Cost-Effective Abatement of Tetrabromobisphenol A from Contaminated Water by a Visible-Light-Driven Photochemical System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Catalyst Preparation
2.2. Experimental Procedures
2.3. Analytical Methods
2.4. Statistical Analysis
3. Results and Discussion
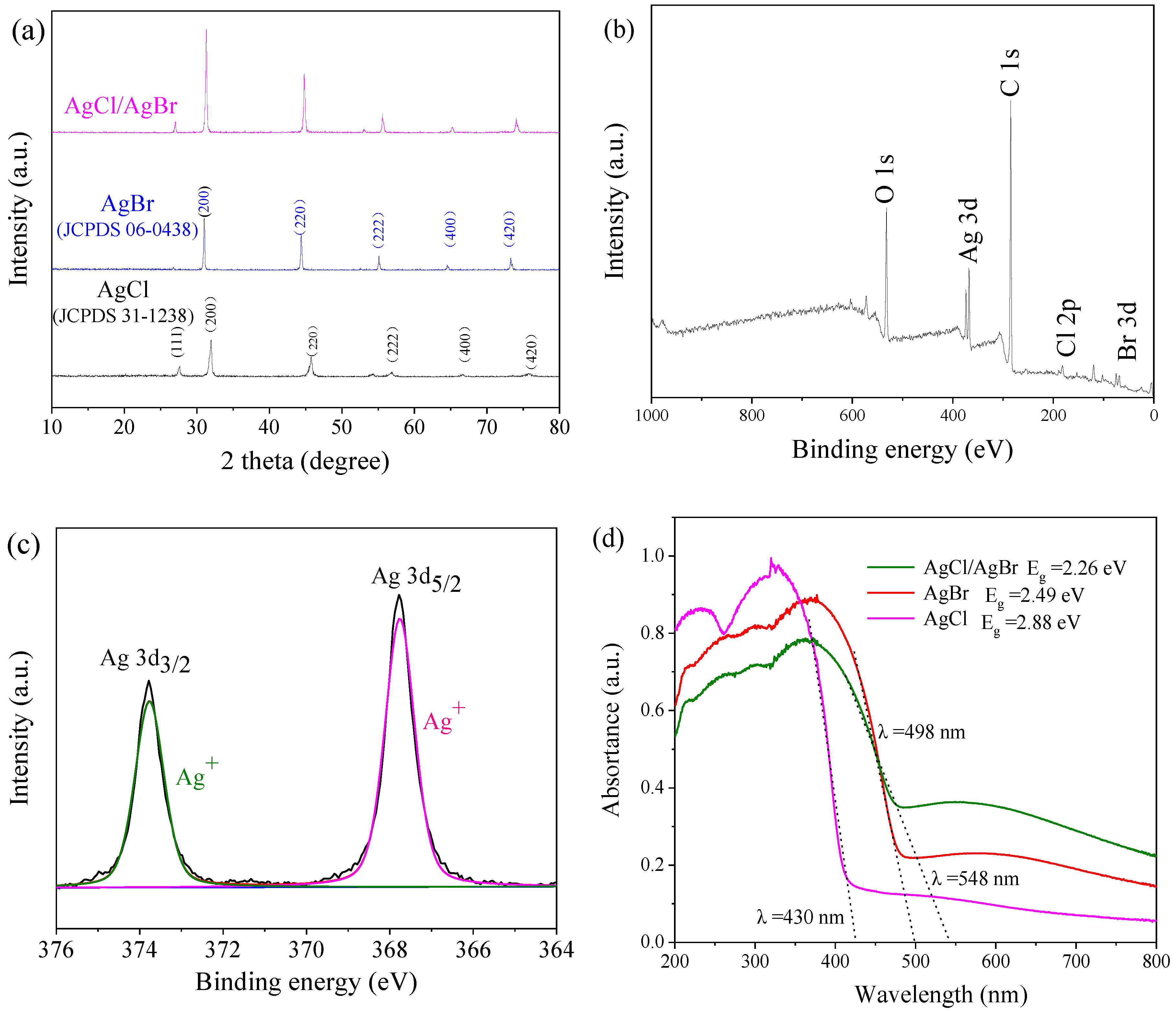
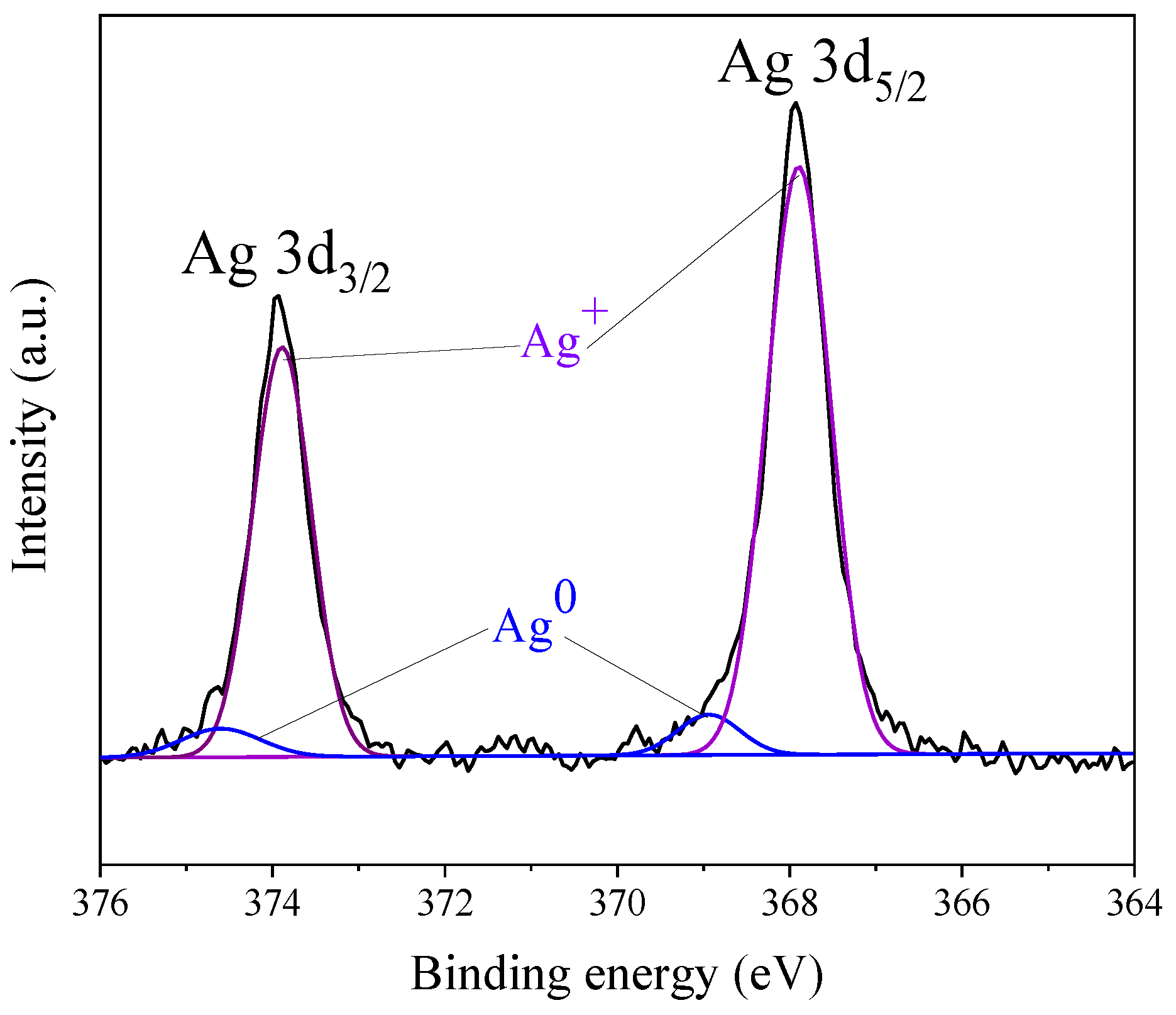
3.1. Catalyst Characterizations
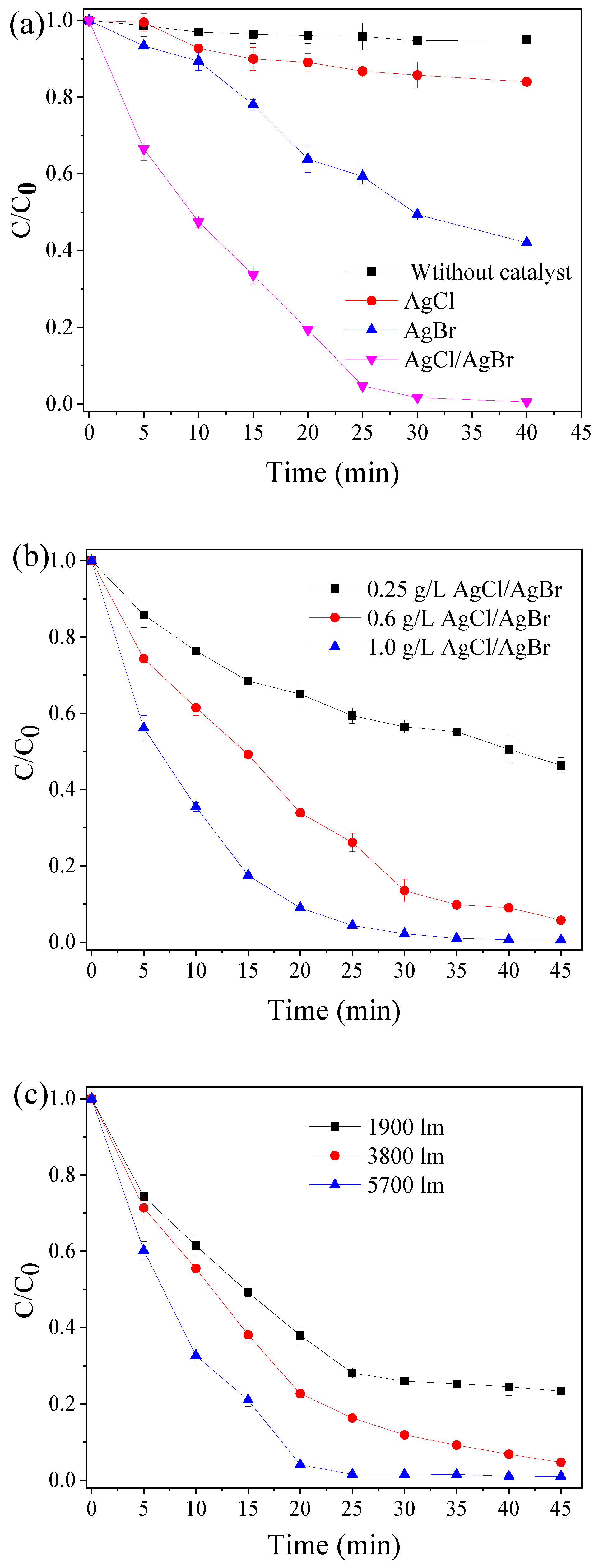
3.2. Degradation of TBBPA by Vis-AgCl/AgBr System
3.3. Reuse of AgCl/AgBr Composites for TBBPA Degradation
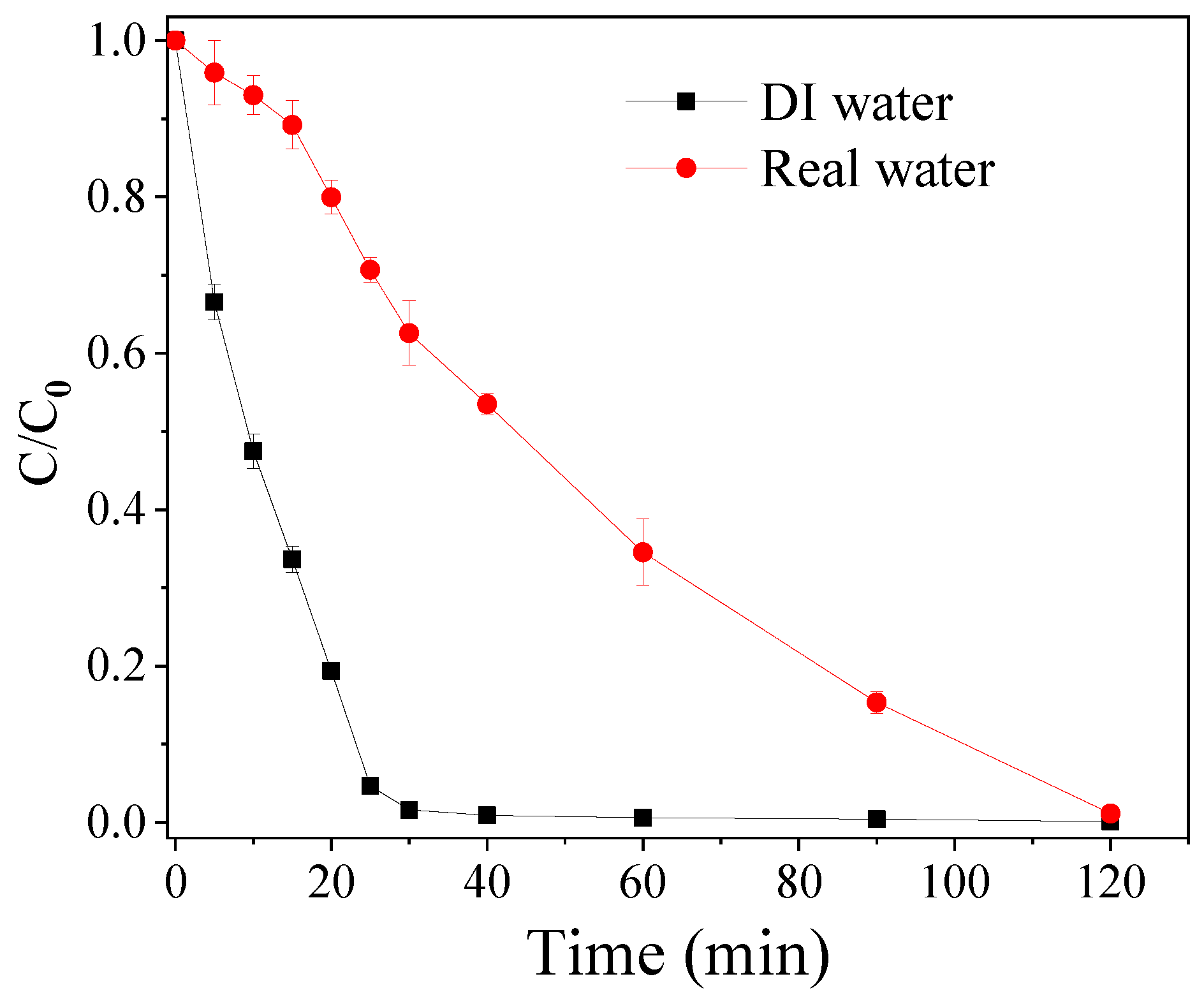
3.4. TBBPA Degradation in Real Water
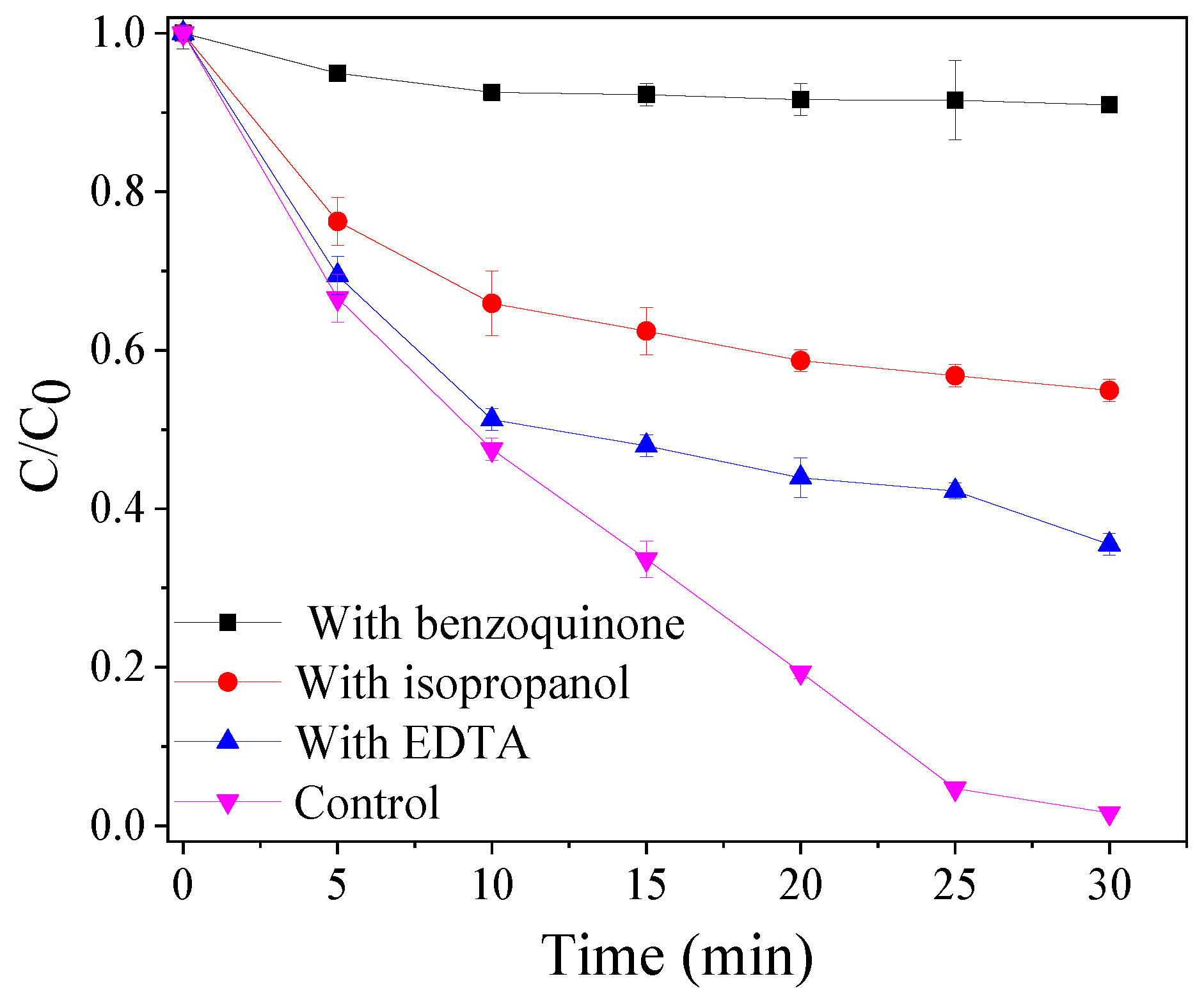
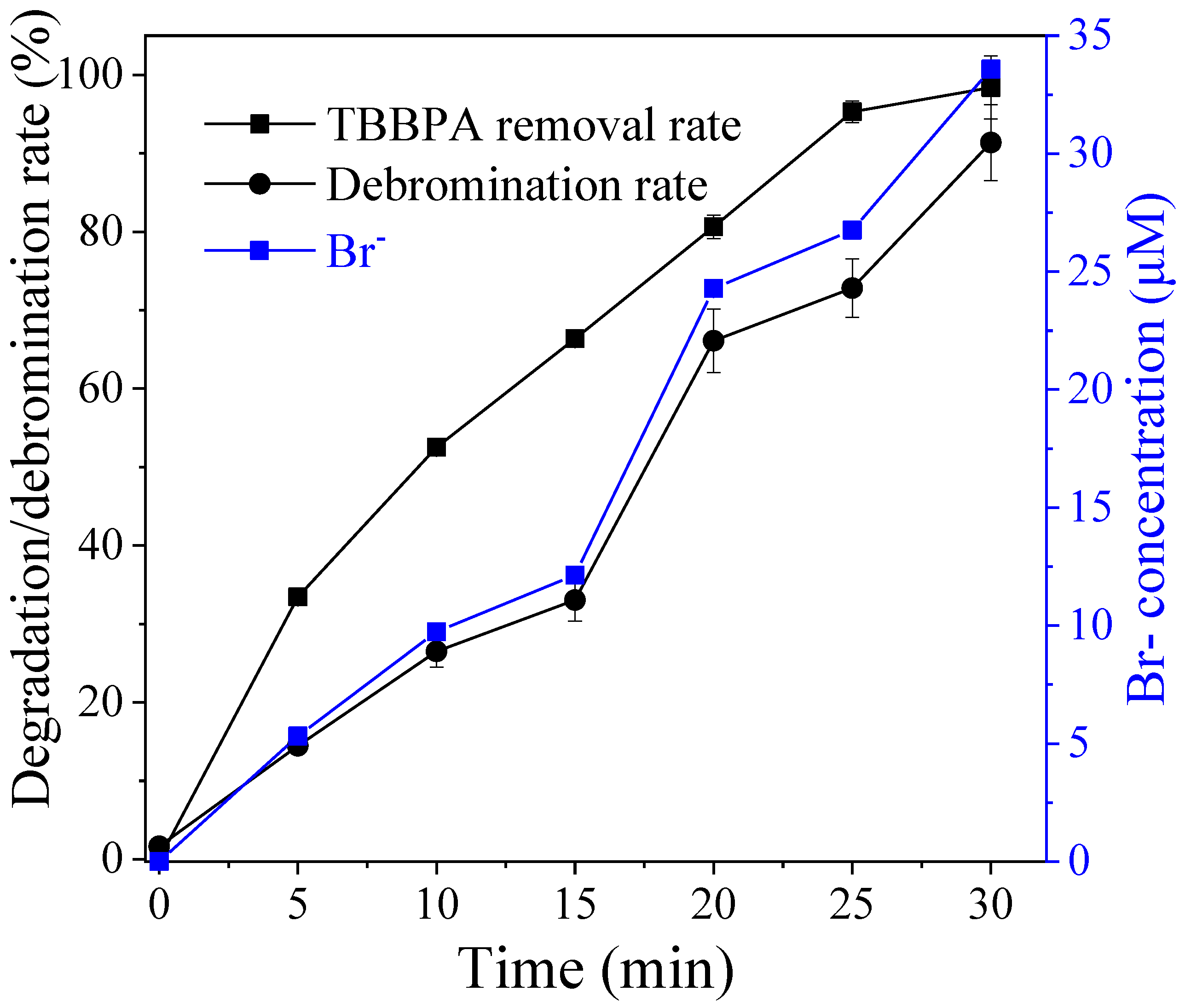
3.5. Contributions of Reactive Species to TBBPA Degradation and Debromination
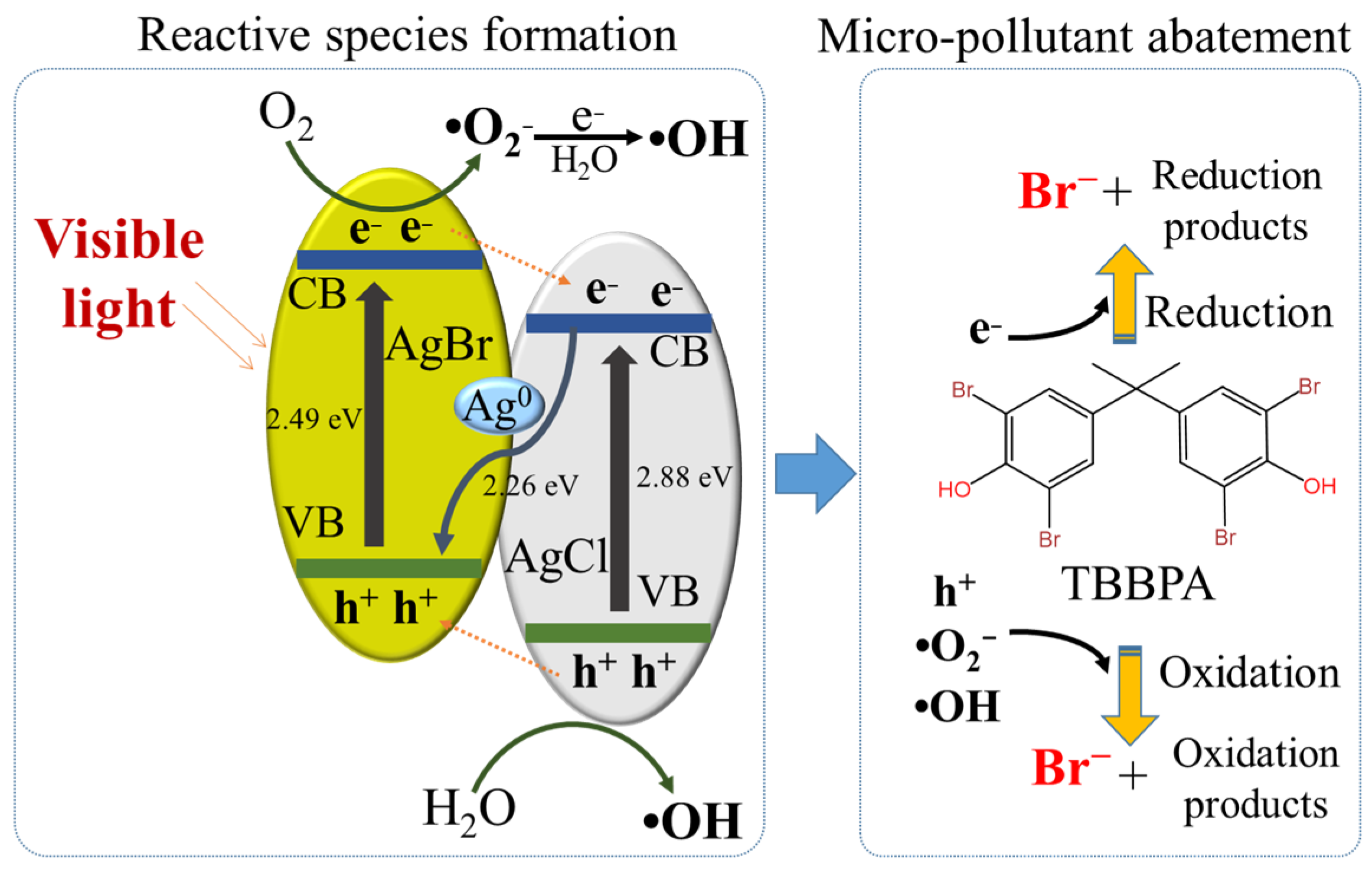
3.6. Proposed Mechanisms of TBBPA Degradation by the Vis-AgCl/AgBr System
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alaee, M.; Arias, P.; Sjödin, A.; Bergman, Å. An overview of commercially used brominated flame retardants, their applications, their use patterns in different countries/regions and possible modes of release. Environ. Int. 2003, 29, 683–689. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, T.; Liu, W.; Yuan, G.; Pan, X.; Zhang, M.; Luan, X.; Cui, Z.; Xin, J. Brominated flame retardants (BFRs) in China over the past half-century: Stocks, flows, fates, and ecological risks. Environ. Sci. Technol. 2024, 58, 13613–13623. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Bu, Q.; Cao, Z.; Du, X.; Xia, J.; Wu, M.; Huang, J. Brominated flame retardants (BFRs): A review on environmental contamination in China. Chemosphere 2016, 150, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Xiong, P.; Yan, X.; Zhu, Q.; Qu, G.; Shi, J.; Liao, C.; Jiang, G. A review of environmental occurrence, fate, and toxicity of novel brominated flame retardants. Environ. Sci. Technol. 2019, 53, 13551–13569. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yu, Z.; Chen, H.; Han, Y.; Xiang, M.; Chen, X.; Ma, R.; Wang, Z. Tetrabromobisphenol A: Disposition, kinetics and toxicity in animals and humans. Environ. Pollut. 2019, 253, 909–917. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, Z.; Luo, W.; Jia, X. Biodegradation of tetrabromobisphenol A by a novel Comamonas sp. strain, JXS-2-02, isolated from anaerobic sludge. Bioresour. Technol. 2013, 128, 173–179. [Google Scholar] [CrossRef]
