Pesticides in Ground and Surface Water from the Rio Preto Hydrographic Basin, an Important Agricultural Area in the Midwestern Region of Brazil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Sample Collection
2.2. Sample Preparation
2.3. Pesticide Analysis
2.4. UHPLC–MS/MS
2.5. Method Validation
2.6. Ecotoxicological Risk Assessment
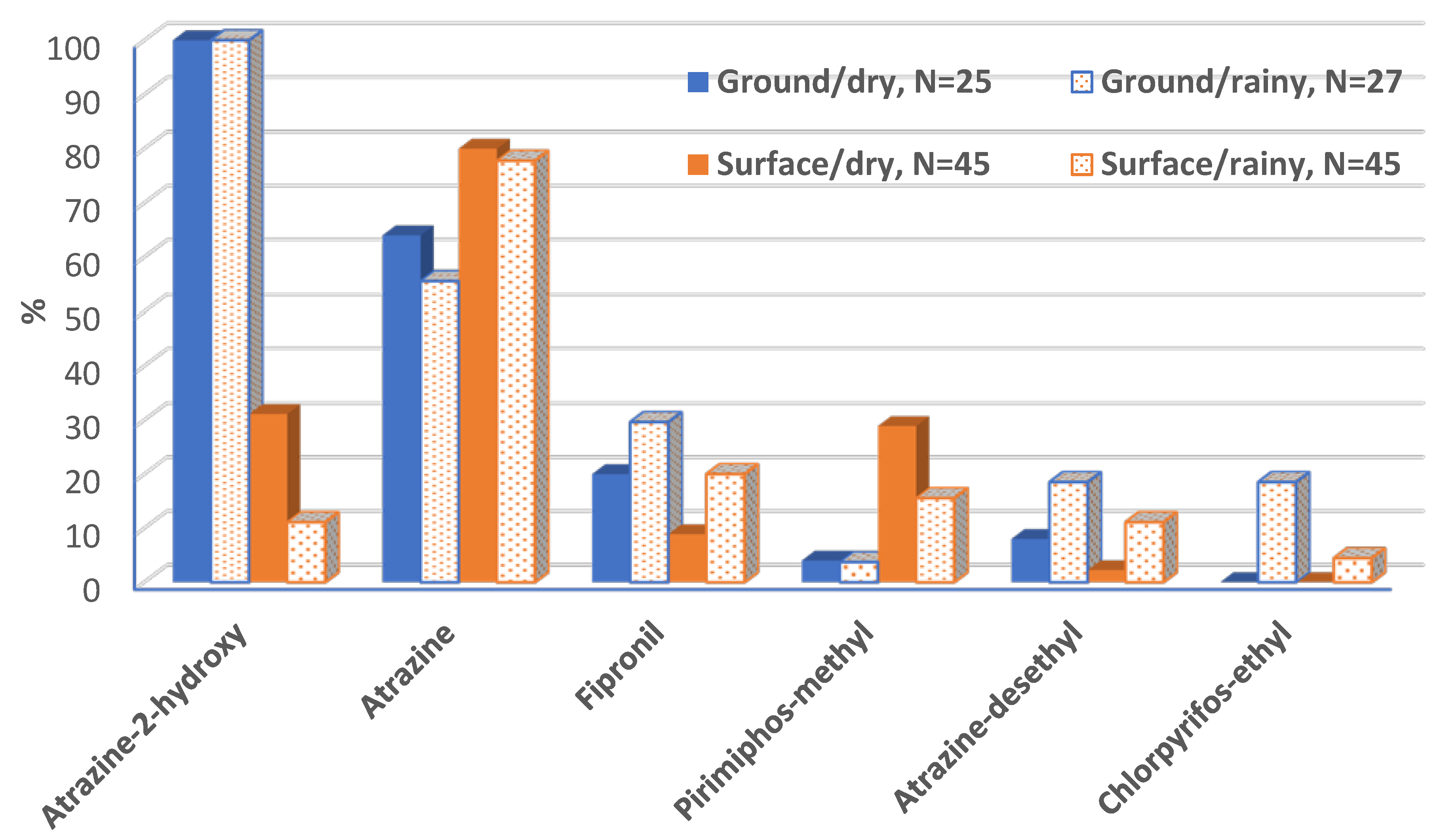
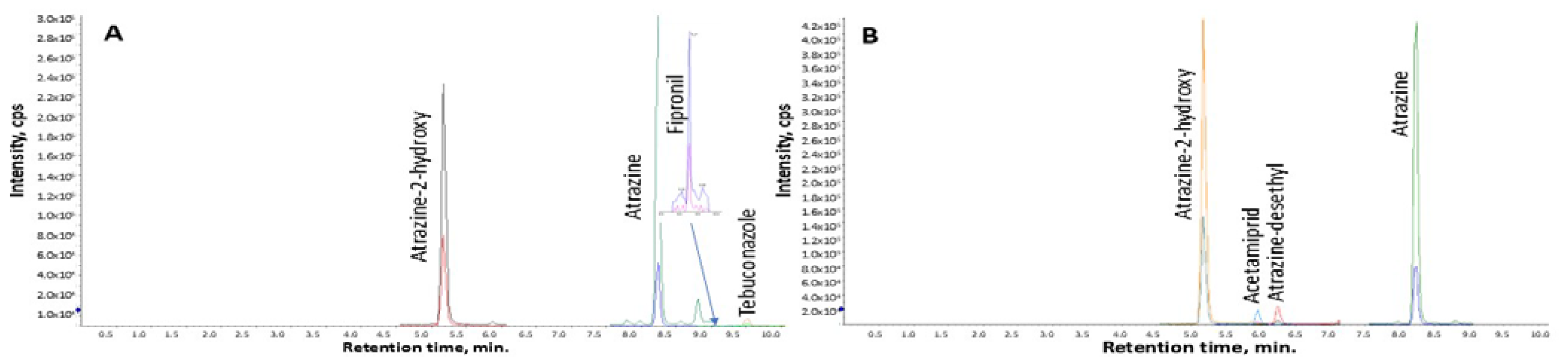
3. Results
3.1. Method Validation
3.2. Analysis of Water Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bahar, N.H.A.; Lo, M.; Sanjaya, M.; Van Vianen, J.; Alexander, P.; Ickowitz, A.; Sunderland, T. Meeting the food security challenge for nine billion people in 2050: What impact on forests? Glob. Environ. Change 2020, 62, 102056. [Google Scholar] [CrossRef]
- FAO. Pesticides Use, Pesticides Trade and Pesticides Indicators, 46th ed.; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Hossini, H.; Asadi, F.; Janjani, H. A systematic review on organochlorine and organophosphorus pesticides content in water resources. Toxin Rev. 2017, 36, 210–221. [Google Scholar] [CrossRef]
- de Araújo, E.P.; Caldas, E.D.; Oliveira-Filho, E.C. Pesticides in surface freshwater: A critical review. Environ. Monit. Assess. 2022, 194, 452. [Google Scholar] [CrossRef]
- Pires, N.L.; Passos, C.J.S.; Morgado, M.G.A.; Mello, D.C.; Infante, C.M.C.; Caldas, E.D. Determination of glyphosate, AMPA and glufosinate by high performance liquid chromatography with fluorescence detection in waters of the Santarém Plateau, Brazilian Amazon. J. Environ. Sci. Health B 2020, 55, 794–802. [Google Scholar] [CrossRef]
