Impact of Iron Minerals on Nitrate Reduction in the Lake–Groundwater Interaction Zone of High-Salinity Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lake Sediment and Water Sample Collection and Testing
2.2. Experimental Design
2.3. Tests of Experimental Indicators
2.3.1. Chemical Indexes
2.3.2. SEM Measurement
2.3.3. Analysis of Microbial Indexes
2.3.4. Microbial Electron Transport System Activity (ETS Activity) Assay
3. Results and Discussion
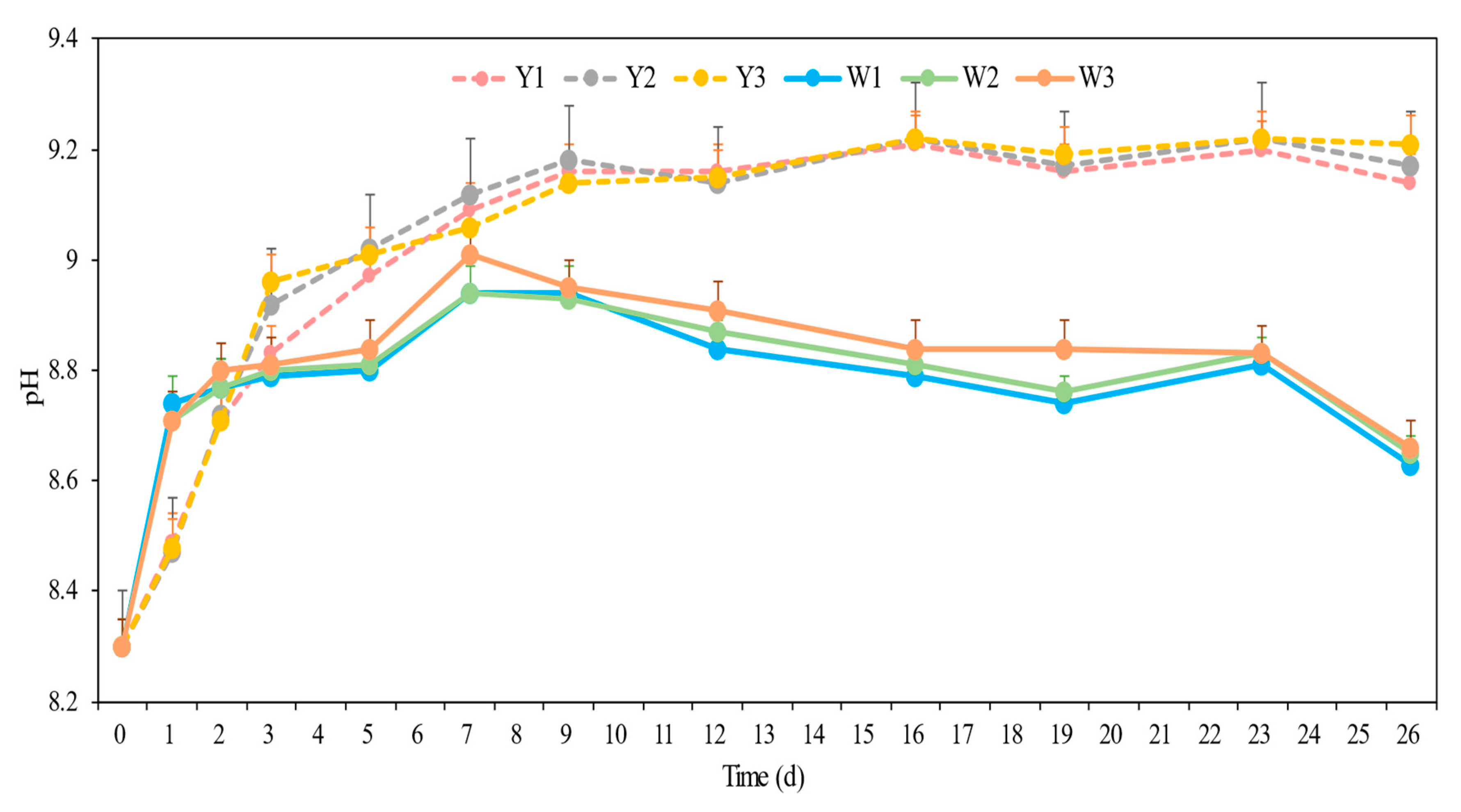
3.1. Changes in Nitrate Reduction Under Different Conditions
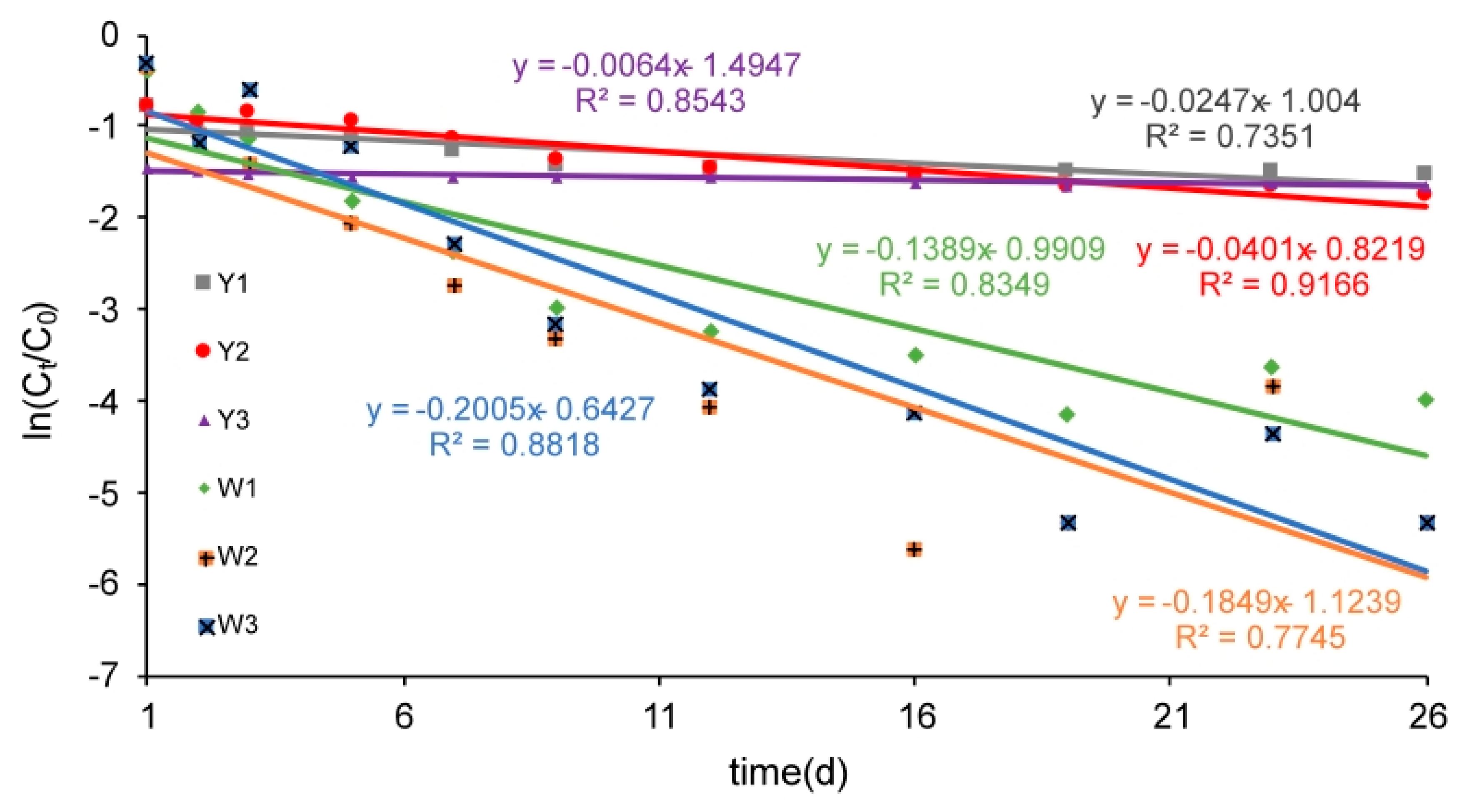
3.1.1. Kinetic Characteristics of Nitrate Reduction
3.1.2. Differences in the Nitrate Reduction Process
