Development of a Continuous Phytoplankton Culture System for Ocean Acidification Experiments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Materials
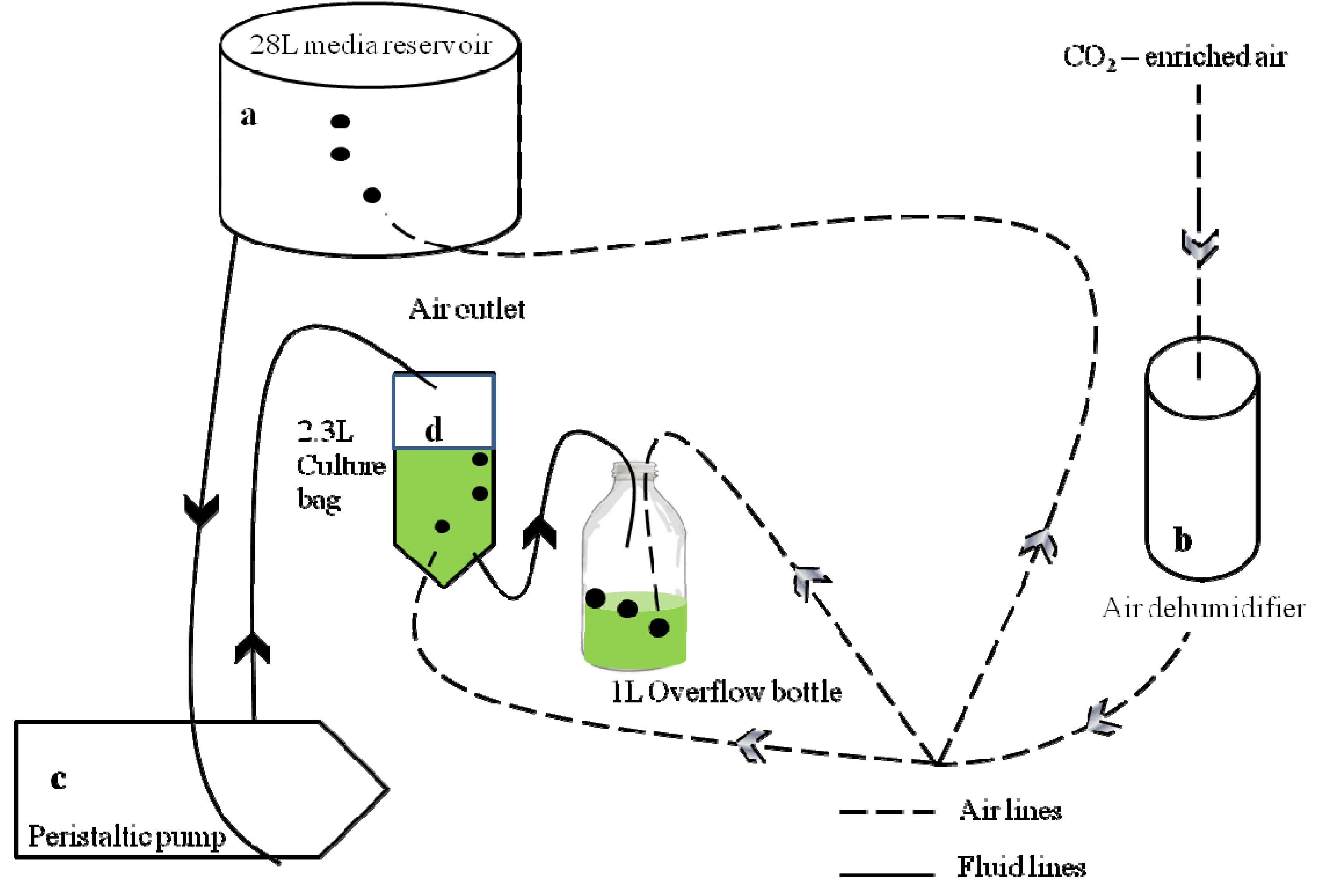

2.2. Experimental Conditions
2.3. Carbonate Chemistry
3. Results and Discussion
3.1. Stability of the Carbonate Chemistry and Cell Densities
| Fragilariopsis cylindrus | 390 | 570 | 750 | 950 |
|---|---|---|---|---|
| Average pH ± SD | 8.02 ± 0.03 | 7.88 ± 0.03 | 7.77 ± 0.04 | 7.69 ± 0.03 |
| pH range | 0.13 | 0.15 | 0.20 | 0.17 |
| Calculated pCO2 ± SD | 428 ± 34 | 590 ± 46 | 771 ± 67 | 950 ± 78 |
| Difference from target | +38 | +20 | +21 | ±0 |
| Pyramimonas gelidicola | 390 | 570 | 750 | 950 |
| Average pH ± SD | 8.04 ± 0.04 | 7.87 ± 0.03 | 7.75 ± 0.04 | 7.67 ± 0.04 |
| pH range | 0.17 | 0.13 | 0.16 | 0.17 |
| Calculated pCO2 ± SD | 400 ± 41 | 612 ± 54 | 806 ± 80 | 977 ± 106 |
| Difference from target | +10 | +42 | +56 | +27 |
| Phaeocystis antarctica | 390 | 570 | 750 | 950 |
| Average pH ± SD | 8.02 ± 0.03 | 7.86 ± 0.04 | 7.76 ± 0.04 | 7.67 ± 0.03 |
| pH range | 0.14 | 0.15 | 0.19 | 0.14 |
| Calculated pCO2 ± SD | 413 ± 31 | 644 ± 62 | 805 ± 76 | 993 ± 83 |
| Difference from target | +23 | +74 | +55 | +43 |
3.2. Discussion

4. Conclusions
Acknowledgments
Authors Contribution
Conflicts of Interest
References
- Bijma, J.; Spero, H.J.; Lea, D.W. Reassessing foraminiferal stable isotope geochemistry: Impact of the oceanic carbonate systems (experimental results). In Use of Proxies in Paleoceanography: Examples from the South Atlantic; Fisher, G., Wefer, G., Eds.; Springer Verlag: New York, NY, USA, 1999. [Google Scholar]
- Riebesell, U.; Zondervan, I.; Rost, B.; Tortell, P.D.; Zeebe, R.E.; Morel, F.M.M. Reduced calcification of marine plankton in response to increased atmospheric CO2. Nature 2000, 407, 364–367. [Google Scholar] [CrossRef]
- Marubini, F.; Atkinson, M.J. Effects of lowered pH and elevated nitrate on coral calcification. Mar. Ecol. Prog. Ser. 1999, 188, 117–121. [Google Scholar] [CrossRef]
