Influence of Inorganic Ions and Organic Substances on the Degradation of Pharmaceutical Compound in Water Matrix
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Study Subject
2.3. Photocatalysis Process
2.4. Analytical Procedure
2.5. Toxic Bioassay
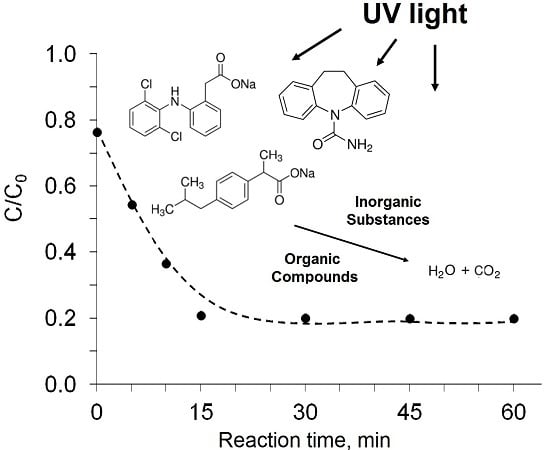
3. Results and Discussion
3.1. Influence of Inorganic Anions
3.2. Influence of Inorganic Cations
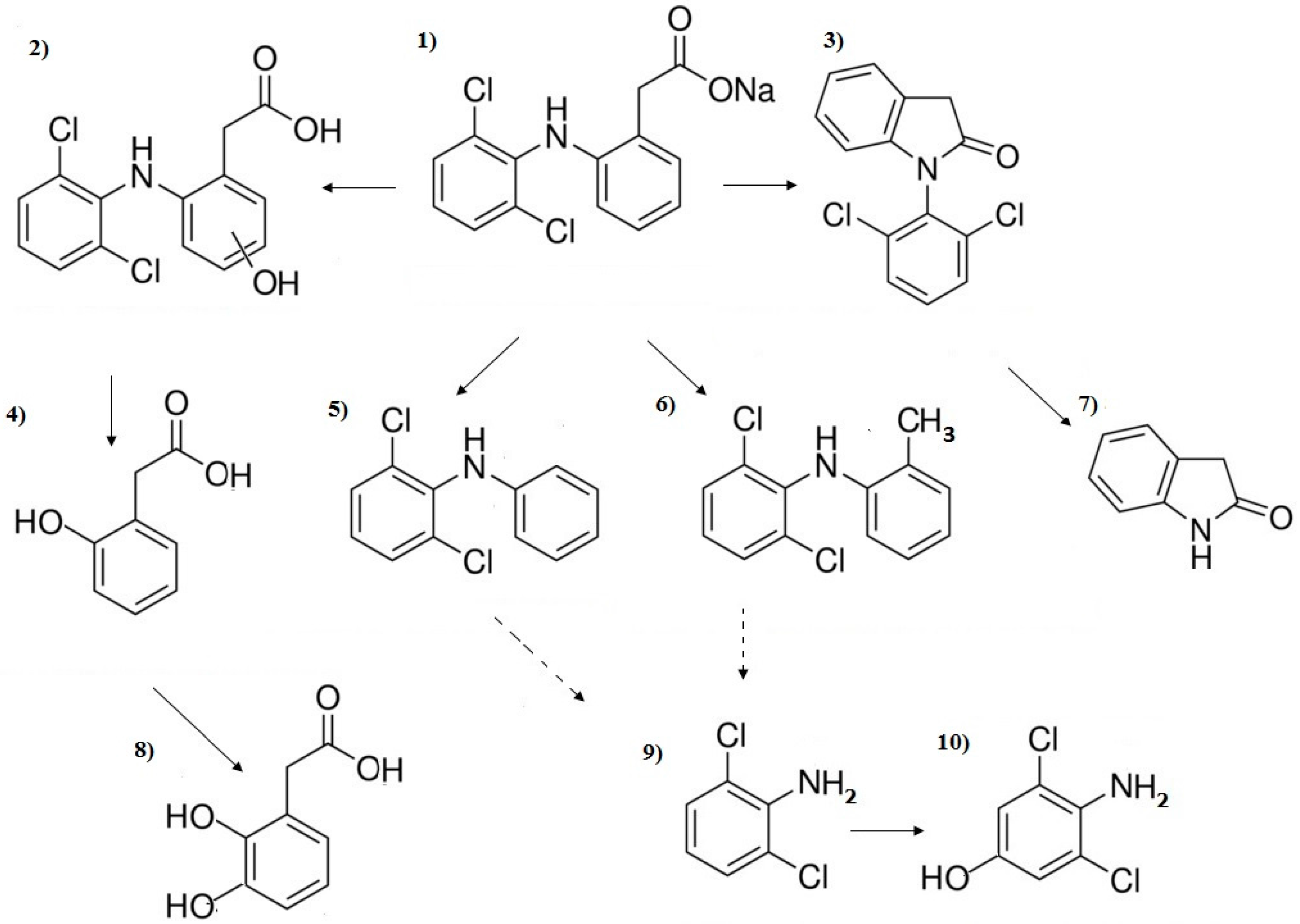
3.3. Influence of Organic Substances
3.4. Toxicological Assessment of Treated Water Solution
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.H.M.L.M.; Araújo, A.N.; Fachini, A.; Pena, A.; Delerue-Matos, C.; Montenegro, M.C.B.S.M. Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. J. Hazard. Mater. 2010, 175, 45–95. [Google Scholar] [CrossRef] [PubMed]
- Oller, I.; Malato, S.; Sánchez-Pérez, J.A. Combination of advanced oxidation processes and biological treatments for wastewater decontamination—A review. Sci. Total Environ. 2011, 409, 4141–4166. [Google Scholar] [CrossRef] [PubMed]
- Gros, M.; Petrović, M.; Ginebreda, A.; Barceló, D. Removal of pharmaceuticals during wastewater treatment and environmental risk assessment using hazard indexes. Environ. Int. 2010, 36, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Jelic, A.; Gros, M.; Ginebreda, A.; Cespedes-Sánchez, R.; Ventura, F.; Petrovic, M.; Barcelo, D. Occurrence, partition and removal of pharmaceuticals in sewage water and sludge during wastewater treatment. Water Res. 2011, 45, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- De Witte, B.; van Langenhove, H.; Demeestere, K.; Dewulf, J. Advanced oxidation of pharmaceuticals: Chemical analysis and biological assessment of degradation products. Crit. Rev. Environ. Sci. Technol. 2011, 41, 215–242. [Google Scholar] [CrossRef]
- Liang, R.; Hu, A.; Li, W.; Zhou, N. Enhanced degradation of persistent pharmaceuticals found in wastewater treatment effluents using TiO2 nanobelt photocatalysts. J. Nanopart. Res. 2013, 15, 1990. [Google Scholar] [CrossRef]
- Bohdziewicz, J.; Kudlek, E.; Dudziak, M. Influence of the catalyst type (TiO2 and ZnO) on the photocatalytic oxidation of pharmaceuticals in the aquatic environment. Desalin. Water Treat. 2016, 57, 1552–1563. [Google Scholar] [CrossRef]
- Czech, B.; Buda, W. Photocatalytic treatment of pharmaceutical wastewater using new multiwall-carbon nanotubes/TiO2/SiO2 nanocomposites. Environ. Res. 2015, 137, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Zhang, X.; Oakes, K.D.; Peng, P.; Zhou, Y.N.; Servos, M.R. Hydrothermal growth of free standing TiO2 nanowire membranes for photocatalytic degradation of pharmaceuticals. J. Hazard. Mater. 