Microplastics in Inland Saline Lakes of the Central Ebro Basin, NE Spain
Abstract
1. Introduction
2. Materials and Methods
2.1. Location
2.2. Sampling in the Field
2.3. Materials and Sample Preparation
2.4. Analytical Procedure
3. Results
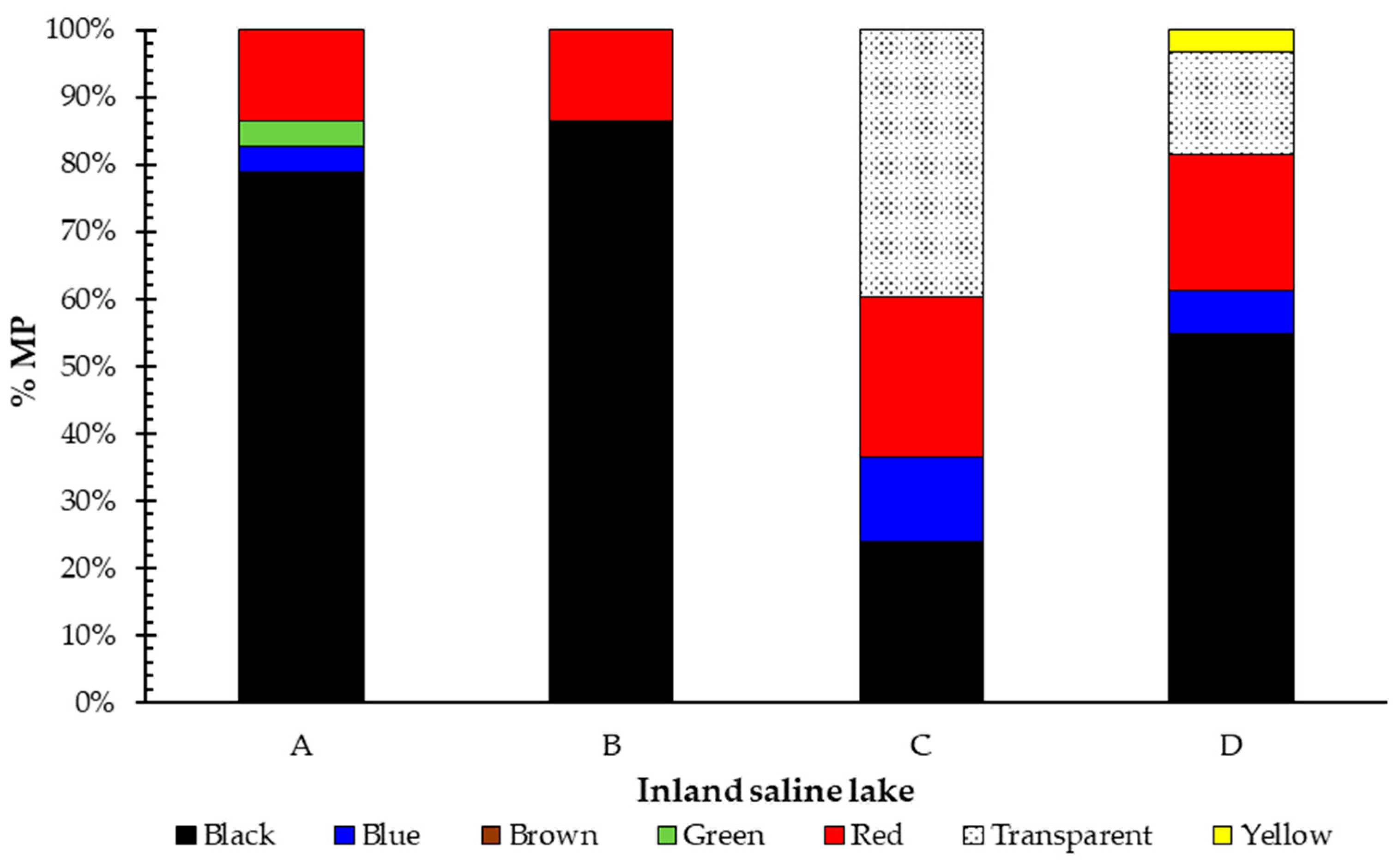
3.1. Presence of MPs
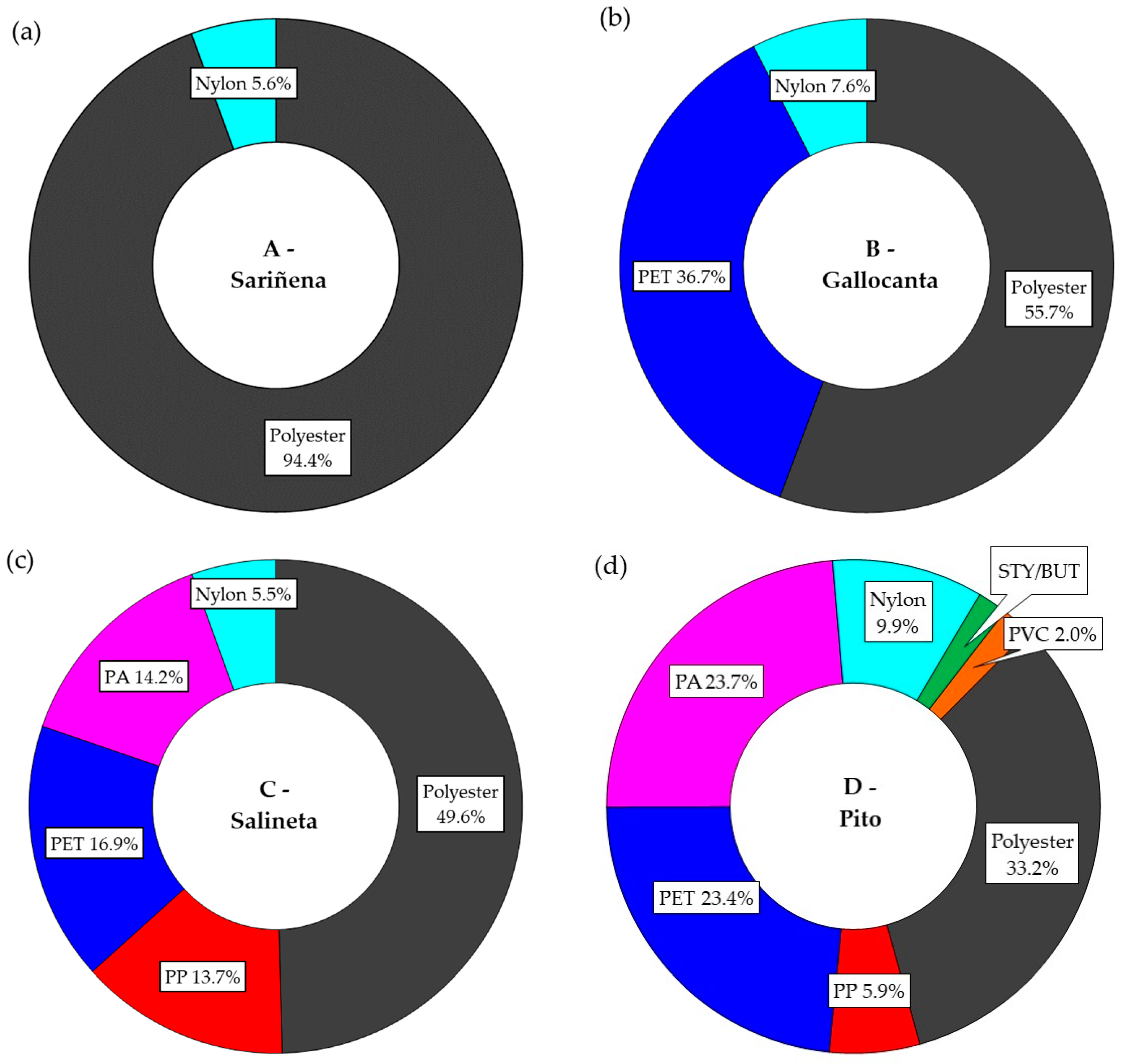
3.2. Polymer Type Characterization
4. Discussion
Justification of Sample Size and Future Research Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MP | Microplastic |
| CEB | Central Ebro Basin |
| FTIR | Fourier Transform Infrared Spectroscopy |
| NUTS | Nomenclature of territorial units for statistics |
| UV | Ultraviolet |
| PP | Polypropylene |
| PE | Polyethylene |
| PET | Polyethylene terephthalate |
| PA | Polyacrylonitrile |
| STY/BUT | Styrene/butadiene copolymer |
| PVC | Polyvinyl chloride |
References
- Shiklomanov, I.A. Global water resources. Nat. Resour. 1998, 26, 34–43. [Google Scholar]
- Williams, W.D. Environmental threats to salt lakes and the likely status of inland saline ecosystems in 2025. Environ. Conserv. 2002, 29, 154–167. [Google Scholar] [CrossRef]
- Menéndez-Serra, M.; Ontiveros, V.J.; Triadó-Margarit, X.; Alonso, D.; Casamayor, E.O. Dynamics and ecological distributions of the Archaea microbiome from inland saline lakes (Monegros Desert, Spain). FEMS Microbiol. Ecol. 2020, 96, fiaa019. [Google Scholar] [CrossRef]
- Bourhane, Z.; Cagnon, C.; Castañeda, C.; Rodríguez, R.; Álvaro-Fuentes, J.; Cravo-Laureau, C.; Duran, R. Vertical organisation of microbial communities in Salineta hypersaline wetland, Spain. Front. Microbiol. Sect. Extrem. Microbiol. 2023, 14, 869907. [Google Scholar] [CrossRef]
- Zadereev, E.; Lipka, O.; Karimov, B.; Krylenko, M.; Elias, V.; Pinto, I.S.; Alizade, V.; Anker, Y.; Feest, A.; Kuznetsova, D.; et al. Overview of past, current, and future ecosystem and biodiversity trends of inland saline lakes of Europe and Central Asia. Inland Waters 2020, 10, 438–452. [Google Scholar] [CrossRef]
- Castañeda, C.; Herrero, J. Assessing the degradation of saline wetlands in an arid agricultural region in Spain. Catena 2008, 72, 205–213. [Google Scholar] [CrossRef]
