Seaweed-Derived Biochar for Effective Treatment of Dye-Contaminated Wastewater
Abstract
1. Introduction
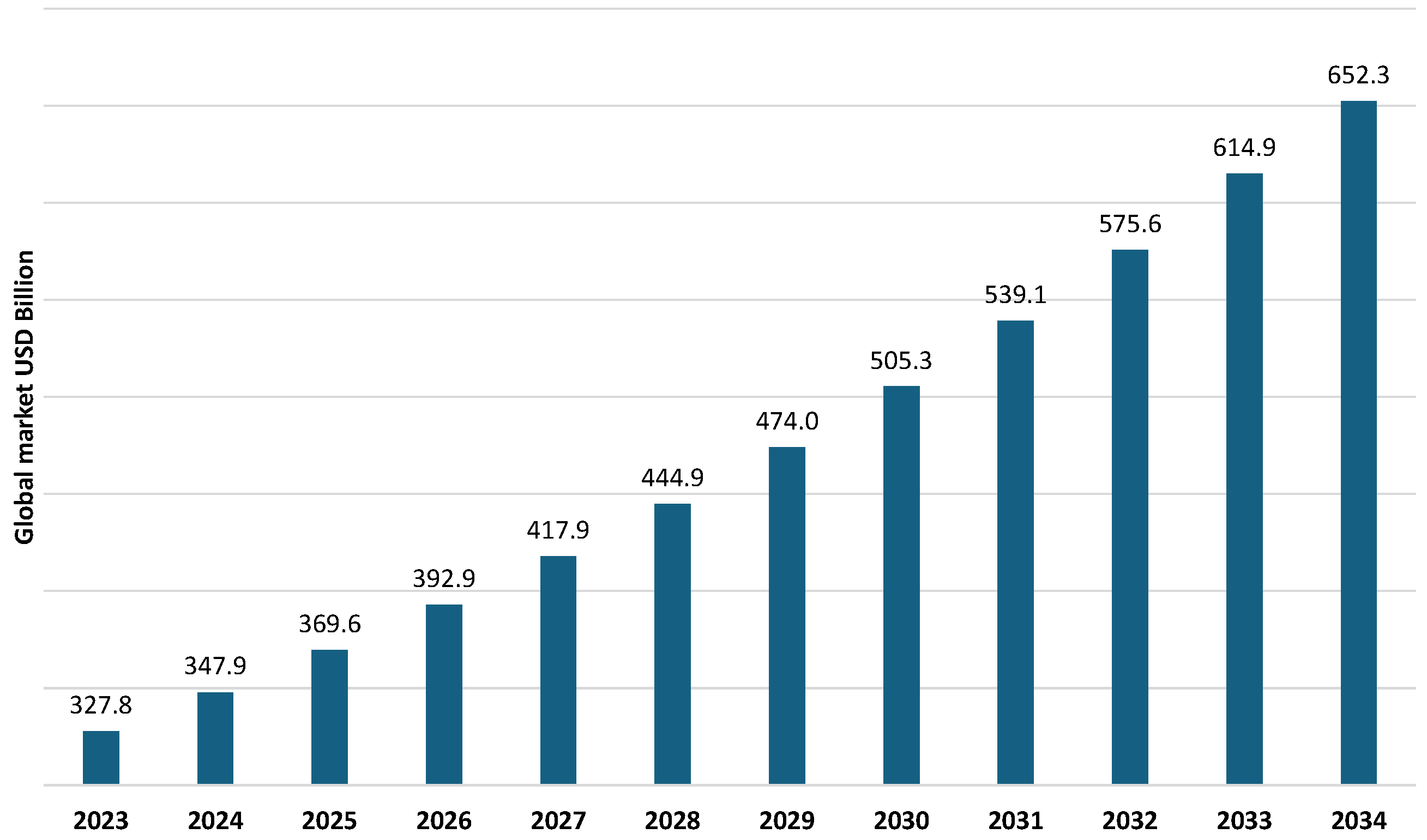
| Biomass | Temperature (°C) | Activation | Washing | qmax (mg/g) * | Reference |
|---|---|---|---|---|---|
| Laminaria digitata | 400 | - | - | 117 | [13] |
| Laminaria digitata | 250 | - | - | 175 | [13] |
| Enteromorpha prolifera | 500 | NaOH, 800 °C | HCl, H2O | 244 | [11] |
| Ulva lactuca | 700 | ZnCl2 | HCl, H2O | 345 | [14] |
| Gelidiella acerosa | 800 | - | HCl, H2O | 513 | [15] |
| Enteromorpha prolifera * Oily sludge | 700 | KOH | HCl, H2O | 910 | [16] |
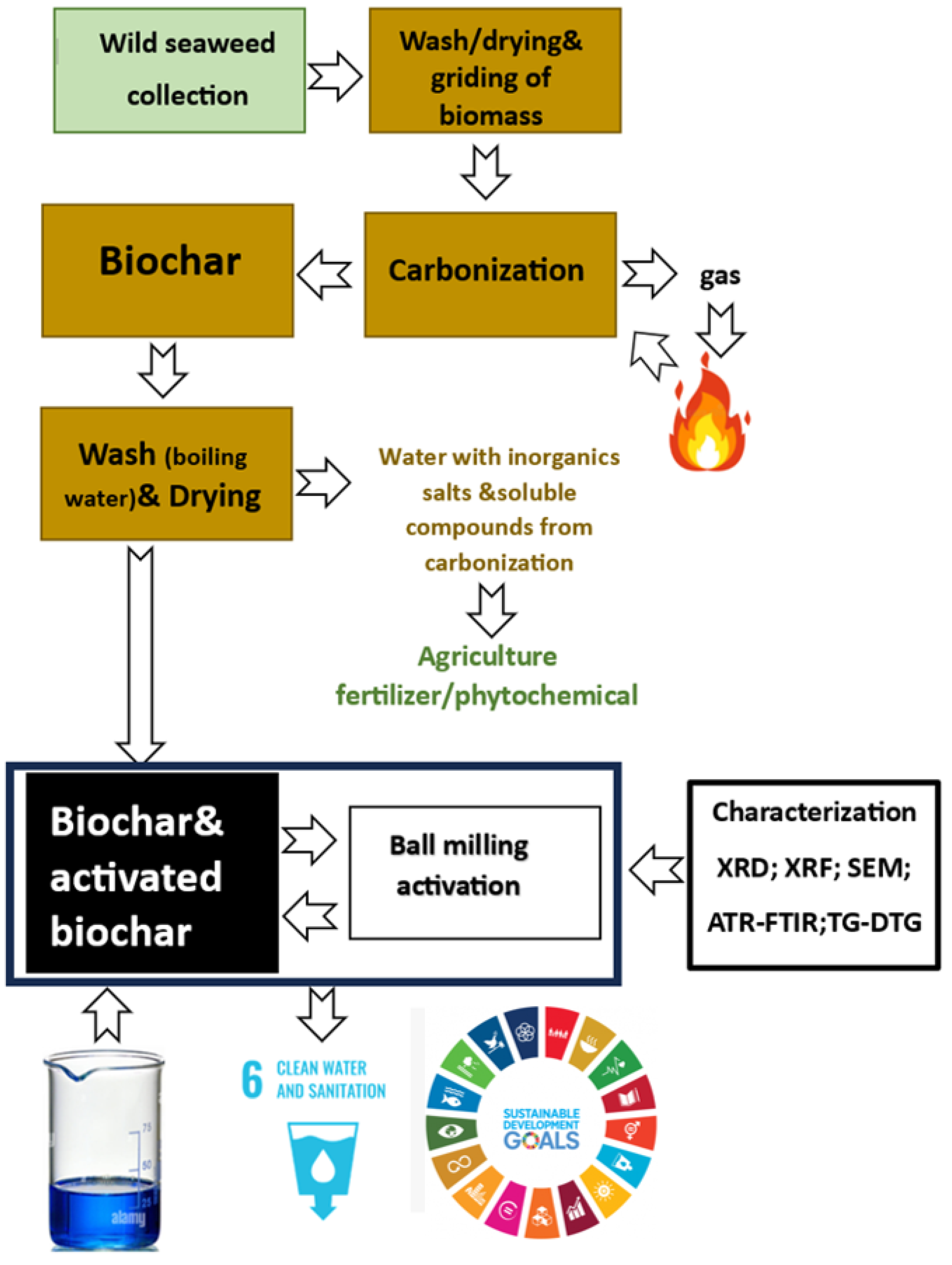
2. Experimental
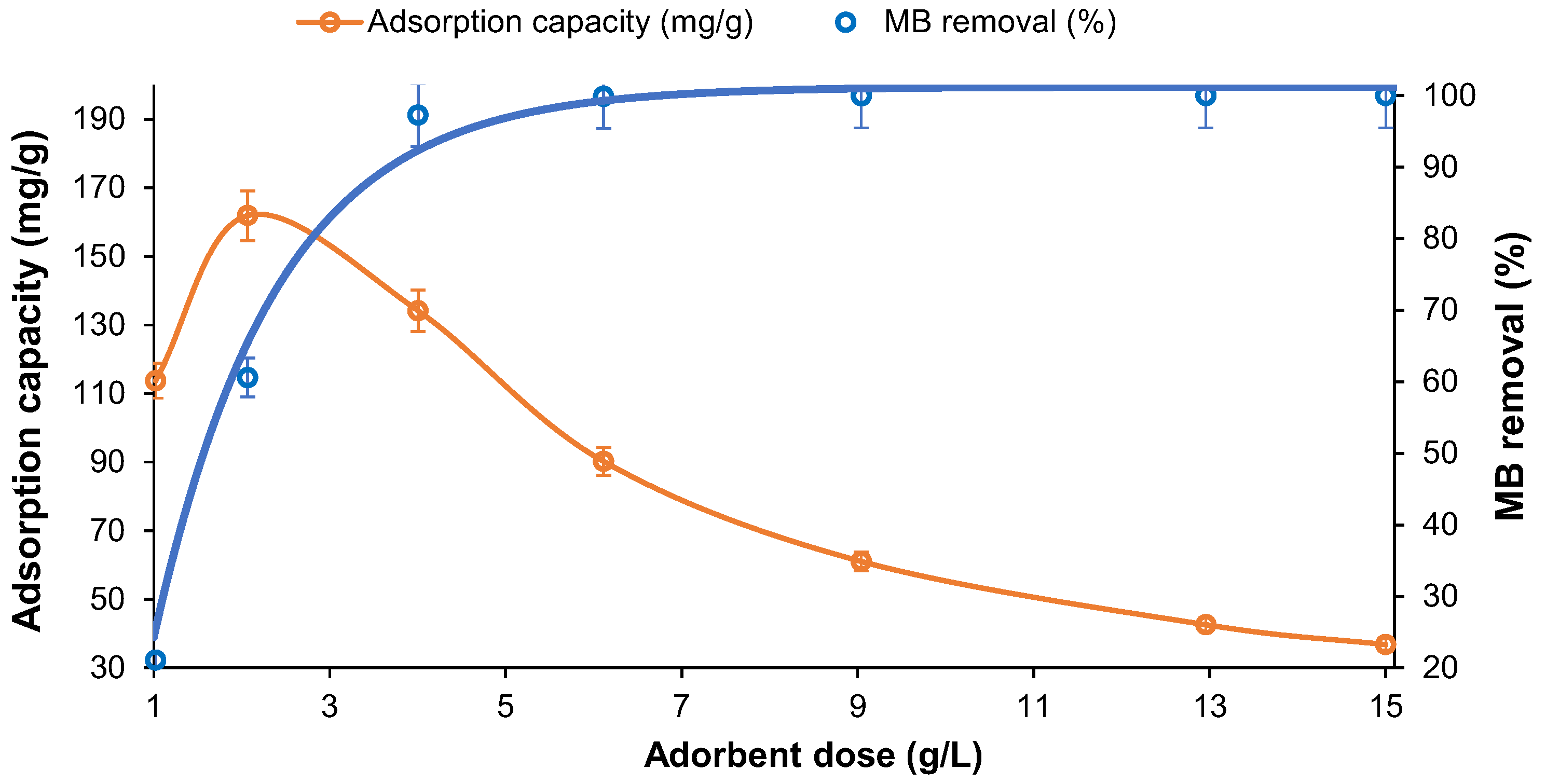
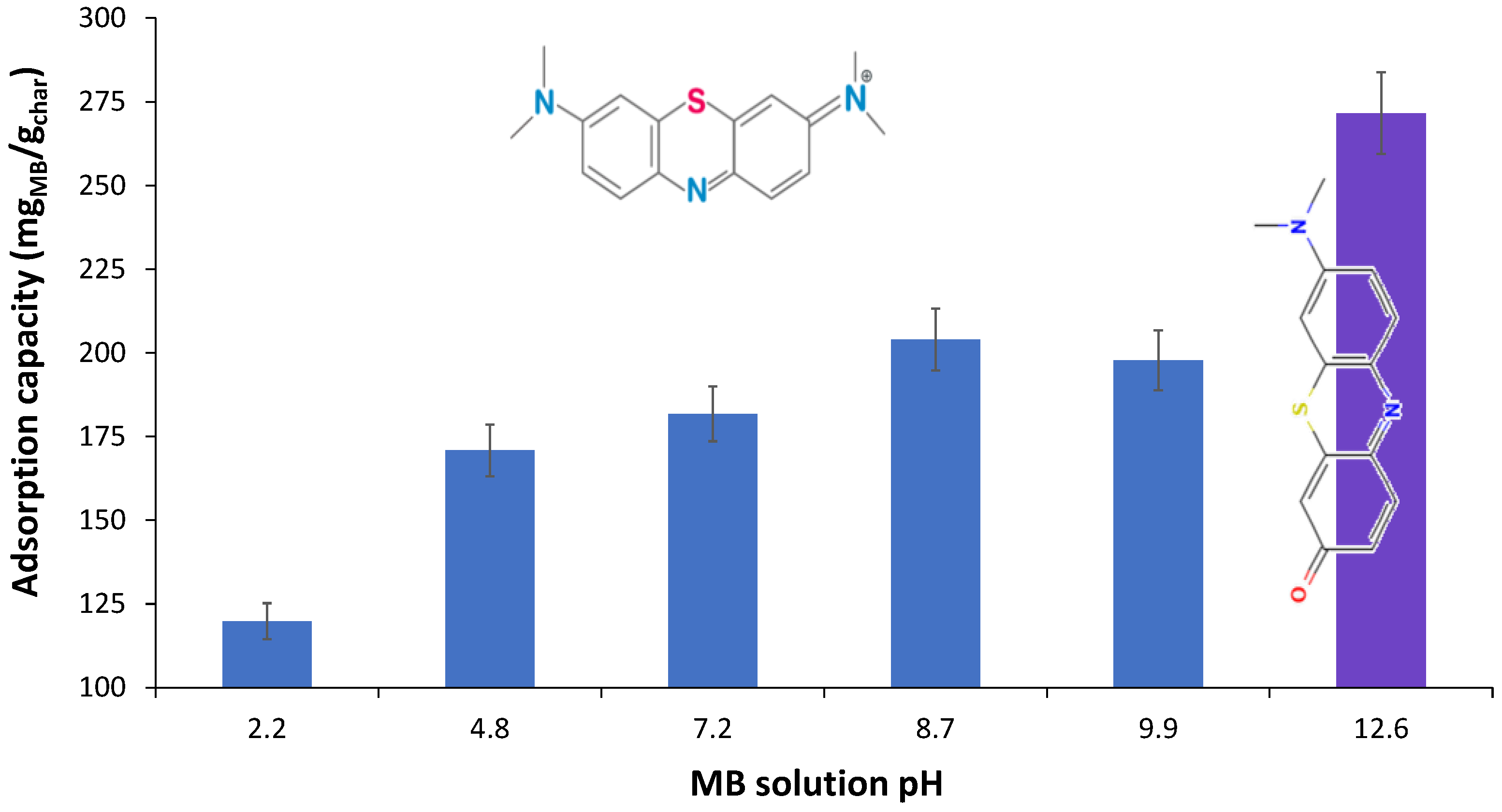
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BM | ball milled |
| Ce | concentration of MB at equilibrium; |
| KF | Freundlich isotherm constant |
| KL | Langmuir isotherm constant |
| MB | methylene blue dye |
| heterogeneity factor | |
| NW | non washed char |
| qe | amount of adsorbate per mass unit of char at equilibrium |
| Qmax | maximum monolayer adsorption capacity |
| S300 | sargaço char produced at 300 °C |
| S400 | sargaço char produced at 400 °C |
| Sargaço | Seaweeds harvested along the Portuguese coastline |
| W | washed char |
References
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Ihsanullah, I.; Hassan Shah, M.U.; Bhaskar Reddy, A.V.; Aminabhavi, T.M. Recent advances in the removal of dyes from wastewater using low-cost adsorbents. J. Environ. Manag. 2022, 321, 115981. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Omoarukhe, F.O.; Ojukwu, V.E.; Iwuozor, K.O.; Igwegbe, C.A. Cost of adsorbent preparation and usage in wastewater treatment: A review. Clean. Chem. Eng. 2022, 3, 100042. [Google Scholar] [CrossRef]
- Water and Wastewater Treatment Market Size to Hit USD 652.30 Billion by 2034. Available online: https://www.precedenceresearch.com/water-and-wastewater-treatment-market (accessed on 14 April 2025).
