Metabolomic Analysis of Both Microcystin-Producing and Microcystin-Free Microcystis aeruginosa Strains in Response to Exogenous Microcystin Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Preparation of Exogenous Microcystins and Exposure Test
2.3. Observation of Density and Morphology of Cells
2.4. Measurement of Chlorophyll a Content and Photosynthetic System Efficiency
2.5. Determination of Enzyme Activity
2.6. Determination of Intracellular Microcystin Content
2.7. Non-Targeted Metabolomics Analysis
2.8. Statistical Analysis
3. Results
3.1. Physiological Responses
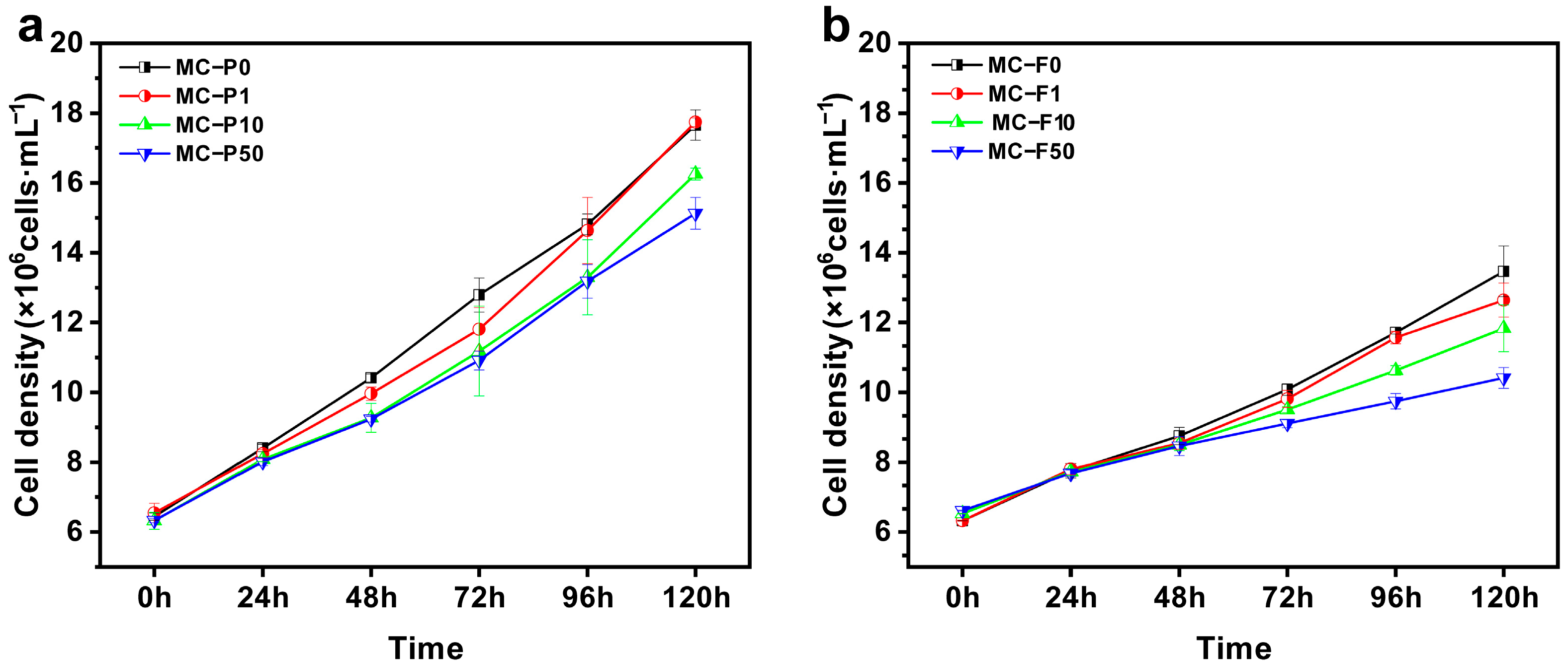
3.1.1. Effects of Exogenous Microcystin Exposure on the Growth of Microcystin-Producing and Microcystin-Free M. aeruginosa Strains
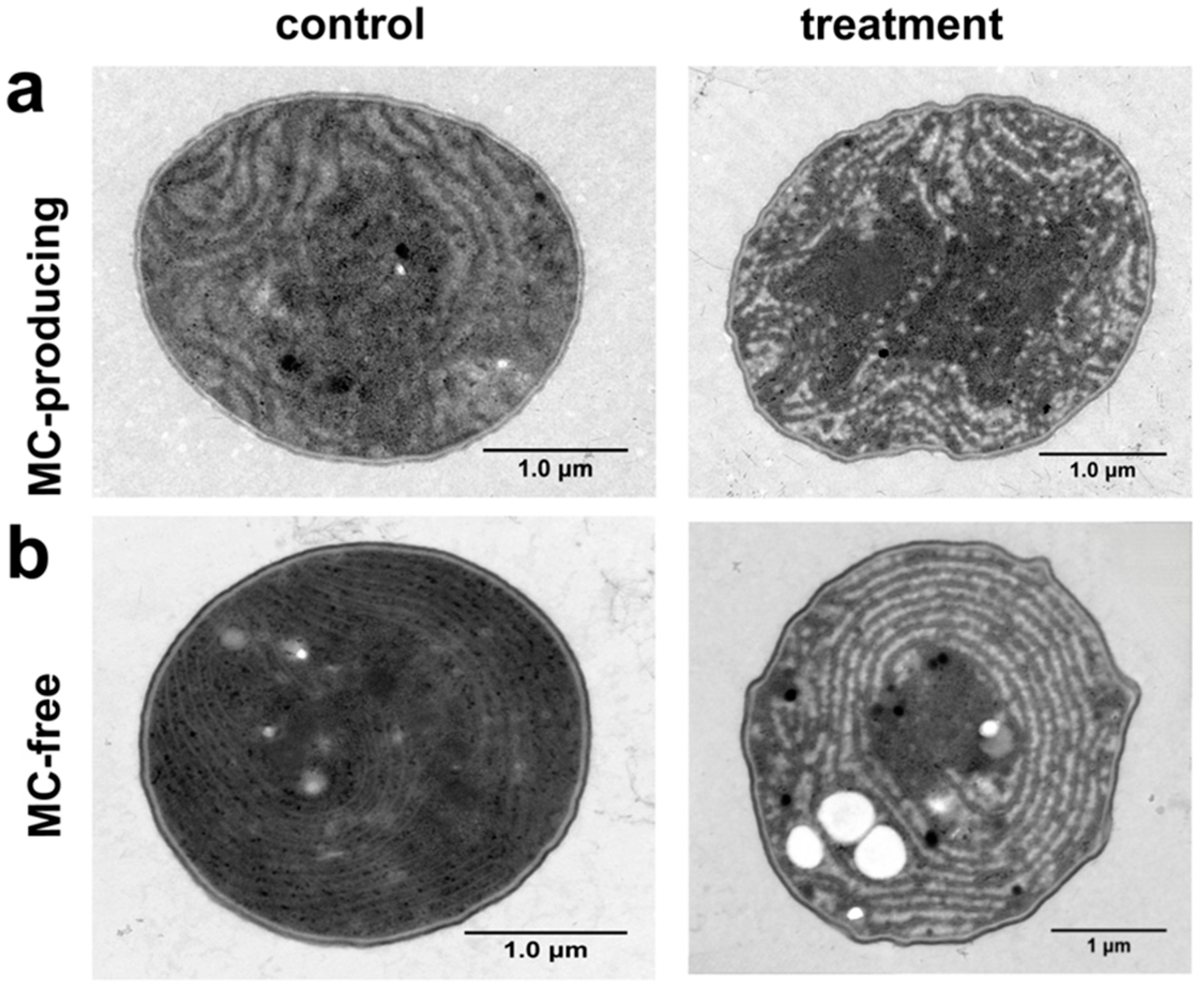
3.1.2. Effects on Cell Morphology
3.1.3. Effects on Chlorophyll a and Photosynthetic System
3.1.4. Impact on Lipid Peroxidation and Antioxidant Enzyme Activities
3.2. Changes in Intracellular Microcystin Concentration
3.3. Effects of Exogenous Microcystin on the Metabolism of M. aeruginosa
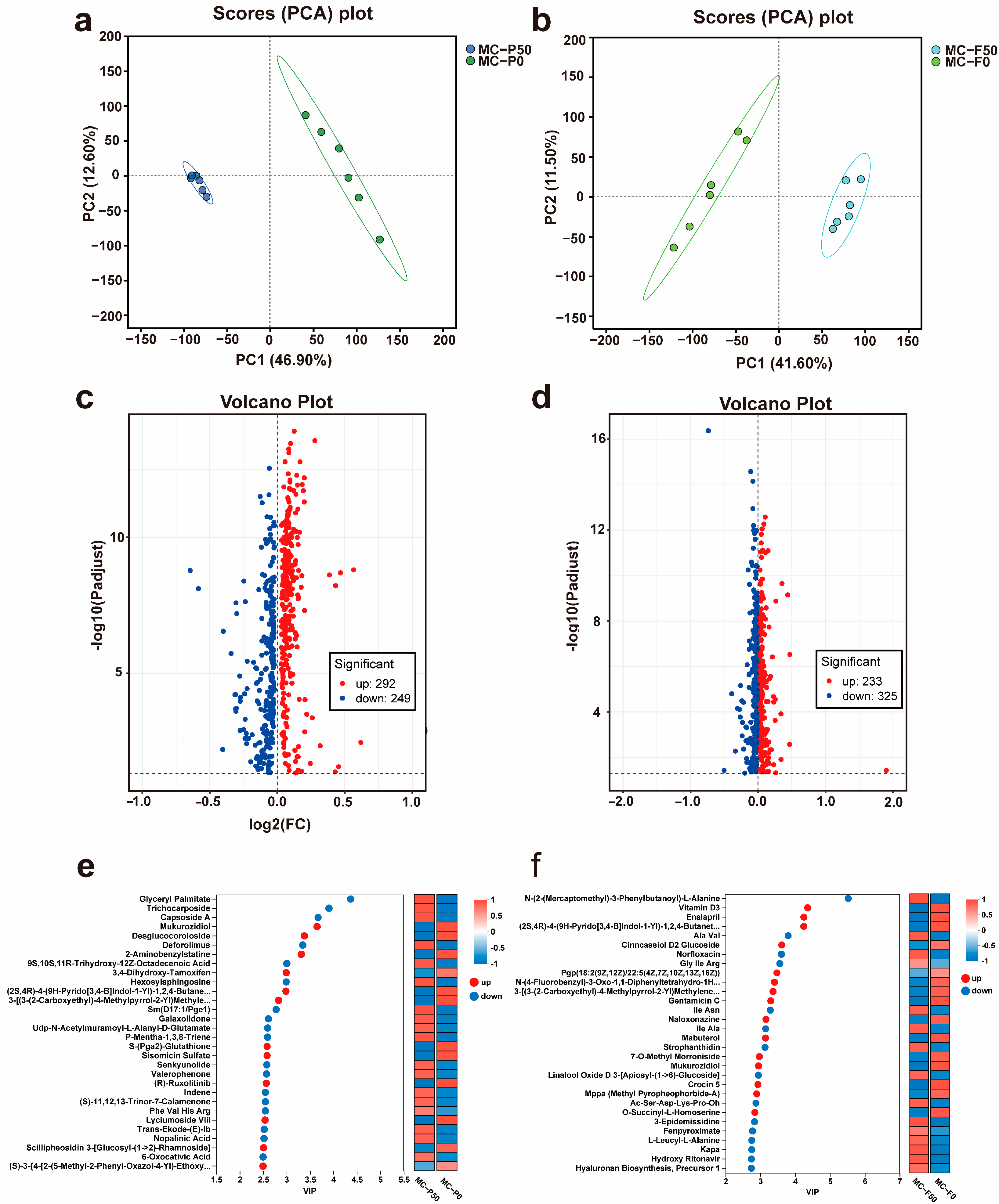
3.3.1. Differentially Expressed Metabolites
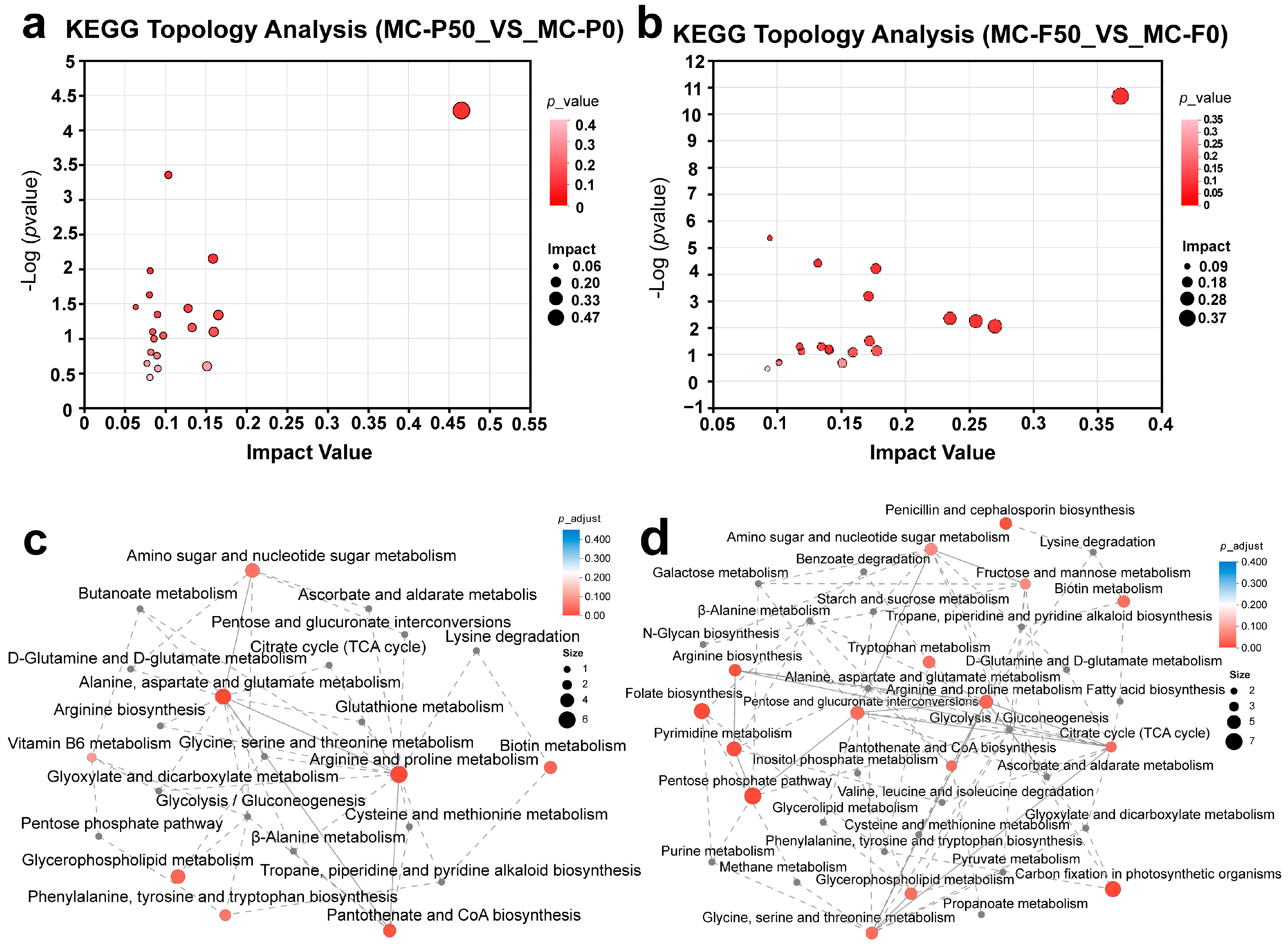
3.3.2. Metabolic Pathway Analysis
4. Discussion
4.1. Impact of Exogenous Microcystin Exposure on Physiological Responses of Microcystin-Producing and Microcystin-Free M. aeruginosa Strains
4.2. Metabolomics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Paerl, H.W.; Xu, H.; McCarthy, M.J.; Zhu, G.; Qin, B.; Li, Y.; Gardner, W.S. Controlling harmful cyanobacterial blooms in a hyper-eutrophic lake (Lake Taihu, China): The need for a dual nutrient (N & P) management strategy. Water Res. 2011, 45, 1973–1983. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.-J.; Sun, X.-D.; Zhu, F.-P.; Feng, Y.; Duan, J.-L.; Xiao, F.; Li, X.-Y.; Shi, Y.; Wang, Q.; Sun, J.-W.; et al. Nanoplastics Promote Microcystin Synthesis and Release from Cyanobacterial Microcystis aeruginosa. Environ. Sci. Technol. 2020, 54, 3386–3394. [Google Scholar] [CrossRef]
