 14, 636. [Google Scholar] [CrossRef]
- Zhao, H.X.; Zhang, T.Y.; Wang, H.; Hu, C.Y.; Tang, Y.L.; Xu, B. Occurrence of Fungal Spores in Drinking Water: A Review of Pathogenicity, Odor, Chlorine Resistance and Control Strategies. Sci. Total Environ. 2022, 853, 158626. [Google Scholar] [CrossRef]
- Nakpan, W.; Yermakov, M.; Indugula, R.; Reponen, T.; Grinshpun, S.A. Inactivation of Bacterial and Fungal Spores by UV Irradiation and Gaseous Iodine Treatment Applied to Air Handling Filters. Sci. Total Environ. 2019, 671, 59–65. [Google Scholar] [CrossRef]
- Pereira, V.J.; Marques, R.; Marques, M.; Benoliel, M.J.; Barreto Crespo, M.T. Free Chlorine Inactivation of Fungi in Drinking Water Sources. Water Res. 2013, 47, 517–523. [Google Scholar] [CrossRef]
- Wu, G.; Zhao, H.; Wan, Q.; Xu, X.; Cao, R.; Li, K.; Wang, J.; Huang, T.; Lu, J.; Wen, G. Inactivation and Subsequent Reactivation of Aspergillus Species by the Combination of UV and Monochloramine: Comparisons with UV/Chlorine. J. Environ. Sci. 2022, 117, 105–118. [Google Scholar] [CrossRef]
- Oliveira, B.R.; Barreto Crespo, M.T.; San Romão, M.V.; Benoliel, M.J.; Samson, R.A.; Pereira, V.J. New Insights Concerning the Occurrence of Fungi in Water Sources and Their Potential Pathogenicity. Water Res. 2013, 47, 6338–6347. [Google Scholar] [CrossRef]
- Wan, Q.; Wen, G.; Cui, Y.; Cao, R.; Xu, X.; Wu, G.; Wang, J.; Huang, T. Occurrence and Control of Fungi in Water: New Challenges in Biological Risk and Safety Assurance. Sci. Total Environ. 2023, 860, 160536. [Google Scholar] [CrossRef]
- Pereira, V.J.; Basílio, M.C.; Fernandes, D.; Domingues, M.; Paiva, J.M.; Benoliel, M.J.; Crespo, M.T.; San Romão, M.V. Occurrence of Filamentous Fungi and Yeasts in Three Different Drinking Water Sources. Water Res. 2009, 43, 3813–3819. [Google Scholar] [CrossRef] [PubMed]
- Hijnen, W.A.M.; Beerendonk, E.F.; Medema, G.J. Inactivation Credit of UV Radiation for Viruses, Bacteria and Protozoan (Oo)Cysts in Water: A Review. Water Res. 2006, 40, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, W.; Kowalski, W. Pulsed UV Systems. In Ultraviolet Germicidal Irradiation Handbook; Springer: Berlin/Heidelberg, Germany, 2009; pp. 383–398. [Google Scholar]
- Lawryshyn, Y.A.; Cairns, B. UV Disinfection of Water: The Need for UV Reactor Validation. Water Supply 2003, 3, 293–300. [Google Scholar] [CrossRef]
- Nakahashi, M.; Mawatari, K.; Hirata, A.; Maetani, M.; Shimohata, T.; Uebanso, T.; Hamada, Y.; Akutagawa, M.; Kinouchi, Y.; Takahashi, A. Simultaneous Irradiation with Different Wavelengths of Ultraviolet Light Has Synergistic Bactericidal Effect on Vibrio Parahaemolyticus. Photochem. Photobiol. 2014, 90, 1397–1403. [Google Scholar] [CrossRef]
- Rivas-Zaballos, I.; Romero-Martínez, L.; Moreno-Garrido, I.; Moreno-Andrés, J.; Acevedo-Merino, A.; Nebot, E. UV-LEDs Combined with Persulfate Salts as a Method to Inactivate Microalgae in Ballast Water. J. Water Process Eng. 2023, 51, 103361. [Google Scholar] [CrossRef]
- Umar, M.; Roddick, F.; Fan, L. Moving from the Traditional Paradigm of Pathogen Inactivation to Controlling Antibiotic Resistance in Water—Role of Ultraviolet Irradiation. Sci. Total Environ. 2019, 662, 923–939. [Google Scholar]