- Singh, S.; Prajapati, A.K.; Chakraborty, J.P.; Mondal, M.K. Adsorption potential of biochar obtained from pyrolysis of raw and torrefied Acacia nilotica towards removal of methylene blue dye from synthetic wastewater. Biomass Convers. Biorefinery 2023, 13, 6083–6104. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Varjani, S.; Saravanan, A. A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar] [CrossRef]
- Jeyasubramanian, K.; Thangagiri, B.; Sakthivel, A.; Dhaveethu Raja, J.; Seenivasan, S.; Vallinayagam, P.; Madhavan, D.; Malathi Devi, S.; Rathika, B. A complete review on biochar: Production, property, multifaceted applications, interaction mechanism and computational approach. Fuel 2021, 292, 120243. [Google Scholar] [CrossRef]
- Dang, B.T.; Ramaraj, R.; Huynh, K.P.H.; Le, M.V.; Tomoaki, I.; Pham, T.T.; Hoang Luan, V.; Thi Le Na, P.; Tran, D.P.H. Current application of seaweed waste for composting and biochar: A review. Bioresour. Technol. 2023, 375, 128830. [Google Scholar] [CrossRef]
- Liu, S.; Shen, C.; Wang, Y.; Huang, Y.; Hu, X.; Li, B.; Karnowo; Zhou, J.; Zhang, S.; Zhang, H. Development of CO2/H2O activated biochar derived from pine pyrolysis: Application in methylene blue adsorption. J. Chem. Technol. Biotechnol. 2022, 97, 885–893. [Google Scholar] [CrossRef]
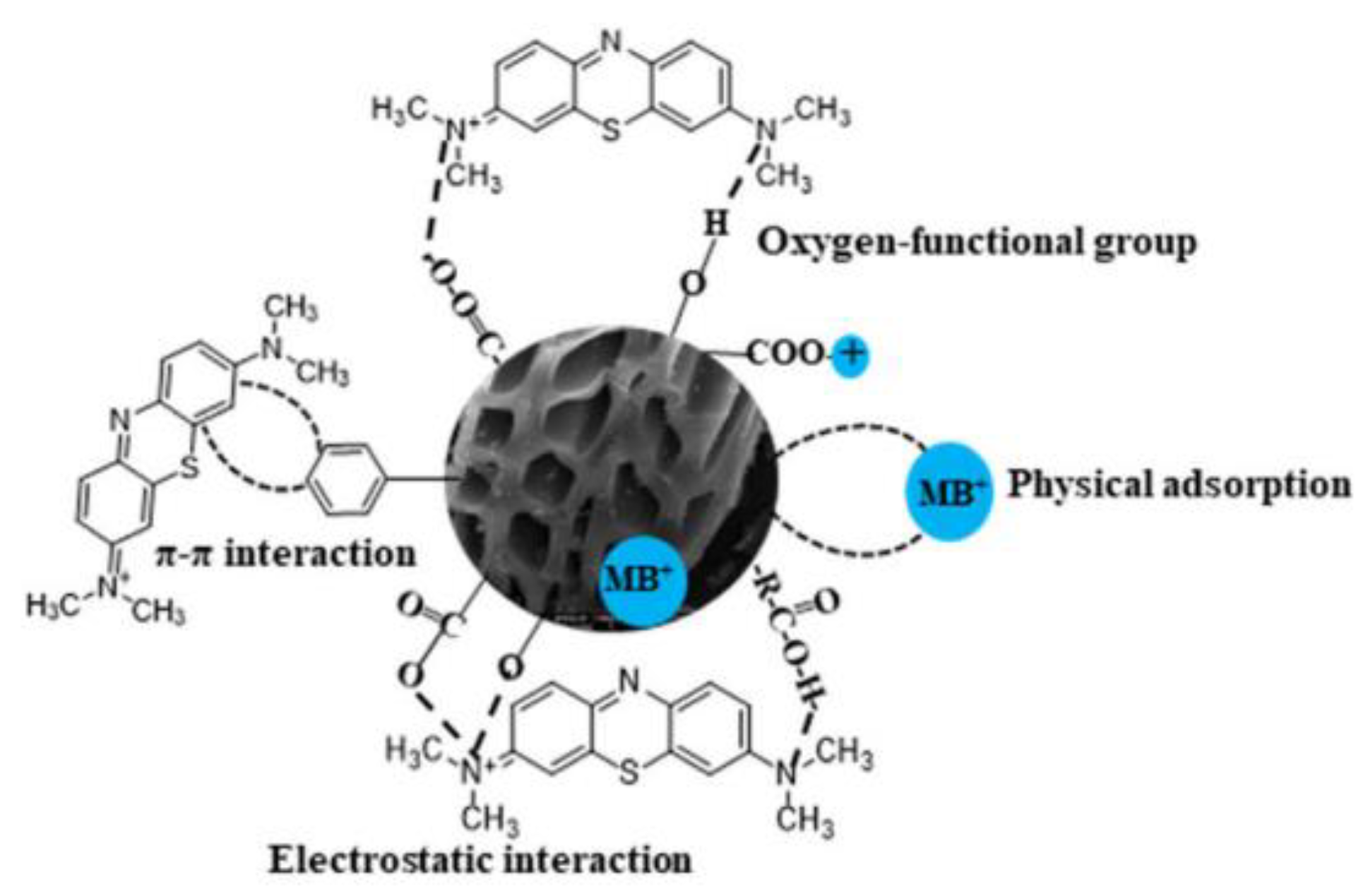
- Jiang, D.; Li, H.; Cheng, X.; Ling, Q.; Chen, H.; Barati, B.; Yao, Q.; Abomohra, A.; Hu, X.; Bartocci, P.; et al. A mechanism study of methylene blue adsorption on seaweed biomass derived carbon: From macroscopic to microscopic scale. Process Saf. Environ. Prot. 2023, 172, 1132–1143. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, C.; Kong, D.; Lian, L.; Liu, Y. Review on application of algae-based biochars in environmental remediation: Progress, challenge and perspectives. J. Environ. Chem. Eng. 2023, 11, 111263. [Google Scholar] [CrossRef]
- Güleç, F.; Williams, O.; Kostas, E.T.; Samson, A.; Stevens, L.A.; Lester, E. A comprehensive comparative study on methylene blue removal from aqueous solution using biochars produced from rapeseed, whitewood, and seaweed via different thermal conversion technologies. Fuel 2022, 330, 125428. [Google Scholar] [CrossRef]