- Segev, O.; Abeliovich, A.; Kushmaro, A. Biodegradation of dibromoneopentyl glycol by a bacterial consortium. Chemosphere 2007, 68, 958–964. [Google Scholar] [CrossRef]
- Ronen, Z.; Abeliovich, A. Anaerobic-aerobic process for microbial degradation of tetrabromobisphenol A. Appl. Environ. Microbiol. 2000, 66, 2372–2377. [Google Scholar] [CrossRef]
- Altarawneh, M.; Saeed, A.; Al-Harahsheh, M.; Dlugogorski, B.Z. Thermal decomposition of brominated flame retardants (BFRs): Products and mechanisms. Prog. Energy Combust. Sci. 2019, 70, 212–259. [Google Scholar] [CrossRef]
- Sun, J.; Chen, Y.; Xiang, Y.; Ling, L.; Fang, J.; Shang, C. Oxidative debromination of 2,2-bis(bromomethyl)-1,3-propanediol by UV/persulfate process and corresponding formation of brominated by-products. Chemosphere 2019, 228, 735–743. [Google Scholar] [CrossRef]
- Huang, X.; Peng, L.; Li, S.; Gu, F.L. Theoretical study on the sequential reduction and oxidation mechanism for tetrabromobisphenol A degradation under photocatalytic UV/Fenton conditions. Theor. Chem. Acc. 2015, 134, 3. [Google Scholar] [CrossRef]
- Zhong, Y.; Liang, X.; Zhong, Y.; Zhu, J.; Zhu, S.; Yuan, P.; He, H.; Zhang, J. Heterogeneous UV/Fenton degradation of TBBPA catalyzed by titanomagnetite: Catalyst characterization, performance and degradation products. Water Res. 2012, 46, 4633–4644. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, J.; Wang, C.; Sun, H. Enhanced photocatalytic degradation of tetrabromobisphenol A by tourmaline–TiO2 composite catalyst. J. Mater. Sci. 2017, 52, 6937–6949. [Google Scholar] [CrossRef]
- Cao, M.; Wang, P.; Ao, Y.; Wang, C.; Hou, J.; Qian, J. Photocatalytic degradation of tetrabromobisphenol A by a magnetically separable graphene–TiO2 composite photocatalyst: Mechanism and intermediates analysis. J. Chem. Eng. 2015, 264, 113–124. [Google Scholar] [CrossRef]
- An, J.; Zhu, L.; Wang, N.; Song, Z.; Yang, Z.; Du, D.; Tang, H. Photo-Fenton like degradation of tetrabromobisphenol A with grapheneBiFeO3 composite as a catalyst. J. Chem. Eng. 2013, 219, 225–237. [Google Scholar] [CrossRef]
- Li, G.; Yang, C.; He, Q.; Liu, J. Ag-based photocatalytic heterostructures: Construction and photocatalytic energy conversion application. J. Environ. Chem. Eng. 2022, 10, 107374. [Google Scholar] [CrossRef]
- Wang, P.; Huang, B.; Lou, Z.; Zhang, X.; Qin, X.; Dai, Y.; Zheng, Z.; Wang, X. Synthesis of Highly Efficient Ag@AgCl Plasmonic Photocatalysts with Various Structures. Chem. Eur. J. 2010, 16, 538–544. [Google Scholar] [CrossRef]