- Vilhunen, S.; Särkkä, H.; Sillanpää, M. Ultraviolet Light-Emitting Diodes in Water Disinfection. Environ. Sci. Pollut. Res. 2009, 16, 439–442. [Google Scholar] [CrossRef]
- Wan, Q.; Wen, G.; Cao, R.; Xu, X.; Zhao, H.; Li, K.; Wang, J.; Huang, T. Comparison of UV-LEDs and LPUV on Inactivation and Subsequent Reactivation of Waterborne Fungal Spores. Water Res. 2020, 173, 115553. [Google Scholar] [CrossRef]
- Duque-Sarango, P.; Romero-Martínez, L.; Pinos-Vélez, V.; Sánchez-Cordero, E.; Samaniego, E. Comparative Study of UV Radiation Resistance and Reactivation Characteristics of E. Coli ATCC 8739 and Native Strains: Implications for Water Disinfection. Sustainability 2023, 15, 9559. [Google Scholar] [CrossRef]
- Harris, T.R.; Pagan, J.G.; Batoni, P. Optical and Fluidic Co-Design of a UV-LED Water Disinfection Chamber. ECS Trans. 2013, 45, 11–18. [Google Scholar] [CrossRef]
- Braga, G.U.L.; Rangel, D.E.N.; Fernandes, É.K.K.; Flint, S.D.; Roberts, D.W. Molecular and Physiological Effects of Environmental UV Radiation on Fungal Conidia. Curr. Genet. 2015, 61, 405–425. [Google Scholar] [CrossRef]
- Guo, M.; Huang, J.; Hu, H.; Liu, W. Growth and Repair Potential of Three Species of Bacteria in Reclaimed Wastewater after UV Disinfection. Biomed. Environ. Sci. 2011, 24, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.; Deng, X.; Wan, Q.; Xu, X.; Huang, T. Photoreactivation of Fungal Spores in Water Following UV Disinfection and Their Control Using UV-Based Advanced Oxidation Processes. Water Res. 2019, 148, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lindenauer, K.G.; Darby, J.L. Ultraviolet Disinfection of Wastewater: Effect of Dose on Subsequent Photoreactivation. Water Res. 1994, 28, 805–817. [Google Scholar] [CrossRef]
- Jing, Z.; Lu, Z.; Santoro, D.; Zhao, Z.; Huang, Y.; Ke, Y.; Wang, X.; Sun, W. Which UV Wavelength Is the Most Effective for Chlorine-Resistant Bacteria in Terms of the Impact of Activity, Cell Membrane and DNA? Chem. Eng. J. 2022, 447, 137584. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, L.; Chen, J.H.; Mao, W.; Li, W.J.; Hu, W.; Wang, S.Y.; Wang, C.M. Role of Ozone in UV-C Disinfection, Demonstrated by Comparison between Wild-Type and Mutant Conidia of Aspergillus niger. Photochem. Photobiol. 2014, 90, 615–621. [Google Scholar] [CrossRef]
- Percival, S.L.; Walker, J.T. Potable Water and Biofilms: A Review of the Public Health Implications. Biofouling 1999, 14, 99–115. [Google Scholar] [CrossRef]
- Brooks, J.; Shaw, G. Sporopollenin: A Review of Its Chemistry, Palaeochemistry and Geochemistry. Grana 2009, 17, 91–97. [Google Scholar] [CrossRef]
- Hoben, H.J.; Somasegaran, P. Comparison of the Pour, Spread, and Drop Plate Methods for Enumeration of Rhizobium spp. in Inoculants Made from Presterilized Peat. Appl. Environ. Microbiol. 1982, 44, 1246–1247. [Google Scholar] [CrossRef]
- Bolton, J.R.; Linden, K.G. Standardization of Methods for Fluence (UV Dose) Determination in Bench-Scale UV Experiments. J. Environ. Eng. 2003, 129, 209–215. [Google Scholar] [CrossRef]
- Björn, L.O. The Measurement of Light. 2015. Available online: https://www.researchgate.net/publication/285657134_The_Measurement_of_Light (accessed on 17 March 2025).
