Optimizing the Nitrogen Removal Efficiency of an Intermittent Biological Sponge Iron Reactor by Immobilizing Aerobic Denitrifying Bacteria in the Biological Sponge Iron System
Abstract
1. Introduction
2. Materials and Methods
2.1. Physicochemical Properties of the Experimental Wastewater
2.2. Isolation and Culturing Aerobic Denitrifying Bacteria
2.3. Preparation of Immobilized Aerobic Denitrification Gel Beads
2.4. Optimization of Immobilized Material Formulation
2.5. Evaluation of the Denitrification Capacity of Immobilized Aerobic Denitrifying Bacteria
2.6. Statistical Analysis
3. Results
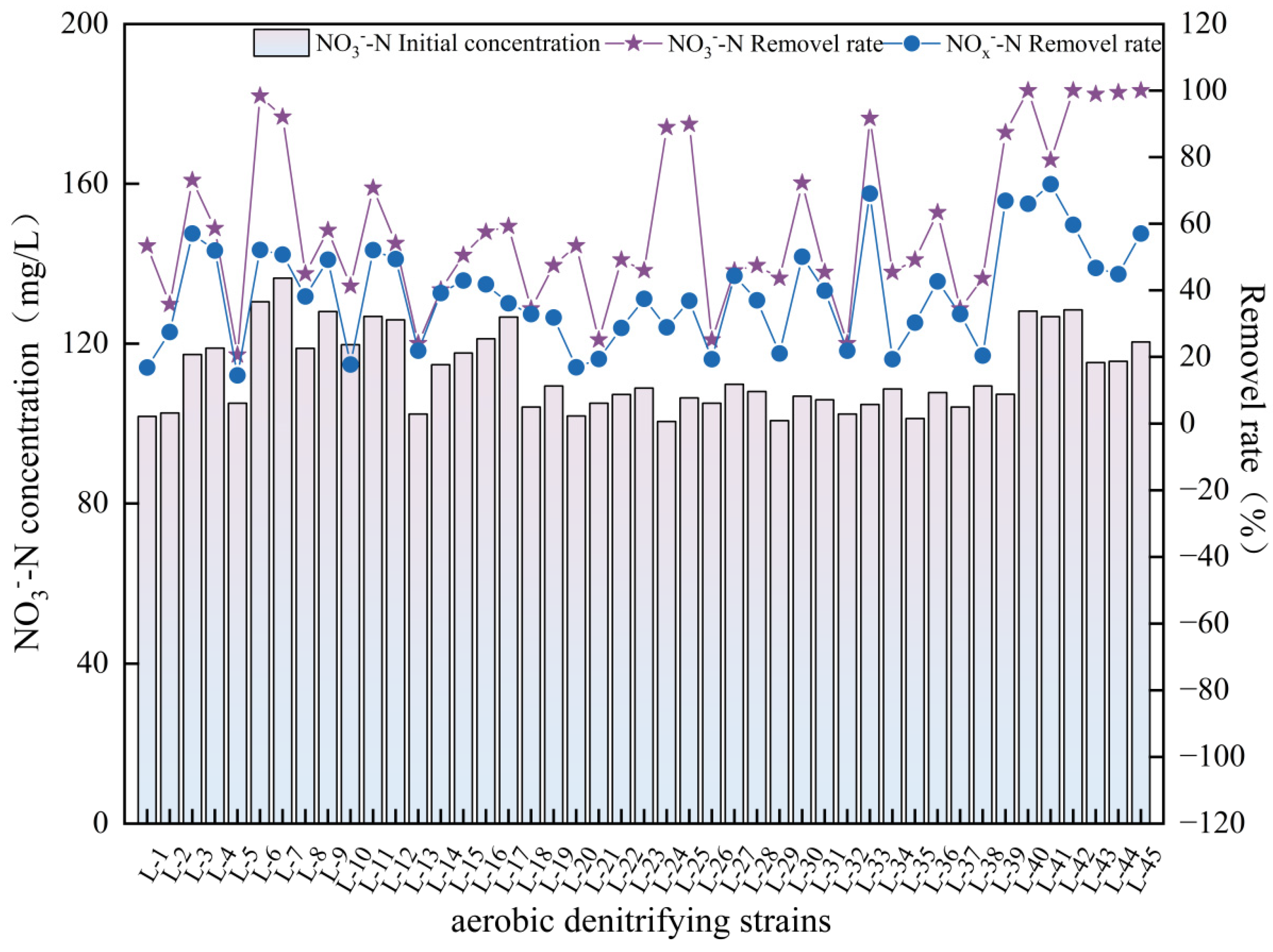
3.1. Isolation of Aerobic Denitrifying Bacterial Communities
3.2. Immobilization Material Ratio Optimization
3.3. Response Surface Methodology Experimental Results
3.3.1. Model Establishment and Predictive Optimization
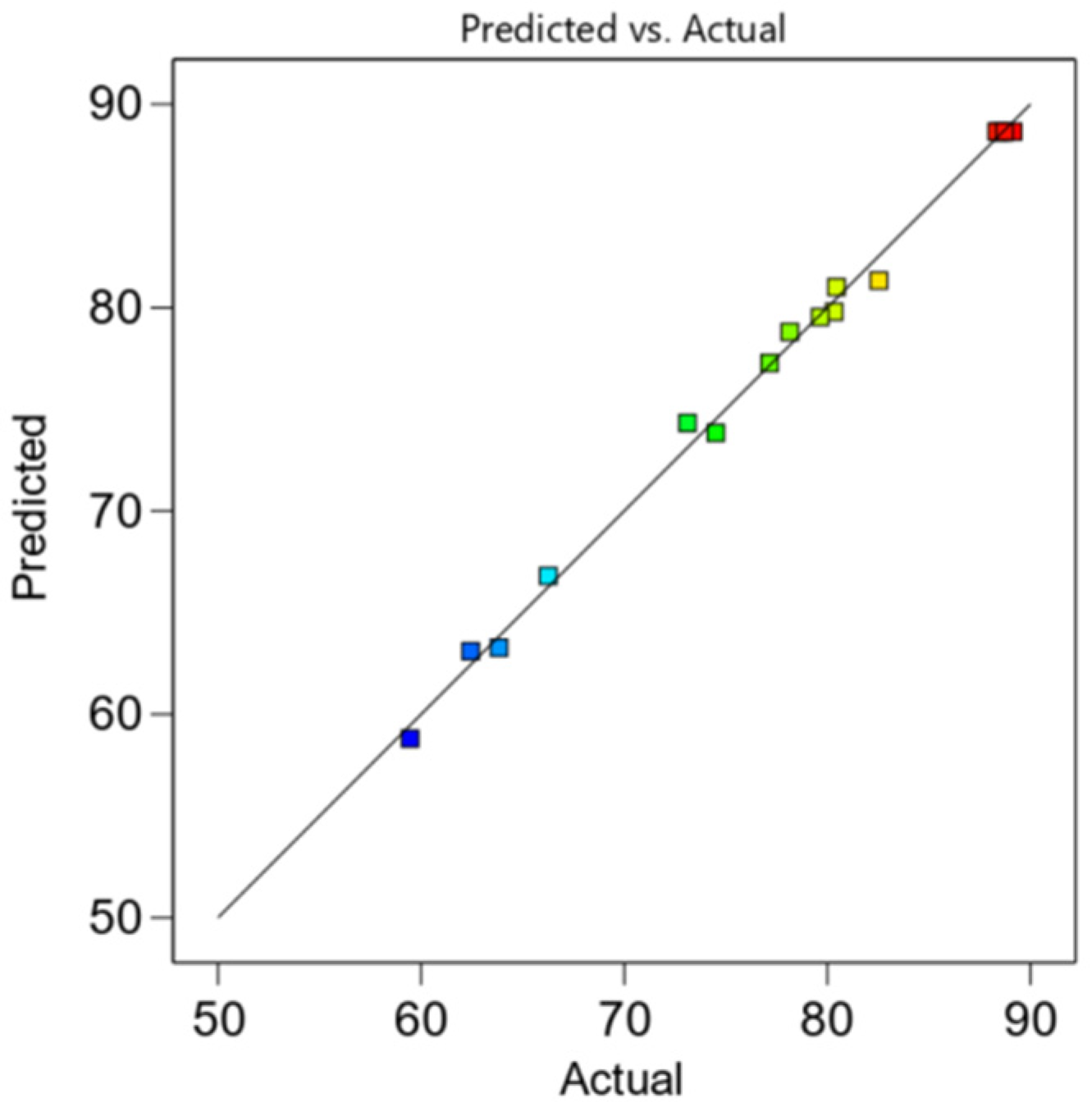
3.3.2. Residual Analysis
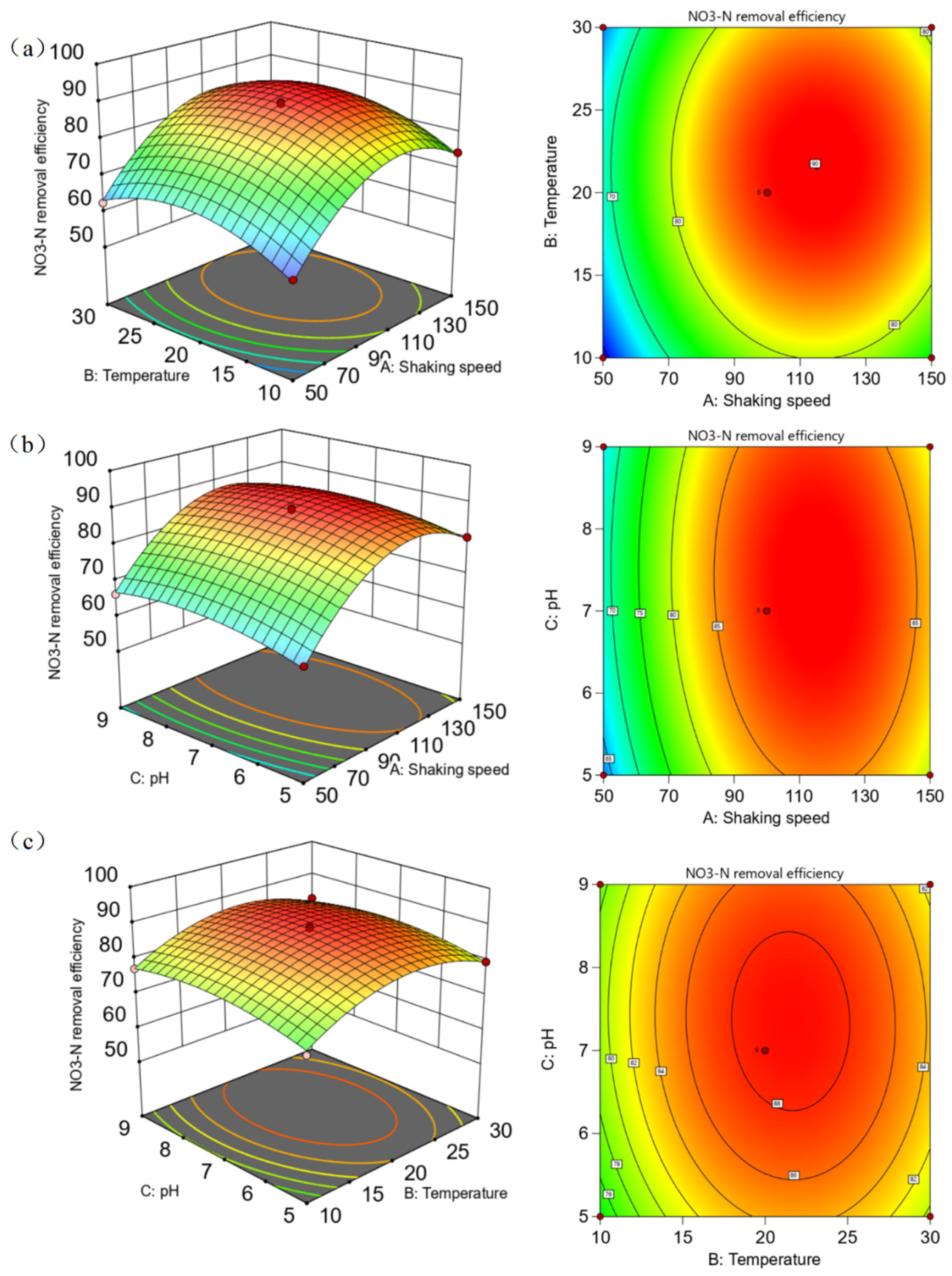
3.3.3. Analysis of Response Surface Plots and Contour Plots Among Various Environmental Factors
3.4. Immobilized Aerobic Denitrifying Bacteria Enhanced the SND Capability of the BSIS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmad, H.A.; Ni, S.-Q.; Ahmad, S.; Zhang, J.; Ali, M.; Ngo, H.H.; Guo, W.; Tan, Z.; Wang, Q. Gel Immobilization: A Strategy to Improve the Performance of Anaerobic Ammonium Oxidation (Anammox) Bacteria for Nitrogen-Rich Wastewater Treatment. Bioresour. Technol. 2020, 313, 123642. [Google Scholar] [CrossRef]
- Bonassa, G.; Bolsan, A.C.; Hollas, C.E.; Venturin, B.; Candido, D.; Chini, A.; De Prá, M.C.; Antes, F.G.; Campos, J.L.; Kunz, A. Organic Carbon Bioavailability: Is It a Good Driver to Choose the Best Biological Nitrogen Removal Process? Sci. Total Environ. 2021, 786, 147390. [Google Scholar] [CrossRef] [PubMed]
- Temkin, A.; Evans, S.; Manidis, T.; Campbell, C.; Naidenko, O.V. Exposure-Based Assessment and Economic Valuation of Adverse Birth Outcomes and Cancer Risk Due to Nitrate in United States Drinking Water. Environ. Res. 2019, 176, 108442. [Google Scholar] [CrossRef] [PubMed]
- Camargo, J.A.; Alonso, Á. Ecological and Toxicological Effects of Inorganic Nitrogen Pollution in Aquatic Ecosystems: A Global Assessment. Environ. Int. 2006, 32, 831–849. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Xie, D.; Li, Z.; Ni, J.; Sun, Q. Ammonium Stimulates Nitrate Reduction during Simultaneous Nitrification and Denitrification Process by Arthrobacter Arilaitensis Y-10. Bioresour. Technol. 2017, 239, 66–73. [Google Scholar] [CrossRef]
