Killing Two Crises with One Spark: Cold Plasma for Antimicrobial Resistance Mitigation and Wastewater Reuse
Abstract
:1. Introduction: Global Water Challenges and the Need for Innovation
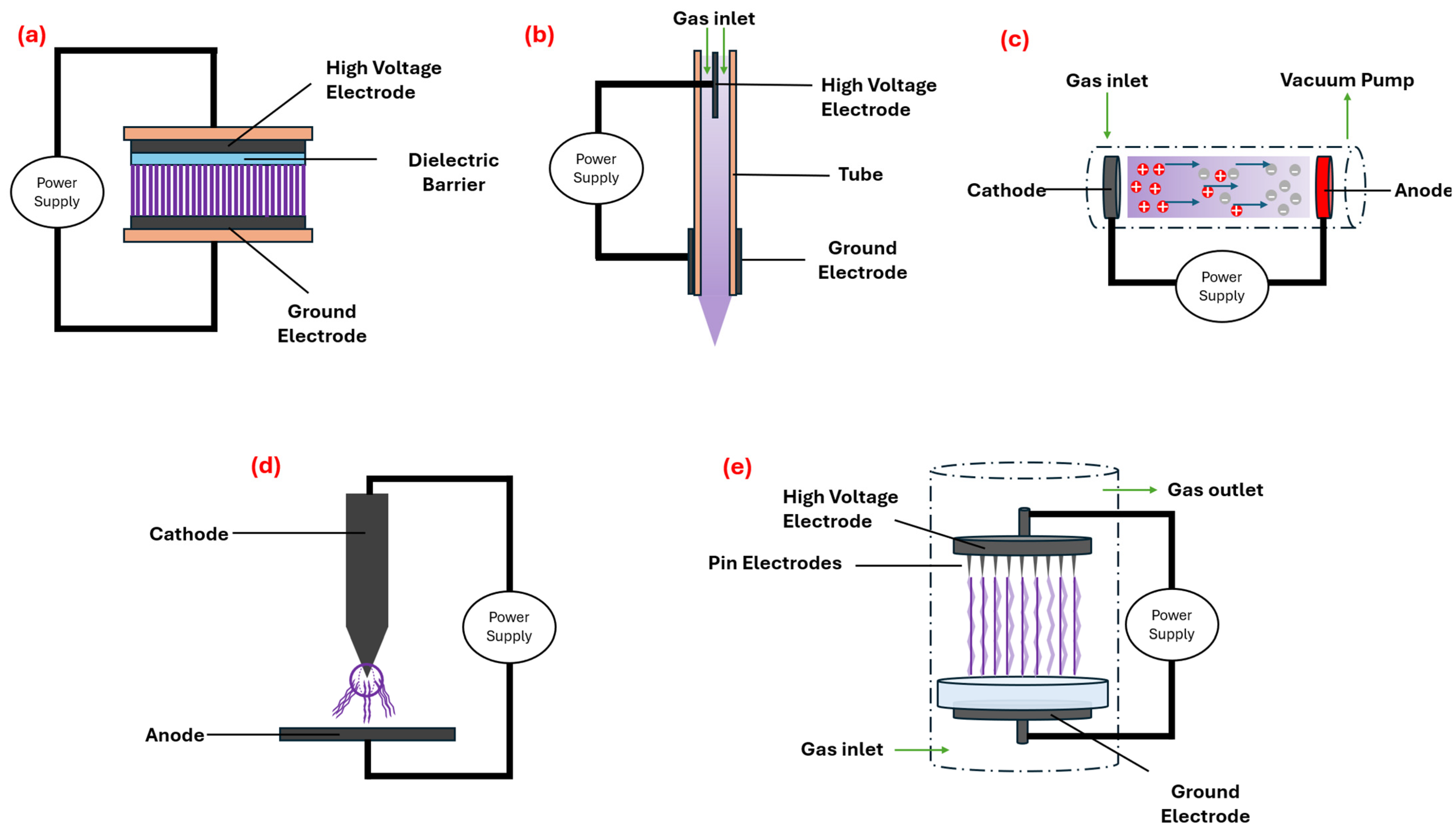
2. Fundamentals of Cold Plasma Technology
3. Bacterial Inactivation Using Cold Plasma Based Technologies
4. Viral Inactivation Using Cold Plasma-Based Technologies
5. Challenges for Cold Plasma Implementation
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ouda, S.; Zohry, A.E.-H. Water-Smart Practices to Manage Water Scarcity. In Climate-Smart Agriculture: Reducing Food Insecurity; Ouda, S., Zohry, A.E.-H., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 3–26. ISBN 978-3-030-93111-7. [Google Scholar]
- Falkenmark, M. Growing Water Scarcity in Agriculture: Future Challenge to Global Water Security. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2013, 371, 20120410. [Google Scholar] [CrossRef]
- Ingrao, C.; Strippoli, R.; Lagioia, G.; Huisingh, D. Water Scarcity in Agriculture: An Overview of Causes, Impacts and Approaches for Reducing the Risks. Heliyon 2023, 9, e18507. [Google Scholar] [CrossRef] [PubMed]
- Adegoke, H.A.; Solihu, H.; Bilewu, S.O. Analysis of Sanitation and Waterborne Disease Occurrence in Ondo State, Nigeria. Environ. Dev. Sustain. 2023, 25, 11885–11903. [Google Scholar] [CrossRef]
- Jofre, J.; Blanch, A.R.; Lucena, F. Water-Borne Infectious Disease Outbreaks Associated with Water Scarcity and Rainfall Events. In Water Scarcity in the Mediterranean: Perspectives Under Global Change; Sabater, S., Barceló, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 147–159. ISBN 978-3-642-03971-3. [Google Scholar]
- Zakar, M.Z.; Zakar, D.R.; Fischer, F. Climate Change-Induced Water Scarcity: A Threat to Human Health. South Asian Stud. 2020, 27, 293–312. [Google Scholar]
- Leal Filho, W.; Totin, E.; Franke, J.A.; Andrew, S.M.; Abubakar, I.R.; Azadi, H.; Nunn, P.D.; Ouweneel, B.; Williams, P.A.; Simpson, N.P. Understanding Responses to Climate-Related Water Scarcity in Africa. Sci. Total Environ. 2022, 806, 150420. [Google Scholar] [CrossRef]
- Wang, M.; Bodirsky, B.L.; Rijneveld, R.; Beier, F.; Bak, M.P.; Batool, M.; Droppers, B.; Popp, A.; van Vliet, M.T.H.; Strokal, M. A Triple Increase in Global River Basins with Water Scarcity Due to Future Pollution. Nat. Commun. 2024, 15, 880. [Google Scholar] [CrossRef]
- Pontius, J.; McIntosh, A. Water Scarcity. In Environmental Problem Solving in an Age of Climate Change: Volume One: Basic Tools and Techniques; Pontius, J., McIntosh, A., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 87–103. ISBN 978-3-031-48762-0. [Google Scholar]
