Mechanism and Risk Control of Chlorine-Resistant Bacteria in Drinking Water Supply Systems: A Comprehensive Bibliometric Analysis
Abstract
1. Introduction
2. Research Data and Methods
2.1. Data Collection and Search Strategy
2.2. Bibliometric Analysis Methods
3. Results
3.1. Bibliometric Analysis
3.1.1. The Number of Published Articles
3.1.2. The Major Countries and Institutions
3.1.3. Literature Co-Citation Analysis
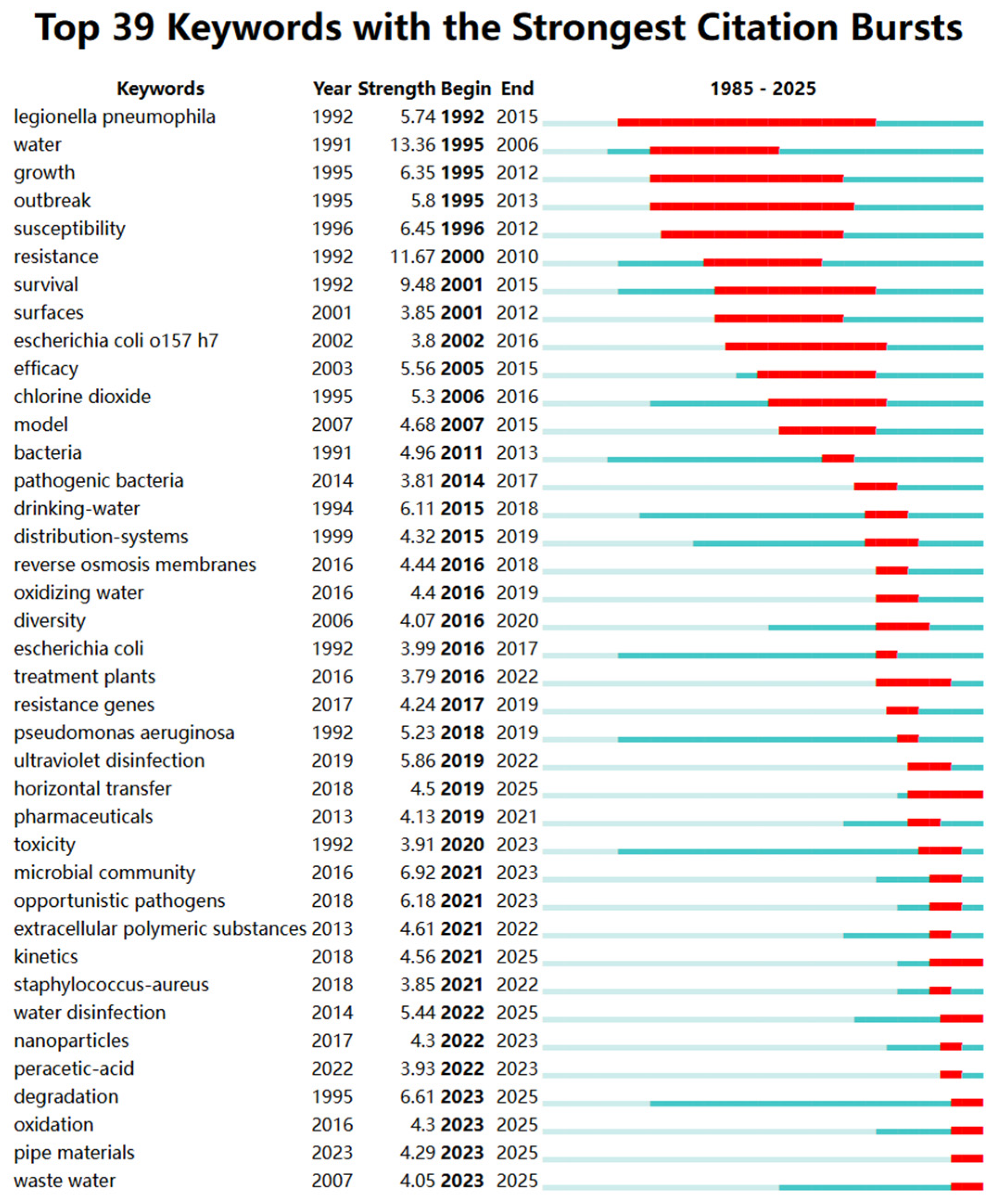
3.1.4. Keywords Co-Occurrence Analysis
3.2. Typical Species of CRB in Drinking Water Supply Systems
3.3. Risks of CRB in Drinking Water
3.3.1. Influence on Public Health
3.3.2. Influence on Water Supply Infrastructure
3.3.3. Influence on Aquatic Environment
3.4. Resistance Mechanism of CRB
3.5. Control Methods of CRB
3.5.1. Physical Method
3.5.2. Chemical Method
3.5.3. Biological Method
3.5.4. Combined Method
4. Future Research Directions
4.1. Overlooked Source of T&O in Drinking Water Caused by CRB
4.2. Internal Intrinsic Chlorine Resistance Determined by Genetic Materials
4.3. Novel and Effective Control Method for CRB Risks
4.4. Monitoring and Operational Management of CRB in Water Networks
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luo, L.W.; Wu, Y.H.; Yu, T.; Wang, Y.H.; Chen, G.Q.; Tong, X.; Bai, Y.; Xu, C.; Wang, H.B.; Ikuno, N.; et al. Evaluating method and potential risks of chlorine-resistant bacteria (CRB): A review. Water Res. 2021, 188, 12. [Google Scholar] [CrossRef]
- Shekhawat, S.S.; Kulshreshtha, N.M.; Gupta, A.B. Investigation of chlorine tolerance profile of dominant gram negative bacteria recovered from secondary treated wastewater in Jaipur, India. J. Environ. Manag. 2020, 255, 109827. [Google Scholar] [CrossRef]
- Khan, S.; Beattie, T.K.; Knapp, C.W. Relationship between antibiotic- and disinfectant-resistance profiles in bacteria harvested from tap water. Chemosphere 2016, 152, 132–141. [Google Scholar] [CrossRef]
- Pang, Y.C.; Xi, J.Y.; Xu, Y.; Huo, Z.Y.; Hu, H.Y. Shifts of live bacterial community in secondary effluent by chlorine disinfection revealed by Miseq high-throughput sequencing combined with propidium monoazide treatment. Appl. Microbiol. Biotechnol. 2016, 100, 6435–6446. [Google Scholar] [CrossRef]
- Zeng, F.Z.; Cao, S.; Jin, W.B.; Zhou, X.; Ding, W.Q.; Tu, R.J.; Han, S.F.; Wang, C.P.; Jiang, Q.J.; Huang, H.; et al. Inactivation of chlorine-resistant bacterial spores in drinking water using UV irradiation, UV/Hydrogen peroxide and UV/Peroxymonosulfate: Efficiency and mechanism. J. Clean. Prod. 2020, 243, 9. [Google Scholar] [CrossRef]
- Farkas-Himsley, H. Killing of chlorine-resistant bacteria by chlorine-bromine solutions. Appl. Microbiol. 1964, 12, 1–6. [Google Scholar] [CrossRef]
- Al-Sulami, A.A.; Al-Taee, A.M.R.; Wida’a, Q.H. Isolation and identification of Mycobacterium avium complex and other nontuberculosis mycobacteria from drinking-water in Basra governorate, Iraq. East. Mediterr. Health J. 2012, 18, 274–278. [Google Scholar] [CrossRef]
- Wang, J.Y.; Sui, M.H.; Yuan, B.J.; Li, H.W.; Lu, H.T. Inactivation of two Mycobacteria by free chlorine: Effectiveness, influencing factors, and mechanisms. Sci. Total Environ. 2019, 648, 271–284. [Google Scholar] [CrossRef]
- Wang, M.Y.; Zhang, Y.; Niu, Z.G.; Miao, Q.K.; Fu, W. Study on the distribution characteristics and metabolic mechanism of chlorine-resistant bacteria in indoor water supply networks. Environ. Pollut. 2023, 328, 9. [Google Scholar] [CrossRef]
- Wang, H.B.; Hu, C.; Shi, B.Y. The control of red water occurrence and opportunistic pathogens risks in drinking water distribution systems: A review. J. Environ. Sci. 2021, 110, 92–98. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, K.; Zhang, T.; Li, C.; Mao, X. An ignored and potential source of taste and odor (T&O) issues-biofilms in drinking water distribution system (DWDS). Appl. Microbiol. Biotechnol. 2017, 101, 3537–3550. [Google Scholar] [CrossRef]
- Zhu, J.; Stuetz, R.M.; Hamilton, L.; Power, K.; Crosbie, N.D.; Tamburic, B. Management of biogenic taste and odour: From source water, through treatment processes and distribution systems, to consumers. J. Environ. Manag. 2022, 323, 17. [Google Scholar] [CrossRef]
- Lanciotti, E.; Santini, C.; Lupi, E.; Burrini, D. Actinomycetes, cyanobacteria and algae causing tastes and odours in water of the River Arno used for the water Supply of Florence. J. Water Supply Res. Technol. AQUA 2003, 52, 489–500. [Google Scholar] [CrossRef]
- Bai, X.Z.; Qu, Z.P.; Li, B.; Li, H.P.; Zhang, T.; Yang, Z.G. Distribution of typical taste and odor compounds and possible formation of 2,4,6-trichloroanisole in drinking water treatment plants. Water Air Soil Pollut. 2017, 228, 10. [Google Scholar] [CrossRef]
- Jia, S.; Shi, P.; Hu, Q.; Li, B.; Zhang, T.; Zhang, X.-X. Bacterial community shift drives antibiotic resistance promotion during drinking water chlorination. Environ. Sci. Technol. 2015, 49, 12271–12279. [Google Scholar] [CrossRef]
- Cai, G.Q.; Liu, T.Z.; Zhang, J.S.; Song, H.R.; Jiang, Q.J.; Zhou, C. Control for chlorine resistant spore forming bacteria by the coupling of pre-oxidation and coagulation sedimentation, and UV-AOPs enhanced inactivation in drinking water treatment. Water Res. 2022, 219, 10. [Google Scholar] [CrossRef]