- El Nemr, A.; Shoaib, A.G.M.; El Sikaily, A.; Mohamed, A.E.D.A.; Hassan, A.F. Evaluation of Cationic Methylene Blue Dye Removal by High Surface Area Mesoporous Activated Carbon Derived from Ulva lactuca. Environ. Process. 2021, 8, 311–332. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Okoye, P.U.; Hummadi, E.H.; Hameed, B.H. High-performance porous biochar from the pyrolysis of natural and renewable seaweed (Gelidiella acerosa) and its application for the adsorption of methylene blue. Bioresour. Technol. 2019, 278, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Han, D.; Xie, J.F.; Wang, Z.B.; Gong, Z.Q.; Li, B. Hierarchical porous activated biochar derived from marine macroalgae wastes (Enteromorpha prolifera): Facile synthesis and its application on Methylene Blue removal. RSC Adv. 2018, 8, 29237–29247. [Google Scholar] [CrossRef] [PubMed]
- EUR-Lex-01998L0083-20151027-EN-EUR-Lex, (n.d.). Available online: https://eur-lex.europa.eu/legal-content/PT/TXT/?uri=CELEX:01998L0083-20151027 (accessed on 16 March 2025).
- Singh, B.; Dolk, M.M.; Shen, Q.; Camps-Arbestain, M. Biochar pH, electrical conductivity and liming potential. In Biochar: A Guide to Analytical Methods; CSIRO Publishing: Clayton, Australia, 2017; pp. 23–38. Available online: https://ebooks.publish.csiro.au/content/ISBN/9781486305100 (accessed on 16 March 2025).
- Rijo, B.; Dias, A.P.S.; Carvalho, J.P.S. Recovery of carbon fibers from aviation epoxy composites by acid solvolysis. Sustain. Mater. Technol. 2023, 35, 14–16. [Google Scholar] [CrossRef]
- Brown, A.B.; McKeogh, B.J.; Tompsett, G.A.; Lewis, R.; Deskins, N.A.; Timko, M.T. Structural analysis of hydrothermal char and its models by density functional theory simulation of vibrational spectroscopy. Carbon N. Y. 2017, 125, 614–629. [Google Scholar] [CrossRef]