- Loftin, K.A.; Clark, J.M.; Journey, C.A.; Kolpin, D.W.; Van Metre, P.C.; Carlisle, D.; Bradley, P.M. Spatial and temporal variation in microcystin occurrence in wadeable streams in the southeastern United States. Toxicol. Environ. Chem. 2016, 35, 2281–2287. [Google Scholar] [CrossRef]
- Wang, Q.; Niu, Y.; Xie, P.; Chen, J.; Ma, Z.; Tao, M.; Qi, M.; Wu, L.; Guo, L. Factors Affecting Temporal and Spatial Variations of Microcystins in Gonghu Bay of Lake Taihu, with Potential Risk of Microcystin Contamination to Human Health. Sci. World J. 2010, 10, 1795–1809. [Google Scholar] [CrossRef]
- Lahti, K.; Rapala, J.; Kivimäki, A.L.; Kukkonen, J.; Niemelä, M.; Sivonen, K. Occurrence of microcystins in raw water sources and treated drinking water of Finnish waterworks. Water Sci. Technol. 2018, 43, 225–228. [Google Scholar] [CrossRef]
- Griffith, A.W.; Doherty, O.M.; Gobler, C.J. Ocean warming along temperate western boundaries of the Northern Hemisphere promotes an expansion of Cochlodinium polykrikoides blooms. Proc. R. Soc. B. 2019, 286, 20190340. [Google Scholar] [CrossRef]
- Brêda-Alves, F.; de Oliveira Fernandes, V.; Cordeiro-Araújo, M.K.; Chia, M.A. The combined effect of clethodim (herbicide) and nitrogen variation on allelopathic interactions between Microcystis aeruginosa and Raphidiopsis raciborskii. Environ. Sci. Pollut. 2020, 28, 11528–11539. [Google Scholar] [CrossRef]
- Wang, S.; Brenner, M.; Curtis, J.H.; Romero-Oliva, C.S.; Dix, M.; Cano, M. Simultaneous Removal of the Freshwater Bloom-Forming Cyanobacterium Microcystis and Cyanotoxin Microcystins via Combined Use of Algicidal Bacterial Filtrate and the Microcystin-Degrading Enzymatic Agent, MlrA. Microorganisms 2021, 9, 1594. [Google Scholar] [CrossRef]