- Li, S.G.; Zheng, S.X.; Zheng, Z.X.; Ling, Y.; Wu, H.M.; Liu, H. Enhancement of chlorine-resistant bacteria inactivation via coupling Magnéli phase Ti4O7-based electrochemical oxidation and chlorination. Chem. Eng. J. 2024, 495, 11. [Google Scholar] [CrossRef]
- Cho, K.; Jeong, D.; Lee, S.; Bae, H. Chlorination caused a shift in marine biofilm niches on microfiltration/ultrafiltration and reverse osmosis membranes and UV irradiation effectively inactivated a chlorine-resistant bacterium. Appl. Microbiol. Biotechnol. 2018, 102, 7183–7194. [Google Scholar] [CrossRef]
- Jing, Z.B.; Lu, Z.D.; Santoro, D.; Zhao, Z.A.; Huang, Y.; Wang, X.H.; Sun, W.J. Which UV wavelength is the most effective for chlorine-resistant bacteria in terms of the impact of activity, cell membrane and DNA? Chem. Eng. J. 2022, 447, 11. [Google Scholar] [CrossRef]
- Ding, W.Q.; Jin, W.B.; Cao, S.; Zhou, X.; Wang, C.P.; Jiang, Q.J.; Huang, H.; Tu, R.J.; Han, S.F.; Wang, Q.L. Ozone disinfection of chlorine-resistant bacteria in drinking water. Water Res. 2019, 160, 339–349. [Google Scholar] [CrossRef]
- He, Z.Q.; Fan, X.M.; Jin, W.B.; Gao, S.H.; Yan, B.W.; Chen, C.; Ding, W.Q.; Yin, S.Y.; Zhou, X.; Liu, H.; et al. Chlorine-resistant bacteria in drinking water: Generation, identification and inactivation using ozone-based technologies. J. Water Process Eng. 2023, 53, 103772. [Google Scholar] [CrossRef]
- Jia, S.Y.; Jia, R.B.; Zhang, K.F.; Sun, S.H.; Lu, N.N.; Wang, M.Q.; Zhao, Q.H. Disinfection characteristics of Pseudomonas peli, a chlorine-resistant bacterium isolated from a water supply network. Environ. Res. 2020, 185, 8. [Google Scholar] [CrossRef]
- Lu, N.N.; Sun, S.H.; Chu, F.M.; Wang, M.Q.; Zhao, Q.H.; Shi, J.M.; Jia, R.B. Identification and inactivation of Gordonia, a new chlorine-resistant bacterium isolated from a drinking water distribution system. J. Water Health 2020, 18, 995–1008. [Google Scholar] [CrossRef]
- Feng, C.M.; Li, J.; Yang, W.Q.; Chen, Z.X. Study on the inactivation effect and mechanism of EGCG disinfectant on Bacillus subtilis. Environ. Pollut. 2024, 356, 12. [Google Scholar] [CrossRef]
- Bhojani, G.; Kumar, S.B.; Saha, N.K.; Haldar, S. Membrane biofouling by chlorine resistant Bacillus spp.: Effect of feedwater chlorination on bacteria and membrane biofouling. Biofouling 2018, 34, 426–439. [Google Scholar] [CrossRef]
- Wang, J.T.; Li, S.L.; Guan, Y.X.; Zhu, C.; Gong, G.H.; Hu, Y.X. Novel RO membranes fabricated by grafting sulfonamide group: Improving water permeability, fouling resistance and chlorine resistant performance. J. Membr. Sci. 2022, 641, 10. [Google Scholar] [CrossRef]
- Sun, H.X.; Chen, Y.H.; Liu, J.H.; Chai, D.D.; Li, P.; Wang, M.; Hou, Y.F.; Niu, Q.J. A novel chlorine-resistant polyacrylate nanofiltration membrane constructed from oligomeric phenolic resin. Sep. Purif. Technol. 2021, 262, 10. [Google Scholar] [CrossRef]
- Wang, C.B.; Wang, H.C.; Li, Y.S.; Feng, Y.Y.; Zhang, K.; Fan, S.J.; Cao, L. Preparation of chlorine-resistant and regenerable antifouling nanofiltration membrane through interfacial polymerization using beta cyclodextrin monomers. Chemosphere 2023, 313, 10. [Google Scholar] [CrossRef]
- Jathar, S.; Dakhni, S.; Shinde, D.; Fernandes, A.; Jha, P.; Desai, N.; Sonawane, T.; Jobby, R. Differential expression of antioxidant enzymes in chlorine-resistant Acinetobacter and Serratia spp. isolated from water distribution sites in Mumbai: A study on mechanisms of chlorine resistance for sustainable water treatment strategies. Sustainability 2023, 15, 8287. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Wu, Y.H.; Luo, L.W.; Huang, B.H.; Chen, Z.; Wang, H.B.; Liu, H.; Ikuno, N.; Koji, N.; Hu, H.Y. Inactivation of chlorine-resistant bacteria (CRB) via various disinfection methods: Resistance mechanism and relation with carbon source metabolism. Water Res. 2023, 244, 9. [Google Scholar] [CrossRef]
- Rajeev, M.; Sushmitha, T.J.; Prasath, K.G.; Toleti, S.R.; Pandian, S.K. Systematic assessment of chlorine tolerance mechanism in a potent biofilm-forming marine bacterium Halomonas boliviensis. Int. Biodeterior. Biodegrad. 2020, 151, 104967. [Google Scholar] [CrossRef]
- Miao, X.C.; Han, X.; Liu, C.X.; Bai, X.H. Intrinsic chlorine resistance of bacteria modulated by glutaminyl-tRNA biosynthesis in drinking water supply systems. Chemosphere 2022, 308, 8. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Pehlivanoglu-Mantas, E.; Hawley, E.L.; Deeb, R.A.; Sedlak, D.L. Formation of nitrosodimethylamine (NDMA) during chlorine disinfection of wastewater effluents prior to use in irrigation systems. Water Res. 2006, 40, 341–347. [Google Scholar] [CrossRef]
- Elsherif, S.M.; Taha, A.F.; Abokifa, A.A. Disinfectant control in drinking water networks: Integrating advection-dispersion-reaction models and byproduct constraints. Water Res. 2024, 267, 122441. [Google Scholar] [CrossRef]
- Clarivate. Available online: https://webofscience.help.clarivate.com/Content/wos-core-collection/wos-core-collection.htm#:~:text=Web%20of%20Science%20Core%20Collection%20is%20the%20world%27s,worldwide%E2%80%94including%20open%20access%20journals%E2%80%94%20conference%20proceedings%20and%20books (accessed on 16 March 2025).
