Phytoplankton and Zooplankton Community Dynamics in an Alpine Reservoir: Environmental Drivers and Ecological Implications in Daqing Reservoir, China
Abstract
:1. Introduction
2. Materials and Methods
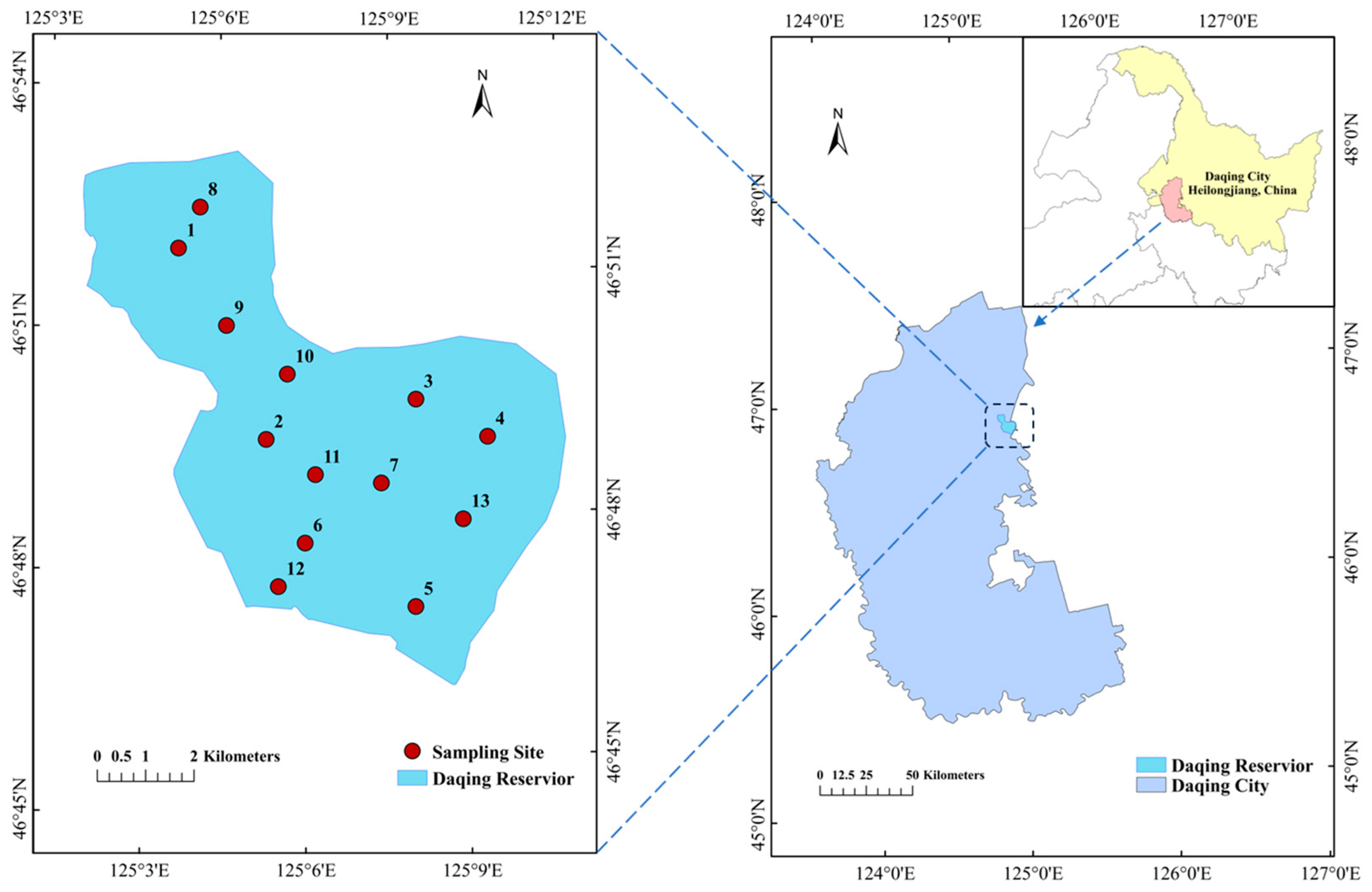
2.1. Sampling Point Setup
2.2. Sample Collection and Analysis Methods
2.2.1. Water Sample Collection and Water Quality Indicator Measurement
2.2.2. Phytoplankton Sample Collection
2.3. Data Analysis
3. Results and Analysis
3.1. Reservoir Water Quality Characteristics
3.2. Phytoplankton Community Characteristics
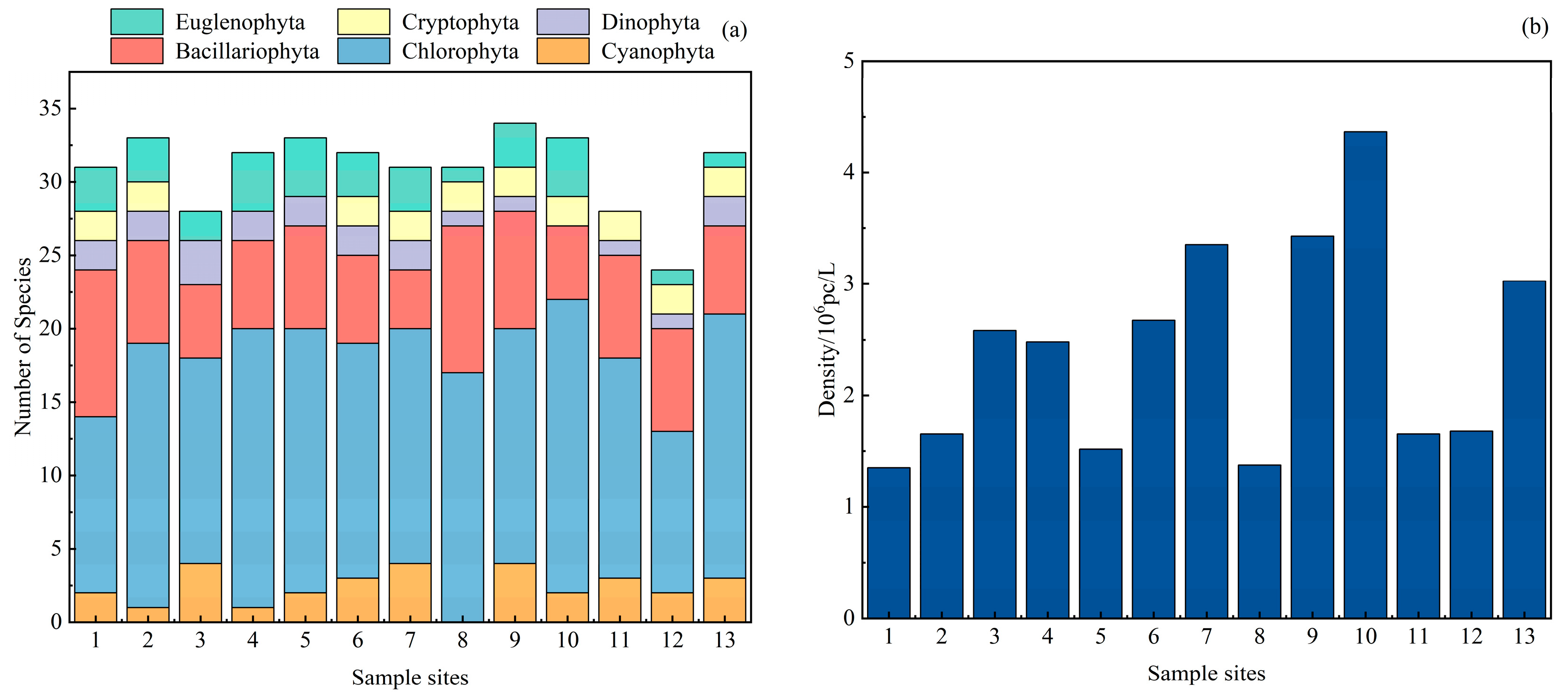
3.2.1. Phytoplankton Species Composition and Density
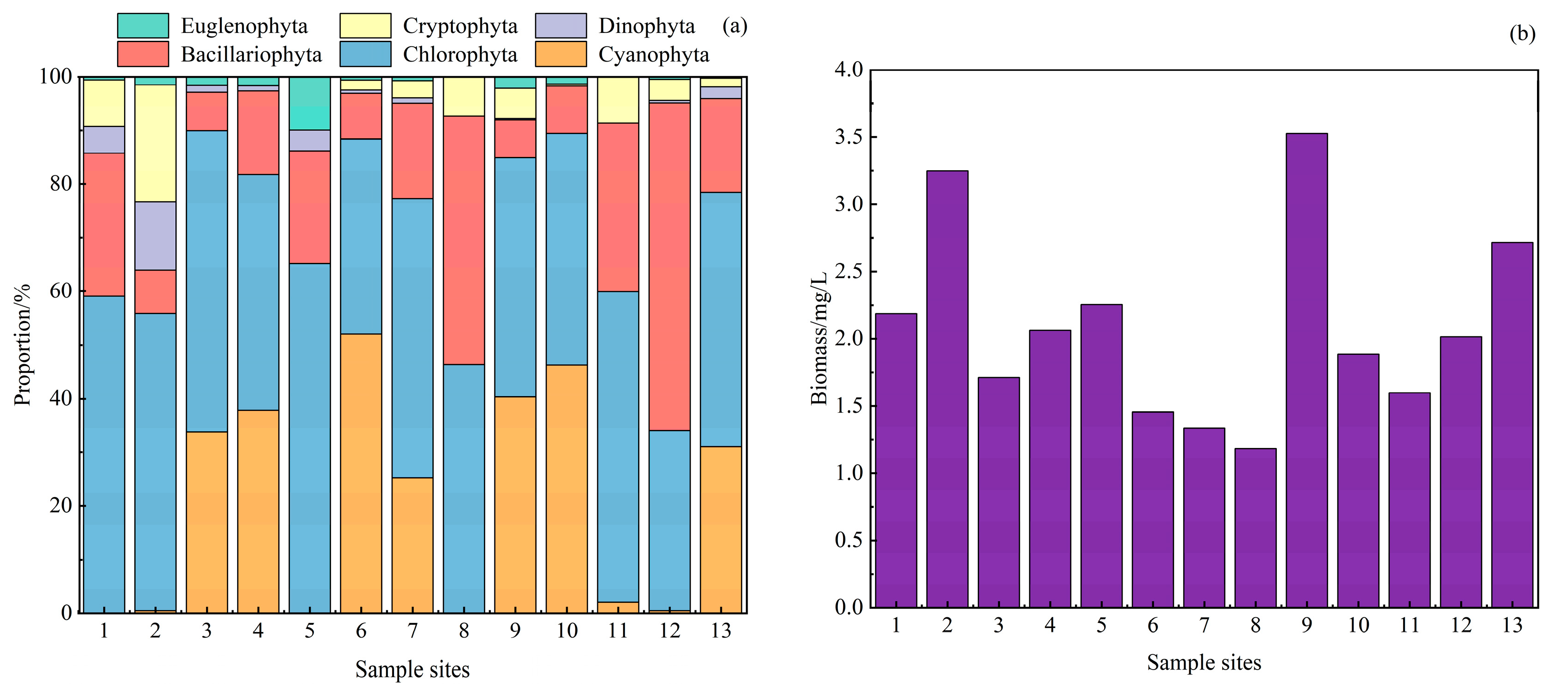
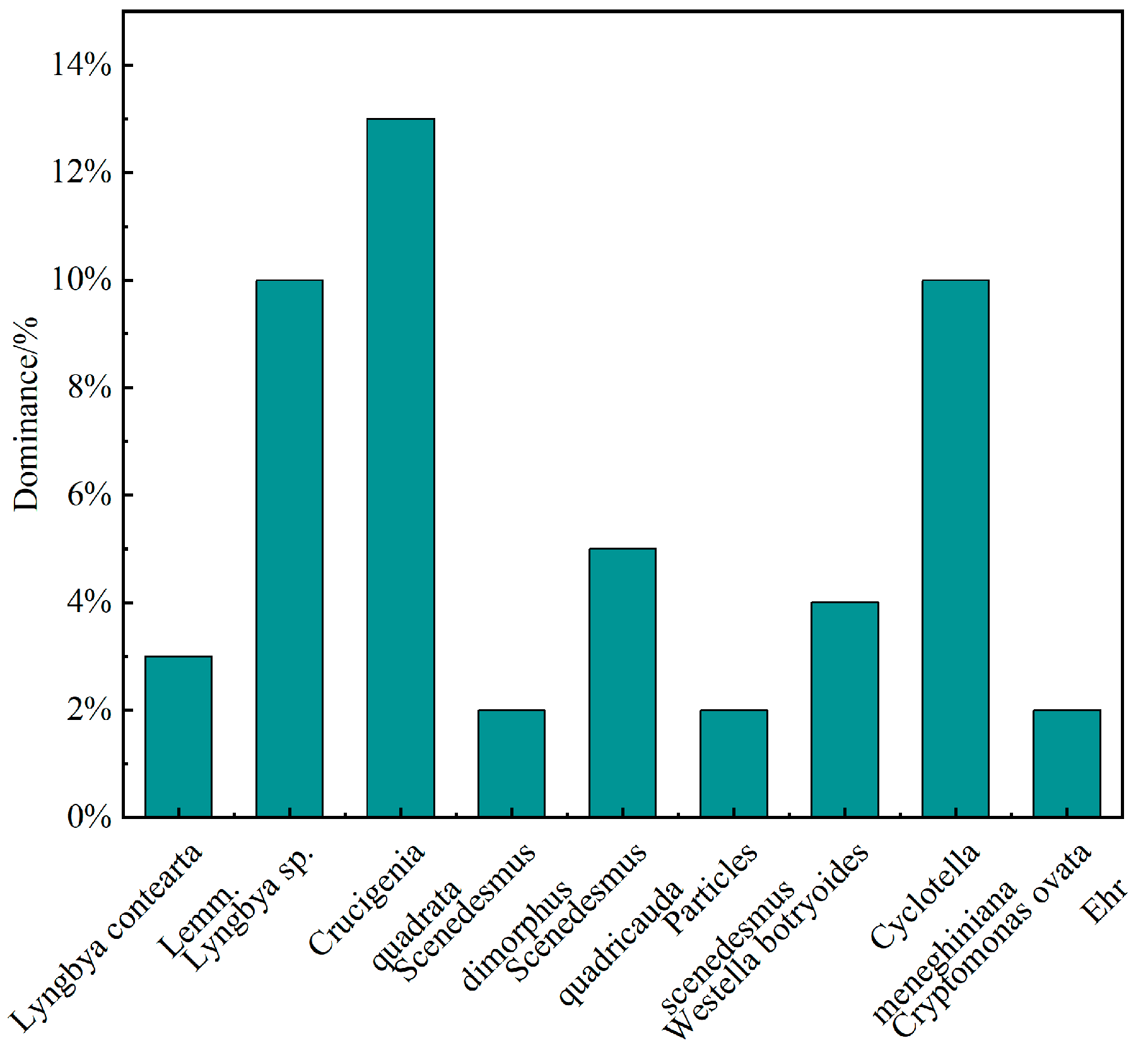
3.2.2. Phytoplankton Dominance and Diversity
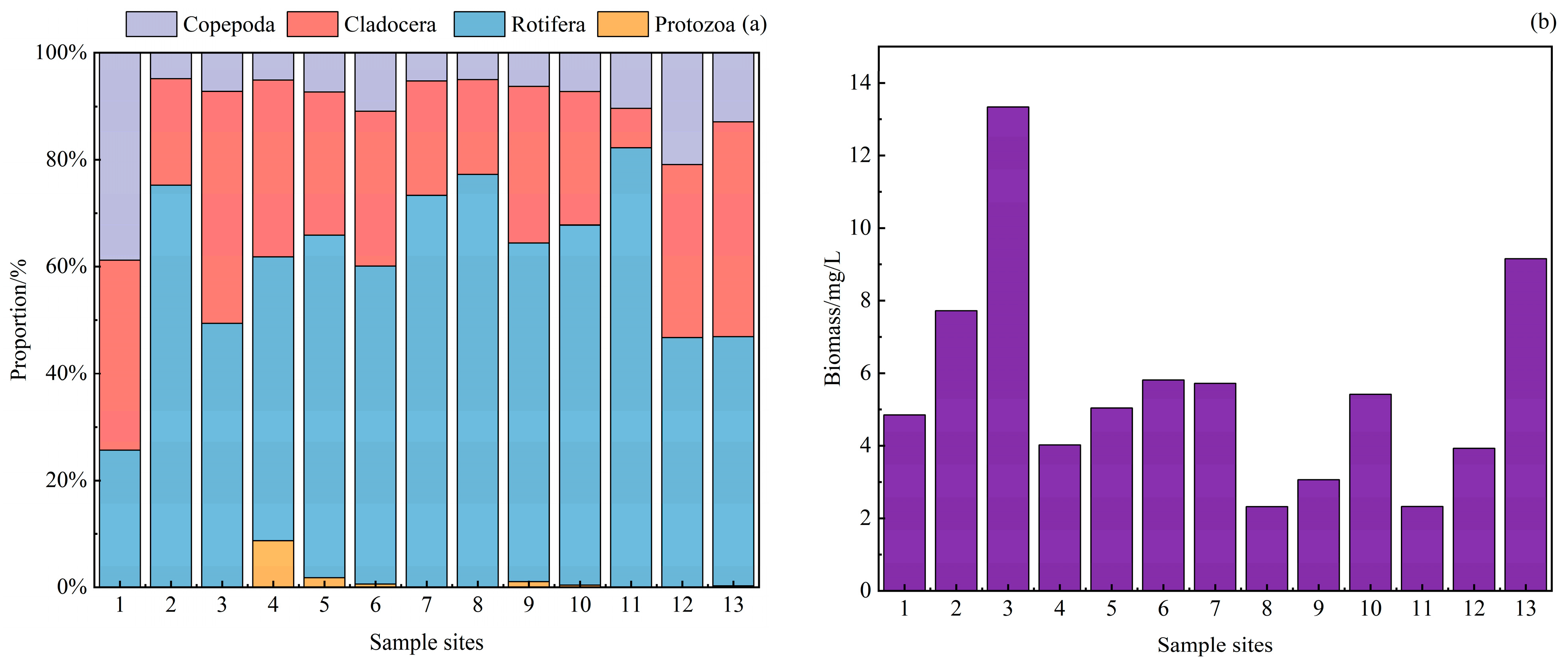
3.3. Zooplankton Community Characteristics
3.3.1. Zooplankton Species Composition and Density
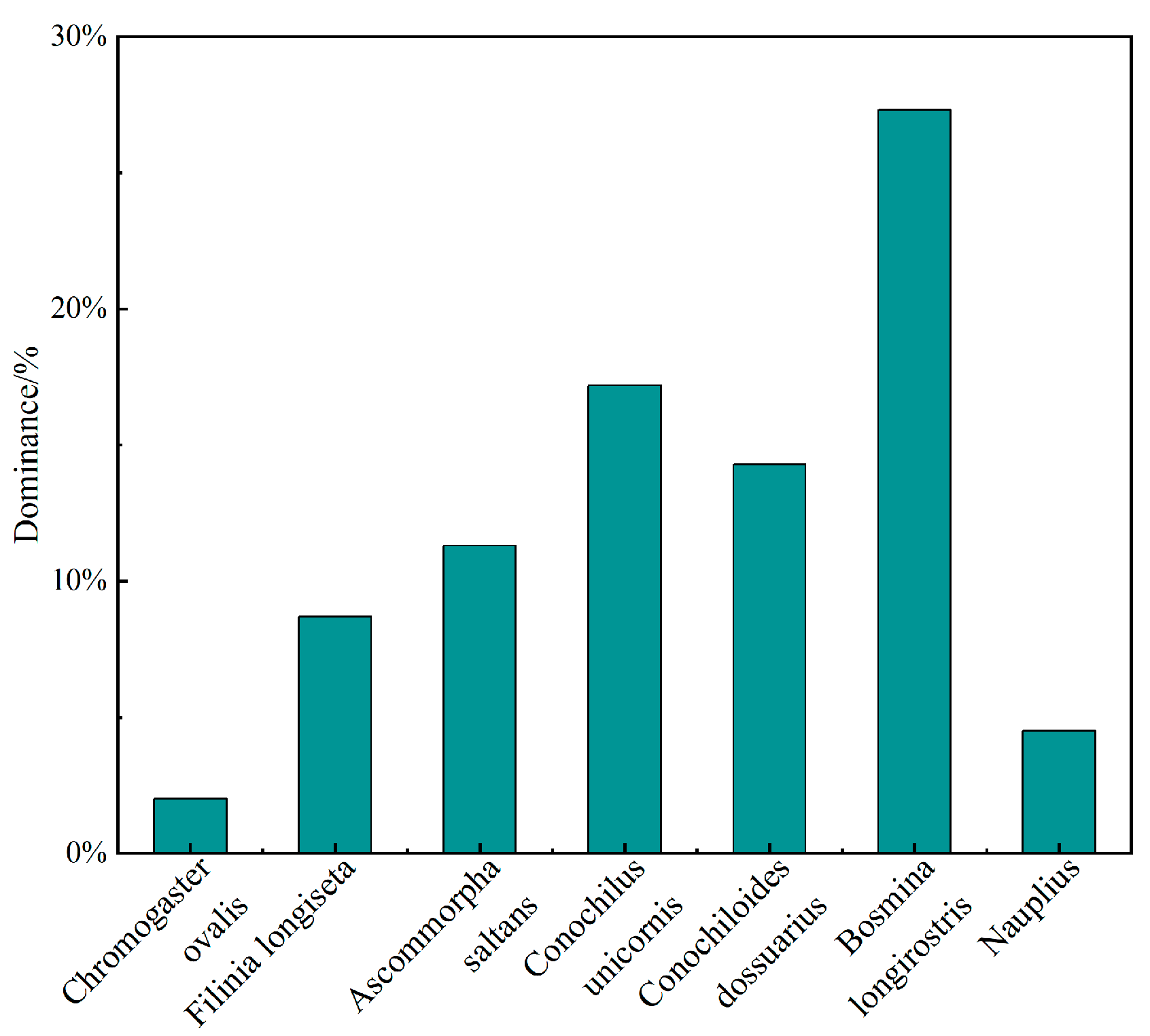
3.3.2. Zooplankton Dominance and Diversity
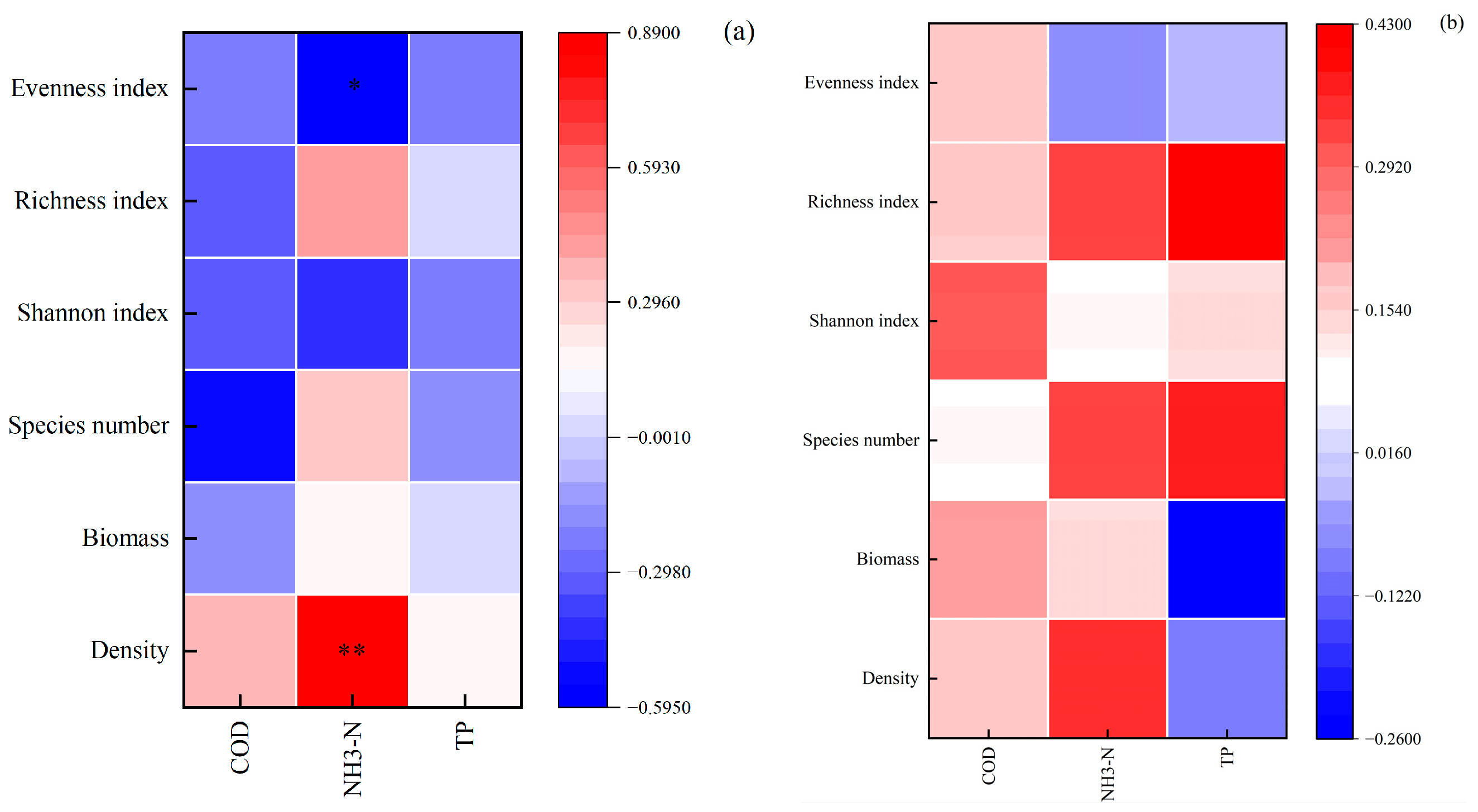
3.4. Relationship Between Plankton and Environmental Factors
4. Discussion
4.1. Analysis of Plankton Community Structure Characteristics
4.2. Analysis of Environmental Influencing Factors on Plankton Communities
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amorim, C.A.; Moura, A.D.N. Ecological impacts of freshwater algal blooms on water quality, plankton biodiversity, structure, and ecosystem functioning. Sci. Total Environ. 2021, 758, 143605. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, E.; Zhang, L.; Wei, H.; Zhang, L.; Wang, Z. Long-term succession characteristics and driving factors of zooplankton communities in a typical subtropical shallow lake, central China. Environ. Sci. Pollut. Res. 2023, 30, 49435–49449. