Analysis of Response Surface and Artificial Neural Network for Cr(Ⅵ) Removal Column Experiment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Material Preparation
2.3. Characterization
2.4. Experiment Design
2.5. Analysis of Experimental Data
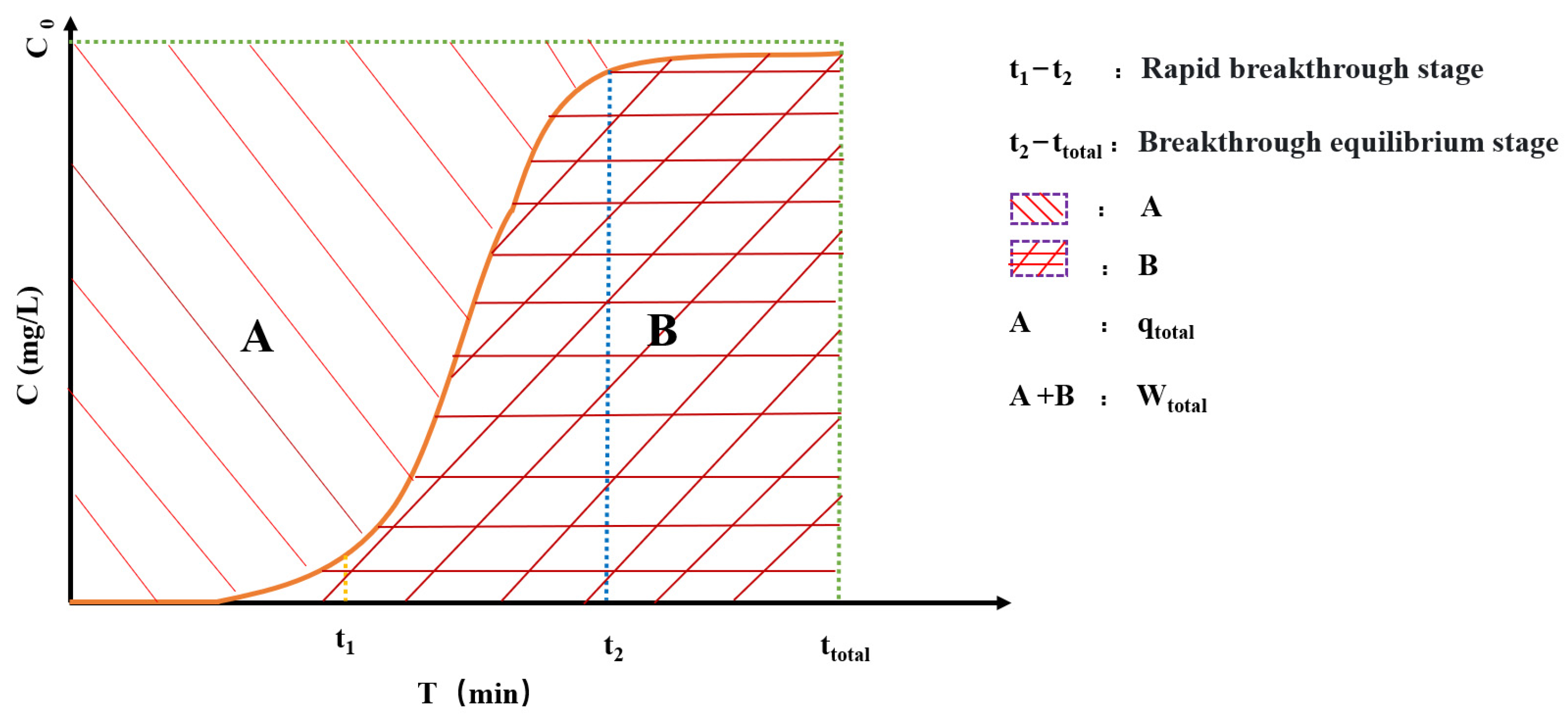
2.5.1. Column Experiment Analysis
2.5.2. Response Surface Methodology
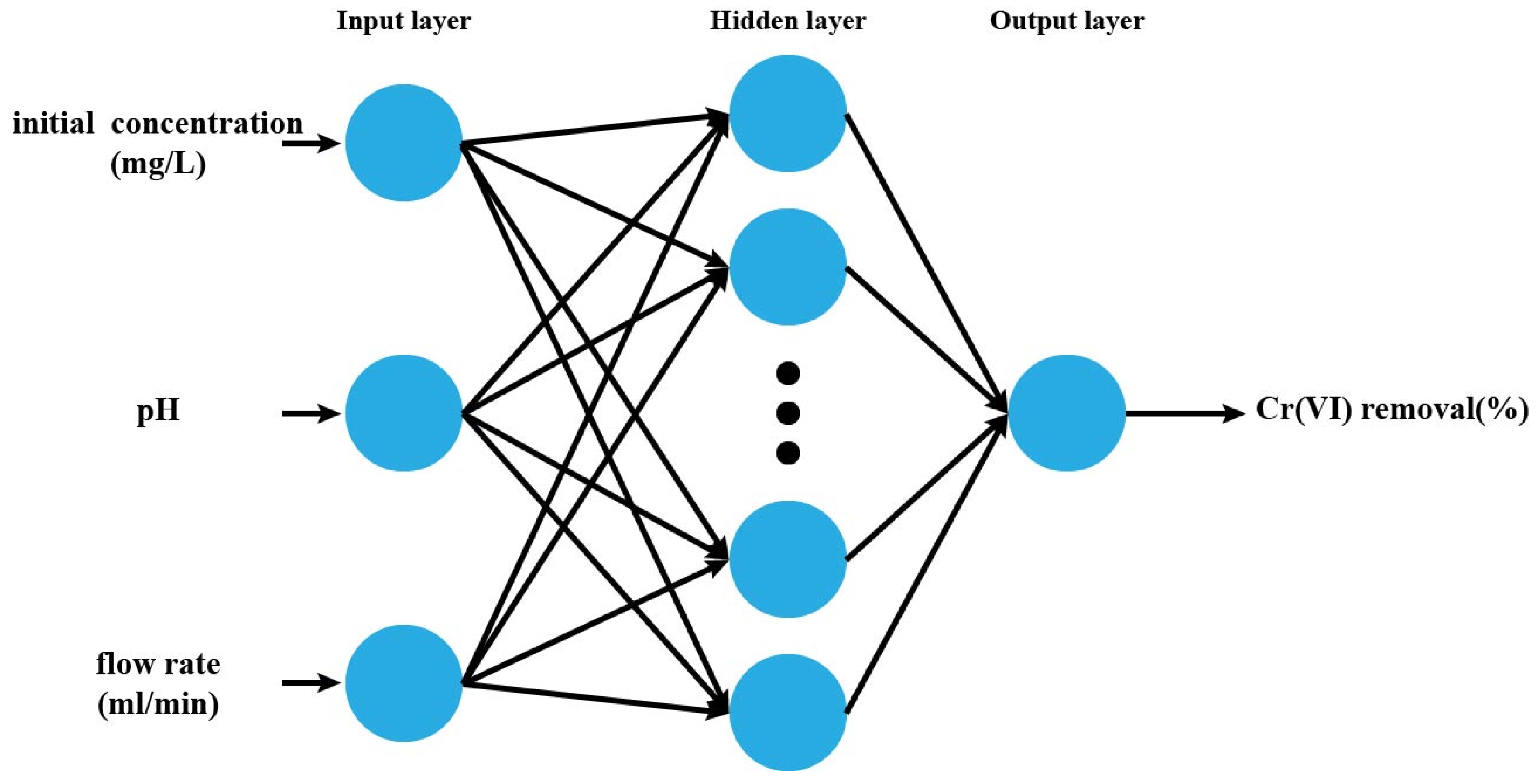
2.5.3. Artificial Neural Network
3. Results and Discussion
3.1. Characterizations
3.1.1. SEM Analysis
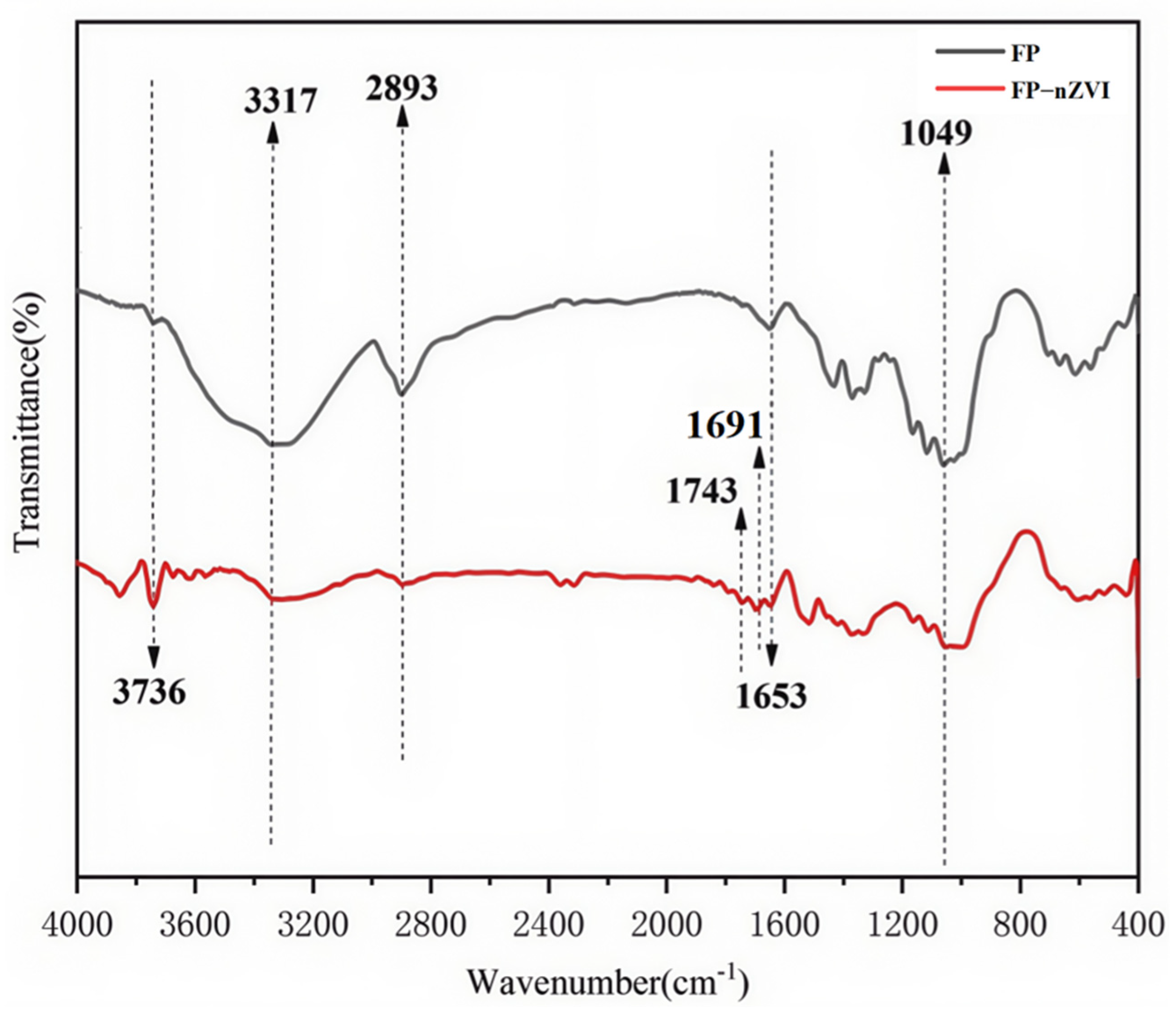
3.1.2. FTIR Analysis
3.1.3. XRD Analysis
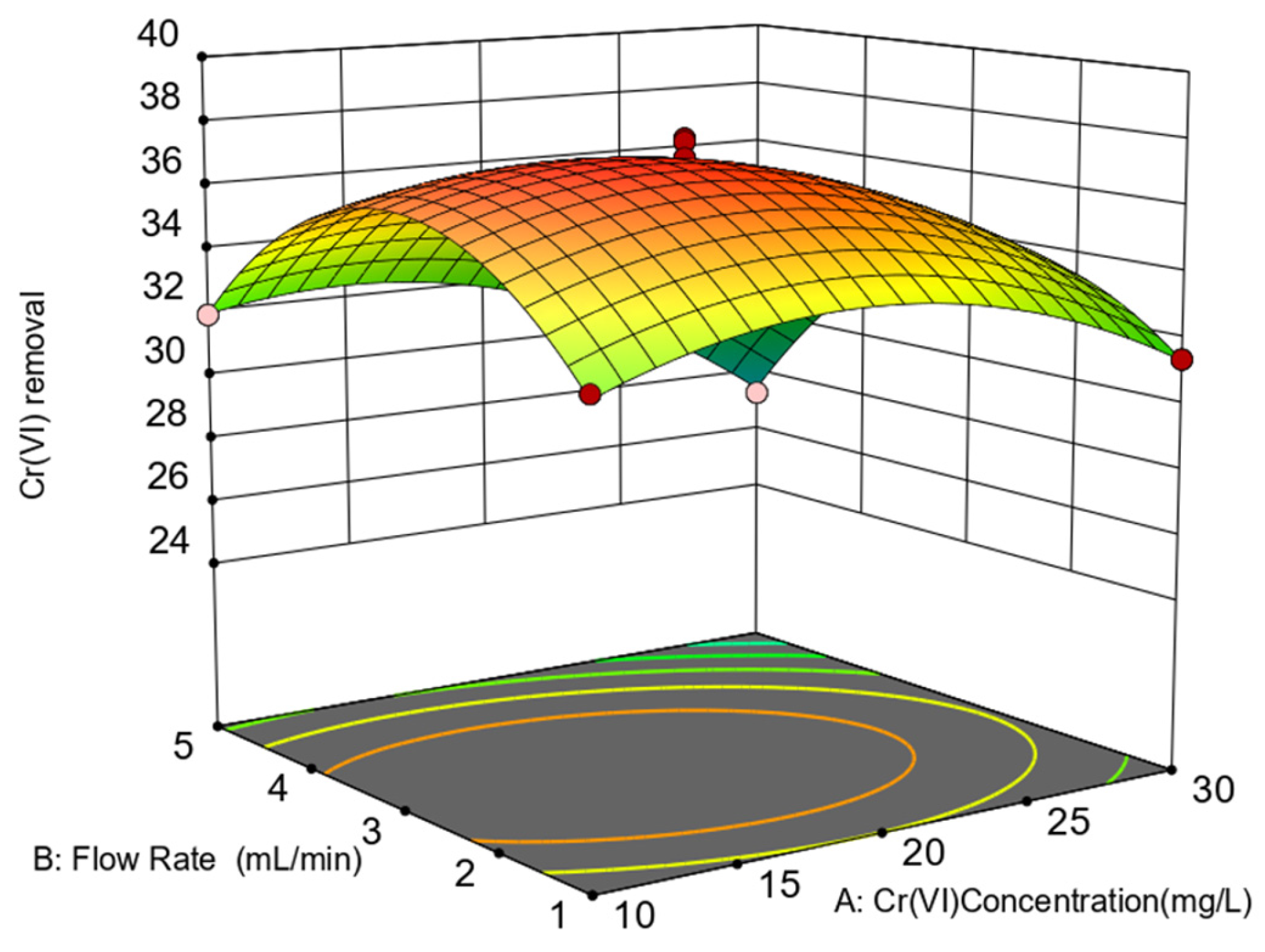
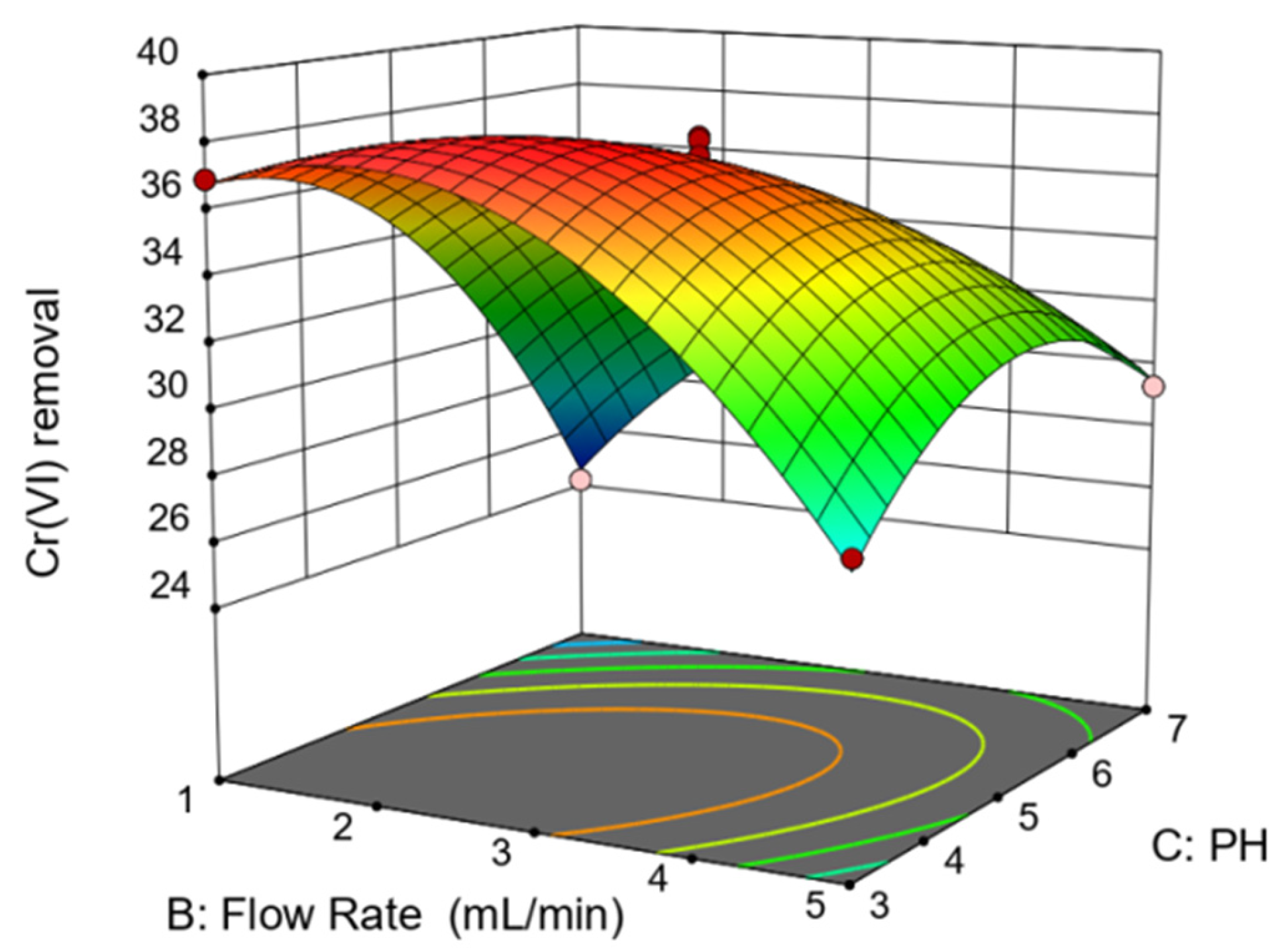
3.2. Response Surface Analysis
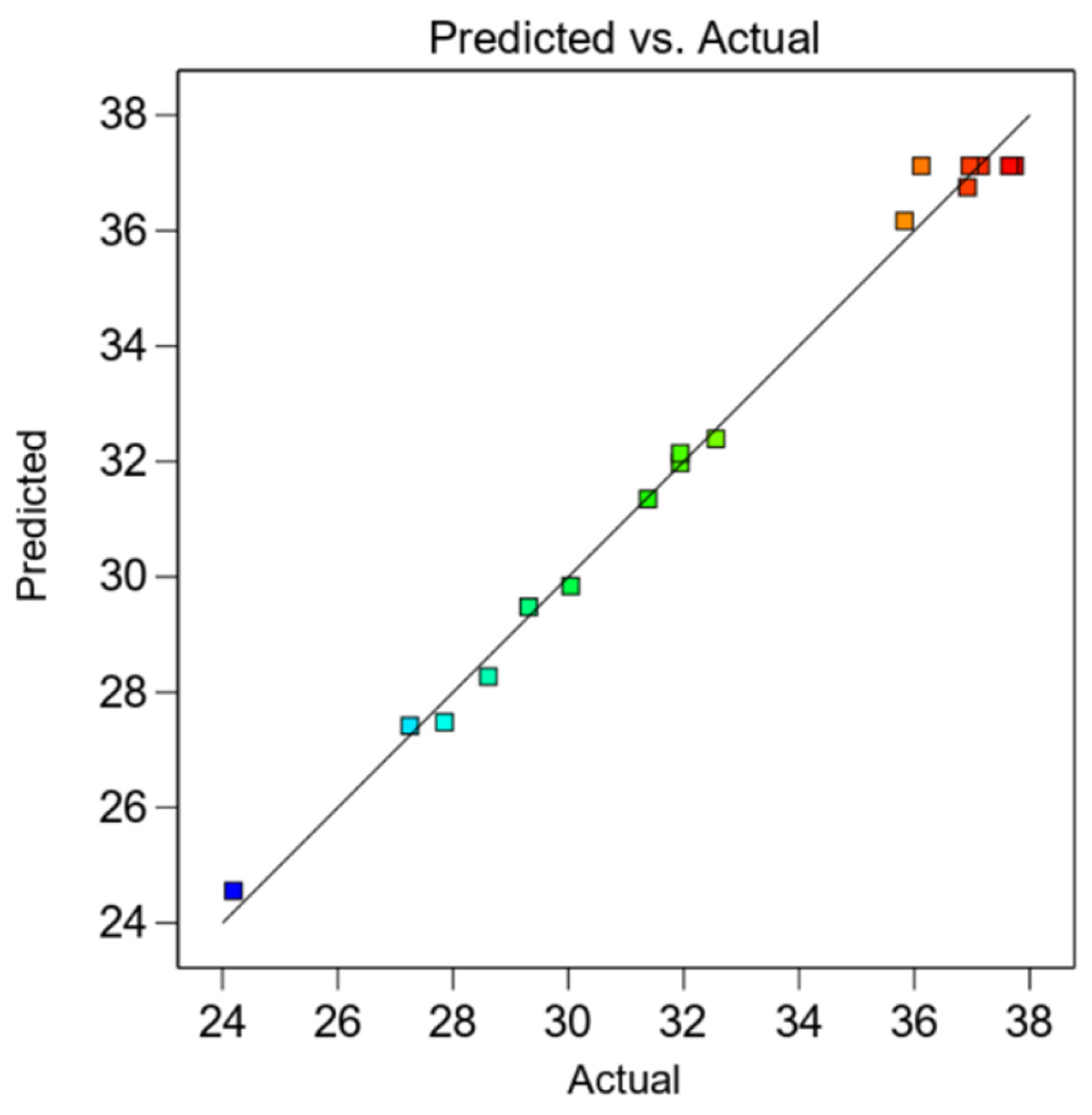
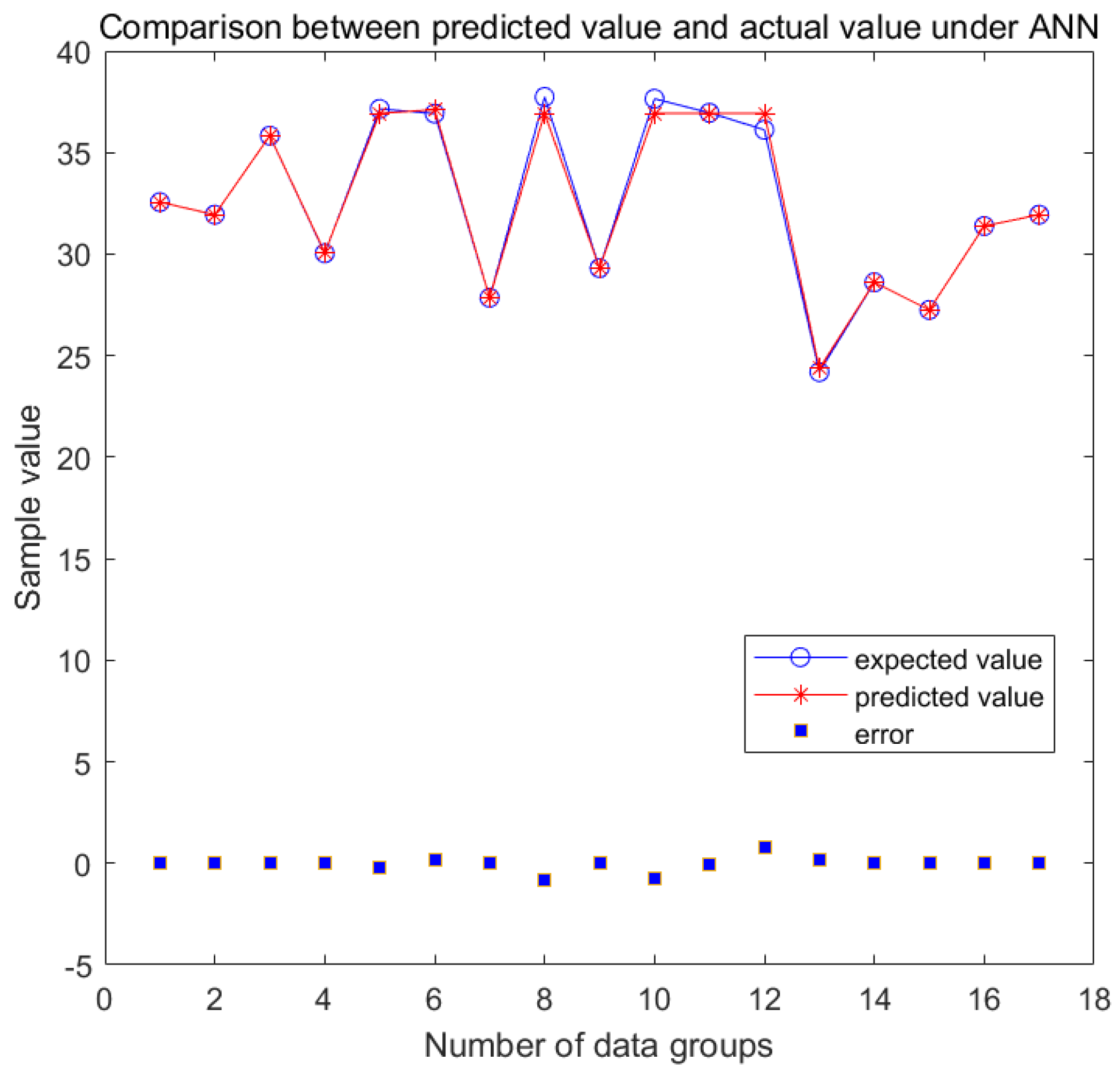
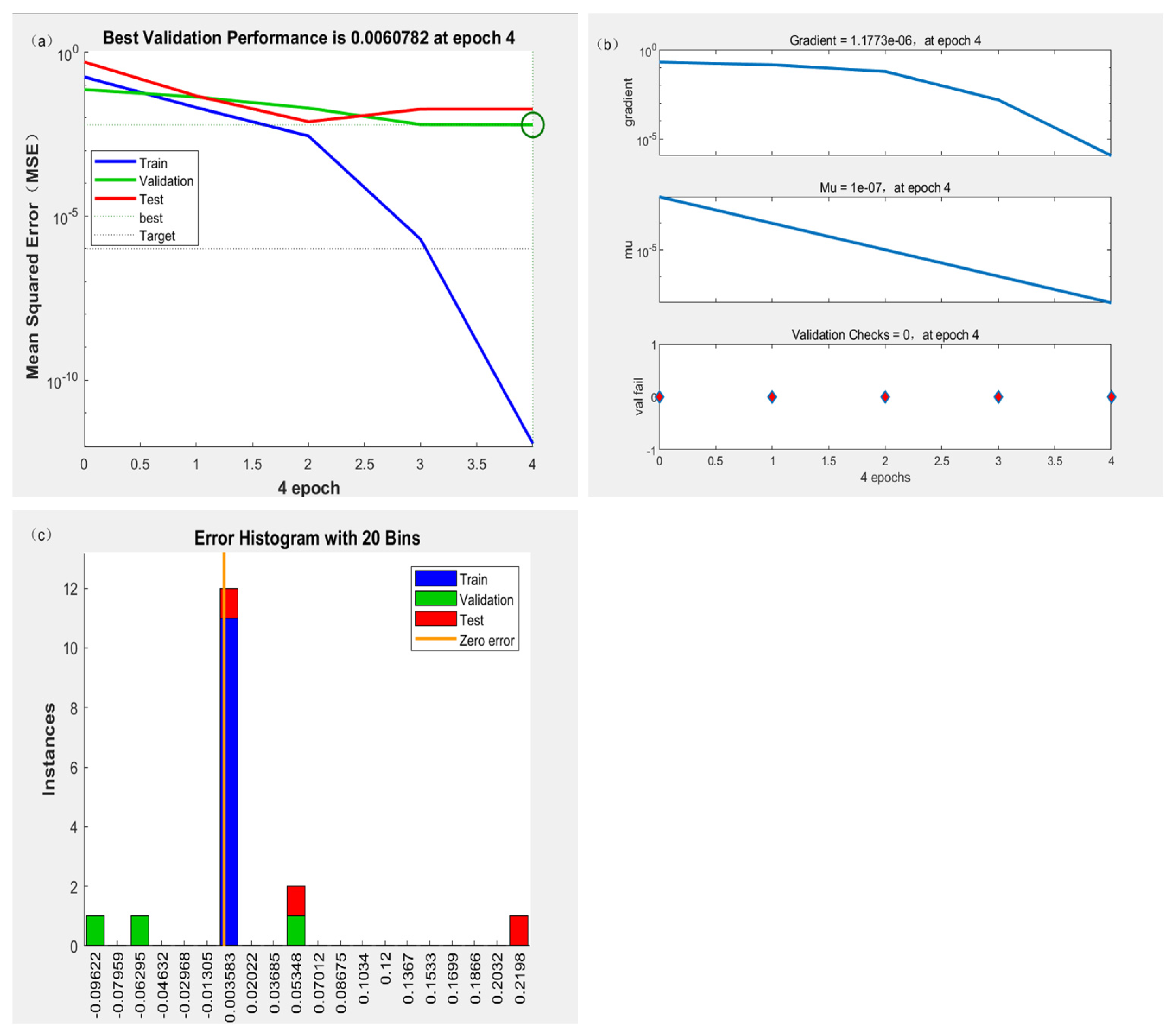
3.3. ANN Evaluation
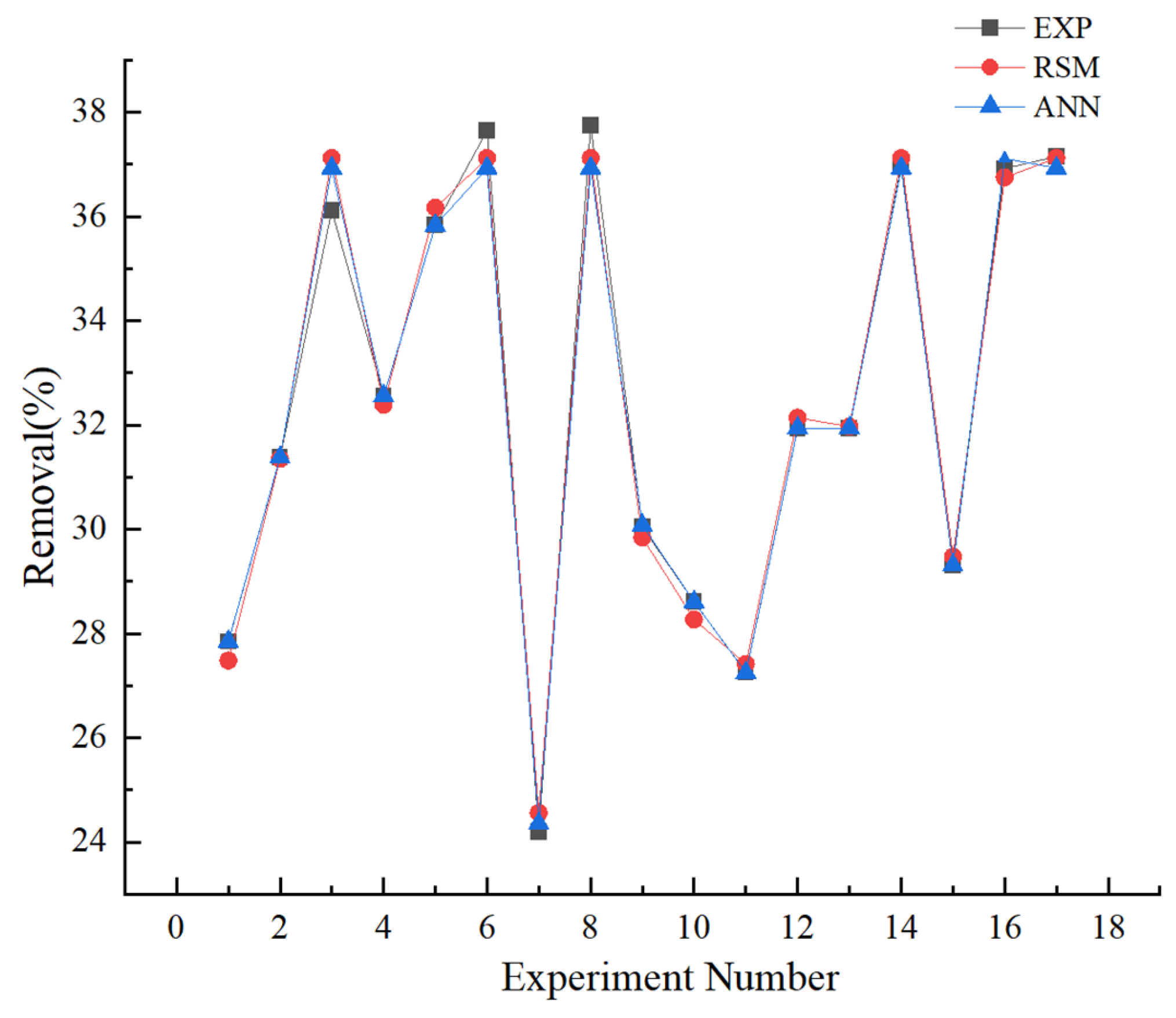
3.4. Comparison of RSM and ANN
4. Conclusions and Suggestions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dotaniya, M.L.; Thakur, J.K.; Meena, V.D.; Jajoria, D.K.; Rathor, G. Chromium pollution: A threat to environment—A review. Agric. Rev. 2014, 35, 153. [Google Scholar] [CrossRef]
- Li, N.; Jiang, J.; Xu, Y.; Pan, H.; Luo, X.; Hu, Y.; Cao, J. Insoluble carbonaceous materials as electron shuttles enhance the anaerobic/anoxic bioremediation of redox pollutants: Recent advances. Chin. Chem. Lett. 2022, 33, 71–79. [Google Scholar] [CrossRef]
- Wang, Y.-P.; Liu, Y.-L.; Tian, S.-Q.; Yang, J.-J.; Wang, L.; Ma, J. Straw biochar enhanced removal of heavy metal by ferrate. J. Hazard. Mater. 2021, 416, 126128. [Google Scholar] [CrossRef] [PubMed]
- Acharya, R.; Sillanpää, M.; Al-Farraj, S. A Comprehensive review on sequestration of aqueous Cr (VI) over graphene based adsorbents. J. Hazard. Mater. Adv. 2025, 18, 100670. [Google Scholar] [CrossRef]
- Hesamedini, S.; Bund, A. Formation of Cr(VI) in cobalt containing Cr(III)-based treatment solution. Surf. Coat. Technol. 2018, 334, 444–449. [Google Scholar] [CrossRef]
- Tu, Y.; Wang, J.; Huang, Y.; Deng, H.; Liu, X.; Lv, J. Transformational fixation of Cr(VI) during Microwave-enhanced reduction of soil iron minerals by tea polyphenols. J. Environ. Manag. 2025, 379, 124889. [Google Scholar] [CrossRef]
- Nizam, T.; Joseph, A.; Krishnan, R.R. Sol-gel derived bismuth-doped nickel ferrite: A promising adsorbent to tackle Cr(VI) pollution—Insights into the thermodynamics, kinetics, and isotherms. J. Alloys Compd. 2025, 1021, 179413. [Google Scholar] [CrossRef]
- Chen, Z.-F.; Guan, X.; Zhao, Y.-S. Morphology analysis of hexavalent chromium reduction to trivalent chromium with syrup under different pH conditions. Appl. Water Sci. 2019, 9, 187. [Google Scholar] [CrossRef]
- Li, T.; Huang, Z.; Hu, M.; Hu, Y.; Quan, K.; Zhou, M.; Chen, Y.; Fu, S. Highly effective adsorption, porous and charged CAP@Ui0-66-NH2@PPy hybrid fibrous membrane based on MOFs for Cr (VI) removal from wastewater. Chem. Eng. J. 2025, 509, 161213. [Google Scholar] [CrossRef]
- Costa, M. Toxicity and carcinogenicity of Cr(VI) in animal models and humans. Crit. Rev. Toxicol. 1997, 27, 431–442. [Google Scholar] [CrossRef]