- Jianlong, W.; Xiangchun, Q.; Libo, W.; Yi, Q.; Hegemann, W. Bioaugmentation as a Tool to Enhance the Removal of Refractory Compound in Coke Plant Wastewater. Process Biochem. 2002, 38, 777–781. [Google Scholar] [CrossRef]
- Amorim, C.L.; Duque, A.F.; Afonso, C.M.M.; Castro, P.M.L. Bioaugmentation for Treating Transient 4-Fluorocinnamic Acid Shock Loads in a Rotating Biological Contactor. Bioresour. Technol. 2013, 144, 554–562. [Google Scholar] [CrossRef]
- Guo, J.; Wang, J.; Cui, D.; Wang, L.; Ma, F.; Chang, C.-C.; Yang, J. Application of Bioaugmentation in the Rapid Start-up and Stable Operation of Biological Processes for Municipal Wastewater Treatment at Low Temperatures. Bioresour. Technol. 2010, 101, 6622–6629. [Google Scholar] [CrossRef]
- Chen, S.; He, S.; Wu, C.; Du, D. Characteristics of Heterotrophic Nitrification and Aerobic Denitrification Bacterium Acinetobacter Sp. T1 and Its Application for Pig Farm Wastewater Treatment. J. Biosci. Bioeng. 2019, 127, 201–205. [Google Scholar] [CrossRef]
- Huang, G.; Huang, Y.; Hu, H.; Liu, F.; Zhang, Y.; Deng, R. Remediation of Nitrate–Nitrogen Contaminated Groundwater Using a Pilot-Scale Two-Layer Heterotrophic–Autotrophic Denitrification Permeable Reactive Barrier with Spongy Iron/Pine Bark. Chemosphere 2015, 130, 8–16. [Google Scholar] [CrossRef]
- Guo, L.; Chen, Q.; Fang, F.; Hu, Z.; Wu, J.; Miao, A.; Xiao, L.; Chen, X.; Yang, L. Application Potential of a Newly Isolated Indigenous Aerobic Denitrifier for Nitrate and Ammonium Removal of Eutrophic Lake Water. Bioresour. Technol. 2013, 142, 45–51. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, Y.; Sun, G.; Gao, X.; Zhang, Q.; Liu, Z. Denitrification Characteristics of a Marine Origin Psychrophilic Aerobic Denitrifying Bacterium. J. Environ. Sci. 2011, 23, 1888–1893. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, L.; Pang, L. Application of a Novel Strain Corynebacterium Pollutisoli Sph6 to Improve Nitrogen Removal in an Anaerobic/Aerobic-Moving Bed Biofilm Reactor (a/O-Mbbr). Bioresour. Technol. 2018, 269, 113–120. [Google Scholar] [CrossRef]