- Kleypas, J.A.; Buddemeier, R.W.; Archer, D.; Gattuso, J.P.; Langdon, C.; Opdyke, B.N. Geochemical consequences of increased atmospheric carbon dioxide on coral reefs. Science 1999, 284, 118–120. [Google Scholar] [CrossRef]
- Spero, H.J.; Bijma, J.; Lea, D.W.; Bemis, B.E. Effect of seawater carbonate concentration on foraminiferal carbon and oxygen isotopes. Nature 1997, 390, 497–500. [Google Scholar]
- Paulino, A.I.; Egge, J.K.; Larsen, A. Effects of increased atmospheric CO2 on small and intermediate sized osmotrophs during a nutrient induced phytoplankton bloom. Biogeosciences 2008, 5, 739–748. [Google Scholar] [CrossRef]
- Shi, D.; Xu, Y.; Morel, F.M.M. Effects of the pH/pCO2 control method on medium chemistry and phytoplankton growth. Biogeosciences 2009, 6, 1199–1207. [Google Scholar] [CrossRef]
- Hurd, C.L.; Hepburn, C.D. Testing the effects of ocean acidification on algal metabolism: Considerations for experimental designs. J. Phycol. 2009, 45, 1236–1251. [Google Scholar] [CrossRef]
- Iglesias-Rodriguez, M.D.; Halloran, P.R.; Rickaby, R.E.M.; Hall, I.R.; Colmenero-Hidalgo, E.; Gittins, J.R.; Green, D.R.H.; Tyrrell, T.; Gibbs, S.J.; von Dassow, P.; et al. Phytoplankton calcification in a high-CO2 world. Science 2008, 320, 336–340. [Google Scholar] [CrossRef]
- Rost, B.; Zondervan, I.; Wolf-Gladrow, D. Sensitivity of phytoplankton to future changes in ocean carbonate chemistry: Current knowledge, contradictions and research directions. Mar. Ecol. Prog. Ser. 2008, 373, 227–237. [Google Scholar] [CrossRef]
- Dickson, A.G.; Sabine, C.L.; Christian, J.R. (Eds.) Guide to best practices for ocean CO2 measurements; PICES Special Publication: Luxembourg, 2007; Volumn 3; p. 191.
- Riebesell, U.; Fabry, V.J.; Hansson, L.; Gattuso, J.-P. (Eds.) Guide to Best Practices for Ocean Acidification Research and Data Reporting; Publications Office of the European Union: Luxembourg, 2010; p. 260.