- Fernandez-Perez, A.; Marban, G. Visible light spectroscopic analysis of methylene blue in water; what comes after dimer? ACS Omega 2020, 5, 29801–29815. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, S.; Uzoejinwa, B.B.; Zheng, A.; Wang, Q.; Huang, J.; Abomohra, A.E.F. A state-of-the-art review on dual purpose seaweeds utilization for wastewater treatment and crude bio-oil production. Energy Convers. Manag. 2020, 222, 113253. [Google Scholar] [CrossRef]
- Soares Dias, A.P.; Rego, F.; Fonseca, F.; Casquilho, M.; Rosa, F.; Rodrigues, A. Catalyzed pyrolysis of SRC poplar biomass. Alkaline carbonates and zeolites catalysts. Energy 2019, 183, 1114–1122. [Google Scholar] [CrossRef]
- Chung, B.Y.H.; Ang, J.C.; Tang, J.Y.; Chong, J.W.; Tan, R.R.; Aviso, K.B.; Chemmangattuvalappil, N.G.; Thangalazhy-Gopakumar, S. Rough set approach to predict biochar stability and pH from pyrolysis conditions and feedstock characteristics. Chem. Eng. Res. Des. 2023, 198, 221–233. [Google Scholar] [CrossRef]
- Wang, K.; Peng, N.; Zhang, D.; Zhou, H.; Gu, J.; Huang, J.; Liu, C.; Chen, Y.; Liu, Y.; Sun, J. Efficient removal of methylene blue using Ca(OH)2 modified biochar derived from rice straw. Environ. Technol. Innov. 2023, 31, 103145. [Google Scholar] [CrossRef]
- Sangha, J.S.; Kelloway, S.; Critchley, A.T.; Prithiviraj, B. Seaweeds (Macroalgae) and Their Extracts as Contributors of Plant Productivity and Quality: The Current Status of Our Understanding. Adv. Bot. Res. 2014, 71, 189–219. [Google Scholar] [CrossRef]
- Singh, M.; Vander Wal, R.L. Carbon Composites—Graphene-Oxide-Catalyzed Sugar Graphitization. C 2022, 8, 15. [Google Scholar] [CrossRef]
- Yuan, R.; Dong, Y.; Hou, R.; Shang, L.; Zhang, J.; Zhang, S.; Chen, X.; Song, H. Structural transformation of porous and disordered carbon during ball-milling. Chem. Eng. J. 2023, 454, 140418. [Google Scholar] [CrossRef]
- Ayiania, M.; Weiss-Hortala, E.; Smith, M.; McEwen, J.S.; Garcia-Perez, M. Microstructural analysis of nitrogen-doped char by Raman spectroscopy: Raman shift analysis from first principles. Carbon N. Y. 2020, 167, 559–574. [Google Scholar] [CrossRef]