- Zou, W.; Zhou, F.; Zhang, Z.; Ruan, Q.; Zhou, J.; Li, Z.; Chen, G.; Chen, Z. Unveiling the Distinctive characterization and adsorption behaviors of MnFe2O4 hydrochar/pyrochar composites for Cr(VI) removal. J. Environ. Chem. Eng. 2025, 13, 116052. [Google Scholar] [CrossRef]
- Mortazavian, S.; Murph, S.E.H.; Moon, J. Biochar Nanocomposite as an Inexpensive and Highly efficient carbonaceous adsorbent for hexavalent chromium removal. Materials 2022, 15, 6055. [Google Scholar] [CrossRef] [PubMed]
- Jamil, W.; Memon, Z.; Memon, S.Q.; Samon, M.K.; Taha, M.; Khan, K.M. Environmental friendly synthetic modification of amberlite XAD-2 resin for the removal of highly toxic hexavalent chromium from water. Acta Chim. Slov. 2020, 67, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Liu, X.; Zhang, M.; Wang, J.; Zhong, J.; Ma, D.; Tang, C.; Hu, X. Lab-scale evaluation of the microbial bioremediation of Cr(VI): Contributions of biosorption, bioreduction, and biomineralization. Environ. Sci. Pollut. Res. 2021, 28, 22359–22371. [Google Scholar] [CrossRef]
- Li, J.; Fan, M.; Li, M.; Liu, X. Cr(VI) removal from groundwater using double surfactant-modified nanoscale zero-valent iron (nZVI): Effects of materials in different status. Sci. Total Environ. 2020, 717, 137112. [Google Scholar] [CrossRef]
- Fang, Y.; Wen, J.; Zhang, H.; Wang, Q.; Hu, X. Enhancing Cr(VI) reduction and immobilization by magnetic core-shell structured NZVI@MOF derivative hybrids. Environ. Pollut. 2020, 260, 114021. [Google Scholar] [CrossRef]
- Ren, Y.; Han, Y.; Lei, X.; Lu, C.; Liu, J.; Zhang, G.; Zhang, B.; Zhang, Q. A Magnetic ion exchange resin with high efficiency of removing Cr (VI). Colloids Surf. A Physicochem. Eng. Asp. 2020, 604, 125279. [Google Scholar] [CrossRef]
- Rivero, M.J.; Primo, O.; Ortiz, M.I. Modelling of Cr(VI) removal from polluted groundwaters by ion exchange. J. Chem. Technol. Biotechnol. 2004, 79, 822–829. [Google Scholar] [CrossRef]
- Roy Choudhury, P.; Majumdar, S.; Sahoo, G.C.; Saha, S.; Mondal, P. High pressure ultrafiltration CuO/Hydroxyethyl cellulose composite ceramic membrane for separation of Cr (VI) and Pb (II) from contaminated water. Chem. Eng. J. 2018, 336, 570–578. [Google Scholar] [CrossRef]
- El Gaayda, J.; Rachid, Y.; Titchou, F.E.; Barra, I.; Hsini, A.; Yap, P.-S.; Oh, W.-D.; Swanson, C.; Hamdani, M.; Akbour, R.A. Optimizing removal of chromium (VI) ions from water by coagulation process using central composite design: Effectiveness of grape seed as a green coagulant. Sep. Purif. Technol. 2023, 307, 122805. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, Z.; Dang, Z.; Li, F.; Zhang, L. Simultaneous redox transformation and removal of Cr(VI) and As(III) by polyethyleneimine modified magnetic mesoporous polydopamine nanocomposite: Insights into synergistic effects and mechanisms. J. Hazard. Mater. 2022, 439, 129581. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Li, T.; Tang, Y.; Luo, T.; Huang, D.; He, P.; Cai, M.; Gan, M. Insights into the mechanism of Cr(VI) removal by biogenic jarosite-biomass derived Fe-C material with anti-environmental interference performance. J. Anal. Appl. Pyrolysis 2025, 189, 107066. [Google Scholar] [CrossRef]