2011, 189, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Chládková, B.; Evgenidou, E.; Kvítek, L.; Panáček, A.; Zbořil, R.; Kovář, P.; Lambropoulou, D. Adsorption and photocatalysis of nanocrystalline TiO2 particles for Reactive Red 195 removal: Effect of humic acids, anions and scavengers. Environ. Sci. Pollut. Res. 2015, 22, 16514–16524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Wang, J.; Wang, J.; Ji, Z. Effect of sulfate ions on the crystallization and photocatalytic activity of TiO2/diatomite composite photocatalyst. Chem. Phys. Lett. 2016, 643, 53–60. [Google Scholar] [CrossRef]
- Li, S.-W.; Lin, A.Y. Increased acute toxicity to fish caused by pharmaceuticals in hospital effluents in a pharmaceutical mixture and after solar irradiation. Chemosphere 2015, 139, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Villain, J.; Minguez, L.; Halm-Lemeille, M.-P.; Durrieu, G.; Bureau, R. Acute toxicities of pharmaceuticals toward green algae. mode of action, biopharmaceutical drug disposition classification system and quantile regression models. Ecotoxicol. Environ. Saf. 2016, 124, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Libralato, G.; Prato, E.; Migliore, L.; Cicero, A.M.; Manfra, L. A review of toxicity testing protocols and endpoints with Artemia spp. Ecol. Indic. 2016, 69, 35–49. [Google Scholar] [CrossRef]
- Dolezalova, J.; Rumlova, L. A new biological test of water toxicity–yeast Saccharomyces cerevisiae conductometric test. Environ. Toxicol. Pharmacol. 2014, 38, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Czech, B.; Jośko, I.; Oleszczuk, P. Ecotoxicological evaluation of selected pharmaceuticals to Vibrio fischeri and Daphnia magna before and after photooxidation process. Ecotoxicol. Environ. Saf. 2014, 104, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zuo, J.; Tang, X.; Li, R.; Li, Z.; Zhang, F. Toxicity evaluation of pharmaceutical wastewaters using the alga Scenedesmus obliquus and the bacterium Vibrio fischeri. J. Hazard. Mater. 2014, 266, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Roldan, R.; Kazlauskaite, L.; Ribo, J.; Riva, M.C.; González, S.; Cortina, J.L. Evaluation of an automated luminescent bacteria assay for in situ aquatic toxicity determination. Sci. Total Environ. 2012, 440, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Zhang, X.; Luong, D.; Oakes, K.D.; Servos, M.R.; Liang, R.; Kurdi, S.; Peng, P.; Zhou, Y. Adsorption and photocatalytic degradation kinetics of pharmaceuticals by TiO2 nanowires during water treatment. Waste Biomass Valoriz. 2012, 3, 443–449. [Google Scholar] [CrossRef]
- Chiou, C.-H.; Wu, C.-Y.; Juang, R.-S. Photocatalytic degradation of phenol and m-nitrophenol using irradiated TiO2 in aqueous solutions. Sep. Purif. Technol. 2008, 62, 559–564. [Google Scholar] [CrossRef]