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zeng, G.; Liang, J.; Huang, L.; Hua, S.; Li, F.; Zhu, Y.; Wu, H.; Liu, J.; He, X.; et al. Variation of water level in Dong-ting Lake over a 50-year period: Implications for the impacts of anthropogenic and climatic factors. J. Hydrol. 2015, 525, 450–456. [Google Scholar] [CrossRef]
- Yang, Y.X.; Du, C.Y.; Qian, Z.; Jiang, C.B.; Chen, H.; Yu, G.L.; Li, Y.J. Phytoplankton community structure and its influencing factors in Nanhan polder area of Dongting Lake. Res. Environ. Sci. 2020, 33, 147–154. [Google Scholar]
- Wang, R.; Wang, J.; Chen, W. The coordinated development of ecosystem services and farming household livelihood security: A case study of the dongting lake area in China. Sustainability 2023, 15, 11121. [Google Scholar] [CrossRef]
- Liu, X.; Qian, K.; Chen, Y.; Gao, J. A comparison of factors influencing the summer phytoplankton biomass in China’s three largest freshwater lakes: Poyang, Dongting, and Taihu. Hydrobiologia 2017, 792, 283–302. [Google Scholar] [CrossRef]
- Wang, C.M.; Wu, K.; Zhang, Y.; Huang, D.; Tian, Q. The analysis of spatial and temporal variations characteristics and driving factors of phytoplankton in Dongting Lake. Freshw. Fish. 2018, 48, 52–57. [Google Scholar]
- Wang, M.Q.; Wang, J.C.; Wang, Q.; Yang, C.Y.; Zou, Z.H.; Qian, B. Characteristics of plankton community structure and eutrophication status in Dongting Lake in the season with normal water level. Chin. J. Ecol. 2018, 37, 2418–2429. [Google Scholar]
- Tian, Y.; Jiang, Y.; Liu, Q.; Xu, D.; Liu, Y.; Song, J. The impacts of local and regional factors on the phytoplankton community dynamics in a temperate river, northern China. Ecol. Indic. 2018, 123, 107352. [Google Scholar] [CrossRef]