- He, T.-X.; Ye, Q.; Sun, Q.; Cai, X.; Ni, J.-p.; Li, Z.; Xie, D.-T. Removal of Nitrate in Simulated Water at Low Temperature by a Novel Psychrotrophic and Aerobic Bacterium, Pseudomonas taiwanensis Strain J. BioMed Res. Int. 2018, 2018, 4984087. [Google Scholar] [CrossRef]
- Ji, B.; Wang, H.; Yang, K. Nitrate and Cod Removal in an Upflow Biofilter under an Aerobic Atmosphere. Bioresour. Technol. 2014, 158, 156–160. [Google Scholar] [CrossRef]
- Guo, K.-H.; Li, W.-X.; Wang, Y.-E.; Zheng, Y.; Ji, B.; Mao, F.-J.; Xie, H.-N.; Li, J. Substantially Enhanced Degradation of Nitrobenzene by Biosponge Iron System: Process and Mechanisms. Process Biochem. 2021, 108, 146–152. [Google Scholar] [CrossRef]
- Tang, Y.; Li, M.; Xu, D.; Huang, J.; Sun, J. Application Potential of Aerobic Denitrifiers Coupled with a Biostimulant for Nitrogen Removal from Urban River Sediment. Environ. Sci. Pollut. Res. 2018, 25, 5980–5993. [Google Scholar] [CrossRef]
- Li, W.; Chen, X.; Yang, T.; Zhu, H.; He, Z.; Zhao, R.; Chen, Y. Sponge Iron Enriches Autotrophic/Aerobic Denitrifying Bacteria to Enhance Denitrification in Sequencing Batch Reactor. Bioresour. Technol. 2024, 407, 131097. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Li, W.; Hu, K.; Zhu, H.; Ren, S.; Peng, Y.; Hei, S.; Li, J.; Quan, H. Characterization of Achromobacter Denitrificans Qhr-5 for Heterotrophic Nitrification-Aerobic Denitrification with Iron Oxidation Function Isolated from Bsis: Nitrogen Removal Performance and Enhanced Snd Capability of Bsis. Biochem. Eng. J. 2023, 191, 108759. [Google Scholar] [CrossRef]
- He, Z.; Geng, S.; Pan, Y.; Cai, C.; Wang, J.; Wang, L.; Liu, S.; Zheng, P.; Xu, X.; Hu, B. Improvement of the Trace Metal Composition of Medium for Nitrite-Dependent Anaerobic Methane Oxidation Bacteria: Iron (Ii) and Copper (Ii) Make a Difference. Water Res. 2015, 85, 235–243. [Google Scholar] [CrossRef]
- Kong, Q.; Ngo, H.H.; Shu, L.; Fu, R.-S.; Jiang, C.-H.; Miao, M.-S. Enhancement of Aerobic Granulation by Zero-Valent Iron in Sequencing Batch Airlift Reactor. J. Hazard. Mater. 2014, 279, 511–517. [Google Scholar] [CrossRef]