- Yi, L.; Chen, S.; Chen, Y. Intra/extracellular electron transfer and energy-dependent Cr(VI) efflux for gram-negative/positive bacteria mediated by PMo12. J. Hazard. Mater. 2025, 491, 137872. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, C.; Peng, X.; Liang, Z.; Hou, S.; Chen, W.; Zhang, T. Modulating D-orbital electronic configuration of magnetic iron sulfide nanocrystals for maximized treatment efficiency of chromium-contaminated water. Water Res. 2025, 280, 123477. [Google Scholar] [CrossRef]
- He, H.; Qiu, S.; Yan, Z.; Shao, D.; Sun, L.; Yuan, X. Photoreduction of Cr(VI) to Cr(III) using reduced graphene oxide/CuOx composites. Mater. Chem. Phys. 2025, 338, 130645. [Google Scholar] [CrossRef]
- Fan, M.; Li, X.; Liu, L.; Yang, H.; Wu, M.; Yang, J. Synthesis, Characterization, and photocatalytic activity of a carboxymethyl cellulose sodium-based hybrid material for efficient degradation of hexavalent chromium. Int. J. Biol. Macromol. 2025, 306, 141536. [Google Scholar] [CrossRef]
- Wang, T.; He, J.; Lu, J.; Zhou, Y.; Wang, Z.; Zhou, Y. Adsorptive Removal of PPCPs from Aqueous Solution Using Carbon-Based Composites: A Review. Chin. Chem. Lett. 2022, 33, 3585–3593. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Ayati, A.; Ghanbari, S.; Orooji, Y.; Tanhaei, B.; Karimi, F.; Sillanpää, M. Recent advances in removal techniques of Cr(VI) toxic ion from aqueous solution: A comprehensive review. J. Mol. Liq. 2021, 329, 115062. [Google Scholar] [CrossRef]
- Wołowiec, M.; Komorowska-Kaufman, M.; Pruss, A.; Rzepa, G.; Bajda, T. Removal of heavy metals and metalloids from water using drinking water treatment residuals as adsorbents: A review. Minerals 2019, 9, 487. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Duwiejuah, A.B.; Abubakari, A.H.; Quainoo, A.K.; Amadu, Y. Review of Biochar properties and remediation of metal pollution of water and soil. J. Health Pollut. 2020, 10, 200902. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, R.; Chen, K.; Zhao, X.; Gu, X.; Lu, J. Enhanced adsorption and photo-degradation of bisphenol a by β-cyclodextrin modified pine sawdust in an aquatic environment. J. Taiwan Inst. Chem. Eng. 2017, 78, 510–516. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, D.; Wang, T.; Hu, X. Leaching of graphene oxide nanosheets in simulated soil and their influences on microbial communities. J. Hazard. Mater. 2021, 404, 124046. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Tang, Q.; Yang, Z.; Dai, Z.; Cheng, C.; Xu, Y.; Chen, X.; Zhang, X.; Su, J. Effects of Iron oxide nanoparticles on phenotype and metabolite changes in hemp clones (Cannabis sativa L.). Front. Environ. Sci. Eng. 2022, 16, 134. [Google Scholar] [CrossRef]
- Ling, L.; Huang, X.; Zhang, W. Enrichment of precious metals from wastewater with core–shell nanoparticles of iron. Adv. Mater. 2018, 30, 1705703. [Google Scholar] [CrossRef]
- Ma, Z.; Cao, H.; Lv, F.; Yang, Y.; Chen, C.; Yang, T.; Zheng, H.; Wu, D. Preparation of nZVI embedded modified mesoporous carbon for catalytic persulfate to degradation of reactive black 5. Front. Environ. Sci. Eng. 2021, 15, 1–10. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, Y.; Jing, Q.; Ren, Z. Comigration behavior of Cr(VI) and microplastics and remediation of microplastics-facilitated Cr(VI) transportation in saturated porous media. Polymers 2024, 16, 3271. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, C.; Liu, M.; Lin, Q.; Lei, M.; Wang, D.; Yang, M.; Yang, Y.; Ma, J.; Jia, Z. One-pot synthesis of spherical nanoscale zero-valent iron/biochar composites for efficient removal of Pb(II). RSC Adv. 2021, 11, 36826–36835. [Google Scholar] [CrossRef]