- Lopez-Tenllado, F.J.; Motta, I.L.; Hill, J.M. Modification of biochar with high-energy ball milling: Development of porosity and surface acid functional groups. Bioresour. Technol. Rep. 2021, 15, 100704. [Google Scholar] [CrossRef]
- Timko, M.T.; Maag, A.R.; Venegas, J.M.; McKeogh, B.; Yang, Z.; Tompsett, G.A.; Escapa, S.; Toto, J.; Heckley, E.; Greenaway, F.T. Spectroscopic tracking of mechanochemical reactivity and modification of a hydrothermal char. RSC Adv. 2016, 6, 12021–12031. [Google Scholar] [CrossRef]
- Soares Dias, A.P.; Rijo, B.; Santos, F.; Galhanos dos Santos, R.; Frade, T. Overview on biofuels production in a seaweed biorefinery. Sci. Total Environ. 2023, 884, 163714. [Google Scholar] [CrossRef]
- Sun, J.; Norouzi, O.; Mašek, O. A state-of-the-art review on algae pyrolysis for bioenergy and biochar production. Bioresour. Technol. 2022, 346, 126258. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, L.; Gao, J.; Zhang, S.; Liu, Q.; Duan, P.; Hu, X. Evolution of the functional groups/structures of biochar and heteroatoms during the pyrolysis of seaweed. Algal Res. 2020, 48, 101900. [Google Scholar] [CrossRef]
- Bakshi, S.; Banik, C.; Laird, D.A. Estimating the organic oxygen content of biochar. Sci. Rep. 2020, 10, 13082. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikov, O.V.; Evtukhova, A.V.; Kondratenko, T.S.; Smirnov, M.S.; Khokhlov, V.Y.; Erina, O.V. Manifestation of intermolecular interactions in FTIR spectra of methylene blue molecules. Vib. Spectrosc. 2016, 86, 181–189. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Cai, J.; Wilson, K.; Lee, A.F. Bio/hydrochar Sorbents for Environmental Remediation. Energy Environ. Mater. 2020, 3, 453–468. [Google Scholar] [CrossRef]
- Gayathri, K.; Palanisamy, N. Methylene blue adsorption onto an eco-friendly modified polyacrylamide/graphite composites: Investigation of kinetics, equilibrium, and thermodynamic studies. Sep. Sci. Technol. 2020, 55, 266–277. [Google Scholar] [CrossRef]