- Caldas, E.D.; Coelho, R.; Souza, L.C.K.R.; Silva, S.C. Organochlorine Pesticides in Water, Sediment, and Fish of Paranoá Lake of Brasilia, Brazil. Bull. Environ. Contam. Toxicol. 1999, 62, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Sodré, F.F.; Santana, J.S.; Sampaio, T.R.; Brandão, C.C.S. Seasonal and spatial distribution of caffeine, atrazine, atenolol and deet in surface and drinking waters from the brazilian federal district. J. Braz. Chem. Soc. 2018, 29, 1854–1865. [Google Scholar] [CrossRef]
- Correia, N.M.; Carbonari, C.A.; Velini, E.D. Detection of herbicides in water bodies of the Samambaia River sub-basin in the Federal District and eastern Goiás. J. Environ. Sci. Health B 2020, 55, 574–582. [Google Scholar] [CrossRef]
- Pires, N.L.; de Araújo, E.P.; Oliveira-Filho, E.C.; Caldas, E.D. An ultrasensitive LC-MS/MS method for the determination of glyphosate, AMPA and glufosinate in water—Analysis of surface and groundwater from a hydrographic basin in the Midwestern region of Brazil. Sci. Total Environ. 2023, 875, 162499. [Google Scholar] [CrossRef]
- Issaka, E.; Wariboko, M.A.; Johnson, N.A.N.; Aniagyei, O.N. Advanced visual sensing techniques for on-site detection of pesticide residue in water environments. Heliyon 2023, 9, e13986. [Google Scholar] [CrossRef]
- Caldas, S.S.; Gonçalves, F.F.; Primel, E.G.; Prestes, O.D.; Martins, M.L.; Zanella, R. Modern techniques of sample preparation for pesticide residues determination in water by liquid chromatography with detection by diode array and mass spectrometry. Quim. Nova 2011, 34, 1604–1617. [Google Scholar] [CrossRef]
- Campanale, C.; Massarelli, C.; Losacco, D.; Bisaccia, D.; Triozzi, M.; Uricchio, V.F. The monitoring of pesticides in water matrices and the analytical criticalities: A review. TrAC Trends Anal. Chem. 2021, 144, 116423. [Google Scholar] [CrossRef]