- Oren, A. Halophilic archaea on Earth and in space: Growth and survival under extreme conditions. Phil. Trans. R. Soc. A 2014, 372, 20140194. [Google Scholar] [CrossRef] [PubMed]
- Samper-Calvete, F.J.; García-Vera, M.A. Inverse modeling of groundwater flow in the semiarid evaporitic closed basin of Los Monegros, Spain. Hydrogeol. J. 1998, 6, 33–49. [Google Scholar] [CrossRef]
- Castañeda, C.; García-Vera, M.Á. Water balance in the playa-lakes of an arid environment, Monegros, NE Spain. Hydrogeol. J. 2008, 16, 87–102. [Google Scholar] [CrossRef]
- Wurtsbaugh, W.A.; Miller, C.; Null, S.E.; DeRose, R.J.; Wilcock, P.; Hahnenberger, M.; Howe, F.; Moore, J. Decline of the world’s saline lakes. Nat. Geosci. 2017, 10, 816–821. [Google Scholar] [CrossRef]
- Saini, J.; Pandey, S. Environmental threat and change detection in saline lakes from 1960 to 2021: Background, present, and future. Environ. Sci. Pollut. Res. 2023, 30, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Hueso-Kortekaas, K.; Carrasco-Vayá, J.-F. Los Paisajes Ibéricos de la Sal. Humedales Salinos de Interior; Asociación de Amigos de las Salinas de Interior: Guadalajara, Spain, 2009. [Google Scholar]
- Herrero, J.; Snyder, R.L. Aridity and irrigation in Aragon, Spain. J. Arid Environ. 1997, 35, 535–547. [Google Scholar] [CrossRef]
- Castañeda, C.; Herrero, J. Measuring the condition of saline wetlands threatened by agricultural intensification. Pedosphere 2008, 18, 11–23. [Google Scholar] [CrossRef]
- Gracia, F.J.; Castañeda, C.; Simarro, G.; Calvete, D. Water level control on coastal landform distribution and associated processes in a highly fluctuating shallow lake (Gallocanta Lake, NE Spain). Earth Surf. Process. Landf. 2023, 49, 770–786. [Google Scholar] [CrossRef]
- Lamichhane, G.; Acharya, A.; Marahatha, R.; Modi, B.; Paudel, R.; Adhikari, A.; Raut, B.K.; Aryal, S.; Parajuli, N. Microplastics in environment: Global concern, challenges, and controlling measures. Int. J. Environ. Sci. Technol. 2023, 20, 4673–4694. [Google Scholar] [CrossRef]
- Nava, V.; Chandra, S.; Aherne, J.; Alfonso, M.B.; Antão-Geraldes, A.M.; Attermeyer, K.; Bao, R.; Bartrons, M.; Berger, S.A.; Biernaczyk, M.; et al. Plastic debris in lakes and reservoirs. Nature 2023, 619, 317–322. [Google Scholar] [CrossRef]