3.2. Reasons for the Differences in Nitrate Reduction Process
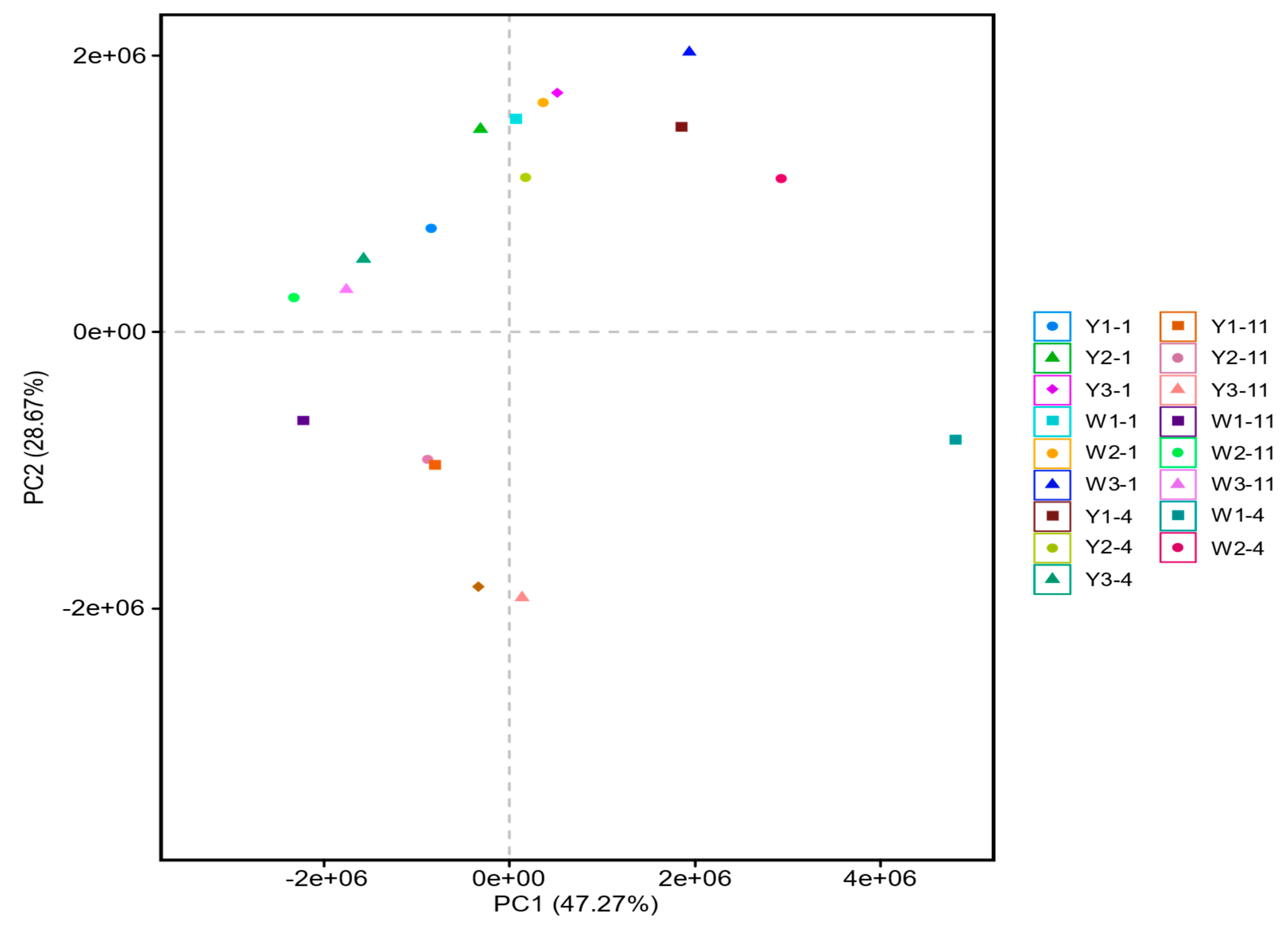
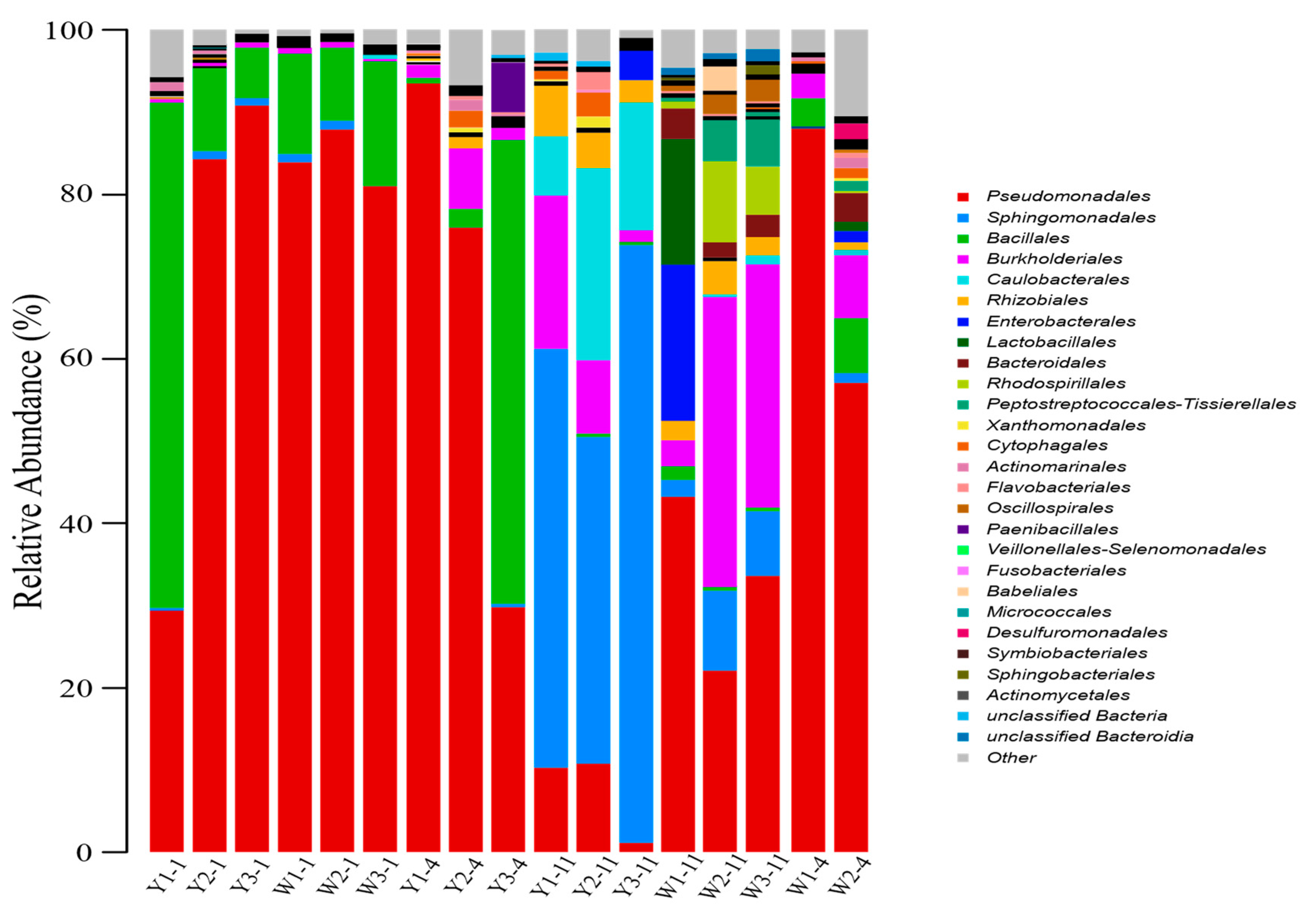
3.2.1. The Influence of Hematite and Siderite on Microbial Community Structure
3.2.2. Effects of Hematite and Siderite on Microbial Biomass
Temporal Changes in Iron Ion Concentrations
The Changes in OD600 Values over Time