- Pérez-Fernández, V.; Rocca, L.M.; Tomai, P.; Fanali, S.; Gentili, A. Recent Advancements and Future Trends in Environmental Analysis: Sample Preparation, Liquid Chromatography and Mass Spectrometry. Anal. Chim. Acta 2017, 983, 9–41. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, S.; Sun, Q. Research Progress on Lyophilization for Pretreatment of Emerging Organic Contaminants in Environmental Samples. Chin. J. Chromatogr. 2021, 39, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, C.E.; Bellmund, S.; Gardinali, P.R. A Simple Method for Routine Monitoring of Glyphosate and Its Main Metabolite in Surface Waters Using Lyophilization and LC-FLD + MS/MS. Case Study: Canals with Influence on Biscayne National Park. Sci. Total Environ. 2014, 496, 389–401. [Google Scholar] [CrossRef]
- Salles, L.d.A.; Lima, J.E.F.W.; Roig, H.L.; Malaquias, J.V. Environmental factors and groundwater behavior in an agricultural experimental basin of the Brazilian central plateau. Appl. Geogr. 2018, 94, 272–281. [Google Scholar] [CrossRef]
- MAPBIOMAS. Projeto MapBiomas—Coleção [7.0] da Série Anual de Mapas de Cobertura e Uso da Terra do Brasil. MapBiomas. 2022. Available online: https://mapbiomas.org/ (accessed on 8 November 2024).
- SIEG. Sistema Estadual de Geoinformação. Shapefile de Pivôs Centrais. 2015. Available online: http://www.sieg.go.gov.br/ (accessed on 8 November 2024).
- Conselho Nacional do Meio Ambiente (CONAMA). Resolução n° 396. Brazil, 2008. Available online: https://conama.mma.gov.br/?option=com_sisconama&task=arquivo.download&id=545 (accessed on 10 April 2025).
- Conselho Nacional do Meio Ambiente (CONAMA). Resolução n° 357. Brazil. 2005. Available online: https://conama.mma.gov.br/?option=com_sisconama&task=arquivo.download&id=450 (accessed on 10 April 2025).