- Fonseca, A.; Andrade, C.; Santos, J.A. Agricultural Water Security under Climate Change in the Iberian Peninsula. Water 2022, 14, 768. [Google Scholar] [CrossRef]
- Lorenzo, M.N.; Alvarez, I.; Taboada, J.J. Drought Evolution in the NW Iberian Peninsula over a 60 Year Period (1960–2020). J. Hydrol. 2022, 610, 127923. [Google Scholar] [CrossRef]
- Caretta, M.A.; Mukherji, A.; Arfanuzzaman, M.; Betts, R.A.; Gelfan, A.; Hirabayashi, Y.; Lissner, T.K.; Lopez Gunn, E.; Liu, J.; Morgan, R.; et al. Water. In Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Pörtner, H.-O., Roberts, D.C., Tignor, M.M.B., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., et al., Eds.; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar]
- De Coninck, H.C.; van Vuuren, D.P.; Al, E. Climate Change 2022: Impacts, Adaptation and Vulnerability: Summary for Policymakers. Available online: https://www.ipcc.ch/report/ar6/wg2/downloads/report/IPCC_AR6_WGII_FinalDraft_FullReport.pdf (accessed on 3 April 2025).
- Boretti, A.; Rosa, L. Reassessing the Projections of the World Water Development Report. Npj Clean Water 2019, 2, 15. [Google Scholar] [CrossRef]
- Capodaglio, A.G. Urban Water Supply Sustainability and Resilience under Climate Variability: Innovative Paradigms, Approaches and Technologies. ACS EST Water 2024, 4, 5185–5206. [Google Scholar] [CrossRef]
- Edokpayi, J.N.; Enitan-Folami, A.M.; Adeeyo, A.O.; Durowoju, O.S.; Jegede, A.O.; Odiyo, J.O. Chapter 9—Recent Trends and National Policies for Water Provision and Wastewater Treatment in South Africa. In Water Conservation and Wastewater Treatment in BRICS Nations; Singh, P., Milshina, Y., Tian, K., Gusain, D., Bassin, J.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 187–211. ISBN 978-0-12-818339-7. [Google Scholar]
- Connors, K.A.; Dyer, S.D.; Belanger, S.E. Advancing the Quality of Environmental Microplastic Research. Environ. Toxicol. Chem. 2017, 36, 1697–1703. [Google Scholar] [CrossRef]
- United Nations. Progress on Wastewater Treatment—2024 Update; United Nations: New York, NY, USA, 2024. [Google Scholar]
- FAO; UN-Water. Progress on the Level of Water Stress: Global Status and Acceleration Needs for SDG Indicator 6.4.2, 2021; Food & Agriculture Organization: Rome, Italy, 2021; ISBN 978-92-5-134826-0. [Google Scholar]
- Wastewater Safely Treated Globally by Region. Available online: https://www.statista.com/statistics/746428/wastewater-treatment-global-share-by-region/ (accessed on 11 April 2025).