- Zhang, M.; Guan, Z.; Li, B.; You, Y.; Cao, G.; Fan, Y.; Wu, J.; Ji, J. Denitrification Enhancement, Kinetic Simulation and Microbial Assessment in the Sponge Iron-Mediated Anammox Process: A Method for Low-Strength Nitrogen Wastewater Treatment. J. Water Process Eng. 2025, 72, 107550. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, X.; Cheng, Y.; Jiang, M.; Li, X.; Xue, G. Iron Robustly Stimulates Simultaneous Nitrification and Denitrification under Aerobic Conditions. Environ. Sci. Technol. 2018, 52, 1404–1412. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, X.; Yang, Z.; Liu, Z.; Chen, Y.; Wang, Y.-E.; Xie, H. Denitrification Performance and Mechanism of Sequencing Batch Reactor with a Novel Iron-Polyurethane Foam Composite Carrier. Biochem. Eng. J. 2021, 176, 108209. [Google Scholar] [CrossRef]
- Liu, Q.; Dai, H.; Song, Y.; Li, H. Magnetite Enhances as Immobilization during Nitrate Reduction and Fe(Ii) Oxidation by Acidovorax Sp. Strain Bofen1. Sci. Total Environ. 2024, 946, 173946. [Google Scholar] [CrossRef]
- Addison, H.; Glatter, T.; KA Hochberg, G.; Rebelein, J.G. Two Distinct Ferredoxins Are Essential for Nitrogen Fixation by the Iron Nitrogenase in Rhodobacter Capsulatus. mBio 2024, 15, e03314-23. [Google Scholar] [CrossRef]
- Bouabidi, Z.B.; El-Naas, M.H.; Zhang, Z. Immobilization of Microbial Cells for the Biotreatment of Wastewater: A Review. Environ. Chem. Lett. 2019, 17, 241–257. [Google Scholar] [CrossRef]
- Wang, J.; Liang, J.; Sun, L.; Li, G.; Temmink, H.; Rijnaarts, H.H.M. Granule-Based Immobilization and Activity Enhancement of Anammox Biomass Via Pva/Cs and Pva/Cs/Fe Gel Beads. Bioresour. Technol. 2020, 309, 123448. [Google Scholar] [CrossRef]
- Dong, H.; Wang, W.; Song, Z.; Dong, H.; Wang, J.; Sun, S.; Zhang, Z.; Ke, M.; Zhang, Z.; Wu, W.-M.; et al. A High-Efficiency Denitrification Bioreactor for the Treatment of Acrylonitrile Wastewater Using Waterborne Polyurethane Immobilized Activated Sludge. Bioresour. Technol. 2017, 239, 472–481. [Google Scholar] [CrossRef]
- Hou, L.-G.; Li, J.; Sun, F.-Y.; Zhang, X.-Y.; Liu, Y. High-Efficiency Denitrification for Steel Wastewater Treatment by Immobilized Bacteria. Desalination Water Treat. 2021, 211, 117–122. [Google Scholar] [CrossRef]
- Kumar, A.; Sood, A.; Han, S.S. Poly (Vinyl Alcohol)-Alginate as Potential Matrix for Various Applications: A Focused Review. Carbohydr. Polym. 2022, 277, 118881. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, Z.; Hu, Y.; Song, C.; Li, F.; He, W.; Wang, X.; Li, Z.; Lin, H. Immobilization of Nitrifying Bacteria in Magnetic Pva–Sa-Diatomite Carrier for Efficient Removal of Nh4+-N from Effluents. Environ. Technol. Innov. 2021, 22, 101407. [Google Scholar] [CrossRef]