- Ministério da Saúde. In: Portaria GM/MS Nº 888. Brazil, 2021. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/gm/2021/prt0888_07_05_2021.html (accessed on 13 April 2025).
- INMETRO. Orientação Sobre Validação de Métodos Analíticos. Instituto Nacional de Metrologia, Qualidade e Tecnologia (INMETRO). DOQ-CGCRE-008. 2020. Available online: https://www.gov.br/cdtn/pt-br/assuntos/documentos-cgcre-abnt-nbr-iso-iec-17025/doq-cgcre-008/view (accessed on 10 April 2025).
- SANTE. Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed (SANTE 12682/2019); European Commission Directorate-General for Health and Food Safety: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Umbuzeiro, G.d.A.; de Simone, S.; de Deus, A.C.C.R.; Altafin, L.; Veiga, L.F.; Alves, L.d.S.N.; Castro, M.L.M.P.; Lamparelli, M.C.; von der Ohe, P.; Araujo, R.P.d.A.; et al. Protocolo para Derivação de Critérios de Qualidade da Água para Proteção da Vida Aquática no Brasil. Sociedade Brasileira de Mutagênese, Carcinogese e Tetratogênese Ambiental. 2011. Available online: https://wordpress.ft.unicamp.br/laeg/wp-content/uploads/sites/33/2017/10/Protocolo-Para-Deriva%C3%A7%C3%A3o-de-Crit%C3%A9rios-de-Qualidade-da-%C3%81gua-Para-Prote%C3%A7%C3%A3o-da-Vida-Aqu%C3%A1tica-no-Brasil.pdf (accessed on 13 April 2025).
- Chris, L.-S. Environmental Risk Assessment Guidance Manual for Agricultural and Veterinary Chemicals. Australian Environment Agency Pty Ltd. 2009. Available online: https://www.nepc.gov.au/sites/default/files/2022-09/cmgt-nchem-eragm-agricultural-and-veterinary-chemicals-200902.pdf (accessed on 13 April 2025).
- NORMAN. NORMAN Ecotoxicology Database. 2024. Available online: https://www.norman-network.com/nds/ecotox/ (accessed on 8 November 2024).
- USEPA. Aquatic Life Benchmarks and Ecological Risk Assessments for Registered Pesticides United States Environmental Protection Agency. 2024. Available online: https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/aquatic-life-benchmarks-and-ecological-risk (accessed on 8 November 2024).
- Kawasaki, H.; Shimanouchi, T.; Kimura, Y. Recent Development of Optimization of Lyophilization Process. J. Chem. 2019, 2019, 9502856. [Google Scholar] [CrossRef]
- Nakhjavan, B.; Bland, J.; Khosravifard, M. Optimization of a Multiresidue Analysis of 65 Pesticides in Surface Water Using Solid-Phase Extraction by LC-MS/MS. Molecules 2021, 26, 6627. [Google Scholar] [CrossRef]
- Sinha, N.S.; Vasudev, K.; Rao, M.V.V.; Odetokun, M. Quantification of Organophosphate Insecticides in Drinking Water in Urban Areas Using Lyophilization and High-Performance Liquid Chromatography—Electrospray Ionization-Mass Spectrometry Techniques. Int. J. Mass. Spectrom. 2011, 300, 12–20. [Google Scholar] [CrossRef]
- Mendonça, C.F.R.; Boroski, M.; Cordeiro, G.A.; Toci, A.T. Glyphosate and AMPA Occurrence in Agricultural Watershed: The Case of Paraná Basin 3, Brazil. J. Environ. Sci. Health B 2020, 55, 909–920. [Google Scholar] [CrossRef]
- ANA. Atlas Irrigação: Uso Da Água na Agricultura Irrigada, 2nd ed; Agência Nacional de Águas e Saneamento Básico, Brasília, 2021. Available online: https://portal1.snirh.gov.br/ana/apps/storymaps/stories/a874e62f27544c6a986da1702a911c6b (accessed on 8 November 2024).