- Yan, G.; Yin, X.; Wang, X.; Wang, L.; Li, Y.; Li, H.; Chen, W. Effects of environmental factors on the composition of phytoplankton community in Sankou of the Yangtze River and the Western Dongting Lake. China Environ. Sci. 2019, 39, 2532–2540. [Google Scholar]
- Richter, D.J.; Watteaux, R.; Vannier, T.; Leconte, J.; Frémont, P.; Reygondeau, G.; Maillet, N.; Henry, N.; Benoit, G.; Da Silva, O.; et al. Genomic evidence for global ocean plankton biogeography shaped by large-scale current systems. eLife 2022, 11, e78129. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Xiao, Y.; Xie, Y.; Luo, H.; Liu, J.; Xu, J.; Wu, X.; Chen, D.; Niu, Y. Structural characteristics of plankton community in Dongting Lake and its relationship with water environmental factors. Sci. Rep. 2024, 14, 28189. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.M.S.; Tagde, J.P.; Singh, P.R.; Dutta, S.; Sangolkar, L.N.; Kumar, M.S. Impact of Port and harbour activities on plankton distribution and dynamics: A multivariate approach. Mar. Pollut. Bull. 2021, 165, 112105. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Li, M.; Yu, R.; Sun, H.; Zhang, L.; Sun, L.; Lv, C.; Xu, J. Response of planktonic diversity and stability to environmental drivers in a shallow eutrophic lake. Ecol. Indic. 2022, 144, 109560. [Google Scholar] [CrossRef]
- Kim, H.; Kwak, I. Evaluating the necessity of geographical locality for patterning biological integrity and its responses to multiple stressors in river systems. Ecol. Indic. 2022, 142, 109285. [Google Scholar] [CrossRef]
