Using Dual Isotopes and a Bayesian Isotope Mixing Model to Evaluate Nitrate Sources of Surface Water in a Drinking Water Source Watershed, East China
Abstract
:1. Introduction
2. Materials and Methods
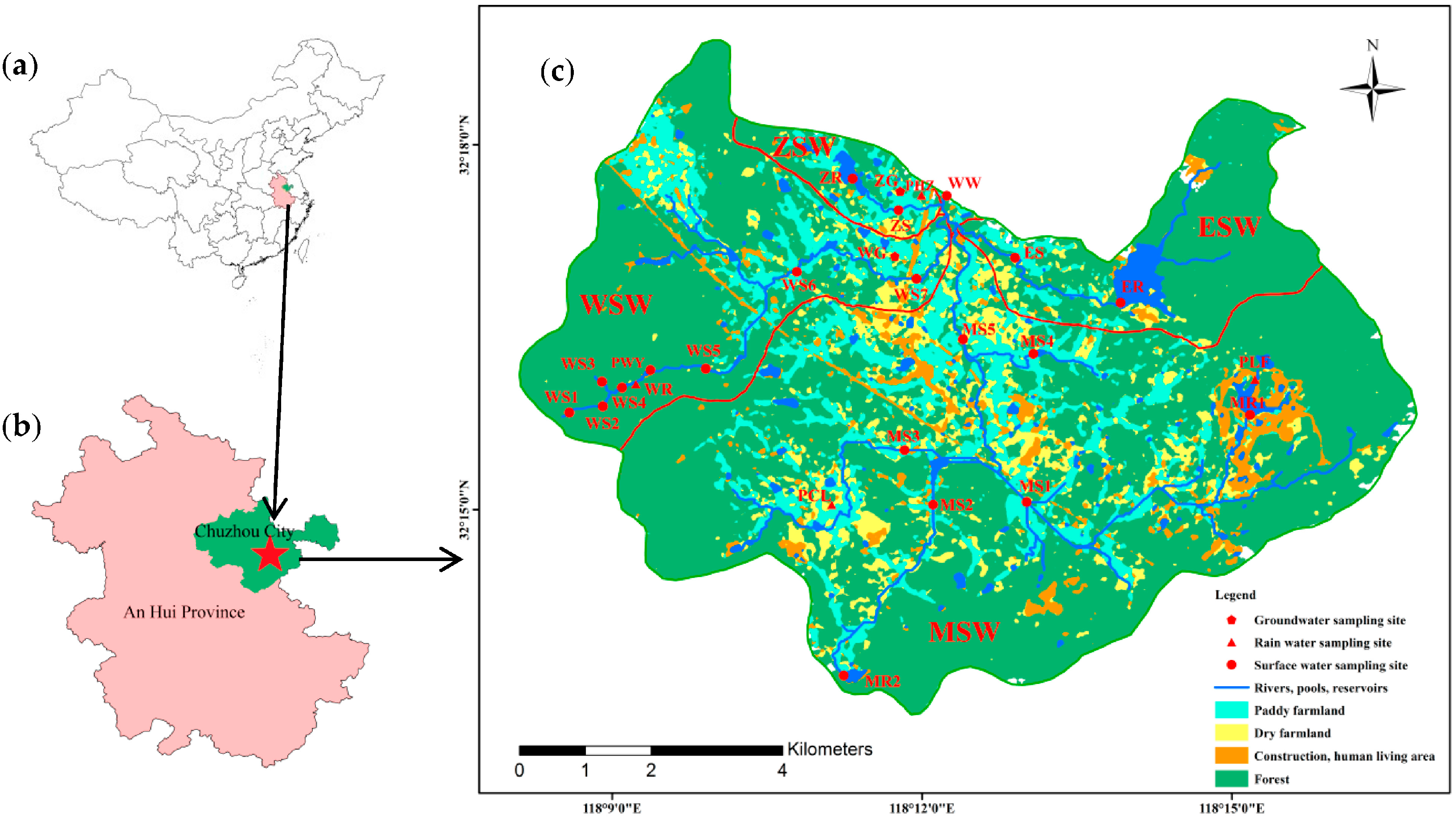
2.1. Study Area Description
2.2. Samplings Collection
2.3. Analytical Methods
2.3.1. Chemistry and Isotopes Analysis Methods
2.3.2. Multivariate Statistics
- One-way analysis of variance (ANOVA) was performed using SPSS version 20 (SPSS Inc., Chicago, IL, USA) to evaluate whether there are any significant differences between the means among three or more independent groups.