- Waters, M.N.; Brenner, M.; Curtis, J.H.; Romero-Oliva, C.S.; Dix, M.; Cano, M. Harmful algal blooms and cyanotoxins in Lake Amatitlán, Guatemala, coincided with ancient Maya occupation in the watershed. PNAS 2021, 118, e2109919118. [Google Scholar] [CrossRef]
- Cai, W.; MacIsaac, H.J.; Xu, R.; Zhang, J.; Pan, X.; Zhang, Y.; Yang, J.; Dixon, B.; Li, J.; Zi, Y.; et al. Abnormal neurobehavior in fish early life stages after exposure to cyanobacterial exudates. Ecotoxicol. Environ. Saf. 2022, 245, 114119. [Google Scholar] [CrossRef]
- Omidi, A.; Pflugmacher, S.; Kaplan, A.; Kim, Y.J.; Esterhuizen, M. Reviewing Interspecies Interactions as a Driving Force Affecting the Community Structure in Lakes via Cyanotoxins. Microorganisms 2021, 9, 1583. [Google Scholar] [CrossRef]
- Zilliges, Y.; Kehr, J.-C.; Meissner, S.; Ishida, K.; Mikkat, S.; Hagemann, M.; Kaplan, A.; Börner, T.; Dittmann, E. The Cyanobacterial Hepatotoxin Microcystin Binds to Proteins and Increases the Fitness of Microcystis under Oxidative Stress Conditions. PLoS ONE 2011, 6, e17615. [Google Scholar] [CrossRef] [PubMed]
- García-Espín, L.; Cantoral, E.A.; Asencio, A.D.; Aboal, M. Microcystins and cyanophyte extracts inhibit or promote the photosynthesis of fluvial algae. Ecological and management implications. Ecotoxicology 2017, 26, 658–666. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, Y.; Ma, H.; Cui, F. Microcystin-LR (MC-LR) inhibits green algae growth by regulating antioxidant and photosynthetic systems. Harmful Algae 2024, 134, 102623. [Google Scholar] [CrossRef] [PubMed]
- Kurmayer, R.; Christiansen, G.; Chorus, I. The Abundance of Microcystin-Producing Genotypes Correlates Positively with Colony Size in Microcystis sp. and Determines Its Microcystin Net Production in Lake Wannsee. Appl. Environ. 2003, 69, 787–795. [Google Scholar] [CrossRef]