- Yazdani, R.; Harirchi, S.; Lak, M.; Mirshafiei, M.; Nojoumi, S.A.; Ramezani, M.; Rasekh, B.; Yazdian, F. Recent Advances in Conventional and Modern Wastewater Treatment Approaches. In Microbial Nexus for Sustainable Wastewater Treatment; CRC Press: Boca Raton, FL, USA, 2024; ISBN 978-1-00-344106-9. [Google Scholar]
- Krzeminski, P.; Tomei, M.C.; Karaolia, P.; Langenhoff, A.; Almeida, C.M.R.; Felis, E.; Gritten, F.; Andersen, H.R.; Fernandes, T.; Manaia, C.M.; et al. Performance of Secondary Wastewater Treatment Methods for the Removal of Contaminants of Emerging Concern Implicated in Crop Uptake and Antibiotic Resistance Spread: A Review. Sci. Total Environ. 2019, 648, 1052–1081. [Google Scholar] [CrossRef] [PubMed]
- Petala, M.; Tsiridis, V.; Samaras, P.; Zouboulis, A.; Sakellaropoulos, G.P. Wastewater Reclamation by Advanced Treatment of Secondary Effluents. Desalination 2006, 195, 109–118. [Google Scholar] [CrossRef]
- Bhatt, P.; Mathur, N.; Singh, A.; Pareek, H.; Bhatnagar, P. Evaluation of Factors Influencing the Environmental Spread of Pathogens by Wastewater Treatment Plants. Water Air Soil. Pollut. 2020, 231, 440. [Google Scholar] [CrossRef]
- Gonçalves, J.; Díaz, I.; Torres-Franco, A.; Rodríguez, E.; da Silva, P.G.; Mesquita, J.R.; Muñoz, R.; Garcia-Encina, P.A. Microbial Contamination of Environmental Waters and Wastewater: Detection Methods and Treatment Technologies. In Modern Approaches in Waste Bioremediation: Environmental Microbiology; Shah, M.P., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 461–483. ISBN 978-3-031-24086-7. [Google Scholar]
- Obotey Ezugbe, E.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Capodaglio, A.G. Critical Perspective on Advanced Treatment Processes for Water and Wastewater: AOPs, ARPs, and AORPs. Appl. Sci. 2020, 10, 4549. [Google Scholar] [CrossRef]
- Manikandan, S.; Subbaiya, R.; Saravanan, M.; Ponraj, M.; Selvam, M.; Pugazhendhi, A. A Critical Review of Advanced Nanotechnology and Hybrid Membrane Based Water Recycling, Reuse, and Wastewater Treatment Processes. Chemosphere 2022, 289, 132867. [Google Scholar] [CrossRef]
- European Commission; Directorate General for Environment; VVA; Toegepast Natuurwetenschappelijk Onderzoek; Tecnalia; ANOTEC; Universitat Autònoma de Barcelona. Assessment of Potential Health Benefits of Noise Abatement Measures in the EU: Phenomena Project; Publications Office: Luxembourg, 2021. [Google Scholar]
- Capodaglio, A. High-Energy Oxidation Process: An Efficient Alternative for Wastewater Organic Contaminants Removal. Clean. Technol. Environ. Policy 2017, 19, 1995–2006. [Google Scholar] [CrossRef]
- Grandclément, C.; Seyssiecq, I.; Piram, A.; Wong-Wah-Chung, P.; Vanot, G.; Tiliacos, N.; Roche, N.; Doumenq, P. From the Conventional Biological Wastewater Treatment to Hybrid Processes, the Evaluation of Organic Micropollutant Removal: A Review. Water Res. 2017, 111, 297–317. [Google Scholar] [CrossRef]
- Zhang, S. Adsorption of Persistent and Mobile Substances on Activated Carbon. Nat. Water 2023, 1, 311. [Google Scholar] [CrossRef]
- Derco, J.; Žgajnar Gotvajn, A.; Guľašová, P.; Šoltýsová, N.; Kassai, A. Selected Micropollutant Removal from Municipal Wastewater. Processes 2024, 12, 888. [Google Scholar] [CrossRef]
- Li, S.; Dang, X.; Yu, X.; Abbas, G.; Zhang, Q.; Cao, L. The Application of Dielectric Barrier Discharge Non-Thermal Plasma in VOCs Abatement: A Review. Chem. Eng. J. 2020, 388, 124275. [Google Scholar] [CrossRef]
- Thirumdas, R.; Sarangapani, C.; Annapure, U.S. Cold Plasma: A Novel Non-Thermal Technology for Food Processing. Food Biophysics 2015, 10, 1–11. [Google Scholar] [CrossRef]
- Gururani, P.; Bhatnagar, P.; Bisht, B.; Kumar, V.; Joshi, N.C.; Tomar, M.S.; Pathak, B. Cold Plasma Technology: Advanced and Sustainable Approach for Wastewater Treatment. Environ. Sci. Pollut. Res. 2021, 28, 65062–65082. [Google Scholar] [CrossRef]
- Mir, S.A.; Siddiqui, M.W.; Dar, B.N.; Shah, M.A.; Wani, M.H.; Roohinejad, S.; Annor, G.A.; Mallikarjunan, K.; Chin, C.F.; Ali, A. Promising Applications of Cold Plasma for Microbial Safety, Chemical Decontamination and Quality Enhancement in Fruits. J. Appl. Microbiol. 2020, 129, 474–485. [Google Scholar] [CrossRef]