- Yang, J.; Wang, S.; Xu, N.; Ye, Z.; Yang, H.; Huangfu, X. Synthesis of montmorillonite-supported nano-zero-valent iron via green tea extract: Enhanced transport and application for hexavalent chromium removal from water and soil. J. Hazard. Mater. 2021, 419, 126461. [Google Scholar] [CrossRef]
- Ma, B.; Yao, J.; Knudsen, T.Š.; Chen, Z.; Liu, B.; Zhao, C.; Zhu, X. Simultaneous removal of typical flotation reagent 8-hydroxyquinoline and Cr(VI) through heterogeneous fenton-like processes mediated by polydopamine functionalized ATP supported nZVI. J. Hazard. Mater. 2022, 424, 126698. [Google Scholar] [CrossRef]
- Lubphoo, Y.; Chyan, J.M.; Grisdanurak, N.; Liao, C.H. Influence of Pd–Cu on nanoscale zero–valent iron supported for selective reduction of nitrate. J. Taiwan Inst. Chem. Eng. 2016, 59, 285–294. [Google Scholar] [CrossRef]
- Taghizadeh, S.M.; Berenjian, A.; Taghizadeh, S.; Ghasemi, Y.; Taherpour, A.; Sarmah, A.K.; Ebrahiminezhad, A. One-put green synthesis of multifunctional silver iron core-shell nanostructure with antimicrobial and catalytic properties. Ind. Crops Prod. 2019, 130, 230–236. [Google Scholar] [CrossRef]
- Chang, C.; Lian, F.; Zhu, L. Simultaneous Adsorption and Degradation of γ-HCH by nZVI/Cu Bimetallic Nanoparticles with Activated Carbon Support. Environ. Pollut. 2011, 159, 2507–2514. [Google Scholar] [CrossRef]
- Di, L.; Chen, X.; Lu, J.; Zhou, Y.; Zhou, Y. Removal of heavy metals in water using nano zero-valent iron composites: A review. J. Water Process Eng. 2023, 53, 103913. [Google Scholar] [CrossRef]
- Yu, Q.; Guo, J.; Muhammad, Y.; Li, Q.; Lu, Z.; Yun, J.; Liang, Y. Mechanisms of enhanced hexavalent chromium removal from groundwater by sodium carboxymethyl cellulose stabilized zerovalent iron nanoparticles. J. Environ. Manag. 2020, 276, 111245. [Google Scholar] [CrossRef]
- Ren, L.; Dong, J.; Chi, Z.; Huang, H. Reduced graphene oxide-nano zero value iron (rGO-nZVI) micro-electrolysis accelerating Cr(VI) removal in aquifer. J. Environ. Sci. 2018, 73, 96–106. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.; Zhang, X.; Guo, H.; Cao, X.; Lou, Z.; Zhang, W.; Wang, C. A Novel lignin hydrogel supported nZVI for efficient removal of Cr(VI). Chemosphere 2022, 301, 134781. [Google Scholar] [CrossRef]
- Yu, P.; Yu, H.; Sun, Q.; Ma, B. Filter paper supported nZVI for continuous treatment of simulated dyeing wastewater. Sci. Rep. 2019, 9, 11322. [Google Scholar] [CrossRef]
- Kamal, T.; Khan, S.B.; Asiri, A.M. Synthesis of zero-valent Cu nanoparticles in the chitosan coating layer on cellulose microfibers: Evaluation of azo dyes catalytic reduction. Cellulose 2016, 23, 1911–1923. [Google Scholar] [CrossRef]
- Ren, Z.; Tang, H.; Li, H.; Jing, Q. Column experimental study on the removal of hexavalent chromium from water by modified cellulose filter paper loaded with nano zero-valent iron. J. Water Process Eng. 2024, 59, 104920. [Google Scholar] [CrossRef]