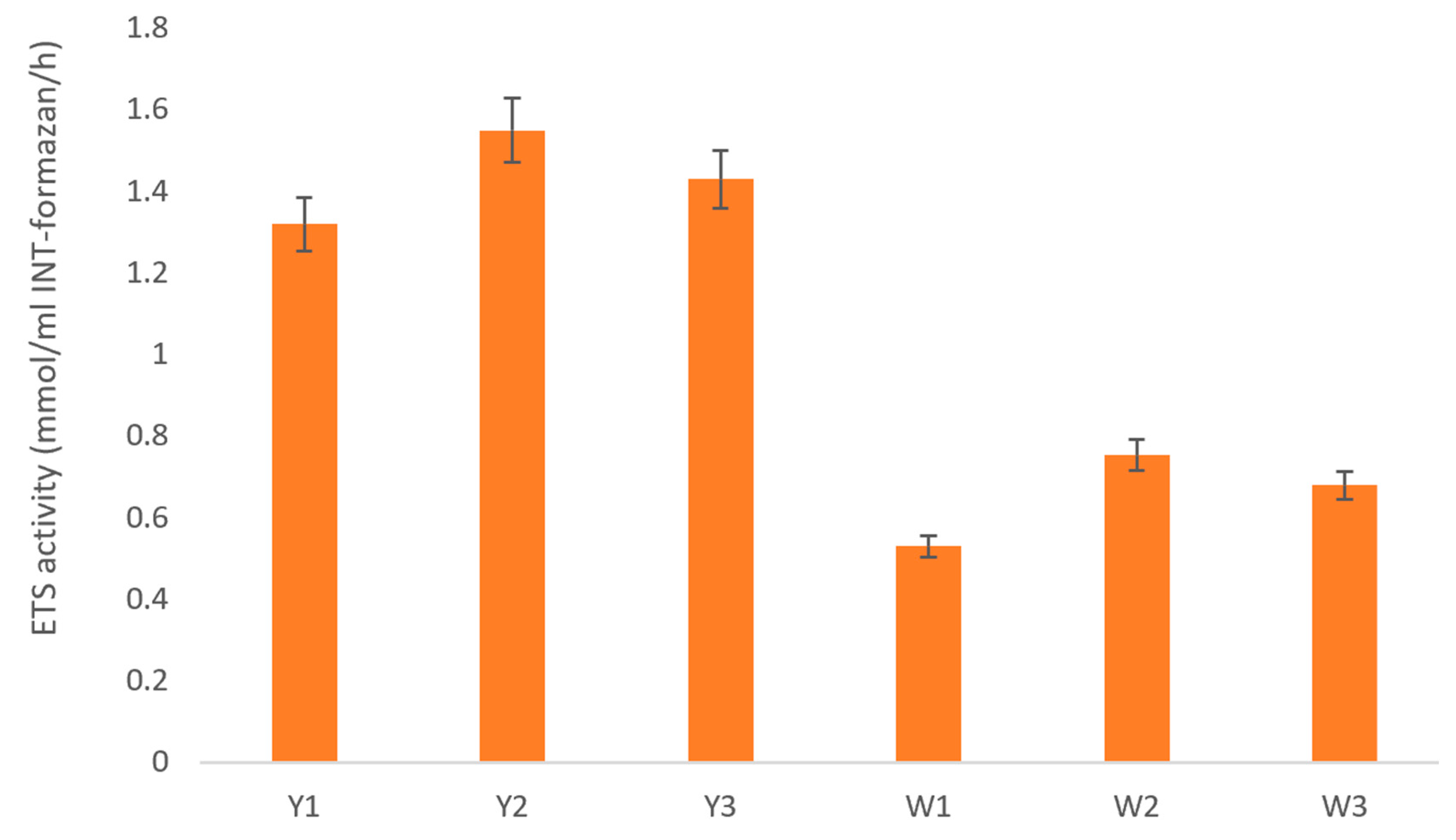
3.2.3. Effects of Hematite and Siderite on Microbial Activity
3.2.4. Interactions Between Iron Minerals and Microorganisms
3.3. Effect Mechanisms of Hematite and Siderite in Nitrate Reduction
3.3.1. Fundamental Differences Between Open and Closed Systems
3.3.2. Mechanism Analysis of Different Iron Minerals
- Mechanism of Hematite
- 2.