- Kleypas, J.A.; Feely, R.A.; Fabry, V.J.; Langdon, C.; Sabine, C.L.; Robbins, L.L. Impacts of Ocean Acidification on Coral Reefs and Other Marine Calcifiers: A Guide for Future Research; Report for the workshop Sponsored by the National Science Foundation, the National Oceanic and Atmospheric Administration, and the U.S. Geological Survey. St. Petersburg, FL, USA, 18–20 April 2005; pp. 1–88. Available online: http://www.ucar.edu/communications/Final_acidification.pdf (accessed on 12 June 2014).
- Gattuso, J.-P.; Lavigne, H. Technical note: Approaches and software tools to investigate the impact of ocean acidification. Biogeosciences 2009, 6, 2121–2133. [Google Scholar] [CrossRef]
- Berge, T.; Daugbjerg, N.; Andersen, B.B.; Hansen, P.J. Effect of lowered pH on marine phytoplankton growth rates. Mar. Ecol. Prog. Ser. 2010, 416, 79–91. [Google Scholar] [CrossRef]
- Boelen, P.; van de Poll, W.H.; van der Strate, H.J.; Neven, I.A.; Beardall, J.; Buma, A.G.J. Neither elevated nor reduced CO2 affects the photophysiological performance of the marine Antarctic diatom. Chaetoceros brevis. J. Exp. Mar. Biol. Ecol. 2011, 406, 38–45. [Google Scholar] [CrossRef]
- Arnold, H.E.; Kerrison, P.; Steinke, M. Interacting effects of ocean acidification and warming on growth and DMS-production in the haptophyte coccolithophore. Emiliania huxleyi. Glob. Chang. Biol. 2013, 19, 1007–1016. [Google Scholar] [CrossRef]
- Thornton, D.C.O. Effect of low pH on carbohydrate production by a marine planktonic diatom (Chaetoceros muelleri). Res. Lett. Ecol. 2009, 2009, 105901. [Google Scholar]
- Holland, D.; Roberts, S.; Beardall, J. Assessment of the nutrient status of phytoplankton: A comparison between conventional bioassays and nutrient-induced fluorescence transients (NIFTs). Ecol. Indic. 2004, 4, 149–159. [Google Scholar] [CrossRef]
- Bonachela, J.A.; Raghib, M.; Leving, S.A. Dynamic model of flexible phytoplankton nutrient uptake. Proc. Natl. Acad. Sci. USA 2011, 108, 20633–20638. [Google Scholar] [CrossRef]
- Guillard, R.R.L.; Ryther, J.H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacae Cleve. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of phyoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Smith, W.L., Chanley, M.H., Eds.; Plenum Press: New York, NY, USA, 1975; pp. 26–60. [Google Scholar]
- Gibson, J.A. Carbon Flow through Marine Environments of the Vestfold Hills, East Antarctica; ANARE Reports 139; Australian Antarctic Division, Hobart: Kingston, Australia, 1998; pp. 174–178. [Google Scholar]
- Roden, N.P.; Shadwick, E.H.; Tilbrook, B.; Trull, T.W. Annual cycle of carbonate chemistry and decadal change in coastal Prydz Bay, East Antarctica. Mar. Chem. 2013, 155, 135–147. [Google Scholar] [CrossRef]
- Egge, J.K.; Aksnes, D.L. Silicate as regulating nutrient in phytoplankton competition. Mar. Ecol. Prog. Ser. 1992, 83, 281–289. [Google Scholar] [CrossRef]
- Harrison, P.J.; Conway, H.L.; Holmes, R.W.; Davis, C.O. Marine diatoms grown in chemostats under silicate or ammonium limitation. III. Cellular composition and morphology of Chaetoceros debilis, Skeletonema costatum, and Thalassiosira gravida. Mar. Biol. 1977, 43, 19–31. [Google Scholar] [CrossRef]
- Wynn-Edwards, C.; King, R.; Davidson, A.; Wright, S.; Nichols, D.P.; Wotherspoon, S.; Kawaguchi, S.; Virtue, P. Species-specific variations in the nutritional quality of Southern Ocean phytoplankton in response to elevated pCO2. Water 2014, 6, 1840–1859. [Google Scholar]
- Thomson, P.G.; Davidson, A.T.; Cadman, N. Seasonal changes in effects of ambient UVR on natural communities of Antarctic marine protists. Aquat. Microb. Ecol. 2008, 52, 131–147. [Google Scholar] [CrossRef]
- Lewis, E.; Wallace, D.W.R. Program Developed for CO2 System Calculations; Oak Ridge National Laboratory, U.S. Department of Energy: Oak Ridge, TN, USA, 1998. [Google Scholar]