- Syafrudin, M.; Kristanti, R.A.; Yuniarto, A.; Hadibarata, T.; Rhee, J.; Al-Onazi, W.A.; Algarni, T.S.; Almarri, A.H.; Al-Mohaimeed, A.M. Pesticides in drinking water—A review. Int. J. Environ. Res. Public Health 2021, 18, 468. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, B.; Yuan, Y.; Wang, S. Spatiotemporal distribution patterns and ecological risk of multi-pesticide residues in the surface water of a typical agriculture area in China. Sci. Total Environ. 2023, 870, 161872. [Google Scholar] [CrossRef] [PubMed]
- Van Opstal, N.V.; Gabioud, E.A.; Seehaus, M.S.; Pighini, R.J.; Repetti, M.R.; Wilson, M.G.; Wingeyer, A.B.; Cuatrin, A.L.; Regaldo, L.M.; Gagneten, A.M.; et al. Spatial distribution of pesticides in surface water of the Estacas stream (Argentine Espinal region) associated with crop production. Environ. Sci. Pollut. Res. Int. 2023, 30, 43573–43585. [Google Scholar] [CrossRef] [PubMed]
- ANVISA. Monografias de agrotóxicos. Agência Nacional de Vigilância Sanitária. 2024. Available online: https://www.gov.br/anvisa/pt-br/setorregulado/regularizacao/agrotoxicos/monografias/monografias-autorizadas-por-letra (accessed on 8 November 2024).
- de Albuquerque, F.P.; de Oliveira, J.L.; Moschini-Carlos, V.; Fraceto, L.F. An overview of the potential impacts of atrazine in aquatic environments: Perspectives for tailored solutions based on nanotechnology. Sci. Total Environ. 2020, 700, 134868. [Google Scholar] [CrossRef]
- Boletins Anuais de Produção, Importação, Exportação e Vendas de Agrotóxicos no Brasil. Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA). Available online: https://www.gov.br/ibama/pt-br/assuntos/quimicos-e-biologicos/agrotoxicos/relatorios-de-comercializacao-de-agrotoxicos#boletinsanuais (accessed on 8 November 2024).
- Vizioli, B.D.C.; da Silva, G.S.; de Medeiros, J.F.; Montagner, C.C. Atrazine and its degradation products in drinking water source and supply: Risk assessment for environmental and human health in Campinas, Brazil. Chemosphere 2023, 336, 139289. [Google Scholar] [CrossRef]
- Urseler, N.; Bachetti, R.; Biolé, F.; Morgante, V.; Morgante, C. Atrazine pollution in groundwater and raw bovine milk: Water quality, bioaccumulation and human risk assessment. Sci. Total Environ. 2022, 852, 158498. [Google Scholar] [CrossRef]
- Montagner, C.C.; Sodré, F.F.; Acayaba, R.D.; Vidal, C.; Campestrini, I.; Locatelli, M.A.; Pescara, I.C.; Albuquerque, A.F.; Umbuzeiro, G.A.; Jardim, W.F. Ten years-snapshot of the occurrence of emerging contaminants in drinking, surface and ground waters and wastewaters from São Paulo State, Brazil. J. Braz. Chem. Soc. 2019, 30, 614–632. [Google Scholar] [CrossRef]
- Brovini, E.M.; Quadra, G.R.; Paranaíba, J.R.; Carvalho, L.; Pereira, R.d.O.; de Aquino, S.F. Occurrence and environmental risk assessment of 22 pesticides in Brazilian freshwaters. Aquat. Toxicol. 2023, 260, 106566. [Google Scholar] [CrossRef]
- Albuquerque, A.F.; Ribeiro, J.S.; Kummrow, F.; Nogueira, A.J.A.; Montagner, C.C.; Umbuzeiro, G.A. Pesticides in Brazilian freshwaters: A critical review. Environ. Sci. Process Impacts 2016, 18, 779–787. [Google Scholar] [CrossRef]
- PPDB. Pesticide Properties DataBase, Agriculture and Environment Research Unit (AERU) at the University of Hertfordshire. 2024. Available online: https://sitem.herts.ac.uk/aeru/ppdb/en/ (accessed on 8 November 2024).