- Phelan, R.R.; Downing, T.G. The localization of exogenous microcystin LR taken up by a non-microcystin producing cyanobacterium. Toxicon 2014, 89, 87–90. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, J.; MacIsaac, H.J.; McKay, R.M.; Xu, R.; Pei, Y.; Zi, Y.; Li, J.; Qian, Y.; Chang, X. Comparative metabolomic analysis of exudates of microcystin-producing and microcystin-free Microcystis aeruginosa strains. Front. Microbiol. 2023, 13, 1075621. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Ban, M.; Wu, Z.; Huang, L.; Wang, Z.; Cheng, Y. Morchella Effectively Removes Microcystins Produced by Microcystis aeruginosa. Microbes Environ. 2024, 39, 23101. [Google Scholar] [CrossRef]
- Luter, H.M.; Gibb, K.; Webster, N.S. Eutrophication has no short-term effect on the Cymbastela stipitata holobiont. Front. Microbiol. 2014, 5, 216. [Google Scholar] [CrossRef]
- Wang, X.; Luo, Y.; Zhang, S.; Zhou, L. Acetylacetone effectively controlled the secondary metabolites of Microcystis aeruginosa under simulated sunlight irradiation. J. Environ. Sci. 2022, 135, 285–295. [Google Scholar] [CrossRef]
- Dai, R.; Li, Z.; Yan, F.; An, L.; Du, W.; Li, X. Evaluation of changes in M. aeruginosa growth and microcystin production under phosphorus starvation via transcriptomic surveys. Sci. Total Environ. 2023, 893, 164848. [Google Scholar] [CrossRef]
- Maqbool, A.; Ali, S.; Rizwan, M.; Ishaque, W.; Rasool, N.; Rehman, M.Z.U.; Bashir, A.; Abid, M.; Wu, L. Management of tannery wastewater for improving growth attributes and reducing chromium uptake in spinach through citric acid application. Environ. Sci. Pollut. 2018, 25, 10848–10856. [Google Scholar] [CrossRef]
- Sang, W.; Du, C.; Ni, L.; Li, S.; Hamad, A.A.A.; Xu, C.; Shao, C. Physiological and molecular mechanisms of the inhibitory effects of artemisinin on Microcystis aeruginosa and Chlorella pyrenoidosa. J. Hazard. Mater. 2024, 470, 134241. [Google Scholar] [CrossRef] [PubMed]
- Metzler, D.M.; Erdem, A.; Tseng, Y.H.; Huang, C.P. Responses of Algal Cells to Engineered Nanoparticles Measured as Algal Cell Population, Chlorophyll a, and Lipid Peroxidation: Effect of Particle Size and Type. J. Nanotechnol. 2012, 2012, 237284. [Google Scholar] [CrossRef]