- McQuatters-Gollop, A.; Stern, R.F.; Atkinson, A.; Best, M.; Bresnan, E.; Creach, V.; Devlin, M.; Holland, M.; Ostle, C.; Schmidt, K.; et al. The silent majority: Pico- and nanoplankton as ecosystem health indicators for marine policy. Ecol. Indic. 2024, 159, 111650. [Google Scholar] [CrossRef]
- Hering, D.; Borja, A.; Jones, J.; Pont, D.; Boets, P.; Bouchez, A.; Bruce, K.; Drakare, S.; Hänfling, B.; Kahlert, M.; et al. Implementation options for DNA-based identification into ecological status assessment under the European Water Framework Directive. Water Res. 2018, 138, 192–205. [Google Scholar] [CrossRef]
- Rock, L.A.; Collins, S.M.A. Broad-Scale Look at Nutrient Limitation and a Shift toward Co-limitation in United States Lakes. Environ. Sci. Technol. ES&T 2024, 58, 11482–11491. [Google Scholar]
- Zhao, W.; Dai, L.; Chen, X.; Wu, Y.; Sun, Y.; Zhu, L. Characteristics of zooplankton community structure and its relationship with environmental factors in the South Yellow Sea. Mar. Pollut. Bull. 2022, 176, 113471. [Google Scholar] [CrossRef]
- Wan, Y.; Huang, G.; Du, H.; Yang, S.; Yang, W.; Li, W. Effects of waterway regulation structures on the planktonic community in the upper Yangtze River. Ecol. Indic. 2023, 155, 111049. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, H.; Sun, Y.; Yang, Q.; Fan, J. Contrasting the assembly of phytoplankton and zooplankton communities in a polluted semi-closed sea: Effects of marine compartments and environmental selection. Environ. Pollut. 2021, 285, 117256. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Wen, L.; Gao, L.; Zhang, J.; Zhang, H.; Yang, S.; Hu, E.; Deng, J.; Xiao, M.; Zamyadi, A.; et al. Exploring intrinsic distribution of phytoplankton relative abundance and biomass in combination with continental-scale field investigation and microcosm experiment. Water Res. 2023, 248, 120853. [Google Scholar] [CrossRef]