- Enaime, G.; Baçaoui, A.; Yaacoubi, A.; Lübken, M. Biochar for Wastewater Treatment—Conversion Technologies and Applications. Appl. Sci. 2020, 10, 3492. [Google Scholar] [CrossRef]
- Barquilha, C.E.R.; Braga, M.C.B. Adsorption of organic and inorganic pollutants onto biochars: Challenges, operating conditions, and mechanisms. Bioresour. Technol. Rep. 2021, 15, 100728. [Google Scholar] [CrossRef]
- Li, H.; Kong, J.; Zhang, H.; Gao, J.; Fang, Y.; Shi, J.; Ge, T.; Fang, T.; Shi, Y.; Zhang, R.; et al. Mechanisms and adsorption capacities of ball milled biomass fly ash/biochar composites for the adsorption of methylene blue dye from aqueous solution. J. Water Process Eng. 2023, 53, 103713. [Google Scholar] [CrossRef]
- Yang, C.; Miao, S.; Li, T. Influence of water washing treatment on Ulva prolifera-derived biochar properties and sorption characteristics of ofloxacin. Sci. Rep. 2021, 11, 1797. [Google Scholar] [CrossRef]
- Lyu, H.; Gao, B.; He, F.; Zimmerman, A.R.; Ding, C.; Tang, J.; Crittenden, J.C. Experimental and modeling investigations of ball-milled biochar for the removal of aqueous methylene blue. Chem. Eng. J. 2018, 335, 110–119. [Google Scholar] [CrossRef]
- Zhu, Y.; Yi, B.; Yuan, Q.; Wu, Y.; Wang, M.; Yan, S. Removal of methylene blue from aqueous solution by cattle manure-derived low temperature biochar. RSC Adv. 2018, 8, 19917–19929. [Google Scholar] [CrossRef] [PubMed]
- Mani, D.; Elango, D.; Priyadharsan, A.; Al-Humaid, L.A.; Al-Dahmash, N.D.; Ragupathy, S.; Jayanthi, P.; Ahn, Y.H. Groundnut shell chemically treated with KOH to prepare inexpensive activated carbon: Methylene blue adsorption and equilibrium isotherm studies. Environ. Res. 2023, 231, 116026. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Fan, S. Preparation of KOH and H3PO4 Modified Biochar and Its Application in Methylene Blue Removal from Aqueous Solution. Processes 2019, 7, 891. [Google Scholar] [CrossRef]
- Sivaranjanee, R.; Kumar, P.S.; Rangasamy, G. A critical review on biochar for environmental applications. Carbon Lett. 2023, 33, 1407–1432. [Google Scholar] [CrossRef]