- Turan, N.G.; Mesci, B.; Ozgonenel, O. Response surface modeling of Cu(II) removal from electroplating waste by adsorption: Application of Box–Behnken experimental design. CLEAN—Soil Air Water 2013, 41, 304–312. [Google Scholar] [CrossRef]
- Raja, S.; Murty, V.R. Optimization of aqueous two-phase systems for the recovery of soluble proteins from tannery wastewater using response surface methodology. J. Eng. 2013, 2013, 217483. [Google Scholar] [CrossRef]
- Khan, S.A.; Hussain, D.; Abbasi, N.; Khan, T.A. Deciphering the adsorption potential of a functionalized green hydrogel nanocomposite for aspartame from aqueous phase. Chemosphere 2022, 289, 133232. [Google Scholar] [CrossRef]
- Ravinuthala, S.; Settu, S. Multi-response parametric optimization and biofilm studies of low-cost ceramic microbial fuel cell for dairy wastewater treatment. Bioresour. Technol. Rep. 2025, 29, 102029. [Google Scholar] [CrossRef]
- Kumari, B.; Tiwary, R.K.; Yadav, M. Non Linear Regression Analysis and RSM Modeling for Removal of Cr (VI) from Aqueous Solution Using PANI@WH Composites. Mater. Chem. Phys. 2022, 290, 126457. [Google Scholar] [CrossRef]
- Slamani, I.; Bengharez, Z.; Boudouaia, N.; Bendaoudi, A.A.; Jellali, S.; Benhafsa, F.M.; Mahmoudi, H.; Benhadria, N.; Guemra, K.; Jeguirim, M. Cross-linked chitosan/H-ZSM-5 zeolite composite film for chromium removal from aqueous solutions: Optimization using response surface methodology and adsorption mechanism assessment. Environ. Sci. Pollut. Res. 2025. [Google Scholar] [CrossRef]
- Saawarn, B.; Mahanty, B.; Hait, S. Adsorption of perfluorooctanoic acid from aqueous matrices onto chitosan-modified magnetic biochar: Response surface methodology-based modeling, performance, and mechanism. Environ. Pollut. 2025, 368, 125734. [Google Scholar] [CrossRef]
- Bingöl, D.; Hercan, M.; Elevli, S.; Kılıç, E. Comparison of the results of response surface methodology and artificial neural network for the biosorption of lead using black cumin. Bioresour. Technol. 2012, 112, 111–115. [Google Scholar] [CrossRef]
- Wairokpam, R.; Ningthoujam, S.; Kumar, P.A. Artificial neural network (ANN) based prediction and modeling of Fe(II) adsorption from contaminated groundwater using deccan hemp stem-derived activated carbon. Int. J. Environ. Res. 2025, 19, 61. [Google Scholar] [CrossRef]
- Shekoohiyan, S.; Dariyan, F.S.; Mahdavianpour, M.; Pourakbar, M.; Aghayani, E. Comparison of response surface method and artificial neural networks in predicting formaldehyde and methanol removal using moving bed sequential batch reactor (MBSBR) and fixed bed sequential batch reactor (FBSBR): Process optimization and kinetic study. Sep. Purif. Technol. 2025, 363, 132097. [Google Scholar] [CrossRef]
- Akanbi, T.E.; Ajayi, O.; Yusuff, A.S.; Obada, D. Modeling and optimization of photocatalytic degradation of methylene blue via TiO2-CuO/HAp catalyst: The use of response surface methodology and artificial neural network. Asian J. Chem. Sci. 2025, 15, 111–131. [Google Scholar] [CrossRef]