- Guo, J.; Chen, C.; Chen, W.; Jiang, J.; Chen, B.; Zheng, F. Effective Immobilization of Bacillus Subtilis in Chitosan-Sodium Alginate Composite Carrier for Ammonia Removal from Anaerobically Digested Swine Wastewater. Chemosphere 2021, 284, 131266. [Google Scholar] [CrossRef]
- Asranudin; Purnomo, A.S.; Holilah; Prasetyoko, D.; El Messaoudi, N.; Rohmah, A.A.; Putra Hidayat, A.R.; Subagyo, R. Adsorption and Biodegradation of the Azo Dye Methyl Orange Using Ralstonia pickettii Immobilized in Polyvinyl Alcohol (PVA)–Alginate–Hectorite Beads (Bhec-Rp). RSC Adv. 2024, 14, 18277–18290. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Zhao, X.; Senbati, Y.; Song, K.; He, X. Nitrogen Removal by Heterotrophic Nitrifying and Aerobic Denitrifying Bacterium Pseudomonas sp. Dm02: Removal Performance, Mechanism and Immobilized Application for Real Aquaculture Wastewater Treatment. Bioresour. Technol. 2021, 322, 124555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, P.; Hao, B.; Yu, Z. Heterotrophic Nitrification and Aerobic Denitrification by the Bacterium Pseudomonas Stutzeri Yzn-001. Bioresour. Technol. 2011, 102, 9866–9869. [Google Scholar] [CrossRef]
- Gao, M.; Shen, Y.; Peng, Y.; Tan, F.; Lv, Y.; Zhu, C.; Guo, Y.; Liu, X. Bioenhancement Mechanism of PVA-SA Immobilized Composite Strains Alishewanella fetalis and Exiguobacterium profundum in Pyridine Degradation. Biochem. Eng. J. 2025, 213, 109559. [Google Scholar] [CrossRef]
- Li, C.; Zhang, W.; Mao, L.; Yin, D.; Niu, D.; Taoli, H.; Wang, C.; Liu, Q.; Ren, J. Immobilization of Peniophora Incarnata F1 in PVA-SA-Biochar Matrix and Its Degradation Performance and Mechanism for Erythromycin Degradation. J. Environ. Manag. 2025, 375, 124297. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, D.; Xiong, W.; Wu, Z.; Xiao, G.; Wang, S.; Su, H. Enhancing Nitrogen Removal Performance Using Immobilized Aerobic Denitrifying Bacteria by Modified Polyvinyl Alcohol/Sodium Alginate (PVA/SA). Chemosphere 2024, 357, 141954. [Google Scholar] [CrossRef]
- Nabilah, B.; Purnomo, A.S.; Prasetyoko, D.; Rohmah, A.A. Methylene Blue Biodecolorization and Biodegradation by Immobilized Mixed Cultures of Trichoderma viride and Ralstonia pickettii into SA-PVA-Bentonite Matrix. Arab. J. Chem. 2023, 16, 104940. [Google Scholar] [CrossRef]