- Mechanism of Siderite
3.3.3. Salinity-Specific Environmental Effects
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lakes as Sentinels and Integrators for the Effects of Climate Change on Watersheds, Airsheds, and Landscapes-Schindler-2009-Limnology and Oceanography-Wiley Online Library. Available online: https://aslopubs.onlinelibrary.wiley.com/doi/abs/10.4319/lo.2009.54.6_part_2.2349 (accessed on 6 March 2025).
- Costanza, R.; d’ Arge, R.; de Groot, R.; Farber, S.; Grasso, M.; Hannon, B.; Limburg, K.; Naeem, S.; O’ Neill, R.V.; Paruelo, J.; et al. The Value of the World’s Ecosystem Services and Natural Capital. Nature 1997, 387, 253–260. [Google Scholar] [CrossRef]
- Millennium Ecosystem Assessment (Ed.) Ecosystems and Human Well-Being: Synthesis. In The Millennium Ecosystem Assessment Series; Island Press: Washington, DC, USA, 2005; ISBN 978-1-59726-040-4. [Google Scholar]
- Schlesinger, W.H. On the Fate of Anthropogenic Nitrogen. Proc. Natl. Acad. Sci. USA 2009, 106, 203–208. [Google Scholar] [CrossRef] [PubMed]
- The Evolution and Future of Earth’s Nitrogen Cycle|Science. Available online: https://www.science.org/doi/10.1126/science.1186120 (accessed on 6 March 2025).
- Menció, A.; Mas-Pla, J.; Otero, N.; Regàs, O.; Boy-Roura, M.; Puig, R.; Bach, J.; Domènech, C.; Zamorano, M.; Brusi, D.; et al. Nitrate Pollution of Groundwater; All Right, but Nothing Else? Sci. Total Environ. 2016, 539, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Craswell, E. Fertilizers and Nitrate Pollution of Surface and Ground Water: An Increasingly Pervasive Global Problem. SN Appl. Sci. 2021, 3, 518. [Google Scholar] [CrossRef]
- Guo, W.; Li, P.; Du, Q.; Zhou, Y.; Xu, D.; Zhang, Z. Hydrogeochemical Processes Regulating the Groundwater Geochemistry and Human Health Risk of Groundwater in the Rural Areas of the Wei River Basin, China. Expo. Health 2024, 16, 291–306. [Google Scholar] [CrossRef]
- Fleckenstein, J.H.; Krause, S.; Hannah, D.M.; Boano, F. Groundwater-Surface Water Interactions: New Methods and Models to Improve Understanding of Processes and Dynamics. Adv. Water Resour. 2010, 33, 1291–1295. [Google Scholar] [CrossRef]
- Rivett, M.O.; Buss, S.R.; Morgan, P.; Smith, J.W.N.; Bemment, C.D. Nitrate Attenuation in Groundwater: A Review of Biogeochemical Controlling Processes. Water Res. 2008, 42, 4215–4232. [Google Scholar] [CrossRef]
- Alexander, R.B.; Böhlke, J.K.; Boyer, E.W.; David, M.B.; Harvey, J.W.; Mulholland, P.J.; Seitzinger, S.P.; Tobias, C.R.; Tonitto, C.; Wollheim, W.M. Dynamic Modeling of Nitrogen Losses in River Networks Unravels the Coupled Effects of Hydrological and Biogeochemical Processes. Biogeochemistry 2009, 93, 91–116. [Google Scholar] [CrossRef]
- Burgin, A.J.; Hamilton, S.K. Have We Overemphasized the Role of Denitrification in Aquatic Ecosystems? A Review of Nitrate Removal Pathways. Front. Ecol. Environ. 2007, 5, 89–96. [Google Scholar] [CrossRef]
- Lee, M.-S.; Lee, K.-K.; Hyun, Y.; Clement, T.P.; Hamilton, D. Nitrogen Transformation and Transport Modeling in Groundwater Aquifers. Ecol. Model. 2006, 192, 143–159. [Google Scholar] [CrossRef]
- Huang, Z.; Zhan, X.; Tigabu, M.; He, Y.; Li, Z.; Wang, G.; Guo, F. Rhizosphere Soil Microbial Communities and Nitrogen Transformation Response to Forest Fire Smoke. Appl. Soil. Ecol. 2025, 208, 105990. [Google Scholar] [CrossRef]
- Beyond Carbon and Nitrogen: How the Microbial Energy Economy Couples Elemental Cycles in Diverse Ecosystems-Burgin-2011-Frontiers in Ecology and the Environment-Wiley Online Library. Available online: https://esajournals.onlinelibrary.wiley.com/doi/full/10.1890/090227 (accessed on 6 March 2025).