- Wang, C.; Che, Y.; Xia, M.; Lin, C.; Chen, Y.; Li, X.; Chen, H.; Luo, J.; Fan, G. The Evolution and Future Directions of Green Buildings Research: A Scientometric Analysis. Buildings 2024, 14, 345. [Google Scholar] [CrossRef]
- Liu, W. The data source of this study is Web of Science Core Collection? Not enough. Scientometrics 2019, 121, 1815–1824. [Google Scholar] [CrossRef]
- Gholipour, S.; Nikaeen, M.; Mehdipour, M.; Mohammadi, F.; Rabbani, D. Occurrence of chlorine-resistant Pseudomonas aeruginosa in hospital water systems: Threat of waterborne infections for patients. Antimicrob. Resist. Infect. Control 2024, 13, 10. [Google Scholar] [CrossRef]
- Roy, P.K.; Ghosh, M. Chlorine resistant bacteria isolated from drinking water treatment plants in West Bengal. Desalination Water Treat. 2017, 79, 103–107. [Google Scholar] [CrossRef]
- Shrivastava, R.; Upreti, R.K.; Jain, S.R.; Prasad, K.N.; Seth, P.K.; Chaturvedi, U.C. Suboptimal chlorine treatment of drinking water leads to selection of multidrug-resistant Pseudomonas aeruginosa. Ecotoxicol. Environ. Saf. 2004, 58, 277–283. [Google Scholar] [CrossRef]
- Khan, A.; Joshi, H.M. Combating chlorine-resistant marine Klebsiella pneumoniae biofilms with chlorine-tolerant bacteriophages. Chemosphere 2024, 368, 143782. [Google Scholar] [CrossRef]
- Jathar, S.; Shinde, D.; Dakhni, S.; Fernandes, A.; Jha, P.; Desai, N.; Jobby, R. Identification and characterization of chlorine-resistant bacteria from water distribution sites of Mumbai. Arch. Microbiol. 2021, 203, 5241–5248. [Google Scholar] [CrossRef]
- Sun, W.; Liu, W.; Cui, L.; Zhang, M.; Wang, B. Characterization and identification of a chlorine-resistant bacterium, Sphingomonas TS001, from a model drinking water distribution system. Sci. Total Environ. 2013, 458, 169–175. [Google Scholar] [CrossRef]
- Le Dantec, C.; Duguet, J.P.; Montiel, A.; Dumoutier, N.; Dubrou, S.; Vincent, V. Chlorine disinfection of a typical mycobacteria isolated from a water distribution system. Appl. Environ. Microbiol. 2002, 68, 1025–1032. [Google Scholar] [CrossRef]
- Furuhata, K.; Ishizaki, N.; Umekawa, N.; Nishizima, M.; Fukuyama, M. Pulsed-field gel electrophoresis (PFGE) pattern analysis and chlorine-resistance of Legionella pneumophila Isolated from hot spring water samples. Biocontrol Sci. 2014, 19, 33–38. [Google Scholar] [CrossRef]
- Garcia, M.T.; Pelaz, C. Effectiveness of disinfectants used in cooling towers against Legionella pneumophila. Chemotherapy 2008, 54, 107–116. [Google Scholar] [CrossRef]
- Wu, X.F.; Nan, J.; Shen, J.M.; Kang, J.; Li, D.P.; Yan, P.W.; Wang, W.Q.; Wang, B.Y.; Zhao, S.X.; Chen, Z.L. Regrowth potential of chlorine-resistant bacteria in drinking water under chloramination. J. Hazard. Mater. 2022, 428, 11. [Google Scholar] [CrossRef]
- Du, B.; Wang, S.D.; Chen, G.W.; Wang, G.; Liu, L. Nutrient starvation intensifies chlorine disinfection-stressed biofilm formation. Chemosphere 2022, 295, 11. [Google Scholar] [CrossRef]
- Shan, L.L.; Xu, S.Y.; Pei, Y.Y.; Zhu, Z.B.; Xu, L.Y.; Liu, X.H.; Yuan, Y.X. Effect of domestic pipe materials on microbiological safety of drinking water: Different biofilm formation and chlorination resistance for diverse pipe materials. Process Biochem. 2023, 129, 11–21. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Tian, Y.M.; Kang, M.X.; Chen, C.; Song, Y.R.; Li, H. Effects of chlorination/chlorine dioxide disinfection on biofilm bacterial community and corrosion process in a reclaimed water distribution system. Chemosphere 2019, 215, 62–73. [Google Scholar] [CrossRef]
- Zhong, D.; Zhou, Z.Y.; Ma, W.C.; Ma, J.; Feng, W.A.; Li, J.X.; Du, X. Antibiotic enhances the spread of antibiotic resistance among chlorine-resistant bacteria in drinking water distribution system. Environ. Res. 2022, 211, 9. [Google Scholar] [CrossRef]
- Ma, L.P.; Yang, H.Y.; Guan, L.; Liu, X.Y.; Zhang, T. Risks of antibiotic resistance genes and antimicrobial resistance under chlorination disinfection with public health concerns. Environ. Int. 2022, 158, 106978. [Google Scholar] [CrossRef]
- Shi, P.; Jia, S.Y.; Zhang, X.X.; Zhang, T.; Cheng, S.P.; Li, A.M. Metagenomic insights into chlorination effects on microbial antibiotic resistance in drinking water. Water Res. 2013, 47, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Liu, L.; Wang, D.N.; Yang, D.; Liu, W.L.; Yin, J.; Yang, Z.W.; Wang, H.R.; Qiu, Z.G.; Shen, Z.Q.; et al. Chlorine disinfection promotes the exchange of antibiotic resistance genes across bacterial genera by natural transformation. Isme J. 2020, 14, 1847–1856. [Google Scholar] [CrossRef]
- Schwering, M.; Song, J.; Louie, M.; Turner, R.J.; Ceri, H. Multi-species biofilms defined from drinking water microorganisms provide increased protection against chlorine disinfection. Biofouling 2013, 29, 917–928. [Google Scholar] [CrossRef]