- Choi, M.; Shin, K.-H.; Jang, J. Plasmonic photocatalytic system using silver chloride/silver nanostructures under visible light. J. Colloid Sci. 2010, 341, 83–87. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Guo, C.; He, Y.; Li, L.; Meng, W. Levels and distribution of tetrabromobisphenol A and hexabromocyclododecane in Taihu Lake, China. Environ. Toxicol. Chem. 2013, 32, 2249–2255. [Google Scholar] [CrossRef]
- Yang, S.; Wang, S.; Liu, H.; Yan, Z. Tetrabromobisphenol A: Tissue distribution in fish, and seasonal variation in water and sediment of Lake Chaohu, China. Environ. Sci. Pollut. Res. 2012, 19, 4090–4096. [Google Scholar] [CrossRef]
- Xiao, J.-Q.; Lin, K.-S.; Yu, Y. Novel Ag@AgCl@AgBr heterostructured nanotubes as high-performance visible-light photocatalysts for decomposition of dyes. Catal. Today 2018, 314, 10–19. [Google Scholar] [CrossRef]
- Landi, S.; Segundo, I.R.; Freitas, E.; Vasilevskiy, M.; Carneiro, J.; Tavares, C.J. Use and misuse of the Kubelka-Munk function to obtain the band gap energy from diffuse reflectance measurements. Solid State Commun. 2022, 341, 114573. [Google Scholar] [CrossRef]
- Huang, M.; Zhu, S.; Wu, Y.; Yu, J.; Wang, Q. Hydrothermal deposition of AgCl/AgBr co-sensitizers to modify TiO2 NTs for enhanced photocatalytic performance. Ceram. Int. 2020, 46, 24008–24017. [Google Scholar] [CrossRef]