- Qin, M.; You, H.; Zhang, W.; Liu, L.; Liu, J.; Xia, L. Effects of Redox Condition on Bacteria-Mediated Hydrochemical Processes and Bacterial Community During Managed Aquifer Recharge. Sustainability 2025, 17, 64. Available online: https://www.mdpi.com/2071-1050/17/1/64 (accessed on 6 March 2025). [CrossRef]
- Santoro, A.E. Microbial Nitrogen Cycling at the Saltwater–Freshwater Interface. Hydrogeol. J. 2010, 18, 187–202. Available online: https://link.springer.com/article/10.1007/s10040-009-0526-z (accessed on 6 March 2025). [CrossRef]
- Cheng, C.; He, Q.; Zhang, J.; Chai, H.; Yang, Y.; Pavlostathis, S.G.; Wu, H. New Insight into Ammonium Oxidation Processes and Mechanisms Mediated by Manganese Oxide in Constructed Wetlands. Water Res. 2022, 215, 118251. [Google Scholar] [CrossRef] [PubMed]
- Braker, G.; Witzel, K.P.; Fesefeldt, A. Development of PCR Primer Systems for Amplification of Nitrite Reductase Genes (nirK and nirS) to Detect Denitrifying Bacteria in Environmental Samples. Appl. Environ. Microbiol. 1998, 64, 3769–3775. [Google Scholar] [CrossRef]
- Horner-Devine, M.C.; Bohannan, B.J.M. Phylogenetic Clustering and Overdispersion in Bacterial Communities. Ecology 2006, 87, S100–S108. [Google Scholar] [CrossRef]
- Jahangir, M.M.R.; Fenton, O.; Müller, C.; Harrington, R.; Johnston, P.; Richards, K.G. In Situ Denitrification and DNRA Rates in Groundwater beneath an Integrated Constructed Wetland. Water Res. 2017, 111, 254–264. [Google Scholar] [CrossRef]
- Wang, C.; He, T.; Zhang, M.; Zheng, C.; Yang, L.; Yang, L. Review of the Mechanisms Involved in Dissimilatory Nitrate Reduction to Ammonium and the Efficacies of These Mechanisms in the Environment. Environ. Pollut. 2024, 345, 123480. [Google Scholar] [CrossRef]
- Knights, D.; Parks, K.C.; Sawyer, A.H.; David, C.H.; Browning, T.N.; Danner, K.M.; Wallace, C.D. Direct Groundwater Discharge and Vulnerability to Hidden Nutrient Loads along the Great Lakes Coast of the United States. J. Hydrol. 2017, 554, 331–341. [Google Scholar] [CrossRef]
- Weber, K.A.; Achenbach, L.A.; Coates, J.D. Microorganisms Pumping Iron: Anaerobic Microbial Iron Oxidation and Reduction. Nat. Rev. Microbiol. 2006, 4, 752–764. Available online: https://www.nature.com/articles/nrmicro1490 (accessed on 6 March 2025). [CrossRef]
- Galloway, J.N.; Aber, J.D.; Erisman, J.W.; Seitzinger, S.P.; Howarth, R.W.; Cowling, E.B.; Cosby, B.J. Nitrogen Cascade. BioScience 2003, 53, 341–356. Available online: https://academic.oup.com/bioscience/article/53/4/341/250178?login=true (accessed on 6 March 2025). [CrossRef]
- Liu, X.; Pei, T.; Xu, G.; Huang, T.; Wu, Y.; Jin, X.; Cao, Y.; Sun, R.; Hu, S.; Manage, P.M.; et al. Suspended Sediment (SPS) Triggers Nitrogen Retention by Altering Microbial Network Stability and Electron Transport Behavior during the Aerobic-Anoxic Transition. J. Environ. Manag. 2025, 373, 123787. [Google Scholar] [CrossRef] [PubMed]
- Baumann, K.B.L.; Thoma, R.; Callbeck, C.M.; Niederdorfer, R.; Schubert, C.J.; Müller, B.; Lever, M.A.; Bürgmann, H. Microbial Nitrogen Transformation Potential in Sediments of Two Contrasting Lakes Is Spatially Structured but Seasonally Stable. mSphere 2022, 7, e01013-21. [Google Scholar] [CrossRef]
- Wang, S.; Liu, C.; Wang, X.; Yuan, D.; Zhu, G. Dissimilatory Nitrate Reduction to Ammonium (DNRA) in Traditional Municipal Wastewater Treatment Plants in China: Widespread but Low Contribution. Water Res. 2020, 179, 115877. [Google Scholar] [CrossRef]
- Nitrogen Removal by an Anaerobic Iron-Dependent Ammonium Oxidation (Feammox) Enrichment: Potential for Wastewater Treatment. Available online: https://www.mdpi.com/2073-4441/13/23/3462 (accessed on 6 March 2025).
- Cheng, X.; Hu, L.; Liu, T.; Cheng, X.; Li, J.; Xu, K.; Zheng, M. High-Level Nitrogen Removal Achieved by Feammox-Based Autotrophic Nitrogen Conversion. Water Res. X 2025, 27, 100292. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Liu, H.; Wang, X. Anaerobic Ammonium Oxidation Coupled to Fe(III) Reduction: Discovery, Mechanism and Application Prospects in Wastewater Treatment. Sci. Total Environ. 2022, 818, 151687. [Google Scholar] [CrossRef]
- Ammonium and Organic Carbon Co-Removal under Feammox-Coupled-with-Heterotrophy Condition as an Efficient Approach for Nitrogen Treatment|Scientific Reports. Available online: https://www.nature.com/articles/s41598-020-80057-y (accessed on 6 March 2025).
- EGUsphere-Evidence of Nitrogen Loss from Anaerobic Ammonium Oxidation Coupled with Ferric Iron Reduction in the Yellow River Wetland. Available online: https://egusphere.copernicus.org/preprints/2023/egusphere-2022-1209/ (accessed on 6 March 2025).