- Stańczyk, B.; Wiśniewski, M. The Promising Potential of Cold Atmospheric Plasma Therapies. Plasma 2024, 7, 465–497. [Google Scholar] [CrossRef]
- Subrahmanyam, K.; Gul, K.; Sehrawat, R.; Tiwari, B.K.; Sahoo, S. Cold Plasma-Mediated Inactivation of Microorganisms for the Shelf-Life Extension of Animal-Based Foods: Efficiency, Mechanism of Inactivation, and Impact on Quality Attributes. Food Control. 2024, 162, 110464. [Google Scholar] [CrossRef]
- Karkhanis, R.P.; Singh, S.P. A Review: Application of Cold Plasma in Food Processing Industry. J. Sci. Res. Rep. 2024, 30, 243–258. [Google Scholar] [CrossRef]
- Jurov, A.; Škoro, N.; Spasić, K.; Modic, M.; Hojnik, N.; Vujošević, D.; Đurović, M.; Petrović, Z.L.; Cvelbar, U. Helium Atmospheric Pressure Plasma Jet Parameters and Their Influence on Bacteria Deactivation in a Medium. Eur. Phys. J. D 2022, 76, 29. [Google Scholar] [CrossRef]
- Rath, S.; Kar, S. Microwave Atmospheric Pressure Plasma Jet: A Review. Contrib. Plasma Phys. 2024, 65, e202400036. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, D.; Guo, Y.; Zhou, Q.; Luo, H.; Tie, J. Surface Decontamination by Atmospheric Pressure Plasma Jet: Key Biological Processes. J. Phys. D: Appl. Phys. 2022, 55, 425203. [Google Scholar] [CrossRef]
- Konchekov, E.M.; Gudkova, V.V.; Burmistrov, D.E.; Konkova, A.S.; Zimina, M.A.; Khatueva, M.D.; Polyakova, V.A.; Stepanenko, A.A.; Pavlik, T.I.; Borzosekov, V.D.; et al. Bacterial Decontamination of Water-Containing Objects Using Piezoelectric Direct Discharge Plasma and Plasma Jet. Biomolecules 2024, 14, 181. [Google Scholar] [CrossRef]
- Kooshki, S.; Pareek, P.; Janda, M.; Machala, Z. Selective Reactive Oxygen and Nitrogen Species Production in Plasma-Activated Water via Dielectric Barrier Discharge Reactor: An Innovative Method for Tuning and Its Impact on Dye Degradation. J. Water Process Eng. 2024, 63, 105477. [Google Scholar] [CrossRef]
- Maybin, J.-A.; McClenaghan, L.A.; Gilmore, B.F.; Thompson, T.P. Cold Plasma for Enhanced Water Purification. Sustain. Microbiol. 2024, 1, qvae032. [Google Scholar] [CrossRef]
- Mouele, E.S.M.; Tijani, J.O.; Badmus, K.O.; Pereao, O.; Babajide, O.; Fatoba, O.O.; Zhang, C.; Shao, T.; Sosnin, E.; Tarasenko, V.; et al. A Critical Review on Ozone and Co-Species, Generation and Reaction Mechanisms in Plasma Induced by Dielectric Barrier Discharge Technologies for Wastewater Remediation. J. Environ. Chem. Eng. 2021, 9, 105758. [Google Scholar] [CrossRef]
- Nguyen, P.T.T.; Nguyen, H.T.; Tran, U.N.P.; Manh Bui, H. Removal of Antibiotics from Real Hospital Wastewater by Cold Plasma Technique. J. Chem. 2021, 2021, 9981738. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, W.; Peng, J.; Xue, L.; He, G. Cold Plasma Activated Ni0/Ni2+ Interface Catalysts for Efficient Electrocatalytic Methane Oxidation to Low-Carbon Alcohols. Green. Chem. 2024, 26, 7091–7100. [Google Scholar] [CrossRef]
- Zhang, Q.; Peng, J.; Xiong, H.; Jiang, S.; Li, W.; Fu, X.; Shang, S.; Xu, J.; He, G. Cold Plasma-Activated Ni-Co Tandem Catalysts with CN Vacancies for Enhancing CH4 Electrocatalytic to Methyl Formate. Appl. Catal. B Environ. Energy 2025, 362, 124759. [Google Scholar] [CrossRef]
- Zhuo, T.; He, L.; Chai, B.; Zhou, S.; Wan, Q.; Lei, X.; Zhou, Z.; Chen, B. Micro-Pressure Promotes Endogenous Phosphorus Release in a Deep Reservoir by Favouring Microbial Phosphate Mineralisation and Solubilisation Coupled with Sulphate Reduction. Water Res. 2023, 245, 120647. [Google Scholar] [CrossRef]
- Hertwig, C.; Steins, V.; Reineke, K.; Rademacher, A.; Klocke, M.; Rauh, C.; Schlüter, O. Impact of Surface Structure and Feed Gas Composition on Bacillus subtilis Endospore Inactivation during Direct Plasma Treatment. Front. Microbiol. 2015, 6, 774. [Google Scholar] [CrossRef]
- Hamid, L.L.; Ali, A.Y.; Ohmayed, M.M.; Ramizy, A.; Mutter, T.Y. Antimicrobial Activity of Silver Nanoparticles and Cold Plasma in the Treatment of Hospital Wastewater. Kuwait J. Sci. 2024, 51, 100212. [Google Scholar] [CrossRef]