- Eriksen, M.; Maximenko, N.; Thiel, M.; Cummins, A.; Lattin, G.; Wilson, S.; Hafner, J.; Zellers, A.; Rifman, S. Plastic pollution in the South Pacific subtropical gyre. Mar. Pollut. Bull. 2013, 68, 71–76. [Google Scholar] [CrossRef]
- Wagner, M.; Scherer, C.; Alvarez-Muñoz, D.; Brennholt, N.; Bourrain, X.; Buchinger, S.; Fries, E.; Grosbois, C.; Klasmeier, J.; Marti, T.; et al. Microplastics in freshwater ecosystems: What we know and what we need to know. Environ. Sci. Eur. 2014, 26, 12. [Google Scholar] [CrossRef]
- Cera, A.; Cesarini, G.; Scalici, M. Microplastics in freshwater: What is the news from the world? Diversity 2020, 12, 276. [Google Scholar] [CrossRef]
- Stabnikova, O.; Stabnikov, V.; Marinin, A.; Klavins, M.; Vaseashta, A. The role of microplastics biofilm in accumulation of trace metals in aquatic environments. World J. Microb. Biot. 2022, 38, 117. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, S.; Xu, W.; Liang, C.; Li, J.; Zhang, H.; Li, Y.; Liu, X.; Jones, D.L.; Chadwick, D.R.; et al. Effects of plastic residues and microplastics on soil ecosystems: A global meta-analysis. J. Hazard. Mater. 2022, 435, 129065. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Okochi, H.; Tani, Y.; Hayami, H.; Minami, Y.; Katsumi, N.; Takeuchi, M.; Sorimachi, A.; Fujii, Y.; Kajino, M.; et al. Airborne hydrophilic microplastics in cloud water at high altitudes and their role in cloud formation. Environ. Chem. Lett. 2023, 21, 3055–3062. [Google Scholar] [CrossRef]
- Alirezazadeh, M.; Nematollahi, M.J.; Keshavarzi, B.; Rezaei, M.; Moore, F.; Busquets, R. Microplastics in abiotic compartments of a hypersaline lacustrine ecosystem. Environ. Toxicol. Chem. 2023, 42, 19–32. [Google Scholar] [CrossRef]
- Cano-Povedano, J.; López-Calderón, C.; Sánchez, M.I.; Hortas, F.; Canuelo-Jurado, B.; Martín-Vélez, V.; Ros, M.; Cózar, A.; Green, A.J. Biovectoring of plastic by white storks from a landfill to a complex of salt ponds and marshes. Mar. Pollut. Bull. 2023, 197, 115773. [Google Scholar] [CrossRef]