- Wei, Q.; Xu, Y.; Ruan, A. Spatial and temporal patterns of phytoplankton community succession and characteristics of realized niches in Lake Taihu, China. Environ. Res. 2024, 243, 117896. [Google Scholar] [CrossRef]
- Park, H.; Shabarova, T.; Salcher, M.M.; Kosová, L.; Rychtecký, P.; Mukherjee, I.; Šimek, K.; Porcal, P.; Seďa, J.; Znachor, P.; et al. In the right place, at the right time: The integration of bacteria into the Plankton Ecology Group model. Microbiome 2023, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.L.; Granek, E.F. Drivers of zooplankton community composition in a novel ecosystem: Hawai’i mangroves as a case study. J. Appl. Ecol. 2021, 58, 3023–3035. [Google Scholar] [CrossRef]
- Li, Z.; Gao, Y.; Wang, S.; Lu, Y.; Sun, K.; Jia, J.; Wang, Y. Phytoplankton community response to nutrients along lake salinity and altitude gradients on the Qinghai-Tibet Plateau. Ecol. Indic. 2021, 128, 107848. [Google Scholar] [CrossRef]
- Hu, M.; Zhu, Y.; Hu, X.; Zhu, B.; Lyu, S.; Wang, G. Assembly mechanism and stability of zooplankton communities affected by China’s south-to-north water diversion project. J. Environ. Manag. 2024, 365, 121497. [Google Scholar] [CrossRef]
- Zhang, Q.; Cai, Y.; Yuan, Q.; Yang, J.; Dong, R.; Gong, Z.; Datry, T.; Qin, B. Hydrological conditions determine the assembly processes of zooplankton in the largest Yangtze River-connected Lake in China. J. Hydrol. 2024, 645, 132252. [Google Scholar] [CrossRef]
- Cicala, F.; Arteaga, M.C.; Herzka, S.Z.; Hereu, C.M.; Jimenez-Rosenberg, S.P.; Saavedra-Flores, A.; Robles-Flores, J.; Gomez, R.; Batta-Lona, P.G.; Galindo-Sánchez, C.E. Environmental conditions drive zooplankton community structure in the epipelagic oceanic water of the southern Gulf of Mexico: A molecular approach. Mol. Ecol. 2022, 31, 546–561. [Google Scholar] [CrossRef]