- Yu, K.; Chai, B.; Zhuo, T.; Tang, Q.; Gao, X.; Wang, J.; He, L.; Lei, X.; Chen, B. Hydrostatic Pressure Drives Microbe-Mediated Biodegradation of Microplastics in Surface Sediments of Deep Reservoirs: Novel Findings from Hydrostatic Pressure Simulation Experiments. Water Res. 2023, 242, 120185. [Google Scholar] [CrossRef] [PubMed]
- Alkawareek, M.Y.; Gorman, S.P.; Graham, W.G.; Gilmore, B.F. Potential Cellular Targets and Antibacterial Efficacy of Atmospheric Pressure Non-Thermal Plasma. Int. J. Antimicrob. Agents 2014, 43, 154–160. [Google Scholar] [CrossRef]
- Patange, A.; O’Byrne, C.; Boehm, D.; Cullen, P.J.; Keener, K.; Bourke, P. The Effect of Atmospheric Cold Plasma on Bacterial Stress Responses and Virulence Using Listeria Monocytogenes Knockout Mutants. Front. Microbiol. 2019, 10, 2841. [Google Scholar] [CrossRef]
- Smet, C.; Baka, M.; Steen, L.; Fraeye, I.; Walsh, J.L.; Valdramidis, V.P.; Van Impe, J.F. Combined Effect of Cold Atmospheric Plasma, Intrinsic and Extrinsic Factors on the Microbial Behavior in/on (Food) Model Systems during Storage. Innov. Food Sci. Emerg. Technol. 2019, 53, 3–17. [Google Scholar] [CrossRef]
- Hertwig, C.; Reineke, K.; Rauh, C.; Schlüter, O. Factors Involved in Bacillus Spore’s Resistance to Cold Atmospheric Pressure Plasma. Innov. Food Sci. Emerg. Technol. 2017, 43, 173–181. [Google Scholar] [CrossRef]
- Kavian, N.; Asadollahfardi, G.; Hasanbeigi, A.; Delnavaz, M.; Samadi, A. Degradation of Phenol in Wastewater through an Integrated Dielectric Barrier Discharge and Fenton/Photo-Fenton Process. Ecotoxicol. Environ. Saf. 2024, 271, 115937. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhu, J.; Ouyang, W.; Ding, C.; Wu, Z.; Ostrikov, K.K. Cold Plasma Turns Mixed-Dye-Contaminated Wastewater Bio-Safe. Environ. Res. 2024, 246, 118125. [Google Scholar] [CrossRef]
- Khlyustova, A.; Sirotkin, N.; Titov, V. Plasma-induced Precipitation of Metal Ions in Aqueous Solutions. J. Chem. Technol. Biotechnol. 2019, 94, 3987–3992. [Google Scholar] [CrossRef]
- Aggelopoulos, C.A. Recent Advances of Cold Plasma Technology for Water and Soil Remediation: A Critical Review. Chem. Eng. J. 2022, 428, 131657. [Google Scholar] [CrossRef]
- Kim, H.-J.; Won, C.-H.; Kim, H.-W. Pathogen Deactivation of Glow Discharge Cold Plasma While Treating Organic and Inorganic Pollutants of Slaughterhouse Wastewater. Water Air Soil. Pollut. 2018, 229, 237. [Google Scholar] [CrossRef]
- Yepez, X.; Illera, A.E.; Baykara, H.; Keener, K. Recent Advances and Potential Applications of Atmospheric Pressure Cold Plasma Technology for Sustainable Food Processing. Foods 2022, 11, 1833. [Google Scholar] [CrossRef] [PubMed]
- Niveditha, A.; Pandiselvam, R.; Prasath, V.A.; Singh, S.K.; Gul, K.; Kothakota, A. Application of Cold Plasma and Ozone Technology for Decontamination of Escherichia Coli in Foods- a Review. Food Control 2021, 130, 108338. [Google Scholar] [CrossRef]
- Triantaphyllidou, I.-E.; Aggelopoulos, C.A. Insights on Bacteria Inactivation in Water by Cold Plasma: Effect of Water Matrix and Pulsed Plasmas Waveform on Physicochemical Water Properties, Species Formation and Inactivation Efficiency of Escherichia Coli. Environ. Res. 2025, 266, 120467. [Google Scholar] [CrossRef]
- Zhao, Y.-M.; Ojha, S.; Burgess, C.M.; Sun, D.-W.; Tiwari, B.K. Inactivation Efficacy and Mechanisms of Plasma Activated Water on Bacteria in Planktonic State. J. Appl. Microbiol. 2020, 129, 1248–1260. [Google Scholar] [CrossRef]
- Chandana, L.; Sangeetha, C.J.; Shashidhar, T.; Subrahmanyam, C. Non-Thermal Atmospheric Pressure Plasma Jet for the Bacterial Inactivation in an Aqueous Medium. Sci. Total Environ. 2018, 640–641, 493–500. [Google Scholar] [CrossRef]
- Kooshki, S.; Pareek, P.; Mentheour, R.; Janda, M.; Machala, Z. Efficient Treatment of Bio-Contaminated Wastewater Using Plasma Technology for Its Reuse in Sustainable Agriculture. Environ. Technol. Innov. 2023, 32, 103287. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Clauson, M.; Hong, J.; Murphy, A.B. Gram Positive and Gram Negative Bacteria Differ in Their Sensitivity to Cold Plasma. Sci. Rep. 2016, 6, 38610. [Google Scholar] [CrossRef]