- Holmberg, J.P.; Ahlberg, E.; Bergenholtz, J.; Hassellöv, M.; Abbas, Z. Surface charge and interfacial potential of titanium dioxide nanoparticles: Experimental and theoretical investigations. J. Colloid Interface Sci. 2013, 407, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Augugliaro, V.; Bellardita, M.; Loddo, V.; Palmisano, G.; Palmisano, L.; Yurdaka, S. Overview on oxidation mechanisms of organic compounds by TiO2 in heterogeneous photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 224–245. [Google Scholar] [CrossRef] [Green Version]
- Surolia, K.P.; Tayade, R.J.; Jasra, R.V. Effect of anions on the photocatalytic activity of Fe(III) salts impregnated TiO2. Ind. Eng. Chem. Res. 2007, 46, 6196–6203. [Google Scholar] [CrossRef]
- Boxall, C.; Kelsall, G.H. Photoelectrophoresis of colloidal semiconductors. Part 1. The technique and its applications. Faraday Trans. 1991, 87, 3537–3545. [Google Scholar] [CrossRef]
- Deborde, M.; von Gunten, U. Reactions of chlorine with inorganic and organic compounds during water treatment—Kinetics and mechanisms: A critical review. Water Res. 2008, 42, 13–51. [Google Scholar] [CrossRef] [PubMed]
- Soufan, M.; Deborde, M.; Legube, B. Aqueous chlorination of diclofenac: Kinetic study and transformation products identification. Water Res. 2012, 46, 3377–3386. [Google Scholar] [CrossRef] [PubMed]
- Liqiang, J.; Xiaoarn, S.; Naifu, X.; Baiqi, W.; Weimain, C.; Honggang, F. The preparation and characterisation of La doped TiO2 nanoparticles and their photocatalytic activity. J. Solid State Chem. 2004, 177, 3375–3382. [Google Scholar] [CrossRef]
- Al-Rasheed, R.; Cardin, D.J. Photocatalytic, degradation of humic acid in saline waters. Part 1. Artificial seawater: Influence of TiO2, temperature, pH, and air-flow. Chemosphere 2003, 51, 925–933. [Google Scholar] [CrossRef]
- Yawalkar, A.A.; Bhatkhande, D.S.; Pangarkar, V.G.; Beenackers, A.A.C.M. Solar-assisted photochemical and photocatalytic degradation of Phenol. J. Chem. Technol. Biotechnol. 2001, 76, 363–370. [Google Scholar] [CrossRef]
- Bhatkhande, D.S.; Pangarkar, V.G.; Beenackers, A.A.C.M. Photocatalytic degradation of nitrobenzene using titanium dioxide and concentrated solar radiation: Chemical effects and scaleup. Water Res. 2003, 37, 1223–1230. [Google Scholar] [CrossRef]
- Ajmera, A.A.; Sawant, S.B.; Pangarkar, V.G.; Beenackers, A.A.C.M. Solar-assisted photocatalytic degradation of benzoic acid using titanium dioxide as a photocatalyst. Chem. Eng. Technol. 2002, 25, 173–180. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, X.; Li, H.; Chen, J.; Wang, Y. Investigation of photocatalytic activity of nano-sized TiO2 with the presence of various inorganic anions. J. Nanosci. Nanotechnol. 2009, 9, 3639–3643. [Google Scholar] [CrossRef] [PubMed]