- Daupor, H.; Wongnawa, S. Urchinlike Ag/AgCl photocatalyst: Synthesis, characterization, and activity. Appl. Catal. A Gen. 2014, 473, 59–69. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mentha, S.S.; Misra, Y.; Dwivedi, N. Emerging pollutants of severe environmental concern in water and wastewater: A comprehensive review on current developments and future research. Water-Energy Nexus 2023, 6, 74–95. [Google Scholar] [CrossRef]
- Puga, F.; Navío, J.A.; Hidalgo, M.C. A critical view about use of scavengers for reactive species in heterogeneous photocatalysis. Appl. Catal. A Gen. 2024, 685, 119879. [Google Scholar] [CrossRef]
- Ahmadian, A.; Ahmadi, S.; Goharrizi, B.A. Roles of reactive species in photocatalysis: Effect of scavengers and inorganic ions on dye removal from wastewater. Int. J. Environ. Sci. Technol. 2023, 20, 6433–6448. [Google Scholar] [CrossRef]
- Wang, R.; Tang, T.; Wei, Y.; Dang, D.; Huang, K.; Chen, X.; Yin, H.; Tao, X.; Lin, Z.; Dang, Z.; et al. Photocatalytic debromination of polybrominated diphenyl ethers (PBDEs) on metal doped TiO2 nanocomposites: Mechanisms and pathways. Environ. Int. 2019, 127, 5–12. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Ding, X.; Wang, S.; Shen, W.; Mu, Y.; Wang, L.; Chen, H.; Zhang, L. Fe@Fe2O3 promoted electrochemical mineralization of atrazine via a triazinon ring opening mechanism. Water Res. 2017, 112, 9–18. [Google Scholar] [CrossRef]
- Tian, M.; Liu, Y.-G.; Hou, J.; Jing, B.; Zhang, Y.; Mu, Y.; Sun, X.; Jiang, H.-Y. Photocatalytic debromination enhancement of Ph-C≡C-Cu by Fe3O4 modification. Appl. Catal. B Environ. 2023, 334, 122866. [Google Scholar] [CrossRef]
- Sun, C.; Zhao, D.; Chen, C.; Ma, W.; Zhao, J. TiO2-mediated photocatalytic debromination of decabromodiphenyl ether: Kinetics and intermediates. Environ. Sci. Technol. 2009, 43, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Chang, W.; Ma, W.; Chen, C.; Zhao, J. Photoreductive debromination of decabromodiphenyl ethers in the presence of carboxylates under visible light irradiation. Environ. Sci. Technol. 2013, 47, 2370–2377. [Google Scholar] [CrossRef]
- Janssen, D.B.; Oppentocht, J.E.; Poelarends, G.J. Microbial dehalogenation. Curr. Opin. Biotechnol. 2001, 12, 254–258. [Google Scholar] [CrossRef]