- Yang, Y.; Wan, K.; Yang, Z.; Li, D.; Li, G.; Zhang, S.; Wang, L.; Yu, X. Inactivation of Antibiotic Resistant Escherichia Coli and Degradation of Its Resistance Genes by Glow Discharge Plasma in an Aqueous Solution. Chemosphere 2020, 252, 126476. [Google Scholar] [CrossRef]
- Polito, J.; Kushner, M.J. A Hierarchal Model for Bacterial Cell Inactivation in Solution by Direct and Indirect Treatment Using Cold Atmospheric Plasmas. J. Phys. D Appl. Phys. 2024, 57, 405207. [Google Scholar] [CrossRef]
- Ruan, Z.; Guo, Y.; Gao, J.; Yang, C.; Lan, Y.; Shen, J.; Xu, Z.; Cheng, C.; Liu, X.; Zhang, S.; et al. Control of Multidrug-Resistant Planktonic Acinetobacter Baumannii: Biocidal Efficacy Study by Atmospheric-Pressure Air Plasma. Plasma Sci. Technol. 2018, 20, 065513. [Google Scholar] [CrossRef]
- Eced-Rodríguez, L.; Beyrer, M.; Rodrigo, D.; Rivas, A.; Esteve, C.; Pina-Pérez, M.C. Sublethal Damage Caused by Cold Plasma on Bacillus Cereus Cells: Impact on Cell Viability and Biofilm-Forming Capacity. Foods 2024, 13, 3251. [Google Scholar] [CrossRef] [PubMed]
- Jungbauer, G.; Moser, D.; Müller, S.; Pfister, W.; Sculean, A.; Eick, S. The Antimicrobial Effect of Cold Atmospheric Plasma against Dental Pathogens—A Systematic Review of In-Vitro Studies. Antibiotics 2021, 10, 211. [Google Scholar] [CrossRef] [PubMed]
- Nwabor, O.F.; Onyeaka, H.; Miri, T.; Obileke, K.; Anumudu, C.; Hart, A. A Cold Plasma Technology for Ensuring the Microbiological Safety and Quality of Foods. Food Eng. Rev. 2022, 14, 535–554. [Google Scholar] [CrossRef]
- Oliulla, H.; Mizan, M.F.R.; Ashrafudoulla, M.; Meghla, N.S.; Ha, A.J.; Park, S.H.; Ha, S.-D. The Challenges and Prospects of Using Cold Plasma to Prevent Bacterial Contamination and Biofilm Formation in the Meat Industry. Meat Sci. 2024, 217, 109596. [Google Scholar] [CrossRef] [PubMed]
- Romero-Rodríguez, A.; Paredes-Sabja, D. Chapter 8—Endospores, Sporulation, and Germination. In Molecular Medical Microbiology, 3rd ed.; Tang, Y.-W., Hindiyeh, M.Y., Liu, D., Sails, A., Spearman, P., Zhang, J.-R., Eds.; Academic Press: Washington, DC, USA, 2024; pp. 141–152. ISBN 978-0-12-818619-0.77. [Google Scholar]
- Lopes, R.P.; Mota, M.J.; Gomes, A.M.; Delgadillo, I.; Saraiva, J.A. Application of High Pressure with Homogenization, Temperature, Carbon Dioxide, and Cold Plasma for the Inactivation of Bacterial Spores: A Review. Compr. Rev. Food Sci. Food Safety 2018, 17, 532–555. [Google Scholar] [CrossRef]
- Tseng, S.; Abramzon, N.; Jackson, J.O.; Lin, W.-J. Gas Discharge Plasmas Are Effective in Inactivating Bacillus and Clostridium Spores. Appl. Microbiol. Biotechnol. 2012, 93, 2563–2570. [Google Scholar] [CrossRef]
- Sun, P.; Wu, H.; Bai, N.; Zhou, H.; Wang, R.; Feng, H.; Zhu, W.; Zhang, J.; Fang, J. Inactivation of Bacillus Subtilis Spores in Water by a Direct-Current, Cold Atmospheric-Pressure Air Plasma Microjet. Plasma Process. Polym. 2012, 9, 157–164. [Google Scholar] [CrossRef]
- Wang, L.-H.; Yan, B.; Wei, G.-F.; Li, J.; Han, Z.; Cheng, J.; Zeng, X.-A. Resistance of Alicyclobacillus Acidoterrestris Spores to Atmospheric Cold Plasma: Insights from Sporulation Temperature and Mechanism Analysis. Innov. Food Sci. Emerg. Technol. 2024, 93, 103629. [Google Scholar] [CrossRef]
- Song, Y.; Liu, D.; Ji, L.; Wang, W.; Zhao, P.; Quan, C.; Niu, J.; Zhang, X. The Inactivation of Resistant Candida Albicans in a Sealed Package by Cold Atmospheric Pressure Plasmas. Plasma Process. Polym. 2012, 9, 17–21. [Google Scholar] [CrossRef]
- Šimončicová, J.; Kaliňáková, B.; Kováčik, D.; Medvecká, V.; Lakatoš, B.; Kryštofová, S.; Hoppanová, L.; Palušková, V.; Hudecová, D.; Ďurina, P.; et al. Cold Plasma Treatment Triggers Antioxidative Defense System and Induces Changes in Hyphal Surface and Subcellular Structures of Aspergillus Flavus. Appl. Microbiol. Biotechnol. 2018, 102, 6647–6658. [Google Scholar] [CrossRef]