- Zhu, Z.; Shan, L.; Li, X.; Hu, F.; Yuan, Y.; Zhong, D.; Zhang, J. Effects of interspecific interactions on biofilm formation potential and chlorine resistance: Evaluation of dual-species biofilm observed in drinking water distribution systems. J. Water Process Eng. 2020, 38, 101564. [Google Scholar] [CrossRef]
- Shan, L.L.; Bao, X.J.; Xu, S.Y.; Zhu, Z.B.; Pei, Y.Y.; Zheng, W.J.; Yuan, Y.X. Biofilm formation and chlorine resistance of microbial communities in household drinking water system: Preliminary idea of using bacteria to control bacteria. Process Biochem. 2024, 141, 179–189. [Google Scholar] [CrossRef]
- Zhu, Z.B.; Xu, S.Y.; Bao, X.J.; Shan, L.L.; Pei, Y.Y.; Zheng, W.J.; Yuan, Y.X. Effect of outdoor pipe materials and community-intrinsic properties on biofilm formation and chlorine resistance: Black sheep or team leader. J. Clean. Prod. 2023, 411, 11. [Google Scholar] [CrossRef]
- Luo, L.W.; Wu, Y.H.; Chen, G.Q.; Wang, H.B.; Wang, Y.H.; Tong, X.; Bai, Y.; Xu, Y.Q.; Zhang, Z.W.; Ikuno, N.; et al. Chlorine-resistant bacteria (CRB) in the reverse osmosis system for wastewater reclamation: Isolation, identification and membrane fouling mechanisms. Water Res. 2022, 209, 13. [Google Scholar] [CrossRef]
- Liu, L.; Hu, Q.Y.; Le, Y.; Chen, G.W.; Tong, Z.L.; Xu, Q.; Wang, G. Chlorination-mediated EPS excretion shapes early-stage biofilm formation in drinking water systems. Process Biochem. 2017, 55, 41–48. [Google Scholar] [CrossRef]
- Xue, Z.; Hessler, C.M.; Panmanee, W.; Hassett, D.J.; Seo, Y. Pseudomonas aeruginosa inactivation mechanism is affected by capsular extracellular polymeric substances reactivity with chlorine and monochloramine. Fems Microbiol. Ecol. 2013, 83, 101–111. [Google Scholar] [CrossRef]
- Parvin, F.; Rahman, M.A.; Deva, A.K.; Vickery, K.; Hu, H.H. Staphylococcus aureus cell wall phenotypic changes associated with biofilm maturation and water availability: A key contributing factor for chlorine resistance. Int. J. Mol. Sci. 2023, 24, 4983. [Google Scholar] [CrossRef]
- Wang, W.; Xiao, X.; Wang, H.; Wang, S.; Xiao, Y.; Yang, H.; Hou, W.; Wang, W. Characterization and comparative transcriptome analyses of Salmonella enterica Enteritidis strains possessing different chlorine tolerance profiles. Lwt-Food Sci. Technol. 2022, 169, 113945. [Google Scholar] [CrossRef]
- Zhou, Z.; Ma, W.; Zhong, D. The stress response mechanisms and resistance change of chlorine-resistant microbial community at multi-phase interface under residual antibiotics in drinking water distribution system. J. Clean. Prod. 2024, 438, 9. [Google Scholar] [CrossRef]
- Li, G.Q.; Wang, W.L.; Huo, Z.Y.; Lu, Y.; Hu, H.Y. Comparison of UV-LED and low pressure UV for water disinfection: Photoreactivation and dark repair of Escherichia coli. Water Res. 2017, 126, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.W.; Liang, X.X.; Wang, C.Y.; Chen, D.; Liu, H. Synergistic nanowire-assisted electroporation and chlorination for inactivation of chlorine-resistant bacteria in drinking water systems via inducing cell pores for chlorine permeation. Water Res. 2023, 229, 9. [Google Scholar] [CrossRef]
- Zhou, W.; Fu, L.; Zhao, L.; Xu, X.J.; Li, W.Y.; Wen, M.; Wu, Q.S. Novel core-sheath Cu/Cu2O-ZnO-Fe3O4 nanocomposites with high-efficiency chlorine-resistant bacteria sterilization and trichloroacetic acid degradation performance. Acs Appl. Mater. Interfaces 2021, 13, 10878–10890. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.Y.; He, Q.; Yang, D.X.; Chen, M.L.; Chen, Y. Dielectric barrier discharge plasma promotes disinfection-residual-bacteria inactivation via electric field and reactive species. Water Res. 2024, 254, 9. [Google Scholar] [CrossRef]
- Feng, C.M.; Yang, W.Q.; Wei, T.; Li, J.; Chen, Z.X.; Yao, X. Factors influencing the inactivation of Bacillus subtilis by epigallocatechin gallate (EGCG). Aqua 2024, 73, 1510–1524. [Google Scholar] [CrossRef]
- Shuai, Y.W.; Zhang, K.J.; Zhu, H.; Lou, J.X.; Zhang, T.Q. Toward the upgrading quality of drinking water from flavor evaluation: Taste, feeling, and retronasal odor issues. ACS EST Eng. 2023, 14, 308–321. [Google Scholar] [CrossRef]
- Skjevrak, I.; Due, A.; Gjerstad, K.O.; Herikstad, H. Volatile organic components migrating from plastic pipes (HDPE, PEX and PVC) into drinking water. Water Res. 2003, 37, 1912–1920. [Google Scholar] [CrossRef]
- Kelley, K.M.; Stenson, A.C.; Cooley, R.; Dey, R.; Whelton, A.J. The cleaning method selected for new PEX pipe installation can affect short-term drinking water quality. J. Water Health 2015, 13, 960–969. [Google Scholar] [CrossRef]
- Dong, Z.Y.; Lin, Y.L.; Zhang, T.Y.; Hu, C.Y.; Pan, Y.; Zheng, Z.X.; Tang, Y.L.; Xu, B.; Gao, N.Y. The formation, analysis, and control of chlor(am)ination-derived odor problems: A review. Water Res. 2021, 203, 11. [Google Scholar] [CrossRef]