- Alkas, T.R.; Purnomo, A.S.; Pratiwi, A.N.; Nurwijayanti, Y.; Ediati, R.; Ersam, T.; Kusumawati, Y. Immobilization of Uio-66/Brown-Rot Fungi (Brf) in PVA-SA Matrix and Its Performance for Methylene Blue Decolorization. Mater. Today Chem. 2023, 29, 101411. [Google Scholar] [CrossRef]
- Ali, M.; Oshiki, M.; Rathnayake, L.; Ishii, S.; Satoh, H.; Okabe, S. Rapid and Successful Start-up of Anammox Process by Immobilizing the Minimal Quantity of Biomass in PVA-SA Gel Beads. Water Res. 2015, 79, 147–157. [Google Scholar] [CrossRef]
- Kim, J.O.; Park, J.K.; Kim, J.H.; Jin, S.G.; Yong, C.S.; Li, D.X.; Choi, J.Y.; Woo, J.S.; Yoo, B.K.; Lyoo, W.S.; et al. Development of Polyvinyl Alcohol–Sodium Alginate Gel-Matrix-Based Wound Dressing System Containing Nitrofurazone. Int. J. Pharm. 2008, 359, 79–86. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.-R.S.; Tamer, T.M.; El-Meligy, M.A.; Mohy Eldin, M.S. Poly (Vinyl Alcohol)-Alginate Physically Crosslinked Hydrogel Membranes for Wound Dressing Applications: Characterization and Bio-Evaluation. Arab. J. Chem. 2015, 8, 38–47. [Google Scholar] [CrossRef]
- Yu, Q.; Yuan, Y.; Feng, L.; Sun, W.; Lin, K.; Zhang, J.; Zhang, Y.; Wang, H.; Wang, N.; Peng, Q. Highly Efficient Immobilization of Environmental Uranium Contamination with Pseudomonas stutzeri by Biosorption, Biomineralization, and Bioreduction. J. Hazard. Mater. 2022, 424, 127758. [Google Scholar] [CrossRef]
- Bae, H.; Choi, M.; Chung, Y.-C.; Lee, S.; Yoo, Y.J. Core-Shell Structured Poly(Vinyl Alcohol)/Sodium Alginate Bead for Single-Stage Autotrophic Nitrogen Removal. Chem. Eng. J. 2017, 322, 408–416. [Google Scholar] [CrossRef]
- Liu, C.; Yu, D.; Wang, Y.; Chen, G.; Tang, P.; Huang, S. A Novel Control Strategy for the Partial Nitrification and Anammox Process (PN/A) of Immobilized Particles: Using Salinity as a Factor. Bioresour. Technol. 2020, 302, 122864. [Google Scholar] [CrossRef] [PubMed]
- Takaya, N.; Catalan-Sakairi Maria Antonina, B.; Sakaguchi, Y.; Kato, I.; Zhou, Z.; Shoun, H. Aerobic Denitrifying Bacteria That Produce Low Levels of Nitrous Oxide. Appl. Environ. Microbiol. 2003, 69, 3152–3157. [Google Scholar] [CrossRef]
- Zhao, B.; Tian, M.; An, Q.; Ye, J.; Guo, J.S. Characteristics of a Heterotrophic Nitrogen Removal Bacterium and Its Potential Application on Treatment of Ammonium-Rich Wastewater. Bioresour. Technol. 2017, 226, 46–54. [Google Scholar] [CrossRef]