- Neishaboori, F.M.; Sohrabi, M.R.; Motiee, F.; Davallo, M. Optimization and modeling of simultaneous removal of reactive Violet 5 and Acid Red 98 using bimetallic copper-zero-valent iron nanoparticles supported on biopolymer chitosan based on a central composite design and artificial neural network. J. Polym. Environ. 2025, 33, 2402–2424. [Google Scholar] [CrossRef]
- Da Costa, M.F.P.; Araújo, R.D.S.; Silva, A.R.; Pereira, L.; Silva, G.M.M. Predictive artificial neural networks as applied tools in the remediation of dyes by adsorption—A review. Appl. Sci. 2025, 15, 2310. [Google Scholar] [CrossRef]
- Kumari, S.; Agarwal, S.; Kumar, M.; Sharma, P.; Kumar, A.; Hashem, A.; Alotaibi, N.H.; Abd-Allah, E.F.; Garg, M.C. An exploration of RSM, ANN, and ANFIS models for methylene blue dye adsorption using oryza sativa straw biomass: A comparative approach. Sci. Rep. 2025, 15, 2979. [Google Scholar] [CrossRef]
- Ahdour, A.; Douihi, N.; Taoufyq, A.; Aneflous, L.; Bakiz, B.; Benlhachemi, A. Electrocatalytic and photoelectrocatalytic degradation of tetracycline using BaHPO4/SSA photoanode: Insights from BBD-RSM and CCD-RSM experimental designs. Surf. Interfaces 2025, 58, 105874. [Google Scholar] [CrossRef]
- Nezhad, P.A.; Dabirian, F.; Derakhshan, A.A.; Rajabi, L.; Heydari, S.; Rostami, A. Optimized electrospun schiff-base Alumoxane/PAN nanofibers for lead and cadmium adsorption: Characterization & RSM analysis. J. Polym. Res. 2025, 32, 51. [Google Scholar] [CrossRef]
- Tunali Akar, S.; Rüstemoğlu, S.; Turkyilmaz, S.; Sayin, F.; Akar, T. ANN-Assisted comprehensive screening of silica gel-alunite composite sorbent system for efficient adsorption of toxic nickel ions: Batch and continuous mode water treatment applications. Chemosphere 2025, 373, 144127. [Google Scholar] [CrossRef]
- Asl, S.H.; Ahmadi, M.; Ghiasvand, M.; Tardast, A.; Katal, R. Artificial Neural Network (ANN) Approach for Modeling of Cr(VI) Adsorption from Aqueous Solution by Zeolite Prepared from Raw Fly Ash (ZFA). J. Ind. Eng. Chem. 2013, 19, 1044–1055. [Google Scholar] [CrossRef]
- Amini-Badr, A.; Behnajady, M.A. Photocatalytic removal of cefazolin in a photoreactor packed with TiO2-P25 nanoparticles Supported on Glass Beads: An Artificial Neural Network Modeling. Int. J. Environ. Anal. Chem. 2024, 104, 5713–5731. [Google Scholar] [CrossRef]
- Li, L.; Hu, J.; Shi, X.; Fan, M.; Luo, J.; Wei, X. Nanoscale zero-valent metals: A review of synthesis, characterization, and applications to environmental remediation. Environ. Sci. Pollut. Res. 2016, 23, 17880–17900. [Google Scholar] [CrossRef]
- Arshadi, M.; Abdolmaleki, M.K.; Eskandarloo, H.; Azizi, M.; Abbaspourrad, A. Synthesis of highly monodispersed, stable, and spherical NZVI of 20–30 Nm on filter paper for the removal of phosphate from wastewater: Batch and column study. ACS Sustain. Chem. Eng. 2018, 6, 11662–11676. [Google Scholar] [CrossRef]
- Wang, X.; Wang, T.; Ma, J.; Liu, H.; Ning, P. Synthesis and characterization of a new hydrophilic boehmite-PVB/PVDF blended membrane supported nano zero-valent iron for removal of Cr(VI). Sep. Purif. Technol. 2018, 205, 74–83. [Google Scholar] [CrossRef]
- Meraj, A.; Jawaid, M.; Karim, Z.; Awayssa, O.; Fouad, H.; Singh, B. Characterization of Carboxymethyl microcrystalline cellulose derived from sustainable kenaf fiber. Carbohydr. Polym. Technol. Appl. 2025, 10, 100745. [Google Scholar] [CrossRef]