- Bouanimba, N.; Laid, N.; Zouaghi, R.; Sehili, T. Effect of pH and inorganic salts on the photocatalytic decolorization of methyl orange in the presence of TiO2 P25 and PC500. Desalin. Water Treat. 2015, 53, 951–963. [Google Scholar]
- Nfodzo, P.; Choi, H. sulfate radicals destroy pharmaceuticals and personal care products. Environ. Eng. Sci. 2011, 28, 605–609. [Google Scholar] [CrossRef]
- Zhu, R.S.; Tian, F.; Dong, W.Y.; Ouyang, F.; Zhang, L.L. Effects of inorganic anions on TiO2 photocatalytic reduction of BrO3. Adv. Mater. Res. 2012, 428, 69–72. [Google Scholar] [CrossRef]
- Ji, Y.; Ferronato, C.; Salvador, A.; Yanga, X.; Chovelon, J.-M. Degradation of ciprofloxacin and sulfamethoxazole by ferrous-activated persulfate: Implications for remediation of groundwater contaminated by antibiotics. Sci. Total Environ. 2014, 472, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Hori, H.; Yamamoto, A.; Hayakawa, E.; Taniyasu, S.; Yamashita, N.; Kutsuna, S. Efficient decomposition of environmentally persistent perfluorocarboxylic acids by use of persulfate as a photochemical oxidant. Environ. Sci. Technol. 2005, 39, 2383–2388. [Google Scholar] [CrossRef] [PubMed]
- Egerton, T.A. The influence of surface alumina and silica on the photocatalytic degradation of organic pollutants. Catalysts 2013, 3, 338–362. [Google Scholar] [CrossRef]
- Zhao, C.; Pelaez, M.; Dionysiou, D.D.; Pillai, S.C.; Byrne, J.A.; O’Shea, K.E. UV and visible light activated TiO2 photocatalysis of 6-hydroxymethyl uracil, a model compound for the potent cyanotoxin cylindrospermopsin. Catal. Today 2014, 224, 70–76. [Google Scholar] [CrossRef]
- Goldstein, S.; Rabani, J. Mechanism of nitrite formation by nitrate photolysis in aqueous solutions: The role of peroxynitrite, nitrogen dioxide, and hydroxyl radical. J. Am. Chem. Soc. 2007, 129, 10597–10601. [Google Scholar] [CrossRef] [PubMed]
- Mack, J.; Bolton, J.R. Photochemistry of nitrite and nitrate in aqueous solution: A review. J. Photochem. Photobiol. A Chem. 1999, 128, 1–13. [Google Scholar] [CrossRef]
- Bianco, A.; Passananti, M.; Perroux, H.; Voyard, G.; Mouchel-Vallon, C.; Chaumerliac, N.; Mailhot, G.; Deguillaume, L.; Brigante, M. A better understanding of hydroxyl radical photochemical sources in cloud waters collected at the puy de Dôme station—Experimental versus modelled formation rates. Atmos. Chem. Phys. 2015, 15, 9191–9202. [Google Scholar] [CrossRef]
- Werle, S.; Dudziak, M. Evaluation of tixicity of sawage sludge and gasification waste-products. Chem. Rev. 2013, 92, 1350–1353. [Google Scholar]
- Hsieh, C.-Y.; Meng-Hsiun, T.; Ryan, D.K.; Pancorbo, O.C. Toxicity of the 13 priority pollutant metals to Vibrio fischeri in the Microtox® chronic toxicity test. Sci. Total Environ. 2004, 320, 37–50. [Google Scholar] [CrossRef]