- PubChem. Explore Chemistry Quickly Find Chemical Information from Authoritative Sources, National Institutes of Health (NIH). 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 8 November 2024).

| G | Analytes | Analytical Curve, μg L−1 | Fortification Level After Lyophilization, μg L−1 |
|---|---|---|---|
| 1 | Aldicarb sulfone, ametrine, atrazine, buprofezin, carbofuran, carbosulfan, dicrotophos, difenoconazole, fipronil, malaoxon, monocrotophos, pirimiphos-ethyl, pirimiphos-methyl, trifloxystrobin | P1: 0.05 P2: 0.5 P3: 2.5 P4: 3.5 P5: 5.0 | N1: 0.0125 N2: 0.025 N3: 0.125 N4: 0.175 N5: 0.25 |
| 2 | Azoxystrobin, chlorfenvinphos, diazinon, dimethoate, metalaxyl-M, pirimicarb, pyraclostrobin, pyrazophos, pyridafenthion, thiabendazole, triazophos, zoxamide | P1: 0.20 P2: 2.0 P3: 5.0 P4: 7.0 P5: 10 | N1: 0.05 N2: 0.10 N3: 0.25 N4: 0.35 N5: 0.50 |
| 3 | Acetamiprid, atrazine–desthyl, atrazine-desisopropyl, atrazine-2-hydroxy, boscalid, carbaryl, carbofuran-3-hydroxy, chlorpyrifos-ethyl, cyromazine, EPN, epoxiconazole, ethion, fenpropathrin, fenpyroximate, fluquinconazole, flutriafol, heptenophos, imazalil, imidacloprid, linuron, malation, MCPA, methamidophos, methomyl, myclobutanil, omethoate, paraoxon-methyl, pencycuron, phentoate, profenophos, propanil, quinalphos, tebuconazole, thiamethoxam, thiobencarb, thiophanate-methyl, trichlorfon | P1: 1.0 P2: 10 P3: 20 P4: 40 P5: 50 | N1: 0.05 N2: 0.50 N3: 1.0 N4: 2.0 N5: 2.5 |
| 4 | Dichlorvos, fenitrothion, fenthion, cresoxim-methyl, methiocarb, metribuzim, oxyflurofem, prochloraz, prothiophos, 2,4-D | P1: 14 P2: 20 P3: 40 P4: 80 P5: 100 | N1: 0.70 N2: 1.0 N3: 2.0 N4: 4.0 N5: 5.0 |
| 5 | Acephate, aldicarb, aldicarb sulfoxide, chlorpyrifos-methyl | P1: 50 P2: 100 P3: 200 P4: 400 P5: 500 | N1: 3.0 N2: 5.0 N3: 10 N4: 20 N5: 25 |
| Compound | LOD, μg L−1 | LOQ, μg L−1 | Compound | LOD, μg L−1 | LOQ, μg L−1 |
|---|---|---|---|---|---|
| 2,4-D | 0.15 | 0.7 | Imidacloprid | 0.015 | 0.05 |
| Acephate | 0.75 | 3 | Kresoxim-methyl | 0.15 | 0.7 |
| Acetamiprid | 0.015 | 0.05 | Linuron | 0.015 | 0.05 |
| Aldicarb | 0.75 | (a) | MCPA | 0.015 | 0.05 |
| Aldicarb sulfone | 0.0005 | 0.0125 | Malaoxon | 0.0005 | (a) |
| Aldicarb sulfoxide | 0.75 | 3 | Malathion | 0.015 | (a) |
| Ametryn | 0.0005 | 0.0125 | Metalaxy-M | 0.003 | 0.05 |
| Atrazine-desethyl | 0.015 | 0.05 | Methamidophos | 0.015 | (a) |
| Atrazine | 0.0005 | 0.0125 | Methiocarb | 0.15 | 0.7 |
| Atrazine-deisopropyl | 0.017 | 0.05 | Methomyl | 0.015 | 0.05 |