- Yao, S.; Ni, J.; Ma, T.; Li, C. Heterotrophic Nitrification and Aerobic Denitrification at Low Temperature by a Newly Isolated Bacterium, Acinetobacter sp. HA2. Bioresour. Technol. 2013, 139, 80–86. [Google Scholar] [CrossRef]
- Xia, L.; Li, X.; Fan, W.; Wang, J. Heterotrophic Nitrification and Aerobic Denitrification by a Novel Acinetobacter Sp. Nd7 Isolated from Municipal Activated Sludge. Bioresour. Technol. 2020, 301, 122749. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.C.; Kao, C.M.; Chen, C.W.; Dong, C.D.; Chien, H.Y. Evaluation of Biological Stability and Corrosion Potential in Drinking Water Distribution Systems: A Case Study. Environ. Monit. Assess. 2009, 153, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Luo, X.; Ma, W.; Lv, P. Biological Nitrogen Removal and Metabolic Characteristics of a Novel Cold-Resistant Heterotrophic Nitrification and Aerobic Denitrification Rhizobium sp. WS7. Bioresour. Technol. 2022, 362, 127756. [Google Scholar] [CrossRef] [PubMed]


| Test Number | SA Dosage (g/100 mL) | PVA Dosage (g/100 mL) | CaCl2 Dosage (g/100 mL) | Bacterial Community Embedding Amount (g/100 mL) | NO3−-N Removal Rate (%) |
|---|---|---|---|---|---|
| 1 | 3(1) | 9(1) | 2(1) | 1(1) | 82.43 |
| 2 | 3(1) | 10(2) | 4(3) | 3(3) | 90.15 |
| 3 | 3(1) | 11(3) | 3(2) | 2(2) | 84.83 |
| 4 | 4(2) | 9(1) | 3(2) | 3(3) | 87.35 |
| 5 | 4(2) | 10(2) | 2(1) | 2(2) | 91.73 |
| 6 | 4(2) | 11(3) | 4(3) | 1(1) | 82.72 |
| 7 | 5(3) | 9(1) | 4(3) | 2(2) | 87.54 |
| 8 | 5(3) | 10(2) | 3(2) | 1(1) | 84.33 |
| 9 | 5(3) | 11(3) | 2(1) | 3(3) | 82.58 |
| K1 | 257.41 | 257.32 | 256.74 | 249.48 | |
| K2 | 261.8 | 266.21 | 256.51 | 264.1 | |
| K3 | 254.45 | 250.13 | 260.41 | 260.08 | |
| k1 | 85.80 | 85.77 | 85.58 | 83.16 | |
| k2 | 87.27 | 88.74 | 85.50 | 88.03 | |
| k3 | 84.82 | 83.38 | 86.80 | 86.69 | |
| R | 2.45 | 5.36 | 1.30 | 4.87 |
| Test Number | Shaking Speed (rpm) | Temperature (°C) | pH | NO3−-N Removal Rate (%) |
|---|---|---|---|---|
| 1 | 50(−1) | 10(−1) | 7(0) | 59.46 |
| 2 | 150(1) | 10(−1) | 7(0) | 74.51 |
| 3 | 50(−1) | 30(1) | 7(0) | 62.44 |
| 4 | 150(1) | 30(1) | 7(0) | 78.15 |
| 5 | 50(−1) | 20(0) | 5(−1) | 63.84 |
| 6 | 150(1) | 20(0) | 5(−1) | 80.34 |
| 7 | 50(−1) | 20(0) | 9(1) | 66.26 |
| 8 | 150(1) | 20(0) | 9(1) | 80.45 |
| 9 | 100(0) | 10(−1) | 5(−1) | 73.12 |