- Mansfield, I.; Reynolds, S.J.; Lynch, I.; Matthews, T.J.; Sadler, J.P. Birds as bioindicators of plastic pollution in terrestrial and freshwater environments: A 30-year review. Environ. Pollut. 2024, 348, 123790. [Google Scholar] [CrossRef]
- Faure, F.; Saini, C.; Potter, G.; Galgani, F.; De Alencastro, L.F.; Hagmann, P. An evaluation of surface micro-and mesoplastic pollution in pelagic ecosystems of the Western Mediterranean Sea. Environ. Sci. Pollut. Res. Int. 2015, 22, 12190–12197. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.K.; Paglialonga, L.; Czech, E.; Tamminga, M. Microplastic pollution in lakes and lake shoreline sediments—A case study on Lake Bolsena and Lake Chiusi (central Italy). Environ. Pollut. 2016, 213, 648–657. [Google Scholar] [CrossRef]
- Liang, Y.; Cao, X.; Mo, A.; Jiang, J.; Zhang, Y.; Gao, W.; He, D. Micro (nano) plastics in plant-derived food: Source, contamination pathways and human exposure risks. TrAC Trends Anal. Chem. 2023, 165, 117138. [Google Scholar] [CrossRef]
- Cledera-Castro, M.D.M.; Hueso-Kortekaas, K.; Sanchez-Mata, C.; Morales-Polo, C.; Calzada-Funes, J.; Delgado-Mellado, N.; Caro-Carretero, R. An exploratory study of fibre microplastics pollution in different process stages of salt production by solar evaporation in Spain. Heliyon 2024, 10, e31609. [Google Scholar] [CrossRef]
- Edo, C.; González-Pleiter, M.; Tamayo-Belda, M.; Ortega-Ojeda, F.E.; Leganés, F.; Fernández-Piñas, F.; Rosal, R. Microplastics in sediments of artificially recharged lagoons: Case study in a Biosphere Reserve. Sci. Total Environ. 2020, 729, 138824. [Google Scholar] [CrossRef]
- Free, C.M.; Jensen, O.P.; Mason, S.A.; Eriksen, M.; Williamson, N.J.; Boldgiv, B. High-levels of microplastic pollution in a large, remote, mountain lake. Mar. Pollut. Bull. 2014, 85, 156–163. [Google Scholar] [CrossRef]
- Anderson, J.C.; Park, B.J.; Palace, V.P. Microplastics in aquatic environments: Implications for Canadian ecosystems. Environ. Pollut. 2016, 218, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.A.; Kammin, L.; Eriksen, M.; Aleid, G.; Wilson, S.; Box, C.; Williamson, N.; Riley, A. Pelagic plastic pollution within the surface waters of Lake Michigan, USA. J. Great Lakes Res. 2016, 42, 753–759. [Google Scholar] [CrossRef]