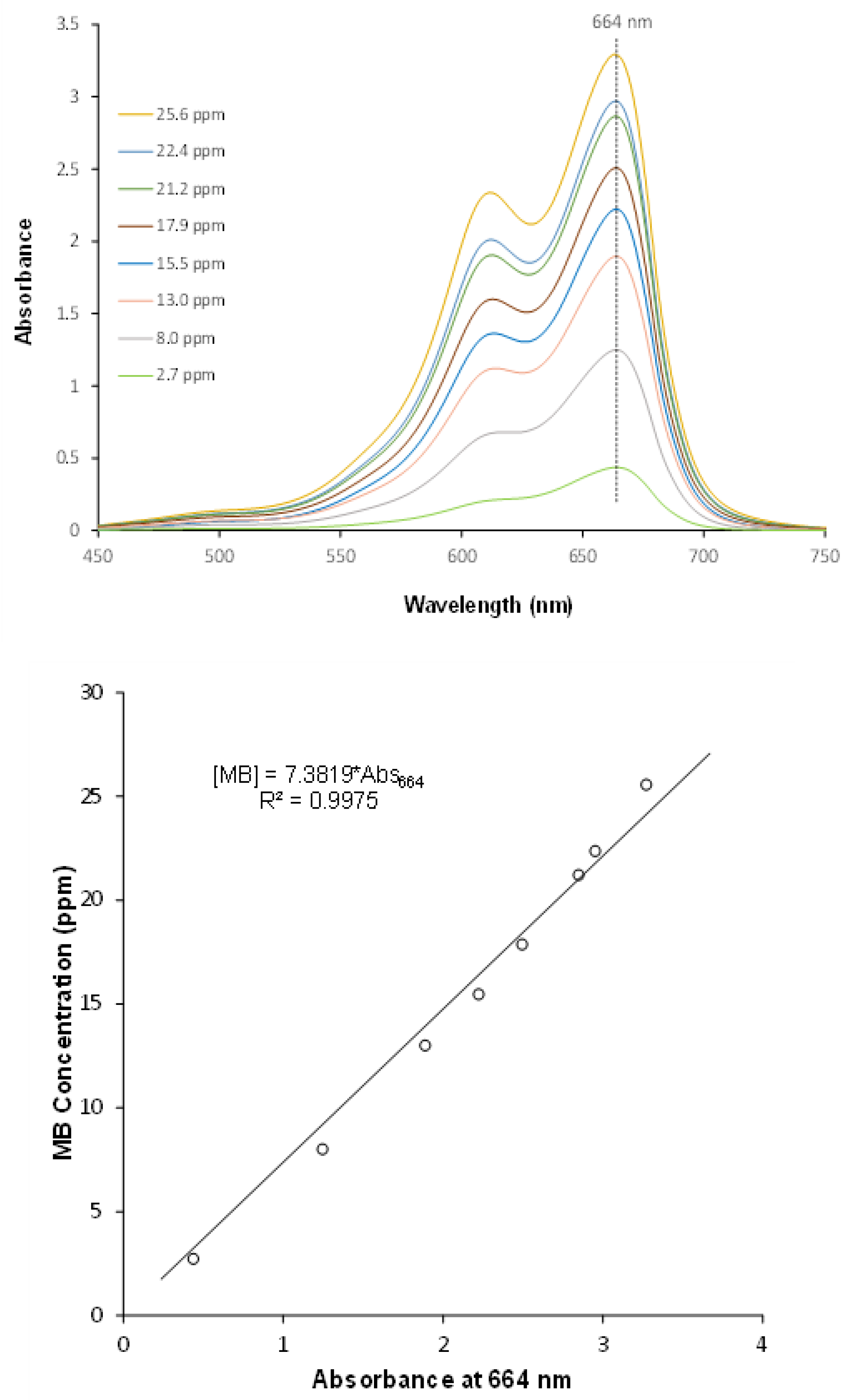

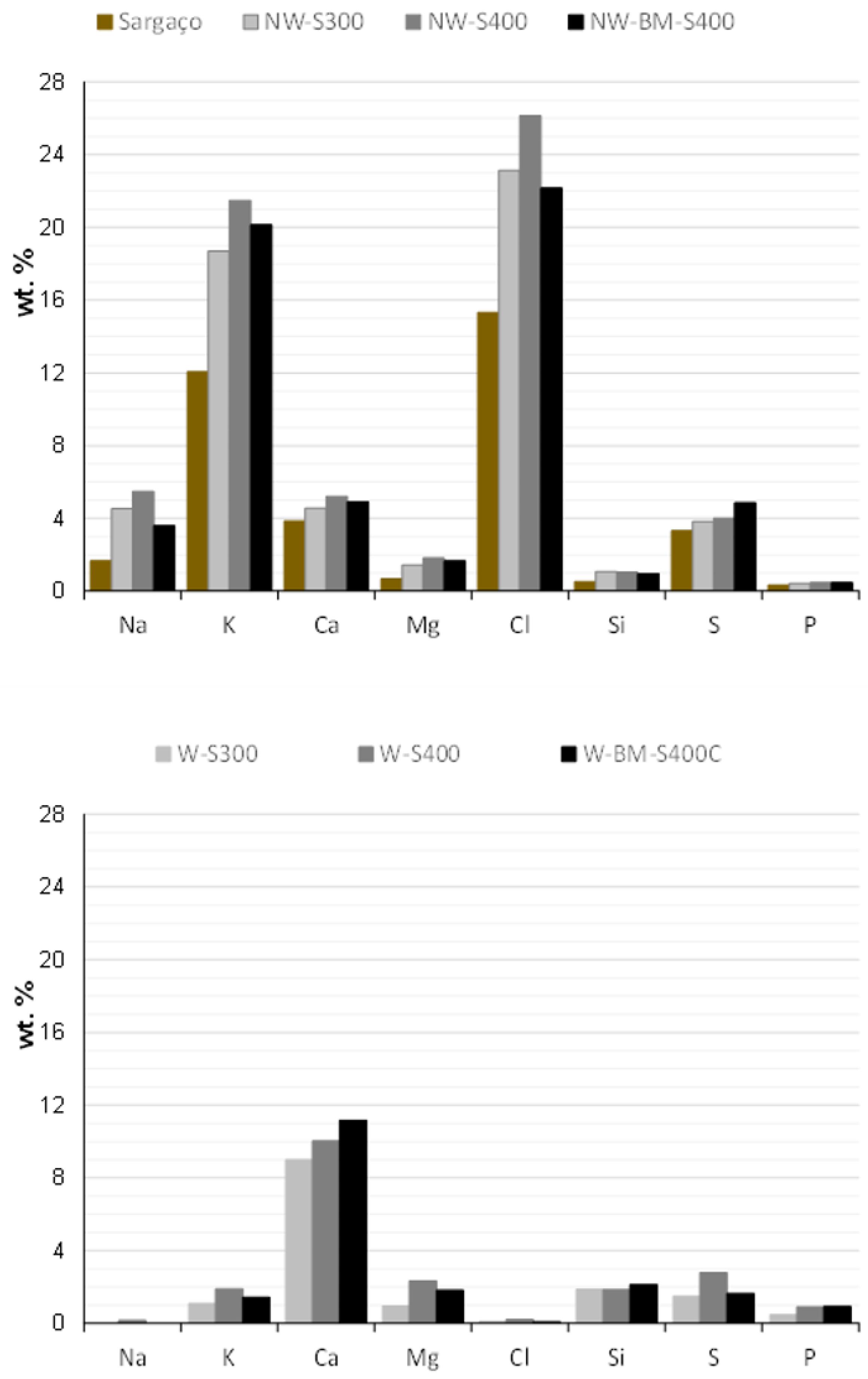


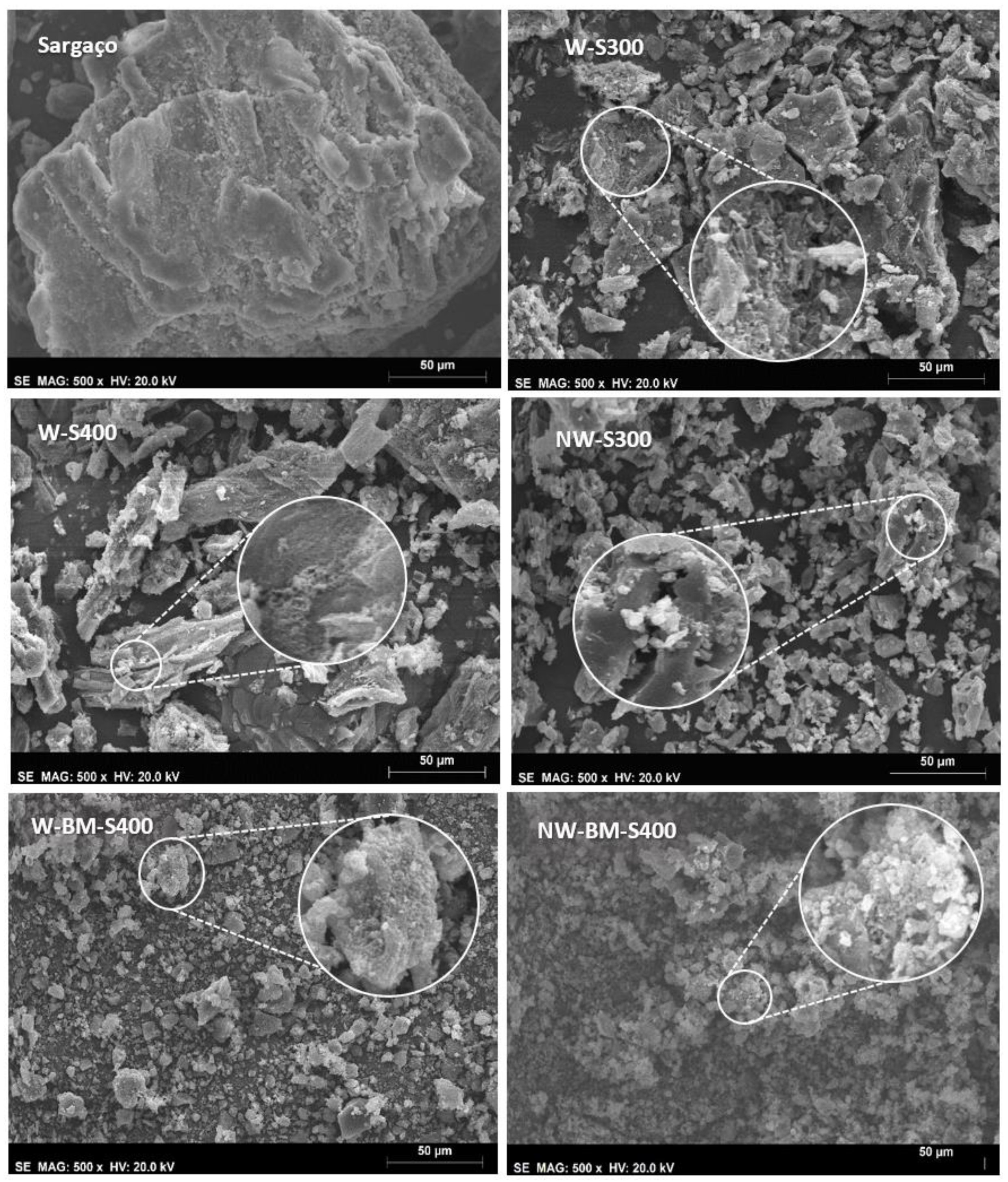


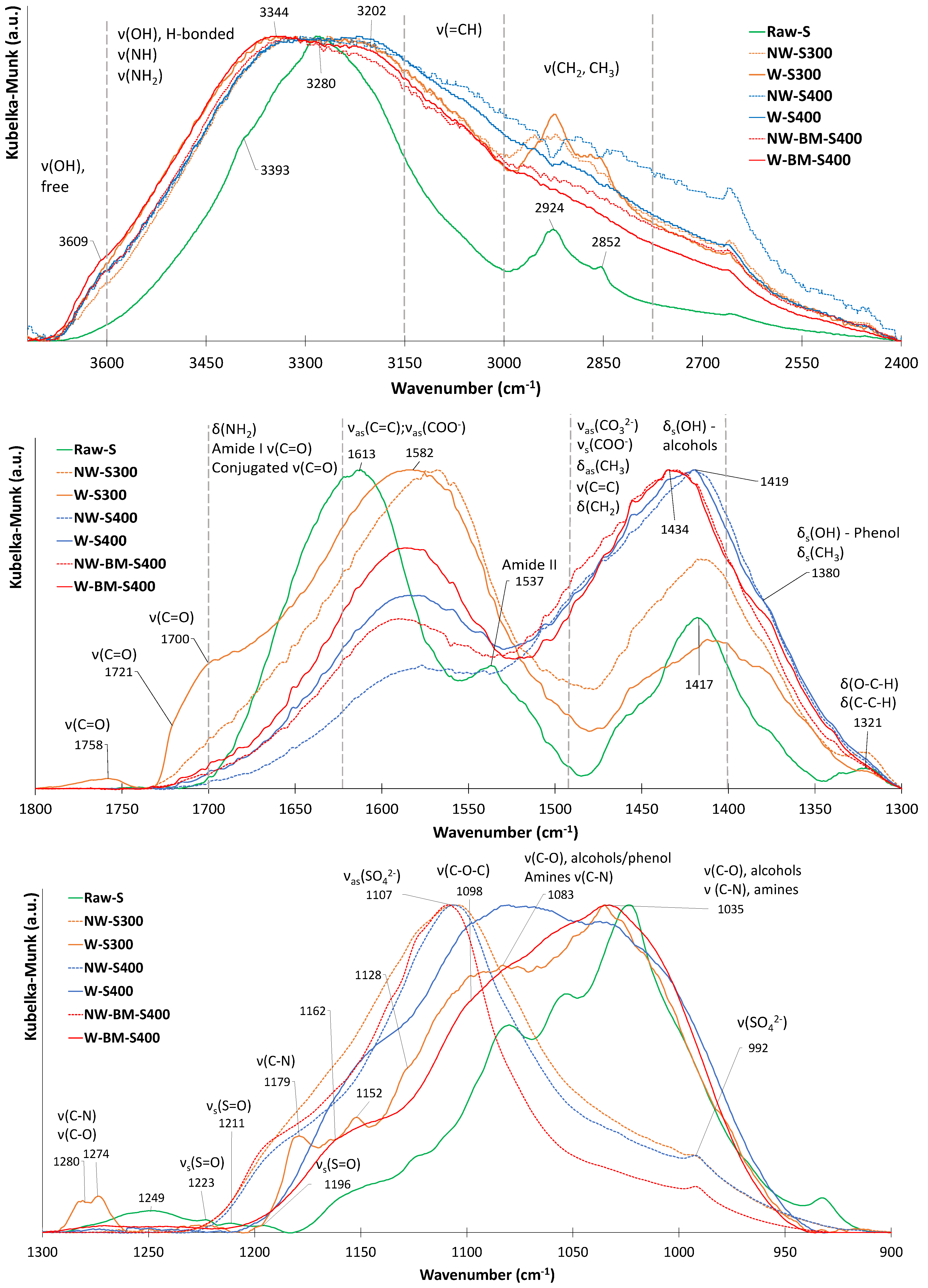



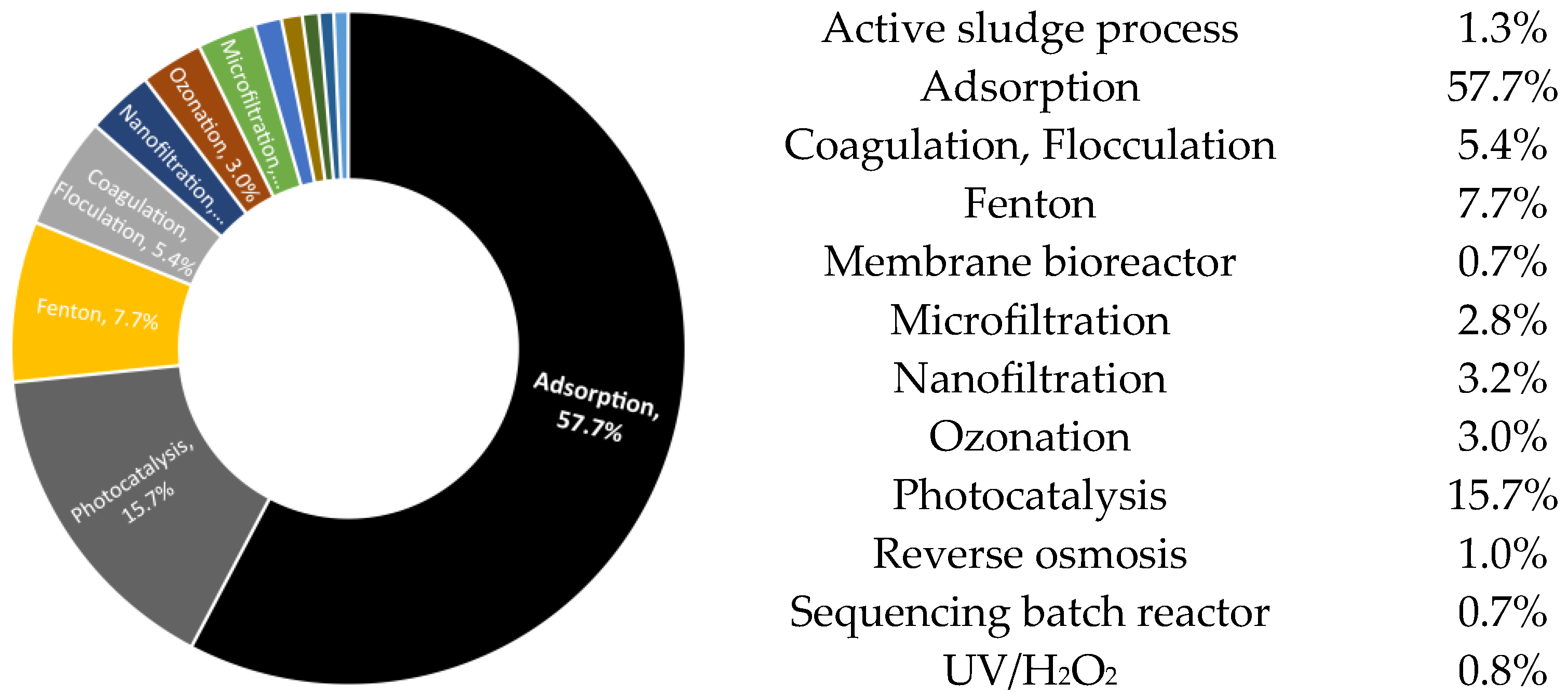
| Technology | Advantages | Disadvantages | |
|---|---|---|---|
| Conventional | Coagulation Flocculation Biodegradation | Simple, economically feasible Economically attractive, publicly acceptable treatment | High sludge production, handling and disposal problems Slow process, necessary to create an optimal favorable environment, maintenance and nutrition requirements |
| Adsorption on activated carbons | The most effective adsorbent, great, capacity, produces a high-quality treated effluent | Ineffective against disperse and vat dyes, the regeneration is expensive and results in loss of the adsorbent, non-destructive process | |
| Established | Membrane separations | Removes all dye types, produces a high-quality treated effluent | High pressures, expensive, incapable of treating large volumes |
| Ion-exchange | No loss of sorbent on regeneration, effective | Economic constraints, not effective for disperse dyes | |
| Oxidation | Rapid and efficient process | High energy cost, chemicals required | |
| Emerging | Advanced oxidation process | No sludge production, little or no consumption of chemicals, efficiency for recalcitrant dyes | Economically unfeasible, formation of by-products, technical constraints |
| Selective bio-adsorbents | Economically attractive, regeneration is not necessary, high selectivity | Requires chemical modification, non-destructive process | |
| Biomass | Low operating cost, good efficiency and selectivity, no toxic effect on microorganisms | Slow process, performance depends on some external factors (pH, salts) |
| Phylum | Species | Species (wt. %; Dried) |
|---|---|---|
| Phaeophyceae | Ascophyllum nodosum | 5.0 |
| Fucus vesiculosus | 15.0 | |
| Gongolaria baccata | 10.1 | |
| Saccorhiza polyschides | 39.9 | |
| Chlorophyta | Ulva lactuca | 7.5 |
| Ulva rigida | 7.5 | |