- Lv, M.; Tang, X.; Zhao, Y.; Li, J.; Zhang, B.; Li, L.; Jiang, Y.; Zhao, Y. The toxicity, bioaccumulation and debromination of BDE-47 and BDE-209 in Chlorella sp. under multiple exposure modes. Sci. Total Environ. 2020, 723, 138086. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Huang, Y.; Gao, D.; Zhuang, J.; Zeng, Y.; Zhao, J.; Peng, Z.; Sun, J. Cost-Effective Abatement of Tetrabromobisphenol A from Contaminated Water by a Visible-Light-Driven Photochemical System. Water 2025, 17, 1311. https://doi.org/10.3390/w17091311
Zhang G, Huang Y, Gao D, Zhuang J, Zeng Y, Zhao J, Peng Z, Sun J. Cost-Effective Abatement of Tetrabromobisphenol A from Contaminated Water by a Visible-Light-Driven Photochemical System. Water. 2025; 17(9):1311. https://doi.org/10.3390/w17091311
Chicago/Turabian StyleZhang, Gang, Yanru Huang, Dafang Gao, Jiaxin Zhuang, Yifan Zeng, Jingjing Zhao, Zhantong Peng, and Jianliang Sun. 2025. "Cost-Effective Abatement of Tetrabromobisphenol A from Contaminated Water by a Visible-Light-Driven Photochemical System" Water 17, no. 9: 1311. https://doi.org/10.3390/w17091311
APA StyleZhang, G., Huang, Y., Gao, D., Zhuang, J., Zeng, Y., Zhao, J., Peng, Z., & Sun, J. (2025). Cost-Effective Abatement of Tetrabromobisphenol A from Contaminated Water by a Visible-Light-Driven Photochemical System. Water, 17(9), 1311. https://doi.org/10.3390/w17091311