- Vaughan, R.; Turner, S.D.; Rose, N.L. Microplastics in the sediments of a UK urban lake. Environ. Pollut. 2017, 229, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.A.; Daily, J.; Aleid, G.; Ricotta, R.; Smith, M.; Donnelly, K.; Knauff, R.; Edwards, W.; Hoffman, M.J. High levels of pelagic plastic pollution within the surface waters of Lakes Erie and Ontario. J. Great Lakes Res. 2020, 46, 277–288. [Google Scholar] [CrossRef]
- Qian, J.; Tang, S.; Wang, P.; Lu, B.; Li, K.; Jin, W.; He, X. From source to sink: Review and prospects of microplastics in wetland ecosystems. Sci. Total Environ. 2021, 758, 143633. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, M.B.; Arias, A.H.; Piccolo, M.C. Microplastics integrating the zooplanktonic fraction in a saline lake of Argentina: Influence of water management. Environ. Monit. Assess. 2020, 192, 117. [Google Scholar] [CrossRef]
- Liu, R.; Liu, F.; Dong, Y.; Jiao, J.; El-Wardany, R.M.; Zhu, L. Microplastic contamination in lacustrine sediments in the Qinghai-Tibet Plateau: Current status and transfer mechanisms. China Geol. 2022, 5, 421–428. [Google Scholar] [CrossRef]
- Tsering, T.; Sillanpää, M.; Viitala, M.; Reinikainen, S.P. Variation of microplastics in the shore sediment of high-altitude lakes of the Indian Himalaya using different pretreatment methods. Sci. Total Environ. 2022, 849, 157870. [Google Scholar] [CrossRef]
- Zhang, F.; Guo, X.; Zhang, J.; Zhang, Z. Microplastics in Qinghai-Tibet Plateau and Yunnan-Guizhou Plateau lakes, China. Sci. Total Environ. 2024, 915, 169978. [Google Scholar] [CrossRef]
- Abbasi, S.; Turner, A. Sources, concentrations, distributions, fluxes and fate of microplastics in a hypersaline lake: Maharloo, south-west Iran. Sci. Total Environ. 2022, 823, 153721. [Google Scholar] [CrossRef] [PubMed]
- Conesa Mor, J.A.; Castañeda del Álamo, C.; Pedrol Solanes, J. Las Saladas de Monegros y su Entorno: Habitats y Paisaje Vegetal; Consejo de Protección de la Naturaleza de Aragón: Zaragoza, Spain, 2011. [Google Scholar]
- Ebro River Basin Authority. Confederación Hidrográfica del Ebro. Available online: https://www.chebro.es/ (accessed on 15 October 2024).