- Li, B.; Yin, Y.; Kang, L.; Feng, L.; Liu, Y.; Du, Z.; Tian, Y.; Zhang, L. A review: Application of allelochemicals in water ecological restoration—Algal inhibition. Chemosphere 2021, 267, 128869. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, M.; Wang, R.; Salam, M.; Yang, Y.; Zhang, Z.; He, Q.; Hu, X.; Li, H. Potassium regulates cadmium toxicity in Microcystis aeruginosa. J. Hazard. Mater. 2021, 413, 125374. [Google Scholar] [CrossRef]
- García–Nieto, P.J.; García–Gonzalo, E.; Alonso Fernández, J.R.; Díaz Muñiz, C. Forecast of chlorophyll-a concentration as an indicator of phytoplankton biomass in El Val reservoir by utilizing various machine learning techniques: A case study in Ebro river basin, Spain. J. Hydrol. 2024, 639, 131639. [Google Scholar] [CrossRef]
- Sun, Z.-C.; Geng, W.J.; Ren, B.Z.; Zhao, B.; Liu, P.; Zhang, J.W. Responses of the photosynthetic characteristics of summer maize to shading stress. J. Agron. Crop Sci. 2023, 209, 330–344. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, G.F.; Ying, K.Z.; Jin, H.; Song, J.T.; Cai, Z.H. Phycosphere Microbial Succession Patterns and Assembly Mechanisms in a Marine Dinoflagellate Bloom. Appl. Environ. 2019, 85, e00349-19. [Google Scholar] [CrossRef]
- Tang, G.-L.; Li, X.-Y.; Lin, L.-S.; Gu, Z.-Y.; Zeng, F.-J. The Chlorophyll a Fluorescence Characteristic in Different Types of Leaf Senescence in Alhagi sparsifolia. J. Plant Growth Regul. 2016, 35, 952–964. [Google Scholar] [CrossRef]
- Maliba, B.G.; Inbaraj, P.M.; Berner, J.M. The effect of ozone and drought on the photosynthetic performance of canola. J. Integr. Agric. 2018, 17, 1137–1144. [Google Scholar] [CrossRef]
- Hermans, C.; Smeyers, M.; Rodriguez, R.M.; Eyletters, M.; Strasser, R.J.; Delhaye, J.-P. Quality assessment of urban trees: A comparative study of physiological characterisation, airborne imaging and on site fluorescence monitoring by the OJIP-test. J. Plant Physiol. 2003, 160, 81–90. [Google Scholar] [CrossRef]