- Zhang, H.H.; Zhao, D.J.; Ma, M.L.; Huang, T.L.; Li, H.Y.; Ni, T.C.; Liu, X.; Ma, B.; Zhang, Y.B.; Li, X.; et al. Actinobacteria produce taste and odor in drinking water reservoir: Community composition dynamics, co-occurrence and inactivation models. J. Hazard. Mater. 2023, 453, 13. [Google Scholar] [CrossRef]
- Asquith, E.; Evans, C.; Dunstan, R.H.; Geary, P.; Cole, B. Distribution, abundance and activity of geosmin and 2-methylisoborneol-producing Streptomyces in drinking water reservoirs. Water Res. 2018, 145, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Zengin, Z.; Köker, L.; Ozbayram, E.G.; Albay, M.; Akçaalan, R. Investigating taste and odour characteristics in a drinking water source: A comprehensive 3-year monitoring study. Environ. Manag. 2024, 13. [Google Scholar] [CrossRef]
- Yamashige, Y.; Chen, S.; Ogawa, Y.; Kawano, T.; Kikuchi, S. Sensitive and real-time monitoring of microbial growth using a dielectric sensor with a 65-GHz LC-oscillator array and polytetrafluoroethylene membrane. Sens. Bio-Sens. Res. 2024, 46, 100703. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, X.; Hao, Z.; Qu, K. Advances in online methods for monitoring microbial growth. Biosens. Bioelectron. 2019, 126, 433–447. [Google Scholar] [CrossRef]
- Mountcastle, S.E.; Vyas, N.; Villapun, V.M.; Cox, S.C.; Jabbari, S.; Sammons, R.L.; Shelton, R.M.; Walmsley, A.D.; Kuehne, S.A. Biofilm viability checker: An open-source tool for automated biofilm viability analysis from confocal microscopy images. Npj Biofilms Microbiomes 2021, 7, 44. [Google Scholar] [CrossRef]
- Pick, F.C.; Fish, K.E.; Husband, S.; Boxall, J.B. Non-invasive biofouling monitoring to assess drinking water distribution system performance. Front. Microbiol. 2021, 12, 730344. [Google Scholar] [CrossRef]
- Seth, A.; Hackebeil, G.A.; Haxton, T.; Murray, R.; Laird, C.D.; Klise, K.A. Evaluation of chlorine booster station placement for water security. In Proceedings of the 9th International Conference on the Foundations of Computer Aided Process Design (FOCAPD), Summit County, CO, USA, 14–18 July 2019; pp. 463–468. [Google Scholar]
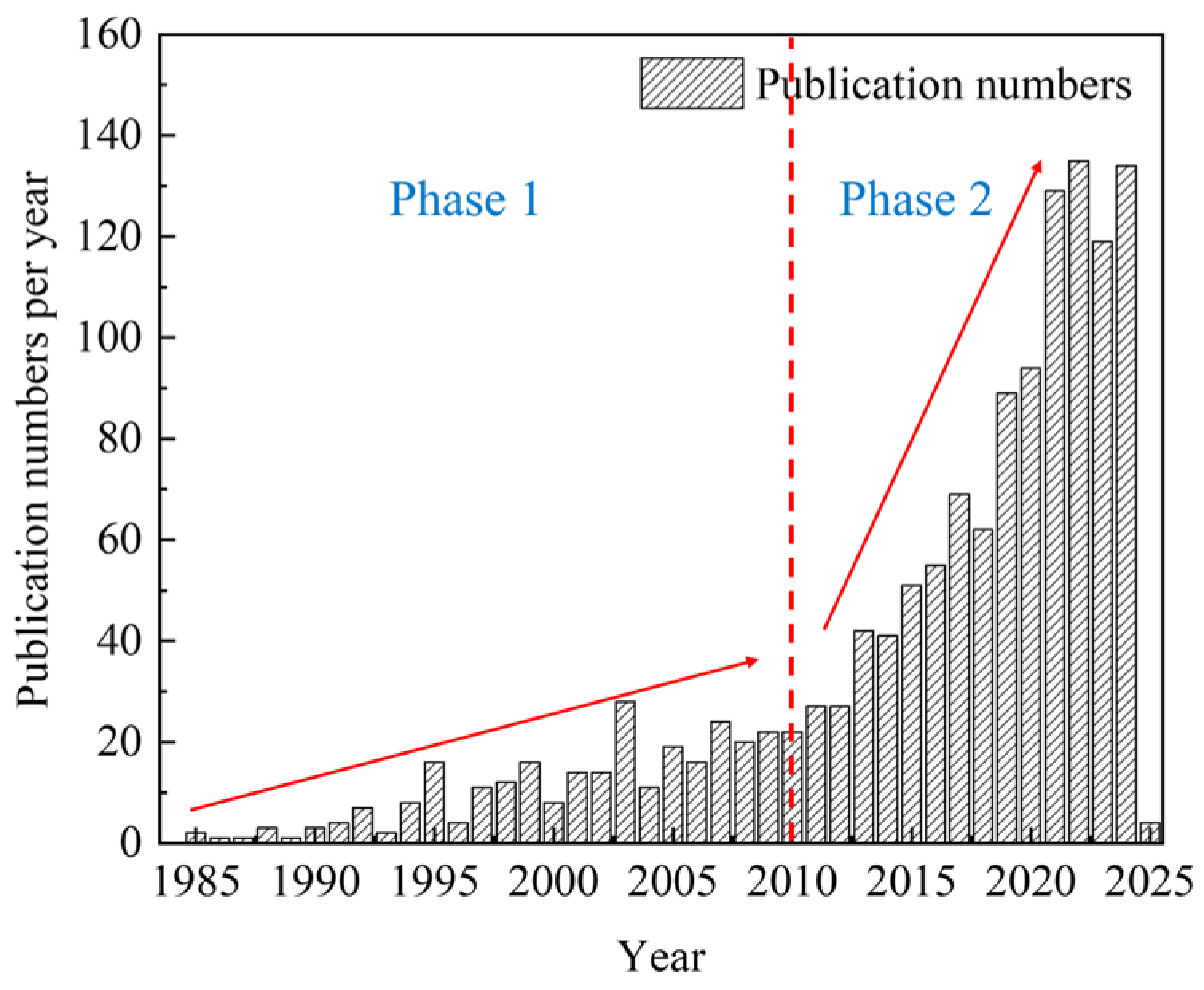

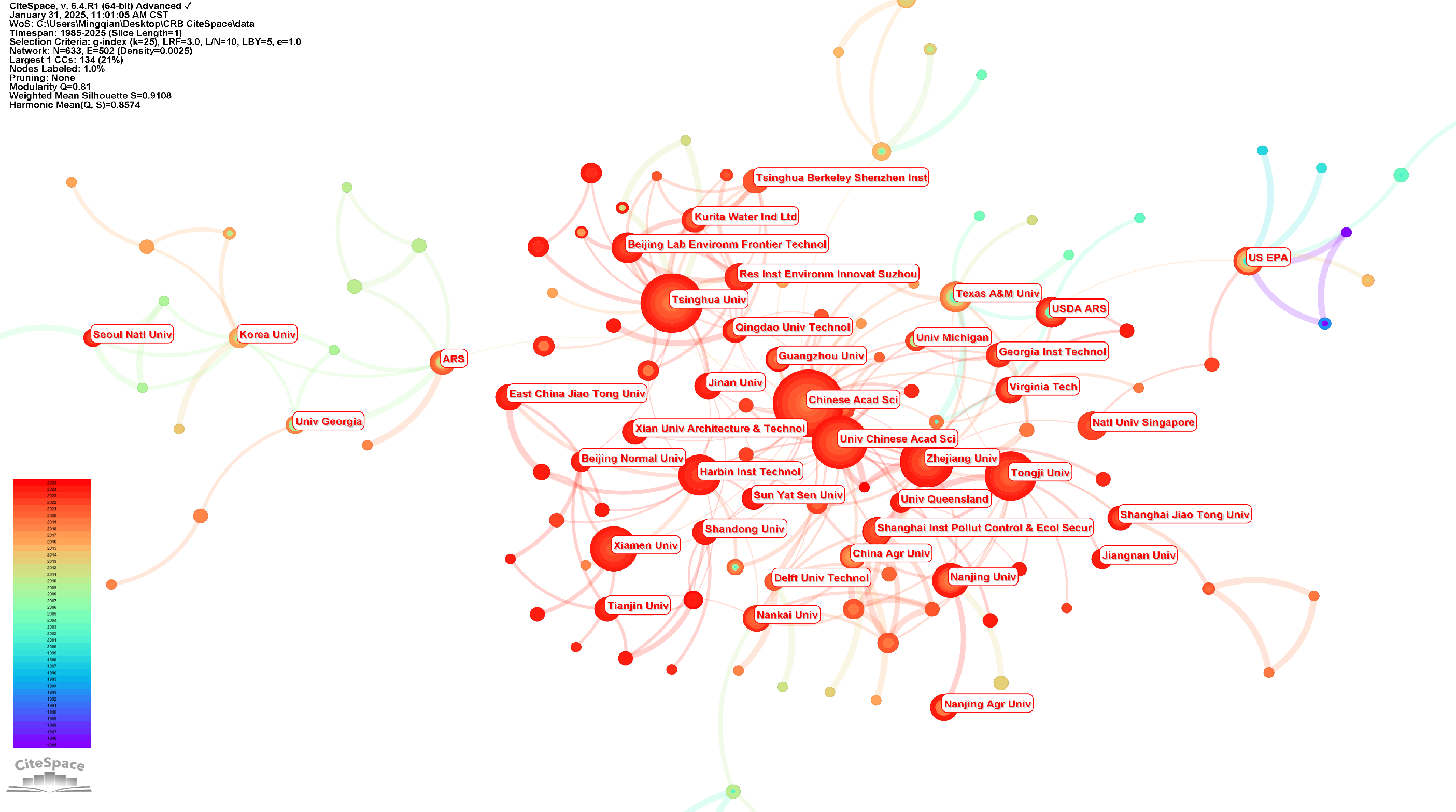


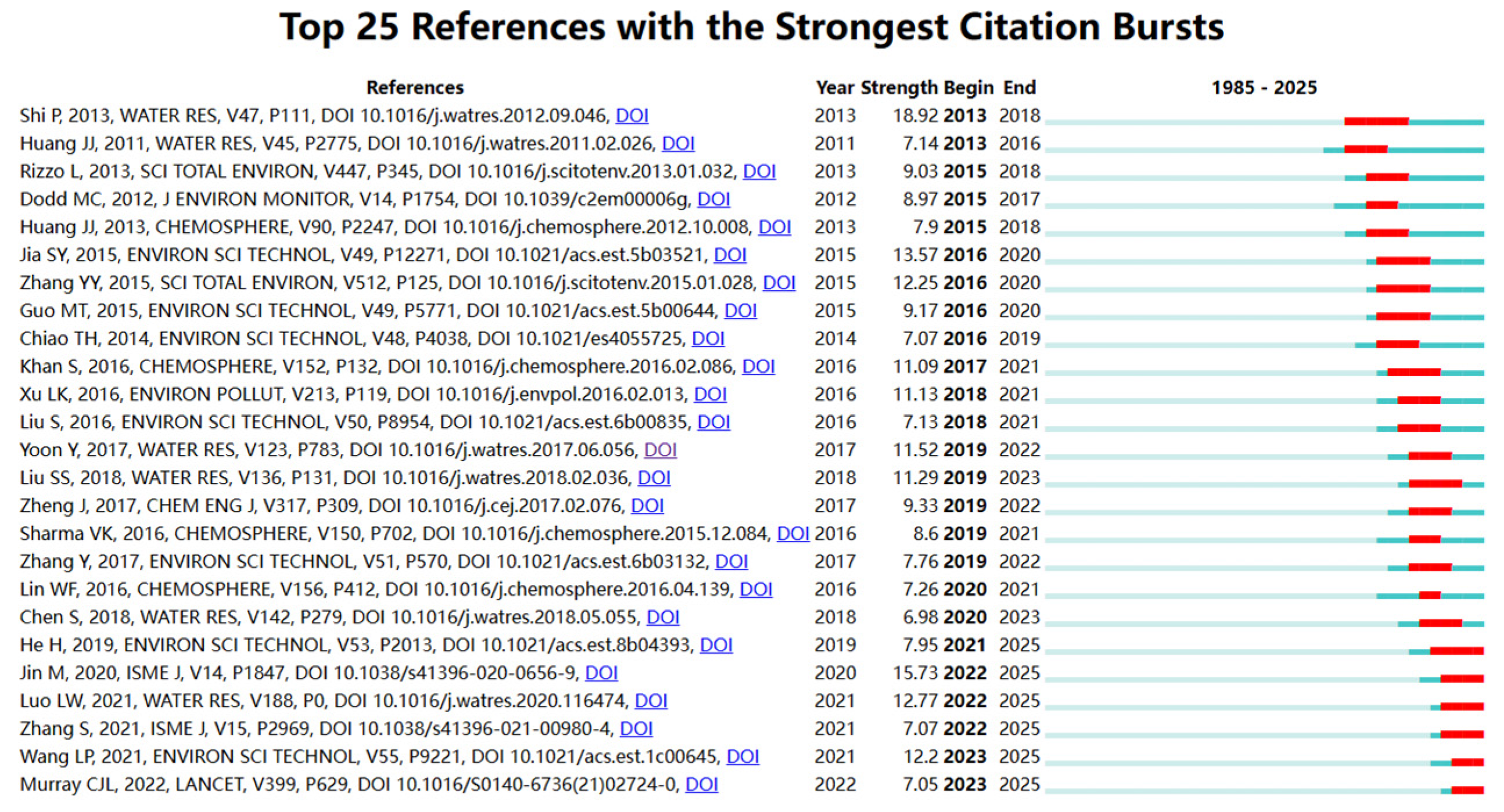
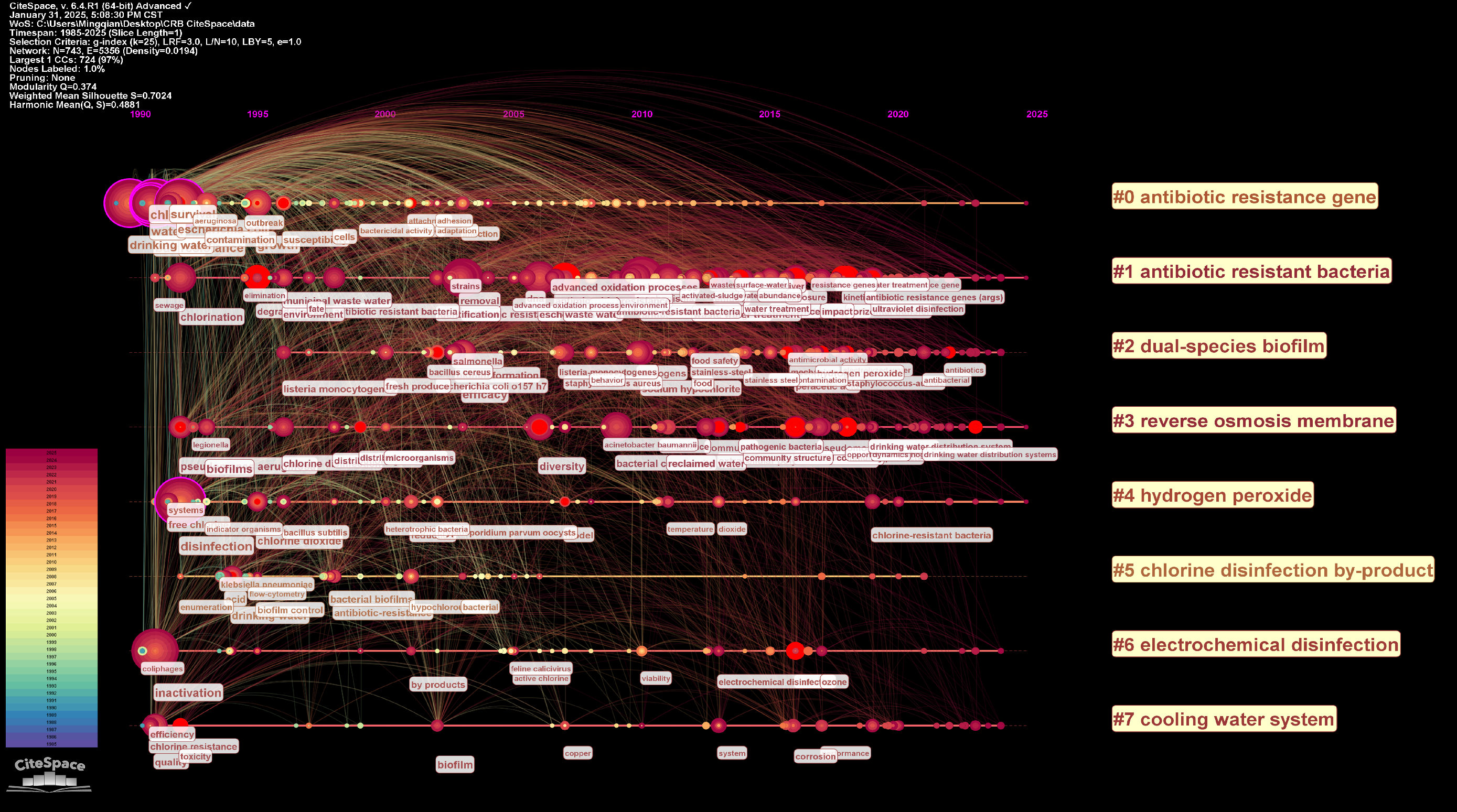

| Rank | Countries | Number of Published Articles | Institutions | Number of Published Articles | Journals | Number of Published Articles | Authors | Number of Published Articles |
|---|---|---|---|---|---|---|---|---|
| 1 | PEOPLES R CHINA | 421 | Chinese Academy of Sciences | 96 | Water Research | 123 | Hu, Hongying | 21 |
| 2 | USA | 346 | Tsinghua University | 46 | Science of the Total Environment | 71 | Ye, Chengsong | 21 |
| 3 | JAPAN | 70 | University of Chinese Academy of Sciences (UCAS) | 39 | Applied and Environmental Microbiology | 47 | Wu, Yinhu | 15 |
| 4 | SPAIN | 52 | Tongji University | 34 | Journal of Hazardous Materials | 41 | Simões, Manuel | 14 |
| 5 | AUSTRALIA | 51 | Harbin Institute of Technology | 33 | Environmental Science Technology | 40 | Wang, Haibo | 13 |
| 6 | ENGLAND | 51 | Research Center for Eco Environmental Sciences (RCEES) | 28 | Chemosphere | 36 | Yuan, Yixing | 12 |
| 7 | SOUTH KOREA | 51 | United States Department of Agriculture (USDA) | 28 | Water Science and Technology | 34 | Feng, Mingbao | 11 |
| 8 | INDIA | 50 | Zhejiang University | 27 | Journal of Food Protection | 30 | Simões, Lúcia C | 11 |
| 9 | FRANCE | 47 | Xiamen University | 23 | Journal of Applied Microbiology | 28 | Peng, Shi | 11 |
| 10 | CANADA | 44 | University System of Georgia | 22 | Food Control | 22 | Yu, Xin | 11 |
| Rank | Co-Citation Count | Author | Years | Publications |
|---|---|---|---|---|
| 1 | 67 | Jin M | 2020 | Jin M, 2020, ISME J, V14, P1847, DOI 10.1038/s41396-020-0656-9 |
| 2 | 61 | Luo LW | 2021 | Luo LW, 2021, WATER RES, V188, P0, DOI 10.1016/j.watres.2020.116474 |
| 3 | 56 | Liu SS | 2018 | Liu SS, 2018, WATER RES, V136, P131, DOI 10.1016/j.watres.2018.02.036 |
| 4 | 47 | Zhang TY | 2019 | Zhang TY, 2019, CHEM ENG J, V358, P589, DOI 10.1016/j.cej.2018.09.218 |
| 5 | 39 | He H | 2019 | He H, 2019, ENVIRON SCI TECHNOL, V53, P2013, DOI 10.1021/acs.est.8b04393 |
| 6 | 37 | Wang LP | 2021 | Wang LP, 2021, ENVIRON SCI TECHNOL, V55, P9221, DOI 10.1021/acs.est.1c00645 |
| 7 | 37 | Yoon Y | 2017 | Yoon Y, 2017, WATER RES, V123, P783, DOI 10.1016/j.watres.2017.06.056 |
| 8 | 36 | Shi P | 2013 | Shi P, 2013, WATER RES, V47, P111, DOI 10.1016/j.watres.2012.09.046 |
| 9 | 36 | Stange C | 2019 | Stange C, 2019, INT J HYG ENVIR HEAL, V222, P541, DOI 10.1016/j.ijheh.2019.02.002 |
| 10 | 34 | Su HC | 2018 | Su HC, 2018, SCI TOTAL ENVIRON, V616, P453, DOI 10.1016/j.scitotenv.2017.10.318 |
| 11 | 33 | Sanganyado E | 2019 | Sanganyado E, 2019, SCI TOTAL ENVIRON, V669, P785, DOI 10.1016/j.scitotenv.2019.03.162 |
| 12 | 31 | Khan S | 2016 | Khan S, 2016, CHEMOSPHERE, V152, P132, DOI 10.1016/j.chemosphere.2016.02.086 |
| 13 | 31 | Jia SY | 2015 | Jia SY, 2015, ENVIRON SCI TECHNOL, V49, P12271, DOI 10.1021/acs.est.5b03521 |