- Miyashiro, T.; Ruby, E.G. Shedding light on bioluminescence regulation in Vibrio fischeri. Mol. Microbiol. 2012, 84, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Yaser, N.A.; Abdullah, M.F.F.; Aris, A.M.; Zainudin, I.I. Isolation and identification of bioluminescent bacteria in squid and water of Malaysia. Int. J. Adv. Agric. Environ. Eng. 2014, 1, 225–228. [Google Scholar]
- Li, Z.; Fenet, H.; Gomez, E.; Chiron, S. Transformation of the antiepileptic drug oxcarbazepine upon different water disinfection processes. Water Res. 2011, 45, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
- Heye, K.; Becker, D.; Lütke Eversloh, C.; Durmaz, V.; Ternes, T.A.; Oetken, M.; Oehlmann, J. Effects of carbamazepine and two of its metabolites on the non-biting midge. Chironomus riparius in a sediment full life cycle toxicity test. Water Res. 2016, 98, 19–27. [Google Scholar]







| Pharmaceutical Compound | Diclofenak Sodium Salt | Ibuprofen Sodium Salt | Carbamazepine |
|---|---|---|---|
| Structural formula | 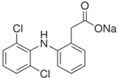 | 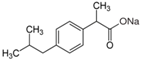 |  |
| Molecular formula | C14H10Cl2NNaO2 | C13H17NaO2 | C16H12N2O |
| CAS No. | 15307-79-6 | 31121-93-4 | 298-46-4 |
| Molecular weight, g/mol | 318.13 | 228.26 | 236.30 |
| Solubility in water, mg/L | 50 | 100 | 17 |
| pKa | 4.15 | 4.91 | 2.30 |
| Indicators of Pollution | Model Effluent |
|---|---|
| pH | 7.10 |
| Conductivity, μS/cm | 772.12 |
| UV-VIS absorbance (λ = 254 nm), cm−1 | 0.063 |
| ChZT, mg O2/L | 32.21 |
| BOD5, mg O2/L | 5.23 |
| N-NH4, mg/L | 1.58 |
| N-NO3, mg/L | 3.50 |
| Total organic nitrogen (TON), mg/L | 6.60 |
| P-PO4, mg/L | 0.40 |
| TOC, mg/L | 21.38 |
| IC, mg/dm3 | 50.54 |
| Pharmaceutical Compound | Ions | Reaction Time, (min) | Reaction Rate Constant k, (min−1) | R2 | Half-Life , (min) |
|---|---|---|---|---|---|
| DCF | - | 0–15 | 0.1395 | 0.89 | 8.4 |
| 15–60 | 0.0039 | 0.93 | 767.9 | ||
| 0–15 | 0.0808 | 0.98 | 11.6 | ||
| 15–60 | 0.0128 | 0.89 | 166.9 | ||
| 0–15 | 0.0209 | 0.87 | 38.0 | ||
| 15–60 | 0.0010 | 0.96 | 1077.8 | ||
| 0–5 | 0.0736 | 0.99 | 11.9 | ||
| 5–15 | 0.0255 | 0.99 | 63.2 | ||
| 15–60 | 0.0330 | 0.88 | 56.4 | ||
| 0–15 | 0.0512 | 0.95 | 14.3 | ||
| 15–60 | 0.0130 | 0.96 | 119.9 | ||
| 0–15 | 0.1375 | 0.94 | 8.1 | ||
| 15–60 | 0.0197 | 0.96 | 150.2 | ||
| IBU | - | 0–15 | 0.0647 | 0.98 | 18.9 |
| 15–60 | 0.0170 | 0.93 | 127.2 | ||
| 0–10 | 0.1603 | 0.99 | 7.7 | ||
| 10–60 | 0.0101 | 0.84 | 293.1 | ||
| 0–15 | 0.0970 | 0.97 | 13.3 | ||
| 15–60 | 0.0147 | 0.95 | 178.8 | ||
| 0–15 | 0.1490 | 0.88 | 10.1 | ||
| 15–60 | 0.0043 | 0.98 | 797.0 | ||
| 0–15 | 0.1231 | 0.91 | 11.2 | ||
| 15–60 | 0.0147 | 0.93 | 206.1 | ||
| 0–15 | 0.1261 | 0.97 | 10.3 | ||
| 15–60 | 0.0245 | 0.95 | 123.5 | ||
| CBZ | - | 0–60 | 0.0153 | 0.98 | 59.6 |
| 0–15 | 0.0384 | 0.97 | 16.07 | ||
| 15–60 | 0.0114 | 0.98 | 17.85 | ||