- Todorenko, D.; Timofeev, N.; Kovalenko, I.; Kukarskikh, G.; Matorin, D.; Antal, T. Chromium effects on photosynthetic electron transport in pea (Pisum sativum L.). Planta 2019, 251, 11. [Google Scholar] [CrossRef]
- Giorio, P.; Sellami, M.H. Polyphasic OKJIP Chlorophyll a Fluorescence Transient in a Landrace and a Commercial Cultivar of Sweet Pepper (Capsicum annuum L.) under Long-Term Salt Stress. Plants 2021, 10, 887. [Google Scholar] [CrossRef]
- Yusuf, M.A.; Kumar, D.; Rajwanshi, R.; Strasser, R.J.; Tsimilli-Michael, M.; Govindjee, n.; Sarin, N.B. Overexpression of γ-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviates abiotic stress: Physiological and chlorophyll a fluorescence measurements. BBA-Bioenergetics 2010, 1797, 1428–1438. [Google Scholar] [CrossRef]
- Kesheri, M.; Kanchan, S.; Sinha, R.P. Responses of antioxidants for resilience to temporal variations in the cyanobacterium Microcystis aeruginosa. S. Afr. J. Bot. 2022, 148, 190–199. [Google Scholar] [CrossRef]
- Ikeda, S.; Matsushima, S.; Okabe, K.; Ishikita, A.; Tadokoro, T.; Enzan, N.; Yamamoto, T.; Sada, M.; Deguchi, H.; Ikeda, M.; et al. P6283Downregulation of Tim44 exacerbates oxidative stress-induced ROS production and cardiomyocytes death by reducing mitochondrial SOD2. Eur. Heart J. 2019, 40 (Suppl. S1), ehz746.0881. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Y.; Guo, J.; Liu, Y. Antioxidative response in leaves and allelochemical changes in root exudates of Ricinus communis under Cu, Zn, and Cd stress. Environ. Sci. Pollut. 2018, 25, 32747–32755. [Google Scholar] [CrossRef]
- Mostafa, I.; Abbas, H.A.; Ashour, M.L.; Yasri, A.; El-Shazly, A.M.; Wink, M.; Sobeh, M. Polyphenols from Salix tetrasperma Impair Virulence and Inhibit Quorum Sensing of Pseudomonas aeruginosa. Molecules 2020, 25, 1341. [Google Scholar] [CrossRef]