| 14 | 30 | Liu LZ | 2019 | Liu LZ, 2019, CHEMOSPHERE, V219, P971, DOI 10.1016/j.chemosphere.2018.12.067 |
| 15 | 30 | Zheng J | 2017 | Zheng J, 2017, CHEM ENG J, V317, P309, DOI 10.1016/j.cej.2017.02.076 |
| 16 | 30 | Ding WQ | 2019 | Ding WQ, 2019, WATER RES, V160, P339, DOI 10.1016/j.watres.2019.05.014 |
| 17 | 30 | Hou AM | 2019 | Hou AM, 2019, WATER RES, V156, P366, DOI 10.1016/j.watres.2019.03.035 |
| 18 | 28 | Zhang HC | 2019 | Zhang HC, 2019, ENVIRON SCI TECHNOL, V53, P2141, DOI 10.1021/acs.est.8b05907 |
| 19 | 28 | Xu LK | 2016 | Xu LK, 2016, ENVIRON POLLUT, V213, P119, DOI 10.1016/j.envpol.2016.02.013 |
| 20 | 28 | Zhang YY, | 2015 | Zhang YY, 2015, SCI TOTAL ENVIRON, V512, P125, DOI 10.1016/j.scitotenv.2015.01.028 |
| 21 | 27 | Wang HC | 2020 | Wang HC, 2020, WATER RES, V185, P0, DOI 10.1016/j.watres.2020.116290 |
| 22 | 27 | Jia SY | 2020 | Jia SY, 2020, WATER RES, V176, P0, DOI 10.1016/j.watres.2020.115721 |
| 23 | 25 | Zhang Y | 2017 | Zhang Y, 2017, ENVIRON SCI TECHNOL, V51, P570, DOI 10.1021/acs.est.6b03132 |
| 24 | 25 | Chen S | 2018 | Chen S, 2018, WATER RES, V142, P279, DOI 10.1016/j.watres.2018.05.055 |
| 25 | 22 | Pazda M | 2019 | Pazda M, 2019, SCI TOTAL ENVIRON, V697, P0, DOI 10.1016/j.scitotenv.2019.134023 |
| Phylum | Genus | Species | Source | Chlorine Resistance | Ref |
|---|---|---|---|---|---|
| Proteobacteria | Pseudomonas | Pseudomonas peli | Drinking water distribution systems (DWDS) | CT value method The CT value to achieve 99.9% inactivation of the P. peli was 51.26–90.36 mg min/L, inversely proportional to the free chlorine concentration. | [22] |
| Pseudomonas aeruginosa | Chlorinated river water for drinking purpose | Treated with various doses of chlorine at room temperature for 24 h and 48 h, Colony count was significantly higher for the resistant strain at higher concentrations of chlorine. | [41] | ||
| Pseudomonas aeruginosa | Hospital drinking water systems | WHO Survival time method P. aeruginosa isolates exhibited resistance to 0.5 mg/L chlorine for both 5- and 30-min exposure durations. When exposed to a higher chlorine concentration (1.5 mg/L), 80% of the isolates were able to survive after a 5-min exposure, with 40% remaining viable even after a 30-min exposure. | [39] | ||
| Pseudomonas sp. | Drinking Water | WHO Survival time method Treated by 2 mg/L free chlorine for 30 min, the bacteria were not completely inactivated. | [40] | ||
| Klebsiella | Klebsiella sp. | ||||
| Klebsiella pneumoniae | Cooling water system | MIC method 80% of K. pneumoniae survival at 2 mg/L chlorine for 30 min at 30 °C. | [42] | ||
| Aeromonas | Aeromonas jandaei | Drinking water treatment plant (DWTP) | Isolated from DWTP water samples with bacterial growth could not be effectively controlled by an increase of sodium hypochlorite disinfectant. Also, B. alvei, B. cereus, and Lysinibacillus fusiformis exhibits spores with strong resistance to common disinfectants. | [20] | |
| Aeromonas sobria | |||||
| Vogesella | Vogesella perlucida | ||||
| Pelomonas | Pelomonas sp. | ||||
| Acinetobacter | Acinetobacter sp. | Water distribution system | WHO Survival time method Resistant to 20 mg/L chlorine for 30 min. | [43] | |
| Serratia | Serratia sp. | ||||
| Sphingomonas | Sphingomonas sp. | Model DWDS | 4 mg/L chlorine with 240 mm retention time provided only approximately 5% viability reduction of the strain. | [44] | |
| Burkholderia | Burkholderia sp. | Tap water | Isolated bacteria as reference + Inhibition zone method Treated by 14.5% standard NaClO, the diameter of the inhibition zone is less than 20 mm. | [3] | |
| Acidovorax | Acidovorax sp. | ||||
| Halomonas | Halomonas boliviensis | Marine biofilm | Reference strain + Logarithmic removal rate method Using the chlorine-sensitive bacterium Pseudoalteromonas espejiana as a reference strain, H. boliviensis showed only a ≤1-fold reduction in viable count, even after prolonged chlorine exposure (4–8 h) at 8 mg/L residual chlorine. | [31] | |
| Phaeobacter | Phaeobacter caeruleus | Marine biofilm | The viable P. caeruleus cell numbers in chlorine-treated samples (0.4 mg Cl2/L for 30 min were higher than that in the control sample, showing the stimulation of microbial growth by chlorine. | [18] | |
| Firmicutes | Bacillus | Bacillus alvei | DWTP | Three bacteria were found survived in and were isolated from a finished water under 0.3 mg/L residual chlorine for 30 min, thus operationally defined as chlorine-resistant bacteria. | [16] |
| Bacillus cereus | |||||
| Bacillus alvei | DWTP | Isolated from DWTP water samples with bacterial growth could not be effectively controlled by an increase of sodium hypochlorite disinfectant. Also, B. alvei, B. cereus, and Lysinibacillus fusiformis exhibits spores with strong resistance to common disinfectants. | [20] | ||
| Bacillus cereus | |||||