- Bergström, A.; Creed, I.F.; Paltsev, A.; De Wit, H.A.; Lau, D.C.P.; Drakare, S.; Vrede, T.; Isles, P.D.F.; Jonsson, A.; Geibrink, E.; et al. Declining calcium concentration drives shifts toward smaller and less nutritious zooplankton in northern lakes. Glob. Change Biol. 2024, 30, e17220. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Jian, S.; Miao, S.; Li, K.; Guan, H.; Mao, Y.; Wang, Z.; Li, C. Hydrodynamics regulate longitudinal plankton community structure in an alpine cascade reservoir system. Front. Microbiol. 2021, 12, 749888. [Google Scholar] [CrossRef] [PubMed]
- Goździejewska, A.M.; Cymes, I.; Glińska-Lewczuk, K. Zooplankton functional diversity as a bioindicator of freshwater ecosystem health across land use gradient. Sci. Rep. 2024, 14, 18456. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Ding, D.; Gu, T.; Jiang, T.; Qu, K.; Sun, J.; Cui, Z. Different responses of phytoplankton and zooplankton communities to current changing coastal environments. Environ. Res. 2022, 215, 114426. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, Y.; Shen, Y.; Liu, Y.; Gao, H.; Guo, P. Driving mechanism of land use and landscape pattern to phytoplankton and zooplankton community and their trophic interactions in river ecosystems. J. Environ. Manag. 2024, 370, 122691. [Google Scholar] [CrossRef]