| Atrazine-2-hydroxy | 0.015 | 0.05 | Metribuzim | 0.15 | 0.7 |
| Azoxystrobin | 0.003 | 0.05 | Monocrotophos | 0.0005 | 0.0125 |
| Boscalid | 0.015 | 0.05 | Myclobutanil | 0.015 | 0.05 |
| Buprofezin | 0.0005 | (a) | Omethoate | 0.015 | 0.05 |
| Carbaryl | 0.015 | 0.05 | Oxyflurofem | 0.15 | (a) |
| Carbofuran | 0.0005 | 0.0125 | Paraoxon-methyl | 0.015 | (a) |
| Carbofuran-3-hydroxy | 0.015 | 0.05 | Pencycuron | 0.015 | 0.05 |
| Carbosulfan | 0.0005 | (a) | Phentoate | 0.015 | (a) |
| Chlorfenvinphos | 0.003 | 0.05 | Pirimicarb | 0.003 | 0.05 |
| Chlorpyrifos-ethyl | 0.015 | (a) | Pirimiphos-ethyl | 0.0005 | (a) |
| Chlorpyrifos-methyl | 0.75 | (a) | Pirimifos-methyl | 0.0005 | (a) |
| Cyromazine | 0.015 | 0.05 | Prochloraz | 0.15 | 0.7 |
| Diazinon | 0.003 | (a) | Profenophos | 0.015 | (a) |
| Dichorvos | 0.15 | (a) | Propanil | 0.015 | 0.05 |
| Dicrotophos | 0.0005 | 0.0125 | Prothiophos | 0.15 | (a) |
| Difenoconazole | 0.0005 | 0.0125 | Pyraclostrobin | 0.003 | 0.05 |
| Dimethoate | 0.003 | 0.05 | Pyrazofos | 0.003 | 0.05 |
| EPN | 0.015 | (a) | Pyridafenthion | 0.003 | 0.05 |
| Epoxiconazole | 0.015 | 0.05 | Quinalphos | 0.015 | (a) |
| Ethion | 0.015 | (a) | Tebuconazole | 0.015 | 0.05 |
| Fenitrothion | 0.15 | (a) | Thiabendazole | 0.003 | 0.05 |
| Fenpropathrin | 0.015 | (a) | Thiamethoxam | 0.015 | 0.05 |
| Fenpyroximate | 0.015 | 0.05 | Thiobencarb | 0.015 | (a) |
| Fenthion | 0.15 | (a) | Thiophanate-methyl | 0.015 | (a) |
| Fluquinconazole | 0.015 | (a) | Trichlorfon | 0.015 | (a) |
| Fipronil | 0.0005 | 0.0125 | Trifloxystrobin | 0.001 | 0.0125 |
| Flutriafol | 0.015 | 0.05 | Triazophos | 0.003 | 0.05 |
| Heptenophos | 0.015 | (a) | Zoxamide | 0.003 | 0.05 |
| Imazalil | 0.015 | 0.05 |
| Campaign, Sample (Sampling Point) | Concentration a, µg L−1 | Risk Assessment | |
|---|---|---|---|
| End Point (PNEC)/SF | RQ | ||
| Atrazine-2-hydroxy | |||
| A, surface, dry (P1) A, surface, dry (P2) | 0.171 0.179 | Algae EC50 164.2 b (0.164)/1000 | 1.04 1.09 |
| E, ground, dry (P11GW) E, ground, dry (P17GW) E, ground, dry (P18GW) E, ground, dry (P20) E, ground, dry (P46) E, ground, dry (P57) | 0.187 0.291 0.168 0.109 0.102 0.102 | NA | |
| F, ground, rainy (P55) | 0.153 | ||
| Atrazine | |||
| A, surface, dry (P13) B, surface, dry (P13) | 0.039 0.022 | Fish NOEAC 5 b (0.5)/10 | 0.08 0.04 |
| E, surface, dry (PS5) E, surface, dry (PS6) | 0.016 0.017 | Fish NOEAC 5 b (0.5)/10 | 0.03 0.03 |