| Bacillus cereus | DWTP | Reference strain + CT value method The inactivation rate of B. cereus species was 2-log lower than that of Escherichia coli at 1 mg/L NaClO. | [5] | ||
| Bacillus sp. | Tap water | Isolated bacteria as reference + Inhibition zone method Treated by 14.5% standard NaClO, the diameter of the inhibition zone is less than 20 mm. | [3] | ||
| Bacillus sp. | Lake Water | Treated by 0.5 mg/L free chlorine for 30 min, the live-to-dead ratio of 8 strains was between 0.3–4.4. | [25] | ||
| Lysinibacillus | Lysinibacillus fusiformis | DWTP | Isolated from DWTP water samples with bacterial growth could not be effectively controlled by an increase of sodium hypochlorite disinfectant. Also, B. alvei, B. cereus, and Lysinibacillus fusiformis exhibits spores with strong resistance to common disinfectants. | [20] | |
| Lysinibacillus fusiformis | DWTP | Three bacteria were found survived in and were isolated from a finished water under 0.3 mg/L residual chlorine for 30 min, thus operationally defined as chlorine-resistant bacteria. | [16] | ||
| Paenibacillus | Paenibacillus sp. | Tap water | Isolated bacteria as reference + Inhibition zone method Treated by 14.5% standard NaClO, the diameter of the inhibition zone is less than 20 mm. | [3] | |
| Clostridium | Clostridium sp. | Drinking Water | WHO Survival time method Treated by 2 mg/L free chlorine for 30 min, the bacteria were not completely inactivated. | [40] | |
| Staphylococcus | Staphylococcus aureus | ||||
| Actinobacteria | Mycobacterium | Mycobacterium fortuitum | Water distribution system | CT value method For a CT value of 60 mg·min/L, frequently used in water treatment lines, chlorine disinfection inactivates over 4 log units of M. gordonae and 1.5 log units of M. fortuitum or M. chelonae. CT values determined under similar conditions show that even the most susceptible species, M. aurum and M. gordonae, are 100 and 330 times more resistant to chlorine than Escherichia coli. | [45] |
| M. chelonae | |||||
| M. gordonae | |||||
| M. aurum | |||||
| M. mucogenicum | Water distribution system | Treated by 2 mg/L free chlorine for 60 min, the inactivation rate is 3.2 log. | [8] | ||
| Legionella | Legionella pneumophila | Spring Water | CT value method The Legionella with the strongest chlorine resistance (of 20 strains) has a CT99.9% of 0.62 mg·min/L. | [46] | |
| Legionella pneumophila | Cooling water | MIC method The MIC50 values of bacteria are between 256 and 1024 mg/L free chlorine. | [47] | ||
| Gordonia | Gordonia | DWDS | CT value method Exhibited high tolerance to chlorine with a CT value of 120 mg min/L for 99.9% reduction. | [23] |
| CRB | Control Methods | Removal Efficiency | Mechanism | Ref |
|---|---|---|---|---|
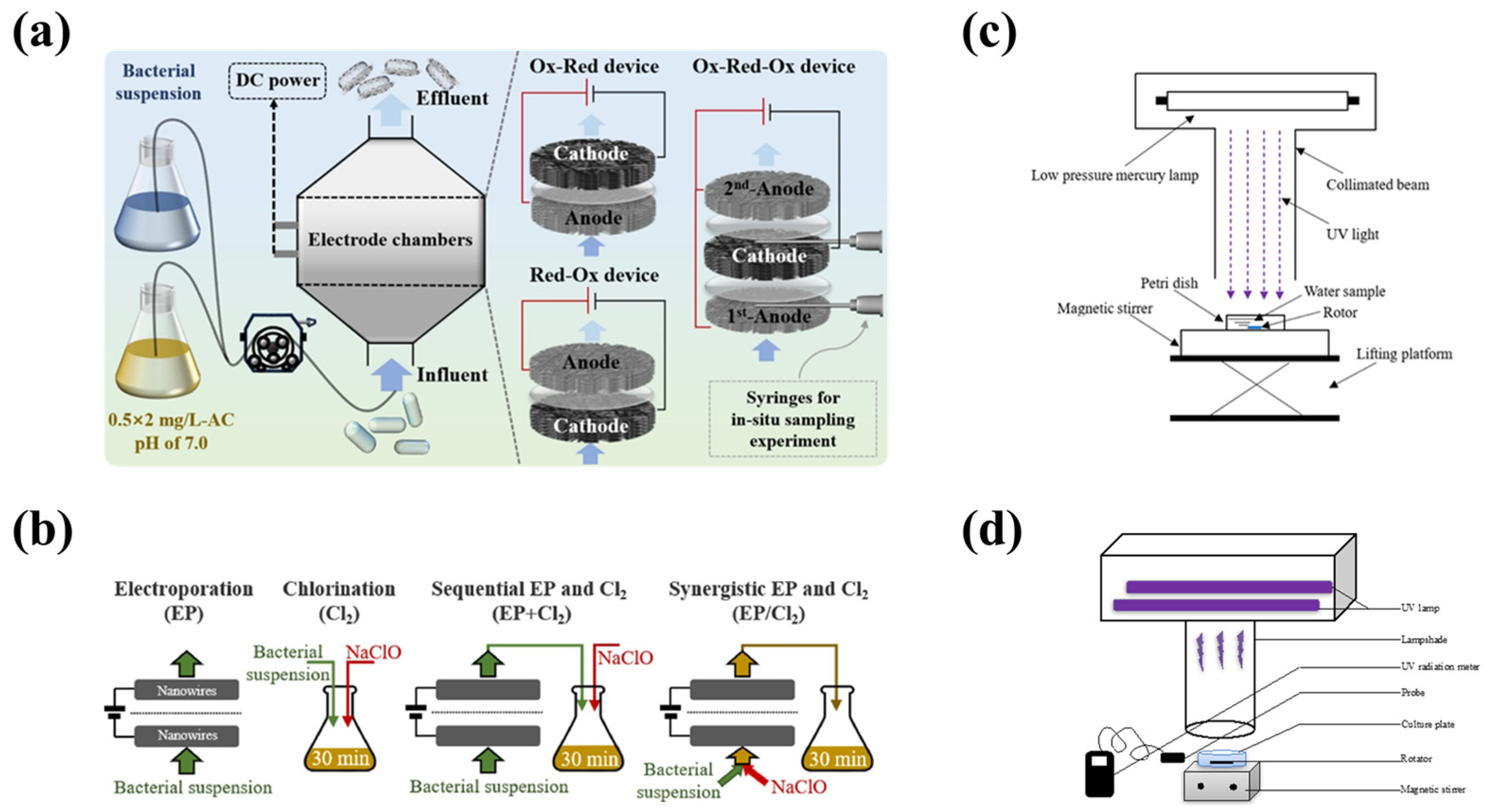
| Phaeobacter caeruleus | Physical method 254 nm of UV light at a dose of 50 mJ/cm2 | UV disinfection reduced viable P. caeruleus by 99.8% (3.3 ± 0.6 × 101 CFU/mL vs. 1.4 ± 0.1 × 10⁴ CFU/mL in the control). | DNA damage | [18] |
| Pseudomonas aeruginosa, Bacillus subtilis, Mycobacterium fortuitum, Pantoea spp., and Stenotrophomonas spp. | Physical method Low-pressure UV (LPUV), medium-pressure UV (MPUV), UV-LEDs (265 and 285 nm), and 222 nm KrCl excilamp irradiation. UV: 0, 5, 10, 20, 40, and 80 mJ/cm2. | Inactivation efficacy of the UV: UV-LED 265 nm > LPUV ≈ MPUV ≈ 222 nm > UV-LED 285 nm. Bacterial resistance to UV: P. aeruginosa < Stenotrophomonas spp. < M. Fortuitum ≈ B. subtilis < Pantoea spp. | CRB inactivation varied by UV type: UV-LED (265/285 nm) and LPUV caused DNA damage and ATP decline, 222 nm induced ROS production, membrane damage, ATP loss, and DNA lesions, while MPUV primarily targeted DNA but also triggered both mechanisms. | [19] |
| Bacillus cereus CR19 | Physical method 40 mJ/cm2 UV Chemical method 2 mg-Cl2/L chlorine, 2 mg-Cl2/L chloramine, and 2 mg/L ozone. | Inactivation efficiency: 40 mJ/cm2 UV (1.90 log), 2 mg-Cl2/L chlorine (0.67 log), 2 mg-Cl2/L chloramine (1.68 log), and 2 mg/L ozone (0.19 log). | EPS primarily contributes to bacterial resistance against chlorine and ozone but not UV or chloramine. Carbon source metabolism is linked to multidrug resistance. | [30] |
| bacteria & spores Bacillus alvei, Lysinibacillus fusiformis, and Bacillus cereus | Chemical method 1.5 mg/L ozone concentration for 1 min | Bacterial inactivation exceeded 3 log, significantly higher than spores. B. alvei, L. fusiformis, and B. cereus spores showed log reductions of 2.33, 2.10, and 1.97, respectively. Over 99.9% of B. cereus spores were inactivated with 3 mg/L ozone in 1 min. | Both cell structures and gene fragments were damaged by ozone disinfection. | [20] |
| Pseudomonas peli 083992 | Physical method UV: 40 mJ/cm2 Chemical method ClO2: 0.38 mg/L | ClO2 (0.38 mg/L, 5 min) inactivated P. peli by 3.6 log. UV (40 mJ/cm2) achieved over 4 log (99.99%) inactivation, with near-total inactivation at 60 mJ/cm2. | Free chlorine and chlorine dioxide inactivated P. peli primarily by disrupting the integrity and permeability of the cell membrane. | [22] |