- Franklin, J. Plant Growth Chamber Handbook. (Iowa Agriculture and Home Economics Experiment Station Special Report No. 99 (SR-99) and North Central Regional Research Publication No. 340.). Ed. by R. W. LANGHANS and T. W. TIBBITS. 21×27·5 Cm. Pp. Viii+240 with 20 Tables and 45 Text-Figures. Ames, IA, USA: Iowa State University, 1997. Price p/b: $15.00, ISBN 0361 199X. New Phytol. 1998, 138, 743–750. [Google Scholar] [CrossRef]
- Geeraerd, A.H.; Valdramidis, V.P.; Van Impe, J.F. GInaFiT, a Freeware Tool to Assess Non-Log-Linear Microbial Survivor Curves. Int. J. Food Microbiol. 2005, 102, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Geeraerd, A.H.; Herremans, C.H.; Van Impe, J.F. Structural Model Requirements to Describe Microbial Inactivation during a Mild Heat Treatment. Int. J. Food Microbiol. 2000, 59, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.; Xu, X.; Zhu, H.; Huang, T.; Ma, J. Inactivation of Four Genera of Dominant Fungal Spores in Groundwater Using UV and UV/PMS: Efficiency and Mechanisms. Chem. Eng. J. 2017, 328, 619–628. [Google Scholar] [CrossRef]
- Mattle, M.J.; Kohn, T. Inactivation and Tailing during UV254 Disinfection of Viruses: Contributions of Viral Aggregation, Light Shielding within Viral Aggregates, and Recombination. Environ. Sci. Technol. 2012, 46, 10022–10030. [Google Scholar] [CrossRef]
- Azimi, Y.; Liu, Y.; Tan, T.C.; Allen, D.G.; Farnood, R.R. The Tail of Two Models: Impact of Circularity and Biomass Non-Homogeneity on UV Disinfection of Wastewater Flocs. Water Res. 2017, 126, 70–78. [Google Scholar] [CrossRef]
- Carré, E.; Pérot, J.; Jauzein, V.; Lopez-Ferber, M. Impact of Suspended Particles on UV Disinfection of Activated-Sludge Effluent with the Aim of Reclamation. J. Water Process Eng. 2018, 22, 87–93. [Google Scholar] [CrossRef]
- Romero-Martínez, L.; Duque-Sarango, P.; González-Martín, C.; Moreno-Andrés, J.; Acevedo-Merino, A.; Nebot, E. Inactivation Efficacy and Reactivation of Fecal Bacteria with a Flow-through LED Ultraviolet Reactor: Intraspecific Response Prevails over Interspecific Differences. J. Water Process Eng. 2023, 52, 103497. [Google Scholar] [CrossRef]
- Duque-Sarango, P.; Delgado-Armijos, N.; Romero-Martínez, L.; Pinos-Vélez, V. Assessing the Potential of Ultraviolet Irradiation for Inactivating Waterborne Fungal Spores: Kinetics and Photoreactivation Studies. Front. Environ. Sci. 2023, 11, 1212807. [Google Scholar] [CrossRef]
- Walker, G.M.; White, N.A. Introduction to Fungal Physiology. In Fungi; Wiley: Hoboken, NJ, USA, 2017; pp. 1–35. [Google Scholar] [CrossRef]
- Clauß, M. Higher Effectiveness of Photoinactivation of Bacterial Spores, UV Resistant Vegetative Bacteria and Mold Spores with 222 Nm Compared to 254 Nm Wavelength. Acta Hydrochim. Hydrobiol. 2006, 34, 525–532. [Google Scholar] [CrossRef]
- Betzalel, Y.; Gerchman, Y.; Cohen-Yaniv, V.; Mamane, H. Multiwell Plates for Obtaining a Rapid Microbial Dose-Response Curve in UV-LED Systems. J. Photochem. Photobiol. B 2020, 207, 111865. [Google Scholar] [CrossRef]
- Duque-Sarango, P.; Pinos, V. Modeling of the Guangarcucho Municipal Wastewater Treatment Plant Using WEST, Cuenca-Ecuador. In Doctoral Symposium on Information and Communication Technologies—DSICT; Berrezueta, S., Abad, K., Eds.; Lecture Notes in Electrical Engineering; Springer: Cham, Switzerland, 2022; Volume 846, pp. 33–46. [Google Scholar] [CrossRef]
- Romero-Martínez, L.; Duque-Sarango, P.; Acevedo-Merino, A.; Nebot, E. Comparing the Inactivating Efficacy of Enteric Bacteria in Seawater Treated with Different Configurations of Continuous Flow-through Ultraviolet Devices: Single-Pass and Recirculation. J. Chem. Technol. Biotechnol. 2019, 94, jctb.6108. [Google Scholar] [CrossRef]
- Li, X.; Cai, M.; Wang, L.; Niu, F.; Yang, D.; Zhang, G. Evaluation Survey of Microbial Disinfection Methods in UV-LED Water Treatment Systems. Sci. Total Environ. 2019, 659, 1415–1427. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.L.; Oliveira, V.; Baptista, I.; Henriques, I.; Gomes, N.C.M.; Almeida, A.; Correia, A.; Cunha, Â. Wavelength Dependence of Biological Damage Induced by UV Radiation on Bacteria. Arch. Microbiol. 2013, 195, 63–74. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).