- García, M.A.; Castañeda, C. Las saladas de Bujaraloz-Sástago. Nat. Aragonesa 2005, 14, 52–58. [Google Scholar]
- Census. Resident Population in Spain on 1 January 2022. Available online: https://www.ine.es/ (accessed on 8 October 2024).
- Liu, K.; Wang, X.; Wei, N.; Song, Z.; Li, D. Accurate quantification and transport estimation of suspended atmospheric microplastics in megacities: Implications for human health. Environ. Int. 2019, 132, 105127. [Google Scholar] [CrossRef]
- Liang, T.; Ho, Y.-W.; Wang, Q.; Wang, P.; Sun, S.; Fang, J.K.-H.; Liu, X. Distribution and risk assessment of microplastics in water, sediment and brine shrimps in a remote salt lake on the Tibetan Plateau, China. J. Hazard. Mater. 2024, 476, 134959. [Google Scholar] [CrossRef]
- Ramamoorthy, S.K.; Skrifvars, M.; Persson, A. A Review of Natural Fibers Used in Biocomposites: Plant, Animal and Regenerated Cellulose Fibers. Polym. Rev. 2015, 55, 107–162. [Google Scholar] [CrossRef]
- Idström, A.; Schantz, S.; Sundberg, J.; Chmelka, B.F.; Gatenholm, P.; Nordstierna, L. 13C NMR assignments of regenerated cellulose from solid-state 2D NMR spectroscopy. Carbohydr. Polymc. 2016, 151, 480–487. [Google Scholar] [CrossRef]
- Neves, R.M.; Ornaghi, H.L.; Zattera, A.J.; Amico, S.C. Recent studies on modified cellulose/nanocellulose epoxy composites: A systematic review. Carbohydr. Polym. 2021, 255, 117366. [Google Scholar] [CrossRef]
- Boixadera, J.; Poch, R.M.; Herrero, C. (Eds.) Tour Guide 88: Soil information for sustainable development. In Guidebook for Field Trip 88 (Catalonia and Aragon, Spain), Proceedings of the 16th World Congress of Soil Science, Montpellier, France, 27 August–2 September 1998; International Union of Soil Science: Lleida, Spain, 1998; L-1068-98. [Google Scholar]