| Bacillus cereus | Chemical method Ox-Red-Ox: 4.0 V and Cl2: 0.5 mg/L | Achieved over 6.7 log removal of B. cereus at 4.0 V and 0.5 mg/L Cl2 | Electrochemical oxidation at high voltage generated HClO and free chlorine radicals, enhancing oxidative damage to bacterial cell structures. | [17] |
| Bacillus cereus | Chemical method EP (electroporation): 1.5 V and Cl2: 0.9 mg/L | EP/Cl2 achieved > 6 log B. cereus inactivation at 1.5 V EP and 0.9 mg/L Cl2, far exceeding EP (1.11 log) or Cl2 (1.13 log) alone. | EP/Cl2 disinfection created reversible pores for chlorine penetration, overcoming the EPS barrier. Chlorine oxidation then expanded these pores, enhancing bacterial inactivation by cell structure destruction. | [67] |
| P. aeruginosa, S. aureus | Chemical method Cu/Cu2O-ZnO-Fe3O4 | Cu/Cu2O-ZnO-Fe3O4 eliminated 106 CFU/mL P. aeruginosa and S. aureus in 30 min at 10 mg/L, 20 min at 25.5 mg/L, and 10 min at 255 mg/L. | These materials took advantage of their nanostructure, ion release, and ROS effects to change and damage the cell wall and membrane, penetrate cells, and trigger apoptosis. | [68] |
| Pseudomonas fluorescens and Bacillus subtilis | Chemical method dielectric barrier discharge (lgDBD): 12 kV | A 12 kV discharge inactivated B. subtilis and P. fluorescens by over 7 log in 6 and 8 min, respectively. | During lgDBD treatment, streamer propagation creates a strong electric field, inducing bacterial electroporation. This disrupts membrane integrity, causing intracellular leakage and allowing ROS/RNS penetration. These reactive species then damage proteins and DNA, preventing bacterial repair. | [69] |
| B. subtilis | Biological method EGCG (800 mg/L) | High concentration of EGCG (800 mg/L) exhibited a significant inactivation effect on B. subtilis vegetative cells (1.3 log). | EGCG disrupts the morphology and energy metabolism of B. subtilis in a concentration-dependent manner. It inhibits multiple gene expressions, impairing material synthesis, energy metabolism, and the antioxidant system. | [24] |
| B. subtilis | Biological method EGCG (400 mg/L) EGCG (800 mg/L) | A 30-min disinfection with 400 mg/L EGCG reduced B. subtilis by 1.07 log, while 800 mg/L achieved a 1.32 log reduction. | EGCG inactivates B. subtilis by disrupting its structure, energy metabolism, and antioxidant system. It lowers SOD, CAT, and GSH levels, weakening defenses and impairing respiration and ATP synthesis. | [70] |
| Bacillus alvei, Bacillus cereus, and Lysinibacillus fusiformis | Physical method UV (40 mJ/cm2) Combined method coupling pre-oxidation (Cl2 or ClO2), coagulation sedimentation (20 mg/L PAC and 0.08 mg/L PAM), and UV-AOPs inactivation | 5 min long Cl2 (0.9 mg/L) or ClO2 (0.5 mg/L) pre-oxidation induced apparent spore transformation (>75%). Coagulation sedimentation can efficiently remove the formed spores up to 3.15-lg. UV-AOPs substantially enhanced SFB inactivation with >2-lg at 40 mJ/cm2 dosage. | UV inactivated bacteria by damaging DNA without breaking the cell structure. UV-AOPs caused intracellular leakage, morphological damage, and structural disruption, leading to bacterial death. | [16] |
| Bacillus cereus spores | Physical method UV Combined method UV/H2O2 or UV/peroxymonosulfate (PMS) | The B. cereus spores showed high chlorine resistance. UV inactivation reaching over 3 log at 180 mJ/cm2. Adding 20 mg/L H2O2 or PMS reduced the required UV dose to 140 mJ/cm2 and 120 mJ/cm2, respectively. | Flow cytometry and SEM showed that UV/H2O2 and UV/PMS disrupted spore structure, damaging membranes and cytoplasm, leading to intracellular leakage. | [5] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, Z.; Xia, M.; Zhang, X.; Lan, R.; Wei, B.; Liu, Y.; Lu, Y.; Fan, G. Mechanism and Risk Control of Chlorine-Resistant Bacteria in Drinking Water Supply Systems: A Comprehensive Bibliometric Analysis. Water 2025, 17, 956. https://doi.org/10.3390/w17070956
Wang Y, Zhang Z, Xia M, Zhang X, Lan R, Wei B, Liu Y, Lu Y, Fan G. Mechanism and Risk Control of Chlorine-Resistant Bacteria in Drinking Water Supply Systems: A Comprehensive Bibliometric Analysis. Water. 2025; 17(7):956. https://doi.org/10.3390/w17070956
Chicago/Turabian StyleWang, Yue, Zhiming Zhang, Mingqian Xia, Xiaomin Zhang, Rongxing Lan, Binqing Wei, Yi Liu, Yi Lu, and Gongduan Fan. 2025. "Mechanism and Risk Control of Chlorine-Resistant Bacteria in Drinking Water Supply Systems: A Comprehensive Bibliometric Analysis" Water 17, no. 7: 956. https://doi.org/10.3390/w17070956
APA StyleWang, Y., Zhang, Z., Xia, M., Zhang, X., Lan, R., Wei, B., Liu, Y., Lu, Y., & Fan, G. (2025). Mechanism and Risk Control of Chlorine-Resistant Bacteria in Drinking Water Supply Systems: A Comprehensive Bibliometric Analysis. Water, 17(7), 956. https://doi.org/10.3390/w17070956