| 10 | 100(0) | 30(1) | 5(−1) | 79.65 |
| 11 | 100(0) | 10(−1) | 9(1) | 77.16 |
| 12 | 100(0) | 30(1) | 9(1) | 82.54 |
| 13 | 100(0) | 20(0) | 7(0) | 88.62 |
| 14 | 100(0) | 20(0) | 7(0) | 89.15 |
| 15 | 100(0) | 20(0) | 7(0) | 88.43 |
| 16 | 100(0) | 20(0) | 7(0) | 88.34 |
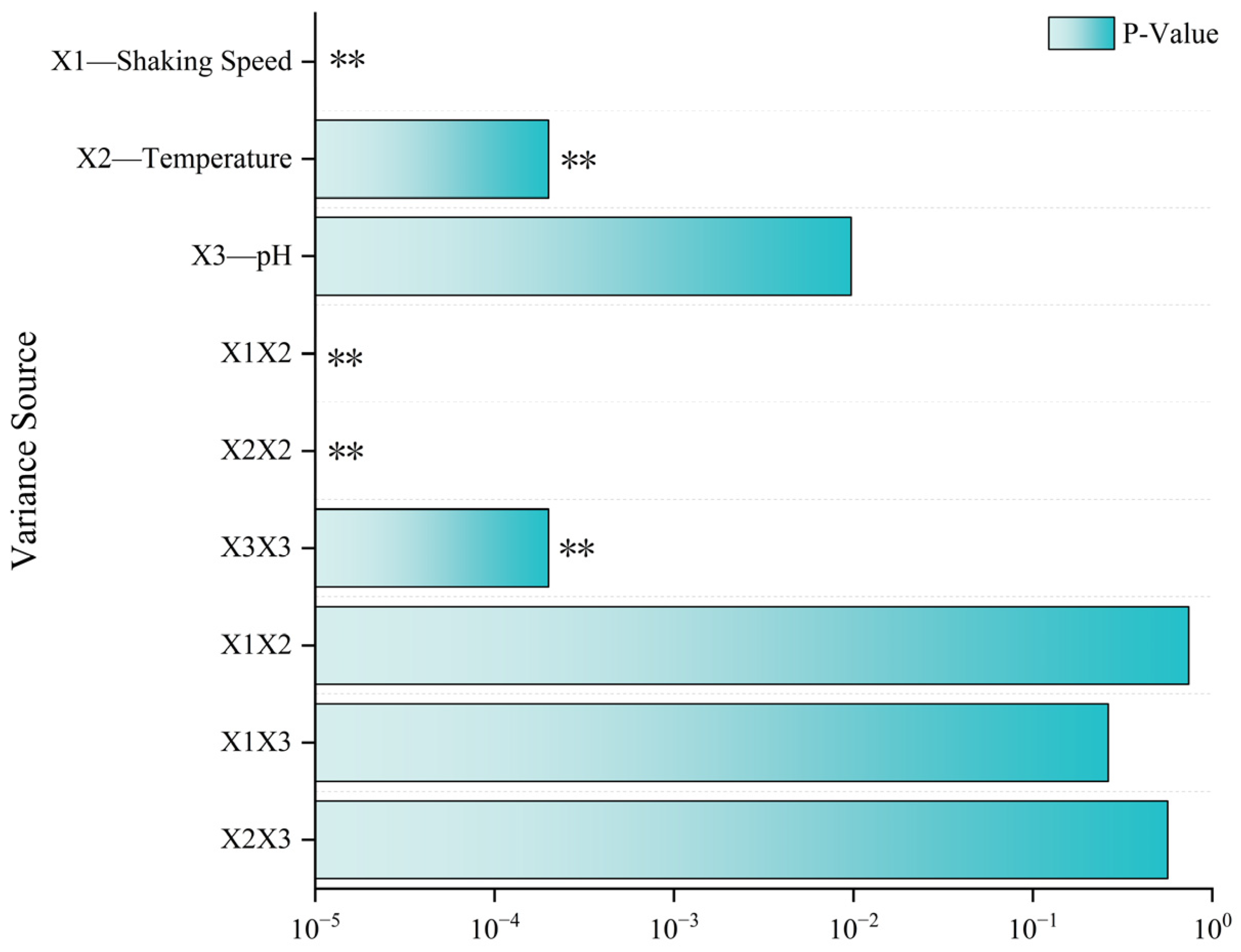
| Variance Source | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 1555.75 | 9 | 172.86 | 191.49 | <0.0001 | ** |
| X1–Shaking Speed | 472.01 | 1 | 472.01 | 522.89 | <0.0001 | ** |
| X2–Temperature | 42.92 | 1 | 42.92 | 47.55 | 0.0002 | ** |
| X3–pH | 11.19 | 1 | 11.19 | 12.39 | 0.0097 | |
| X1X2 | 0.1089 | 1 | 0.1089 | 0.1206 | 0.7386 | |
| X1X3 | 1.33 | 1 | 1.33 | 1.48 | 0.2635 | |
| X2X3 | 0.3306 | 1 | 0.3306 | 0.3663 | 0.5641 | |
| X12 | 679.49 | 1 | 679.49 | 752.73 | <0.0001 | ** |
| X22 | 224.90 | 1 | 224.90 | 249.14 | <0.0001 | ** |
| X32 | 43.82 | 1 | 43.82 | 48.54 | 0.0002 | ** |
| Residual | 6.32 | 7 | 0.9027 | |||
| Lack of Fit | 5.92 | 3 | 1.97 | 19.71 | 0.0074 | * |
| Pure Error | 0.4003 | 4 | 0.1001 | |||
| Total Deviation | 1562.07 | 16 | ||||
| R2 = 0.9960 | Signal-to-Noise Ratio = 40.9559 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Li, J.; Wang, Y.; Mu, H.; Xie, H.; Zhao, W. Optimizing the Nitrogen Removal Efficiency of an Intermittent Biological Sponge Iron Reactor by Immobilizing Aerobic Denitrifying Bacteria in the Biological Sponge Iron System. Water 2025, 17, 1308. https://doi.org/10.3390/w17091308
Li J, Li J, Wang Y, Mu H, Xie H, Zhao W. Optimizing the Nitrogen Removal Efficiency of an Intermittent Biological Sponge Iron Reactor by Immobilizing Aerobic Denitrifying Bacteria in the Biological Sponge Iron System. Water. 2025; 17(9):1308. https://doi.org/10.3390/w17091308
Chicago/Turabian StyleLi, Jing, Jie Li, Yae Wang, Hao Mu, Huina Xie, and Wei Zhao. 2025. "Optimizing the Nitrogen Removal Efficiency of an Intermittent Biological Sponge Iron Reactor by Immobilizing Aerobic Denitrifying Bacteria in the Biological Sponge Iron System" Water 17, no. 9: 1308. https://doi.org/10.3390/w17091308
APA StyleLi, J., Li, J., Wang, Y., Mu, H., Xie, H., & Zhao, W. (2025). Optimizing the Nitrogen Removal Efficiency of an Intermittent Biological Sponge Iron Reactor by Immobilizing Aerobic Denitrifying Bacteria in the Biological Sponge Iron System. Water, 17(9), 1308. https://doi.org/10.3390/w17091308