- Ding, B.-J.; Li, Z.-K.; Zhu, H.-J.; Chen, S.; Qin, Y.-B.; Yang, J.-H.; Hu, Y.-Y. Insight into the Mechanism of Feammox in the Surface Soils of a Riparian Zone. Huan Jing Ke Xue Huanjing Kexue 2018, 39, 1833–1839. [Google Scholar] [CrossRef]
- Zhang, M.; Zheng, P.; Li, W.; Wang, R.; Ding, S.; Abbas, G. Performance of Nitrate-Dependent Anaerobic Ferrous Oxidizing (NAFO) Process: A Novel Prospective Technology for Autotrophic Denitrification. Bioresour. Technol. 2015, 179, 543–548. [Google Scholar] [CrossRef]
- Wang, P.; Tan, J.; Xiao, Z.; Xu, F.; Jin, Q.; He, D. New Insights and Enhancement Mechanisms of Activated Carbon in Autotrophic Denitrification System Utilizing Zero-Valent Iron as Indirect Electron Donors. Bioresour. Technol. 2024, 410, 131237. [Google Scholar] [CrossRef]
- Rahman, M.M.; Roberts, K.L.; Grace, M.R.; Kessler, A.J.; Cook, P.L.M. Role of Organic Carbon, Nitrate and Ferrous Iron on the Partitioning between Denitrification and DNRA in Constructed Stormwater Urban Wetlands. Sci. Total Environ. 2019, 666, 608–617. [Google Scholar] [CrossRef]
- Holtappels, M.; Lavik, G.; Jensen, M.M.; Kuypers, M.M. 15N-Labeling Experiments to Dissect the Contributions of Heterotrophic Denitrification and Anammox to Nitrogen Removal in the OMZ Waters of the Ocean. In Methods in enzymology; Klotz, M.G., Ed.; Research on Nitrification and Related Processes, Part A; Academic Press: Cambridge, MA, USA, 2011; Volume 486, pp. 223–251. [Google Scholar]
- Pang, Y.; Ji, G. Biotic Factors Drive Distinct DNRA Potential Rates and Contributions in Typical Chinese Shallow Lake Sediments. Environ. Pollut. 2019, 254, 112903. [Google Scholar] [CrossRef]
- Li, S.; Liao, Y.; Pang, Y.; Dong, X.; Strous, M.; Ji, G. Denitrification and Dissimilatory Nitrate Reduction to Ammonia in Long-Term Lake Sediment Microcosms with Iron(II). Sci. Total Environ. 2022, 807, 150835. [Google Scholar] [CrossRef]
- Dissimilatory Nitrate Reduction to Ammonium in the Yellow River Estuary: Rates, Abundance, and Community Diversity|Scientific Reports. Available online: https://www.nature.com/articles/s41598-017-06404-8 (accessed on 6 March 2025).
- Zhang, X.; Zhao, Y.; Wang, Y.; Qian, H.; Xing, J.; Joseph, A.; Rene, E.R.; Li, J.; Zhu, N. The Interplay of Hematite and Photic Biofilm Triggers the Acceleration of Biotic Nitrate Removal. Chemosphere 2024, 358, 142136. [Google Scholar] [CrossRef]
- Denitrifier Abundance and Activity across the San: Environmental Microbiology Reports. Available online: https://www.ovid.com/journals/emmr/fulltext/10.1111/j.1758-2229.2010.00156.x~denitrifier-abundance-and-activity-across-the-san-francisco (accessed on 6 March 2025).
- Hettiarachchi, E.; Reynolds, R.L.; Goldstein, H.L.; Moskowitz, B.; Rubasinghege, G. Iron Dissolution and Speciation in Atmospheric Mineral Dust: Metal-Metal Synergistic and Antagonistic Effects. Atmos. Environ. 2018, 187, 417–423. [Google Scholar] [CrossRef]
- Liu, Y.; Wan, Y.; Ma, Z.; Dong, W.; Su, X.; Shen, X.; Yi, X.; Chen, Y. Effects of Magnetite on Microbially Driven Nitrate Reduction Processes in Groundwater. Sci. Total Environ. 2023, 855, 158956. [Google Scholar] [CrossRef]
- Weller, A.; Slater, L. Salinity Dependence of Complex Conductivity of Unconsolidated and Consolidated Materials: Comparisons with Electrical Double Layer Models. Geophysics 2012, 77, D185–D198. [Google Scholar] [CrossRef]
- Gammons, C.H.; Allin, N.C. Stability of Aqueous Fe(III) Chloride Complexes and the Solubility of Hematite between 150 and 300 °C. Geochim. Cosmochim. Acta 2022, 330, 148–164. [Google Scholar] [CrossRef]
- Kwon, S.-K.; Kimijima, K.; Kanie, K.; Muramatsu, A.; Suzuki, S.; Matsubara, E.; Waseda, Y. Inhibition of Conversion Process from Fe(OH)3 to β-FeOOH and α-Fe2O3 by the Addition of Silicate Ions. ISIJ Int. 2005, 45, 77–81. [Google Scholar] [CrossRef]
- Hao, X.; Zeng, W.; Li, J.; Zhan, M.; Miao, H.; Gong, Q. High-Efficient Nitrogen Removal with Low Demand of Fe Source and Mechanism Analysis Driven by Fe(II)/Fe(III) Cycle. Chem. Eng. J. 2024, 481, 148702. [Google Scholar] [CrossRef]