| Rhodophyta | Gelidium corneum | 7.0 |
| Gracilaria gracilis | 4.0 | |
| Plocamium cartilagineum | 4.0 |
| Biochar ID | Yield (%) | pH |
|---|---|---|
| NW-S300 | 57.4 | 8.5 |
| W-S300 | - | 7.1 |
| NW-S400 | 46.0 | 10.3 |
| W-S400 | - | 9.3 |
| NW-BM-S400 | - | 10 |
| W-BM-S400 | - | 9.1 |
| MB Solid (cm−1) | Char (cm−1) | Shift (cm−1) | Band Assignment in MB |
|---|---|---|---|
| 3041 | 3058 | +17 | vhet(C-H) |
| 2921 | 2921 | 0 | νas(CH3) |
| 2850 | 2850 | 0 | νs(CH3) |
| 1585 | 1596 | +11 | vhet(C=C), vhet(C=N) |
| 1484 | 1489 | +5 | vhet(C=S+) |
| 1437 | 1437 | 0 | δas(CH3) |
| 1382 | 1387 | +4 | δ(C-H), γ(C-H) |
| 1349 | 1349 | 0 | v(C=S+) |
| 1313 | 1327 | +14 | v(C-N), in N-CH3 |
| 1244 | 1244 | 0 | δ(C-H), γ(C-H) |
| 1215 | 1223 | +8 | νhet(C-C) |
| 1162 | 1172 | +10 | δhet(C-H) |
| 1132 | 1134 | +2 | δhet(C-N) |
| 1036 | 1036 | 0 | γhet(C-H) |
| 947 | 952 | +5 | Nhet···HO |
| 876 | 884 | +8 | Nhet···HO |
| 821 | 827 | +6 | δhet(C-C) |
| 666 | 668 | +2 | νs(C-S-C) in heterocycle |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares Dias, A.P.; Santos, F.A.; Rijo, B.; Simes, D.C.; Pereira, L.; Pereira, M.F.C. Seaweed-Derived Biochar for Effective Treatment of Dye-Contaminated Wastewater. Water 2025, 17, 1215. https://doi.org/10.3390/w17081215
Soares Dias AP, Santos FA, Rijo B, Simes DC, Pereira L, Pereira MFC. Seaweed-Derived Biochar for Effective Treatment of Dye-Contaminated Wastewater. Water. 2025; 17(8):1215. https://doi.org/10.3390/w17081215
Chicago/Turabian StyleSoares Dias, Ana Paula, Francisco Ascenção Santos, Bruna Rijo, Dina Costa Simes, Leonel Pereira, and Manuel Francisco Costa Pereira. 2025. "Seaweed-Derived Biochar for Effective Treatment of Dye-Contaminated Wastewater" Water 17, no. 8: 1215. https://doi.org/10.3390/w17081215
APA StyleSoares Dias, A. P., Santos, F. A., Rijo, B., Simes, D. C., Pereira, L., & Pereira, M. F. C. (2025). Seaweed-Derived Biochar for Effective Treatment of Dye-Contaminated Wastewater. Water, 17(8), 1215. https://doi.org/10.3390/w17081215