- García-Vargas, M.; Munguía-Quintero, M.F.; Alcaraz-Cienfuegos, J.; Rosas-Aburto, A.; Valdivia-López, M.D.L.Á.; Hernández-Luna, M.G.; Vivaldo-Lima, E. Synthesis, characterization and evaluation of cellulose-graft-poly(4-vinylpirydine), using cellulose from a new pretreatment process, for heavy metal removal from wastewater. Int. J. Biol. Macromol. 2025, 284, 137986. [Google Scholar] [CrossRef]
- Duchemin, B.; Le Corre, D.; Leray, N.; Dufresne, A.; Staiger, M.P. All-cellulose composites based on microfibrillated cellulose and filter paper via a NaOH-urea solvent system. Cellulose 2016, 23, 593–609. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, G.; Wang, X.; Li, S.; Liu, Y.; Yang, G. Removal of hexavalent chromium by bentonite supported organosolv lignin-stabilized zero-valent iron nanoparticles from wastewater. J. Clean. Prod. 2020, 267, 122009. [Google Scholar] [CrossRef]
- Hamid, H.A.; Jenidi, Y.; Thielemans, W.; Somerfield, C.; Gomes, R.L. Predicting the capability of carboxylated cellulose nanowhiskers for the remediation of copper from water using response surface methodology (RSM) and artificial neural network (ANN) models. Ind. Crops Prod. 2016, 93, 108–120. [Google Scholar] [CrossRef]
- Bayuo, J.; Abukari, M.A.; Pelig-Ba, K.B. Optimization using central composite design (CCD) of response surface methodology (RSM) for biosorption of hexavalent chromium from aqueous media. Appl. Water Sci. 2020, 10, 135. [Google Scholar] [CrossRef]
- Vinayagam, R.; Dave, N.; Varadavenkatesan, T.; Rajamohan, N.; Sillanpää, M.; Nadda, A.K.; Selvaraj, R. Artificial Neural Network and Statistical Modelling of Biosorptive Removal of Hexavalent Chromium Using Macroalgal Spent Biomass. Chemosphere 2022, 296, 133965. [Google Scholar] [CrossRef]
- de Luna, M.D.G.; Sablas, M.M.; Hung, C.M.; Chen, C.W.; Garcia-Segura, S.; Dong, C.D. Modeling and optimization of imidacloprid degradation by catalytic percarbonate oxidation using artificial neural network and box-behnken experimental design. Chemosphere 2020, 251, 126254. [Google Scholar] [CrossRef]
- Geyikçi, F.; Kılıç, E.; Çoruh, S.; Elevli, S. Modelling of lead adsorption from industrial sludge leachate on red mud by using RSM and ANN. Chem. Eng. J. 2012, 183, 53–59. [Google Scholar] [CrossRef]
- Shanmugaprakash, M.; Sivakumar, V. Development of experimental design approach and ANN-based models for determination of Cr(VI) ions uptake rate from aqueous solution onto the solid biodiesel waste residue. Bioresour. Technol. 2013, 148, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Kim, Z.; Shin, Y.; Yu, J.; Kim, G.; Hwang, S. Development of NOx removal process for LNG evaporation system: Comparative assessment between response surface methodology (RSM) and artificial neural network (ANN). J. Ind. Eng. Chem. 2019, 74, 136–147. [Google Scholar] [CrossRef]
- Isiyaka, H.A.; Jumbri, K.; Sambudi, N.S.; Zango, Z.U.; Fathihah Abdullah, N.A.; Saad, B.; Mustapha, A. Adsorption of dicamba and MCPA onto MIL-53(Al) metal–organic framework: Response surface methodology and artificial neural network model studies. RSC Adv. 2020, 10, 43213–43224. [Google Scholar] [CrossRef]





| Factor | Units | Levels | |||
|---|---|---|---|---|---|
| −1 | 0 | +1 | |||