- Wen, W.-R.; Liu, T.-C.; Fan, S.-Q.; Tan, X.; Lu, Y.; Xing, D.-F.; Liu, B.-F.; Ren, N.-Q.; Xie, G.-J. Dissimilatory Nitrate Reduction to Ammonium Driven by Different Electron Donors: Mechanisms, Recent Advances, and Future Perspectives. Chem. Eng. J. 2025, 507, 160625. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, X.; Wan, Y.; Zhang, B.; Wang, Z.; Li, N.; Wang, X. Fe(III)/Fe(II) Cycle Enables Biological Nitrate Ammoniation at Low C/N Ratio for Reactive Nitrogen Recovery. Resour. Conserv. Recycl. 2024, 205, 107587. [Google Scholar] [CrossRef]
- Liu, W.; Etschmann, B.; Brugger, J.; Spiccia, L.; Foran, G.; McInnes, B. UV-Vis Spectrophotometric and XAFS Studies of Ferric Chloride Complexes in Hyper-Saline LiCl Solutions at 25–90 °C. Chem. Geol. 2006, 231, 326–349. [Google Scholar] [CrossRef]
- Persson, I. Ferric Chloride Complexes in Aqueous Solution: An EXAFS Study. J. Solut. Chem. 2018, 47, 797–805. Available online: https://link.springer.com/article/10.1007/s10953-018-0756-6 (accessed on 6 March 2025). [CrossRef] [PubMed]





| Experimental Design | Group | Additives | Experimental Environment |
|---|---|---|---|
| Open Environment | Y1 | Original sediment (3.000 g) | Simulate a shallow water environment, solution untreated and left open |
| Y2 | Original sediment (3.000 g) + hematite (0.119 g) | ||
| Y3 | Original sediment (3.000 g) + siderite (0.172 g) | ||
| Closed Environment | W1 | Original sediment (3.000 g) | Simulate underground environment, solution purged with nitrogen and sealed |
| W2 | Original sediment (3.000 g) + hematite (0.119 g) | ||
| W3 | Original sediment (3.000 g) + siderite (0.172 g) |
| Experimental Groups | Y1 | Y2 | Y3 | W2 | W3 |
|---|---|---|---|---|---|
| W1 | The Influence of Open and Closed Systems on Nitrate Reduction | The Influence of Adding Hematite on Nitrate Reduction | The Influence of Adding Siderite on Nitrate Reduction | ||
| W2 | The Influence of Open and Closed Systems on Nitrate Reduction | The Difference in the Influence of Hematite and Siderite on Nitrate Reduction | |||
| W3 | The Influence of Open and Closed Systems on Nitrate Reduction | ||||
| Y1 | The Influence of Adding Hematite on Nitrate Reduction | The Influence of Adding Siderite on Nitrate Reduction | |||
| Y2 | The Difference in the Influence of Hematite and Siderite on Nitrate Reduction |
| Reaction System | k | R2 |
|---|---|---|
| Y1 | −0.0247 | 0.7351 |
| Y2 | −0.0401 | 0.9166 |
| Y3 | −0.0064 | 0.8543 |
| W1 | −0.1389 | 0.8349 |
| W2 | −0.1849 | 0.7745 |
| W3 | −0.2005 | 0.8818 |
| Sample | Shannon | Simpson | Shannon Evenness | Coverage |
|---|---|---|---|---|
| Y1 | 2.43 | 0.23 | 0.39 | 0.9986 |
| Y2 | 2.83 | 0.16 | 0.45 | 0.9986 |
| Y3 | 2.53 | 0.21 | 0.41 | 0.9994 |
| W1 | 3.29 | 0.15 | 0.52 | 0.9992 |
| W2 | 3.39 | 0.07 | 0.53 | 0.9986 |
| W3 | 3.31 | 0.10 | 0.53 | 0.9991 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wan, Y.; Ma, Z.; Xu, L.; Zhai, Y.; Su, X. Impact of Iron Minerals on Nitrate Reduction in the Lake–Groundwater Interaction Zone of High-Salinity Environment. Water 2025, 17, 1241. https://doi.org/10.3390/w17091241
Wang Z, Wan Y, Ma Z, Xu L, Zhai Y, Su X. Impact of Iron Minerals on Nitrate Reduction in the Lake–Groundwater Interaction Zone of High-Salinity Environment. Water. 2025; 17(9):1241. https://doi.org/10.3390/w17091241
Chicago/Turabian StyleWang, Zhen, Yuyu Wan, Zhe Ma, Luwen Xu, Yuanzheng Zhai, and Xiaosi Su. 2025. "Impact of Iron Minerals on Nitrate Reduction in the Lake–Groundwater Interaction Zone of High-Salinity Environment" Water 17, no. 9: 1241. https://doi.org/10.3390/w17091241
APA StyleWang, Z., Wan, Y., Ma, Z., Xu, L., Zhai, Y., & Su, X. (2025). Impact of Iron Minerals on Nitrate Reduction in the Lake–Groundwater Interaction Zone of High-Salinity Environment. Water, 17(9), 1241. https://doi.org/10.3390/w17091241