- Bosak, T.; Losick, R.M.; Pearson, A. A polycyclic terpenoid that alleviates oxidative stress. PNAS 2008, 105, 6725–6729. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, G.; Xue, H.; Zhang, J.; Wang, Q.; Zhang, Z.; Zhang, B. Metabolite Profile of Xylem Sap in Cotton Seedlings Is Changed by K Deficiency. Front. Plant Sci. 2020, 11, 592591. [Google Scholar] [CrossRef]
- Du, Y.; Wang, J.; Zhu, F.; Mai, D.; Xiang, Z.; Chen, J.; Guo, R. Comprehensive assessment of three typical antibiotics on cyanobacteria (Microcystis aeruginosa): The impact and recovery capability. Ecotoxicol. Environ. Saf. 2018, 160, 84–93. [Google Scholar] [CrossRef]
- Zhang, X.-l.; Zhu, Q.-Q.; Chen, C.-Y.; Xie, B.; Tang, B.-G.; Fan, M.-H.; Hu, Q.-J.; Liao, Z.; Yan, X.-J. The growth inhibitory effects and non-targeted metabolomic profiling of Microcystis aeruginosa treated by Scenedesmus sp. Chemosphere 2023, 338, 139446. [Google Scholar] [CrossRef] [PubMed]
- Katayama, N.; Iwazumi, K.; Suzuki, H.; Osanai, T.; Ito, S. Malic Enzyme, not Malate Dehydrogenase, Mainly Oxidizes Malate That Originates from the Tricarboxylic Acid Cycle in Cyanobacteria. mBio 2022, 13, e02187-22. [Google Scholar] [CrossRef]
- Dao, O.; Kuhnert, F.; Weber, A.P.M.; Peltier, G.; Li-Beisson, Y. Physiological functions of malate shuttles in plants and algae. Trends Plant Sci. 2022, 27, 488–501. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.R. The relationship between carbon-dioxide-limited photosynthetic rate and ribulose-1,5-bisphosphate-carboxylase content in two nuclear-cytoplasm substitution lines of wheat, and the coordination of ribulose-bisphosphate-carboxylation and electron-transport capacities. Planta 1986, 167, 351–358. [Google Scholar] [CrossRef]
- Makino, A.; Mae, T.; Ohira, K. Differences between wheat and rice in the enzymic properties of ribulose-1,5-bisphosphate carboxylase/oxygenase and the relationship to photosynthetic gas exchange. Planta 1988, 174, 30–38. [Google Scholar] [CrossRef]