- Bruna, P.; Gómez, E. El Cultivo en Invernadero en Aragón; Centro de Transferencia Agroalimentaria, Consejería de Desarrollo Rural y Sostenibilidad, Gobierno de Aragón: Zaragoza, Spain, 2018. [Google Scholar]
- Bergmann, M.; Mützel, S.; Primpke, S.; Tekman, M.B.; Trachsel, J.; Gerdts, G. White and wonderful? Microplastics prevail in snow from the Alps to the Arctic. Sci. Adv. 2019, 5, eaax1157. [Google Scholar] [CrossRef]
- Xia, W.; Rao, Q.; Deng, X.; Chen, J.; Xie, P. Rainfall is a significant environmental factor of microplastic pollution in inland waters. Sci. Total Environ. 2020, 732, 139065. [Google Scholar] [CrossRef]
- Xiao, S.; Cui, Y.; Brahney, J.; Mahowald, N.M.; Li, Q. Long-distance atmospheric trasport of microplastic fibres influenced by their shapes. Nat. Geosci. 2023, 16, 863–870. [Google Scholar] [CrossRef]
- Yan, M.; Wang, L.; Dai, Y.; Sun, H.; Liu, C. Behavior of microplastics in inland waters: Aggregation, settlement, and transport. Bull. Environ. Contam. Toxicol. 2021, 107, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Martín-Vélez, V.; Cano-Povedano, J.; Canuelo-Jurado, B.; López-Calderón, C.; Céspedes, V.; Ros, M.; Sánchez, M.I.; Shamoun-Baranes, J.; Müller, W.; Thaxter, C.B.; et al. Leakage of plastics and other debris from landfills to a highly protected lake by wintering gulls. Waste Manag. 2024, 177, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Shi, L.; Huang, H.; Ye, K.; Yang, L.; Wang, Z.; Sun, Y.; Li, D.; Shi, Y.; Xiao, L.; et al. Analysis of aged microplastics: A review. Environ. Chem. Lett. 2024, 22, 1861–1888. [Google Scholar] [CrossRef]


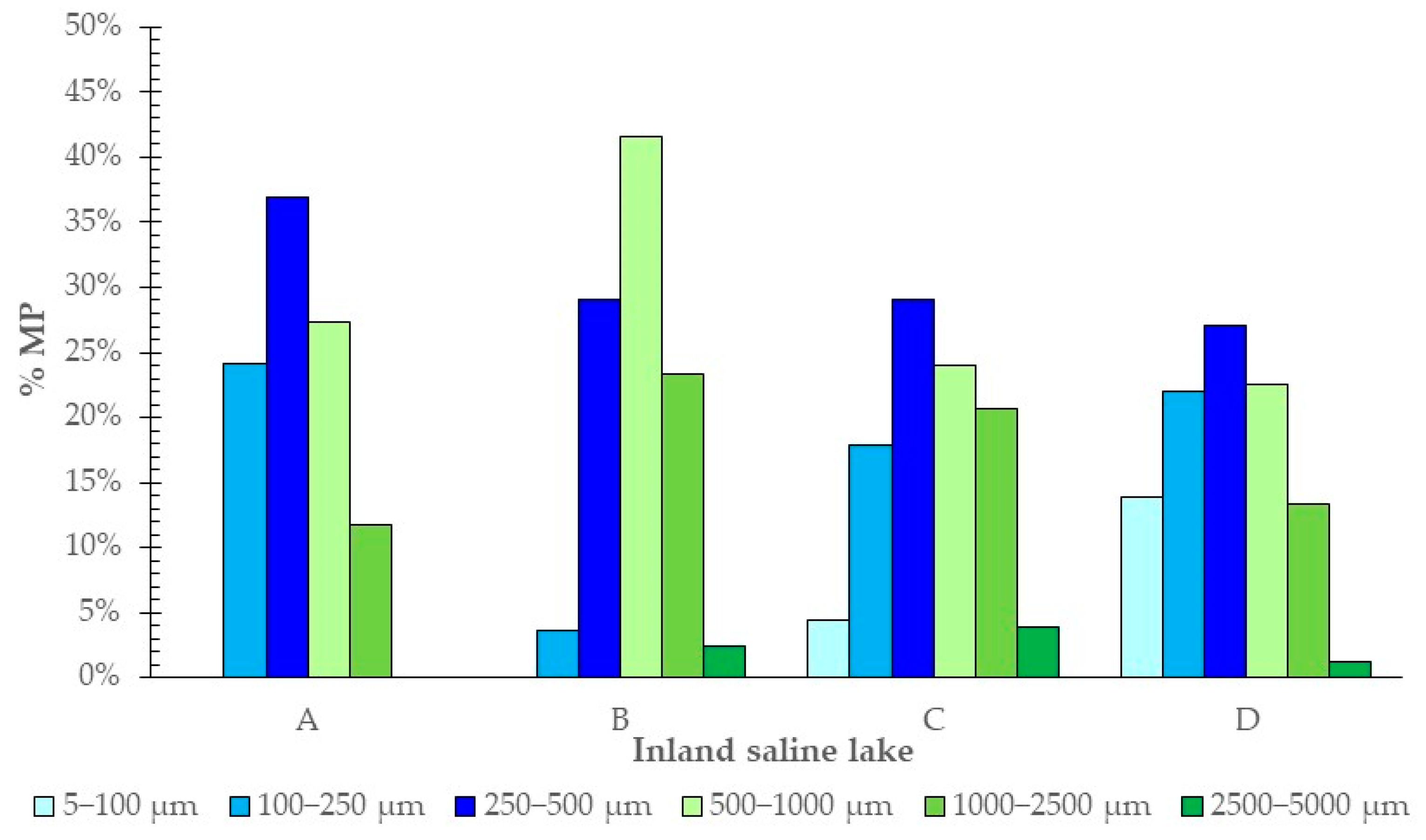
| ID | Lake | Latitude | Longitude | Hydric Regime | Surface Extent (ha) | Electrical Conductivity ** (dS/m) |
|---|---|---|---|---|---|---|
| A | Sariñena | 41.797720 | −0.180270 | Permanent | 137.7 | 2.45 |
| B | Gallocanta | 40.971938 | −1.501162 | Fluctuating | 1450.0 * | 47.30 |
| C | Salineta | 41.482195 | −0.160151 | Highly fluctuating | 23.0 * | 79.40 |