| 0–60 | 0.0165 | 0.99 | 47.4 | ||
| 0–15 | 0.0099 | 0.78 | 59.70 | ||
| 15–60 | 0.0114 | 0.97 | 52.90 | ||
| 0–5 | 0.0558 | 0.99 | 11.53 | ||
| 5–15 | 0.0006 | 0.98 | 612.75 | ||
| 15–60 | 0.0049 | 0.99 | 87.30 | ||
| 0–15 | 0.0134 | 0.94 | 37.40 | ||
| 15–60 | 0.0104 | 0.87 | 50.70 |
| Pharmaceutical Compound | Ions | Reaction Time, (min) | Reaction Rate Constant k, (min−1) | R2 | Half-Life , (min) |
|---|---|---|---|---|---|
| DCF | 0–10 | 0.1760 | 0.98 | 4.5 | |
| 10–60 | 0.0172 | 0.92 | 151.3 | ||
| 0–10 | 0.1904 | 0.93 | 3.8 | ||
| 10–30 | 0.0013 | 0.96 | 2137.1 | ||
| 30–60 | 0.0539 | 0.89 | 50.8 | ||
| 0–15 | 0.2241 | 0.94 | 5.5 | ||
| 15–60 | 0.0243 | 0.88 | 173.3 | ||
| 0–10 | 0.2436 | 0.99 | 3.6 | ||
| 10–60 | 0.0171 | 0.92 | 197.9 | ||
| 0–10 | 0.2365 | 0.94 | 4.7 | ||
| 10–60 | 0.0286 | 0.99 | 115.9 | ||
| IBU | 0–15 | 0.0447 | 0.99 | 20.0 | |
| 15–60 | 0.0165 | 0.88 | 95.1 | ||
| 0–15 | 0.0309 | 0.77 | 30.8 | ||
| 15–60 | 0.0385 | 0.98 | 35.9 | ||
| 0–15 | 0.1023 | 0.94 | 12.3 | ||
| 15–60 | 0.0143 | 0.76 | 182.1 | ||
| 0–10 | 0.1494 | 0.90 | 9.4 | ||
| 10–60 | 0.0267 | 0.93 | 106.0 | ||
| 0–10 | 0.1307 | 0.92 | 10.3 | ||
| 10–60 | 0.0030 | 0.90 | 853.8 | ||
| CBZ | 0–30 | 0.0043 | 0.98 | 116.76 | |
| 30–60 | 0.0002 | 0.97 | 1921.25 | ||
| 0–15 | 0.0161 | 0.95 | 33.49 | ||
| 15–60 | 0.0042 | 0.98 | 89.85 | ||
| 0–60 | 0.0094 | 0.94 | 99.9 | ||
| 0–60 | 0.0160 | 0.97 | 57.6 | ||
| 0–15 | 0.0111 | 0.99 | 72.4 | ||
| 15–60 | 0.0026 | 0.97 | 371.9 |
| Pharmaceutical Compound | Reaction Time, (min) | Reaction Rate Constant k, (min−1) | R2 | Half-Life , (min) |
|---|---|---|---|---|
| DCF | 0–10 | 0.2303 | 0.96 | 7.4 |
| 10–60 | 0.0167 | 0.82 | 253.7 | |
| IBU | 0–15 | 0.1189 | 0.99 | 18.3 |
| 15–60 | 0.0360 | 0.99 | 110.6 | |
| CBZ | 0–15 | 0.0858 | 0.99 | 10.6 |
| 15–60 | 0.0029 | 0.78 | 782.7 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudlek, E.; Dudziak, M.; Bohdziewicz, J. Influence of Inorganic Ions and Organic Substances on the Degradation of Pharmaceutical Compound in Water Matrix. Water 2016, 8, 532. https://doi.org/10.3390/w8110532
Kudlek E, Dudziak M, Bohdziewicz J. Influence of Inorganic Ions and Organic Substances on the Degradation of Pharmaceutical Compound in Water Matrix. Water. 2016; 8(11):532. https://doi.org/10.3390/w8110532
Chicago/Turabian StyleKudlek, Edyta, Mariusz Dudziak, and Jolanta Bohdziewicz. 2016. "Influence of Inorganic Ions and Organic Substances on the Degradation of Pharmaceutical Compound in Water Matrix" Water 8, no. 11: 532. https://doi.org/10.3390/w8110532
APA StyleKudlek, E., Dudziak, M., & Bohdziewicz, J. (2016). Influence of Inorganic Ions and Organic Substances on the Degradation of Pharmaceutical Compound in Water Matrix. Water, 8(11), 532. https://doi.org/10.3390/w8110532