- Suzuki, Y.; Wada, S.; Kondo, E.; Yamori, W.; Makino, A. Effects of co-overproduction of sedoheptulose-1,7-bisphosphatase and Rubisco on photosynthesis in rice. Soil Sci. Plant Nutr. 2018, 65, 36–40. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, X.; Yao, Z.; Qian, Z.; Wang, Y.; Xian, Q. The effects of exogenous amino acids on production of microcystin variants in Microcystis aeruginosa. Aquat. Toxicol. 2023, 259, 106525. [Google Scholar] [CrossRef]
- Dai, R.; Zhou, Y.; Chen, Y.; Zhang, X.; Yan, Y.; An, D. Effects of arginine on the growth and microcystin-LR production of Microcystis aeruginosa in culture. Sci. Total Environ. 2019, 651, 706–712. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, L.; Chen, C.; Wang, B.; Xie, G.; Wang, B.; Li, X.; Wang, W. Metabolomic Analysis of Both Microcystin-Producing and Microcystin-Free Microcystis aeruginosa Strains in Response to Exogenous Microcystin Exposure. Water 2025, 17, 993. https://doi.org/10.3390/w17070993
Cai L, Chen C, Wang B, Xie G, Wang B, Li X, Wang W. Metabolomic Analysis of Both Microcystin-Producing and Microcystin-Free Microcystis aeruginosa Strains in Response to Exogenous Microcystin Exposure. Water. 2025; 17(7):993. https://doi.org/10.3390/w17070993
Chicago/Turabian StyleCai, Lijuan, Chen Chen, Bingqing Wang, Guoao Xie, Baicai Wang, Xiuling Li, and Wenxia Wang. 2025. "Metabolomic Analysis of Both Microcystin-Producing and Microcystin-Free Microcystis aeruginosa Strains in Response to Exogenous Microcystin Exposure" Water 17, no. 7: 993. https://doi.org/10.3390/w17070993
APA StyleCai, L., Chen, C., Wang, B., Xie, G., Wang, B., Li, X., & Wang, W. (2025). Metabolomic Analysis of Both Microcystin-Producing and Microcystin-Free Microcystis aeruginosa Strains in Response to Exogenous Microcystin Exposure. Water, 17(7), 993. https://doi.org/10.3390/w17070993