- Bai, Y.; Idris Muhammad, A.; Hu, Y.; Koseki, S.; Liao, X.; Chen, S.; Ye, X.; Liu, D.; Ding, T. Inactivation Kinetics of Bacillus Cereus Spores by Plasma Activated Water (PAW). Food Res. Int. 2020, 131, 109041. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Muhammad, A.I.; Chen, S.; Hu, Y.; Ye, X.; Liu, D.; Ding, T. Bacterial Spore Inactivation Induced by Cold Plasma. Crit. Rev. Food Sci. Nutr. 2019, 59, 2562–2572. [Google Scholar] [CrossRef]
- Gonçalves, J. The Role of Smart Technologies in Wastewater-Based Epidemiology. JEEA 2023, 2, 18. [Google Scholar] [CrossRef]
- Gonçalves, J.; Gutiérrez-Aguirre, I.; Balasubramanian, M.N.; Zagorščak, M.; Ravnikar, M.; Turk, V. Surveillance of Human Enteric Viruses in Coastal Waters Using Concentration with Methacrylate Monolithic Supports Prior to Detection by RT-qPCR. Mar. Pollut. Bull. 2018, 128, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.; Franco, A.F.; Gomes da Silva, P.; Rodriguez, E.; Diaz, I.; González Peña, M.J.; Mesquita, J.R.; Muñoz, R.; Garcia-Encina, P. Exposure Assessment of Severe Acute Respiratory Syndrome Coronavirus 2 and Norovirus Genogroup I/Genogroup II in Aerosols Generated by a Municipal Wastewater Treatment Plant. CLEAN–Soil Air Water 2024, 52, 2300267. [Google Scholar] [CrossRef]
- Gonçalves, J.; da Silva, P.G.; Koritnik, T.; Bosilj, M.; Torres-Franco, A.; Diaz, I.; Rodriguéz, E.; Marcos, E.; Mesquita, J.R.; García-Encina, P. Quantification and Whole Genome Characterization of SARS-CoV-2 RNA in Wastewater and Air Samples. J. Vis. Exp. 2023, 196, e65053. [Google Scholar] [CrossRef]
- Kranjec, N.; Steyer, A.; Cerar Kišek, T.; Koritnik, T.; Janko, T.; Bolješić, M.; Vedlin, V.; Mioč, V.; Lasecky, B.; Jurša, T.; et al. Wastewater Surveillance of SARS-CoV-2 in Slovenia: Key Public Health Tool in Endemic Time of COVID-19. Microorganisms 2024, 12, 2174. [Google Scholar] [CrossRef]
- Capelli, F.; Tappi, S.; Gritti, T.; de Aguiar Saldanha Pinheiro, A.C.; Laurita, R.; Tylewicz, U.; Spataro, F.; Braschi, G.; Lanciotti, R.; Gómez Galindo, F.; et al. Decontamination of Food Packages from SARS-CoV-2 RNA with a Cold Plasma-Assisted System. Appl. Sci. 2021, 11, 4177. [Google Scholar] [CrossRef]
- Guo, L.; Xu, R.; Gou, L.; Liu, Z.; Zhao, Y.; Liu, D.; Zhang, L.; Chen, H.; Kong, M.G. Mechanism of Virus Inactivation by Cold Atmospheric-Pressure Plasma and Plasma-Activated Water. Appl. Environ. Microbiol. 2018, 84, e00726-18. [Google Scholar] [CrossRef]
- Thirumdas, R. Inactivation of Viruses Related to Foodborne Infections Using Cold Plasma Technology. J. Food Saf. 2022, 42, e12988. [Google Scholar] [CrossRef]
- Wu, Y.; Liang, Y.; Wei, K.; Li, W.; Yao, M.; Zhang, J.; Grinshpun, S.A. MS2 Virus Inactivation by Atmospheric-Pressure Cold Plasma Using Different Gas Carriers and Power Levels. Appl. Environ. Microbiol. 2015, 81, 996–1002. [Google Scholar] [CrossRef]
- Meganck, R.M.; Baric, R.S. Developing Therapeutic Approaches for Twenty-First-Century Emerging Infectious Viral Diseases. Nat. Med. 2021, 27, 401–410. [Google Scholar] [CrossRef]
- Bunz, O.; Mese, K.; Funk, C.; Wulf, M.; Bailer, S.M.; Piwowarczyk, A.; Ehrhardt, A. Cold Atmospheric Plasma as Antiviral Therapy – Effect on Human Herpes Simplex Virus Type 1. J. Gen. Virol. 2020, 101, 208–215. [Google Scholar] [CrossRef]
- Fu, J.; Xu, Y.; Arts, E.J.; Bai, Z.; Chen, Z.; Zheng, Y. Viral Disinfection Using Nonthermal Plasma: A Critical Review and Perspectives on the Plasma-Catalysis System. Chemosphere 2022, 309, 136655. [Google Scholar] [CrossRef]
- Xia, T.; Kleinheksel, A.; Lee, E.M.; Qiao, Z.; Wigginton, K.R.; Clack, H.L. Inactivation of Airborne Viruses Using a Packed Bed Non-Thermal Plasma Reactor. J. Phys. D: Appl. Phys. 2019, 52, 255201. [Google Scholar] [CrossRef] [PubMed]
- Min, S.C.; Roh, S.H.; Niemira, B.A.; Sites, J.E.; Boyd, G.; Lacombe, A. Dielectric Barrier Discharge Atmospheric Cold Plasma Inhibits Escherichia Coli O157:H7, Salmonella, Listeria Monocytogenes, and Tulane Virus in Romaine Lettuce. Int. J. Food Microbiol. 2016, 237, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Panchal, D.; Lu, Q.; Saedi, Z.; Luk, H.; Yu, T.; Zhang, X. Integrate Bubble Flotation and Intermittent Microbubble-Enhanced Cold Plasma Activation for Scalable Disinfection of Food Processing Wastewater. Sep. Purif. Technol. 2025, 362, 131677. [Google Scholar] [CrossRef]