- Choo, S.; Kwak, M.T.; Cho, Y.K.; Yoon, Y.H.; Soh, H.Y. Effects of water masses on the zooplankton community structure in the northern East China Sea during the East Asian Summer Monsoon in 2020. Ecol. Indic. 2023, 154, 110847. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, D.; Zeng, J.; Mao, Z.; Gu, X.; Wu, Q.L. Evaluating the effects of aquaculture on the freshwater lake from the perspective of plankton communities: The diversity, co-occurrence patterns and their underlying mechanisms. Environ. Pollut. 2022, 309, 119741. [Google Scholar] [CrossRef] [PubMed]
- Vezhnavets, V.V.; Kouraev, A.V.; Gukasyan, E.K.; Gabrielyan, B.K. Zooplankton Study of Lake Sevan as an Indicator of Ecosystem Stability in the Context of Global Climate Change. Inland Water Biol. 2024, 17, 48–58. [Google Scholar] [CrossRef]
- Shen, J.; Qin, G.; Gu, X.; Liu, Y.; An, S.; Liu, R.; Leng, X.; Wan, Y. Effects of seasonal hydrological regulation of cascade dams on the functional diversity of zooplankton: Implications for the management of massive reservoirs and dams. J. Hydrol. 2022, 610, 127825. [Google Scholar] [CrossRef]


| Sampling Sites | COD | NH3-N | TP |
|---|---|---|---|
| 1 | 13.22 | 0.34 | 0.01 |
| 2 | 13.35 | 0.37 | 0.02 |
| 3 | 20.39 | 0.40 | 0.01 |
| 4 | 14.56 | 0.38 | 0.03 |
| 5 | 15.66 | 0.22 | 0.02 |
| 6 | 16.20 | 0.41 | 0.03 |
| 7 | 16.54 | 0.53 | 0.02 |
| 8 | 17.11 | 0.20 | 0.02 |
| 9 | 18.22 | 0.54 | 0.03 |
| 10 | 18.55 | 0.69 | 0.02 |
| 11 | 19.34 | 0.45 | 0.05 |
| 12 | 19.83 | 0.25 | 0.03 |
| 13 | 19.59 | 0.47 | 0.05 |
| Sample Sites | No. of Species/Number | Shannon Index | Richness Index | Evenness |
|---|---|---|---|---|
| 1 | 31 | 4.12 | 1.77 | 0.88 |
| 2 | 33 | 4.12 | 1.89 | 0.86 |
| 3 | 28 | 3.51 | 1.49 | 0.78 |
| 4 | 32 | 3.29 | 1.77 | 0.69 |
| 5 | 33 | 4.21 | 1.76 | 0.89 |
| 6 | 32 | 3.07 | 1.49 | 0.68 |
| 7 | 31 | 3.73 | 1.80 | 0.78 |
| 8 | 31 | 3.76 | 1.56 | 0.83 |
| 9 | 34 | 3.33 | 1.86 | 0.69 |