| D | Pito | 41.412354 | −0.151142 | Highly fluctuating | 80.5 * | 27.10 |
| ID | MPs/L | Typology (%) |
|---|---|---|
| A | 916 ± 518 | Fiber (100%) |
| B | 850 ± 271 | Fiber (100%) |
| C | 1486 ± 100 | Fiber (100%) |
| D | 1556 ± 59 | Fiber (98.4%); fragment (1.6%) |
| Black | Blue | Brown | Green | Red | Transparent | Yellow | |
|---|---|---|---|---|---|---|---|
| Polyester | 66.7% | 49.6% | 100.0% | 100.0% | 45.4% | 42.4% | - |
| PP 1 | - | 8.9% | - | - | - | 16.4% | - |
| PET 2 | 24.8% | 41.5% | - | - | 13.1% | 13.2% | - |
| PA 3 | 5.6% | - | - | - | 20.9% | 15.5% | 100.0% |
| Nylon | 1.5% | - | - | - | 16.2% | 12.6% | - |
| STY/BUT 4 | 1.4% | - | - | - | - | - | - |
| PVC 5 | - | - | - | - | 4.4% | - | - |
| TOTAL | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hueso-Kortekaas, K.; Delgado-Mellado, N.; Calzada-Funes, J.; Sanchez-Mata, C.; Castañeda, C.; Cledera-Castro, M.d.M. Microplastics in Inland Saline Lakes of the Central Ebro Basin, NE Spain. Water 2025, 17, 989. https://doi.org/10.3390/w17070989
Hueso-Kortekaas K, Delgado-Mellado N, Calzada-Funes J, Sanchez-Mata C, Castañeda C, Cledera-Castro MdM. Microplastics in Inland Saline Lakes of the Central Ebro Basin, NE Spain. Water. 2025; 17(7):989. https://doi.org/10.3390/w17070989
Chicago/Turabian StyleHueso-Kortekaas, Katia, Noemí Delgado-Mellado, Javier Calzada-Funes, Carlos Sanchez-Mata, Carmen Castañeda, and María del Mar Cledera-Castro. 2025. "Microplastics in Inland Saline Lakes of the Central Ebro Basin, NE Spain" Water 17, no. 7: 989. https://doi.org/10.3390/w17070989
APA StyleHueso-Kortekaas, K., Delgado-Mellado, N., Calzada-Funes, J., Sanchez-Mata, C., Castañeda, C., & Cledera-Castro, M. d. M. (2025). Microplastics in Inland Saline Lakes of the Central Ebro Basin, NE Spain. Water, 17(7), 989. https://doi.org/10.3390/w17070989