- Mehrbach, C.; Culberson, C.; Hawley, J.; Pytkowicz, R. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol. Oceanogr. 1973, 18, 897–907. [Google Scholar] [CrossRef]
- Dickson, A.G.; Millero, F.J. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res. Part A: Oceanogr. Res. Pap. 1987, 34, 1733–1743. [Google Scholar] [CrossRef]
- McNeil, B.I.; Matear, R.J. Southern Ocean acidification: A tipping point at 450-ppm atmospheric CO2. Proc. Natl. Acad. Sci. USA 2008, 104, 18860–18864. [Google Scholar] [CrossRef]
- McNeil, B.I.; Sweeney, C.; Gibson, J.A.E. Short Note: Natural seasonal variability of aragonite saturation state within two Antarctic coastal ocean sites. Antarct. Sci. 2011, 23, 411–412. [Google Scholar] [CrossRef]
- Crawfurd, K.; Raven, J.A.; Wheeler, G.L.; Baxter, E.J.; Joint, I. The response of Thalassiosira pseudonana to long-term exposure to increased CO2 and decreased pH. PLoS ONE 2011, 6, e26695. [Google Scholar]
- Lefebvre, S.C.; Benner, I.; Stillman, J.H.; Parker, A.E.; Drake, M.K.; Rossignol, P.E.; Okimura, K.M.; Komada, T.; Carpenter, E.J. Nitrogen source and pCO2 synergistically affect carbon allocation, growth and morphology of the coccolithophore Emiliania huxleyi: Potential implications of ocean acidification for the carbon cycle. Glob. Chang. Biol. 2012, 18, 493–503. [Google Scholar] [CrossRef]
- Li, G.; Campbell, D.A. Rising CO2 interacts with growth light and growth rate to alter photosystem II photoinactivation of the coastal diatom Thalassiosira pseudonana. PLoS One 2013, 8, e55562. [Google Scholar]
- Hitchcock, G.L. Diel variation in chlorophyll a, carbohydrate and protein content of the marine diatom Skeletonema costatum. Mar. Biol. 1980, 57, 271–278. [Google Scholar] [CrossRef]
- Burkhardt, S.; Zondervan, I.; Riebesell, U. Effect of CO2 concentration on C:N:P ratio in marine phytoplankton: A species comparison. Limnol. Oceanogr. 1999, 44, 683–690. [Google Scholar] [CrossRef]
- McGraw, C.M.; Cornwall, C.E.; Reid, M.R.; Currie, K.I.; Hepburn, C.D.; Boyd, P.; Hurd, C.L.; Hunter, K.A. An automated pH-controlled culture system for laboratory-based ocean acidification experiments. Limnol. Oceanogr. Methods 2010, 8, 686–694. [Google Scholar] [CrossRef]
- Schapira, M.; Seuront, L.; Gentilhomme, V. Effects of small-scale turbulence on Phaeocystis globosa (Prymnesiophyceae) growth and life cycle. J. Exp. Mar. Biol. Ecol. 2006, 335, 27–38. [Google Scholar] [CrossRef]
- Berdalet, E.; Peters, F. Species-specific physiological response of dinoflagellates to quantified small-scale turbulence. J. Phycol. 2007, 43, 965–977. [Google Scholar] [CrossRef]
- Peters, F.; Arin, L.; Marrase, C.; Berdalet, E.; Sala, M.M. Effects of small-scale turbulence on the growth of two diatoms of different size in a phosphorus-limited medium. J. Mar. Syst. 2006, 61, 134–148. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wynn-Edwards, C.; King, R.; Kawaguchi, S.; Davidson, A.; Wright, S.; Nichols, P.D.; Virtue, P. Development of a Continuous Phytoplankton Culture System for Ocean Acidification Experiments. Water 2014, 6, 1860-1872. https://doi.org/10.3390/w6061860
Wynn-Edwards C, King R, Kawaguchi S, Davidson A, Wright S, Nichols PD, Virtue P. Development of a Continuous Phytoplankton Culture System for Ocean Acidification Experiments. Water. 2014; 6(6):1860-1872. https://doi.org/10.3390/w6061860
Chicago/Turabian StyleWynn-Edwards, Cathryn, Rob King, So Kawaguchi, Andrew Davidson, Simon Wright, Peter D. Nichols, and Patti Virtue. 2014. "Development of a Continuous Phytoplankton Culture System for Ocean Acidification Experiments" Water 6, no. 6: 1860-1872. https://doi.org/10.3390/w6061860
APA StyleWynn-Edwards, C., King, R., Kawaguchi, S., Davidson, A., Wright, S., Nichols, P. D., & Virtue, P. (2014). Development of a Continuous Phytoplankton Culture System for Ocean Acidification Experiments. Water, 6(6), 1860-1872. https://doi.org/10.3390/w6061860