| 10 | 33 | 3.49 | 1.83 | 0.72 |
| 11 | 28 | 3.68 | 1.47 | 0.83 |
| 12 | 24 | 3.67 | 1.47 | 0.82 |
| 13 | 32 | 3.83 | 2.01 | 0.77 |
| Sample Sites | Shannon Index | Richness Index | Evenness |
|---|---|---|---|
| 1 | 2.99 | 2.81 | 0.79 |
| 2 | 1.40 | 3.08 | 0.33 |
| 3 | 2.49 | 2.42 | 0.64 |
| 4 | 2.86 | 2.88 | 0.72 |
| 5 | 2.61 | 2.33 | 0.68 |
| 6 | 2.91 | 3.34 | 0.69 |
| 7 | 2.77 | 2.21 | 0.75 |
| 8 | 2.89 | 2.40 | 0.76 |
| 9 | 3.05 | 3.43 | 0.72 |
| 10 | 2.99 | 3.27 | 0.69 |
| 11 | 2.61 | 3.69 | 0.59 |
| 12 | 3.08 | 3.33 | 0.75 |
| 13 | 2.57 | 2.68 | 0.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Bai, M.; Yao, L.; Ma, J.; He, F.; Bian, G.; Li, W. Phytoplankton and Zooplankton Community Dynamics in an Alpine Reservoir: Environmental Drivers and Ecological Implications in Daqing Reservoir, China. Water 2025, 17, 1202. https://doi.org/10.3390/w17081202
Li Z, Bai M, Yao L, Ma J, He F, Bian G, Li W. Phytoplankton and Zooplankton Community Dynamics in an Alpine Reservoir: Environmental Drivers and Ecological Implications in Daqing Reservoir, China. Water. 2025; 17(8):1202. https://doi.org/10.3390/w17081202
Chicago/Turabian StyleLi, Zheng, Minggang Bai, Liangliang Yao, Jie Ma, Fei He, Guodong Bian, and Weixin Li. 2025. "Phytoplankton and Zooplankton Community Dynamics in an Alpine Reservoir: Environmental Drivers and Ecological Implications in Daqing Reservoir, China" Water 17, no. 8: 1202. https://doi.org/10.3390/w17081202
APA StyleLi, Z., Bai, M., Yao, L., Ma, J., He, F., Bian, G., & Li, W. (2025). Phytoplankton and Zooplankton Community Dynamics in an Alpine Reservoir: Environmental Drivers and Ecological Implications in Daqing Reservoir, China. Water, 17(8), 1202. https://doi.org/10.3390/w17081202