- Mercado-Cabrera, A.; Jaramillo-Sierra, B.; Peña-Eguiluz, R.; Rodríguez-Méndez, B.G.; López-Callejas, R.; Valencia-Alvarado, R. Thin Film Water Non-Thermal Plasma Reactor for Acetaminophen Analgesic Oxidation. Int. J. Environ. Sci. Technol. 2025, 22, 1357–1368. [Google Scholar] [CrossRef]
- Phlaengsattra, K.; Kanokkantapong, V.; Sangsanont, J. Trihalomethane Formation Potentials from the Effluent of Different Wastewater Treatment Sources. IOP Conf. Ser.: Earth Environ. Sci. 2024, 1372, 012031. [Google Scholar] [CrossRef]
- Rajcoomar, S.; Amoah, I.D.; Abunama, T.; Mohlomi, N.; Bux, F.; Kumari, S. Biofilm Formation on Microplastics in Wastewater: Insights into Factors, Diversity and Inactivation Strategies. Int. J. Environ. Sci. Technol. 2024, 21, 4429–4444. [Google Scholar] [CrossRef]
- Zhang, L.; Du, P.; Zheng, Q.; Zhao, M.; Zhang, R.; Wang, Z.; Xu, Z.; Li, X.; Thai, P.K. Exposure to Smoking and Greenspace Are Associated with Allergy Medicine Use—A Study of Wastewater in 28 Cities of China. Environ. Int. 2025, 196, 109291. [Google Scholar] [CrossRef] [PubMed]

| Target Contaminant(s) | Wastewater Type | CP Type and Conditions | Removal Efficiency | Comparison to Other Technologies | References |
|---|---|---|---|---|---|
| E. coli (Gram− bacterium) | Synthetic/municipal | Air plasma jet; ~15 min | >7 log10 reduction | Comparable to UV; no DBPs; fast | [45] |
| S. aureus (Gram+ bacterium) | Synthetic/municipal | ~3–4 log10; higher resistance than Gram– | ~3–4 log10 reduction | Less efficient than on Gram–; combo recommended | [6,8] |
| Bacillus spores | Synthetic/lab | Radio-frequency plasma jet; 5–10 min; with O2/N2 | ~4 log10 spores; enhanced by UV/ROS | UV/Cl2 less effective; CP good without heat | [9,50] |
| Viruses (e.g., MS2, PMMoV) | Synthetic/real effluent | Atmospheric plasma jet or submerged DBD; 0.12 s–5 min | >95–99.99% virus inactivation | CP inactivates viruses faster; works on surfaces | [55,56] |
| Antibiotics (e.g., ciprofloxacin) | Hospital wastewater | DBD; 30 kV; 15 min | 100% ciprofloxacin; >72% other antibiotics | Better than biological; no added chemicals | [26] |
| ARGs (tetA, tetR, aphA) | Synthetic saline water | Glow discharge; 15–30 min | ~5.8 log gene reduction | Unlike UV/Cl2, CP degrades DNA | [34] |
| Phenol | Phenol-spiked water | DBD; 100 W; Fe2+ (Fenton); 10–12 min | 86.8% with Fe2+; 33% COD | Plasma–Fenton better than standalone | [57] |
| Mixed wastewater (slaughterhouse) | Slaughterhouse wastewater | Glow discharge; continuous flow; 5 L/min | COD 78–93%; TN 51–92%; TP 35–83% | Outperformed biological + chemical combo | [36] |
| Mixed dyes (textile) | Synthetic textile wastewater | Underwater plasma; pulsed high voltage | 100% mixed dye; enhanced on mixtures | Synergistic degradation in dye mixtures | [52] |
| Heavy metals (Fe, Cu, Zn) | Industrial wastewater | AC diaphragm underwater plasma | >90% removal of Fe, Cu, Zn | No chemicals needed; better than factory system | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, J.; Pequeno, J.; Diaz, I.; Kržišnik, D.; Žigon, J.; Koritnik, T. Killing Two Crises with One Spark: Cold Plasma for Antimicrobial Resistance Mitigation and Wastewater Reuse. Water 2025, 17, 1218. https://doi.org/10.3390/w17081218
Gonçalves J, Pequeno J, Diaz I, Kržišnik D, Žigon J, Koritnik T. Killing Two Crises with One Spark: Cold Plasma for Antimicrobial Resistance Mitigation and Wastewater Reuse. Water. 2025; 17(8):1218. https://doi.org/10.3390/w17081218
Chicago/Turabian StyleGonçalves, José, João Pequeno, Israel Diaz, Davor Kržišnik, Jure Žigon, and Tom Koritnik. 2025. "Killing Two Crises with One Spark: Cold Plasma for Antimicrobial Resistance Mitigation and Wastewater Reuse" Water 17, no. 8: 1218. https://doi.org/10.3390/w17081218
APA StyleGonçalves, J., Pequeno, J., Diaz, I., Kržišnik, D., Žigon, J., & Koritnik, T. (2025). Killing Two Crises with One Spark: Cold Plasma for Antimicrobial Resistance Mitigation and Wastewater Reuse. Water, 17(8), 1218. https://doi.org/10.3390/w17081218