| Flow rate | mL/min | 1 | 3 | 5 | |
| pH | - | 3 | 5 | 7 | |
| Concentration of Cr(VI) | mg/L | 10 | 20 | 30 | |
| Experiment No. | C0 (mg/L) | Q (mL/min) | pH | r Experimental (%) | r RSM (%) | r ANN (%) |
|---|---|---|---|---|---|---|
| 1 | 20 | 5 | 3 | 27.85 | 27.48 | 27.85 |
| 2 | 30 | 1 | 5 | 31.38 | 31.35 | 31.38 |
| 3 | 20 | 3 | 5 | 36.12 | 37.12 | 36.93 |
| 4 | 10 | 1 | 5 | 32.56 | 32.39 | 32.56 |
| 5 | 10 | 3 | 3 | 35.83 | 36.17 | 35.83 |
| 6 | 20 | 3 | 5 | 37.65 | 37.12 | 36.93 |
| 7 | 20 | 1 | 7 | 24.19 | 24.56 | 24.36 |
| 8 | 20 | 3 | 5 | 37.74 | 37.12 | 36.93 |
| 9 | 10 | 3 | 7 | 30.04 | 29.84 | 30.08 |
| 10 | 30 | 3 | 7 | 28.61 | 28.27 | 28.61 |
| 11 | 30 | 5 | 5 | 27.25 | 27.42 | 27.25 |
| 12 | 30 | 3 | 3 | 31.94 | 32.14 | 31.94 |
| 13 | 10 | 5 | 5 | 31.94 | 31.97 | 31.94 |
| 14 | 20 | 3 | 5 | 36.96 | 37.12 | 36.93 |
| 15 | 20 | 5 | 7 | 29.31 | 29.48 | 29.31 |
| 16 | 20 | 1 | 3 | 36.92 | 36.75 | 37.11 |
| 17 | 20 | 3 | 5 | 37.15 | 37.12 | 36.93 |
| Statistical Parameter | Mathematical Expression |
|---|---|
| Root Mean Square Error (RMSE) | |
| Mean square errors (MSE) | |
| Mean Absolute Percentage Error (MAPE) | |
| Mean Absolute Error (MAE) | |
| Absolute Average Deviation (AAD) | |
| Standard Error of Prediction (SEP) |
| Source | Sum of Squares | df | Mean Square | F Value | Prob > F | |
|---|---|---|---|---|---|---|
| Model | 288.27 | 9 | 32.03 | 93.92 | <0.0001 | significant |
| A-Cr(VI) concentration | 15.65 | 1 | 15.65 | 45.89 | 0.0003 | |
| B-Flow rate | 9.46 | 1 | 9.46 | 27.74 | 0.0012 | |
| C-pH | 51.97 | 1 | 51.97 | 152.38 | <0.0001 | |
| AB | 3.08 | 1 | 3.08 | 9.03 | 0.0198 | |
| AC | 1.51 | 1 | 1.51 | 4.44 | 0.0732 | |
| BC | 50.34 | 1 | 50.34 | 147.60 | <0.0001 | |
| A2 | 19.50 | 1 | 19.50 | 57.17 | 0.0001 | |
| B2 | 73.90 | 1 | 73.90 | 216.69 | <0.0001 | |
| C2 | 47.73 | 1 | 47.73 | 139.96 | <0.0001 | |
| Residual | 2.39 | 7 | 0.34 | |||
| Lack of Fit | 0.70 | 3 | 0.23 | 0.55 | 0.6755 | not significant |
| Pure Error | 1.69 | 4 | 0.42 | |||
| Cor Total | 290.66 | 16 | ||||
| R2 = 0.9918 |
| Statistical Parameters | RSM | ANN |
|---|---|---|
| R2 | 0.9918 | 0.9937 |
| MSE | 0.1404 | 0.1146 |
| RMSE | 0.3747 | 0.3385 |
| MAE | 0.2874 | 0.1760 |
| MAPE | 0.8895% | 0.4903% |
| AAD | 0.8795 | 0.4903 |
| SEP | 1.1511 | 1.0399 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Z.; Li, Z.; Tang, H.; Yang, L.; Zhu, J.; Jing, Q. Analysis of Response Surface and Artificial Neural Network for Cr(Ⅵ) Removal Column Experiment. Water 2025, 17, 1211. https://doi.org/10.3390/w17081211
Ren Z, Li Z, Tang H, Yang L, Zhu J, Jing Q. Analysis of Response Surface and Artificial Neural Network for Cr(Ⅵ) Removal Column Experiment. Water. 2025; 17(8):1211. https://doi.org/10.3390/w17081211
Chicago/Turabian StyleRen, Zhongyu, Zhicong Li, Haokai Tang, Lin Yang, Jinrun Zhu, and Qi Jing. 2025. "Analysis of Response Surface and Artificial Neural Network for Cr(Ⅵ) Removal Column Experiment" Water 17, no. 8: 1211. https://doi.org/10.3390/w17081211
APA StyleRen, Z., Li, Z., Tang, H., Yang, L., Zhu, J., & Jing, Q. (2025). Analysis of Response Surface and Artificial Neural Network for Cr(Ⅵ) Removal Column Experiment. Water, 17(8), 1211. https://doi.org/10.3390/w17081211