| E, ground, dry (P17GW) E, ground, dry (P18GW) E, ground, dry (P25) E, ground, dry (P46) E, ground, dry (P55) E, ground, dry (PT) | 0.305 0.159 0.025 0.166 0.075 0.017 | NA | |
| F, ground, rainy (P10GW) F, ground, rainy (P25) F, ground, rainy (P27) F, ground, rainy (PT) | 0.014 0.020 0.026 0.015 | ||
| 2,4-D | |||
| E, ground, dry (P54) E, ground, dry (PT) | 0.913 1.045 | NA | |
| Reference | Analyte (LOQ, µg L−1) | Sample Preparation a; Detection | Recovery, % |
|---|---|---|---|
| Present study | 77 pesticides, validated for 49 (0.0125 to 3) | 10 mL sample, resuspended in 500 μL MeOH–water (1:1), UPHLC–MS/MS | 30 to 100 |
| [31] | 8 pesticides (0.016–0.171) | 5 mL sample, resuspended with 1 mL ACN; HPLC–MS/MS | 96–103 |
| [16] | Glyphosate and AMPA (LOD: 0.058 and 0.108) | 40 mL sample, resuspended with EDTA:FMOC-Cl; LC-FLD + MS/MS | 63–69 |
| [6] | Glyphosate (0.2) and glufosinate (0.07) | 5 mL sample, resuspended with 500 µL water; HPLC-FL | 72–94 |
| [32] | Glyphosate and AMPA (0.3) | 10 mL of sample, resuspended in 2 mL water/240 µL borate buffer/800 µL ACN +120 µL FMOC-Cl; UHPLC-FL | 70–99 |
| [10] | Glyphosate, AMPA and glufosinate (0.0025) | 10 mL sample, resuspended in 500 μL 50 mM ammonium formate (pH 2.9); LC–MS/MS | 79–111 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pires, N.L.; Araújo, E.P.d.; Muniz, D.H.d.F.; Oliveira-Filho, E.C.; Caldas, E.D. Pesticides in Ground and Surface Water from the Rio Preto Hydrographic Basin, an Important Agricultural Area in the Midwestern Region of Brazil. Water 2025, 17, 1186. https://doi.org/10.3390/w17081186
Pires NL, Araújo EPd, Muniz DHdF, Oliveira-Filho EC, Caldas ED. Pesticides in Ground and Surface Water from the Rio Preto Hydrographic Basin, an Important Agricultural Area in the Midwestern Region of Brazil. Water. 2025; 17(8):1186. https://doi.org/10.3390/w17081186
Chicago/Turabian StylePires, Nayara Luiz, Esmeralda Pereira de Araújo, Daphne Heloisa de Freitas Muniz, Eduardo Cyrino Oliveira-Filho, and Eloisa Dutra Caldas. 2025. "Pesticides in Ground and Surface Water from the Rio Preto Hydrographic Basin, an Important Agricultural Area in the Midwestern Region of Brazil" Water 17, no. 8: 1186. https://doi.org/10.3390/w17081186
APA StylePires, N. L., Araújo, E. P. d., Muniz, D. H. d. F., Oliveira-Filho, E. C., & Caldas, E. D. (2025). Pesticides in Ground and Surface Water from the Rio Preto Hydrographic Basin, an Important Agricultural Area in the Midwestern Region of Brazil. Water, 17(8), 1186. https://doi.org/10.3390/w17081186