- Before proportion nitrate sources using Bayesian isotope mixing model, normality test of nitrate sources input data was needed. In this study, Lilliefors normality test of δ15N and δ18O values in NO3− sources were conducted on MATLAB (MATLAB 2015a, The Mathworks Inc., Natick, MA, USA). Lilliefors method was based on the Kolmogorov–Smirnov test and inspects the null hypothesis that data come from a normally distributed population, when the null hypothesis does not specify which normal distribution.
2.3.3. A Bayesian Isotope Mixing Model
3. Results and Discussion
3.1. Spatial-Temporal Characteristics of Nitrogen in the Study Area
3.2. Identification of the Potential Nitrate Sources and Transformation
3.2.1. Nitrate Isotope Characteristics and Qualitative Analysis of Potential Sources
3.2.2. Nitrification Processes of Ammonia
3.2.3. Denitrification Process
3.3. Evaluation of Proportional Contributions of Different Nitrate Sources
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Diaz, R.J. Overview of hypoxia around the world. J. Environ. Qual. 2001, 30, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S. Research on esophageal cancer in china: A review. Cancer Res. 1980, 40, 2633–2644. [Google Scholar] [PubMed]
- Xue, D.; Botte, J.; De Baets, B.; Accoe, F.; Nestler, A.; Taylor, P.; Van Cleemput, O.; Berglund, M.; Boeckx, P. Present limitations and future prospects of stable isotope methods for nitrate source identification in surface- and groundwater. Water Res. 2009, 43, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.A.; Wu, J.L. Tracing the Nitrate Sources of the Yili River in the Taihu Lake Watershed: A Dual Isotope Approach. Water 2015, 7, 188–201. [Google Scholar] [CrossRef]
- Kendall, C.; Elliott, E.M.; Wankel, S.D. Tracing anthropogenic inputs of nitrogen to ecosystems. In Stable Isotopes in Ecology and Environmental Science, 2nd ed.; Blackwell: Oxford, UK, 2007; Chapter 12; pp. 375–449. [Google Scholar]
- Ma, J.; Chen, X.; Huang, B.; Shi, Y.; Chi, G.Y.; Lu, C.Y. Utilizing water characteristics and sediment nitrogen isotopic features to identify non-point nitrogen pollution sources at watershed scale in Liaoning province, china. Environ. Sci. Pollut. Res. 2015, 22, 2699–2707. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.G.; Kang, P.P.; Sun, Y. A stable isotope approach and its application for identifying nitrate source and transformation process in water. Environ. Sci. Pollut. Res. 2016, 23, 1133–1148. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, F.; Michalski, G.; Xia, X.; Liu, S. Using 15N, 17O, and 18O to determine nitrate sources in the Yellow River, China. Environ. Sci. Technol. 2013, 47, 13412–13421. [Google Scholar] [CrossRef] [PubMed]
- Heaton, T. Isotopic studies of nitrogen pollution in the hydrosphere and atmosphere: A review. Chem. Geol. 1986, 59, 87–102. [Google Scholar] [CrossRef]
- Widory, D.; Petelet-Giraud, E.; Négrel, P.; Ladouche, B. Tracking the sources of nitrate in groundwater using coupled nitrogen and boron isotopes: A synthesis. Environ. Sci. Technol. 2005, 39, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Sigman, D.M.; Casciotti, K.L.; Andreani, M.; Barford, C.; Galanter, M.; Bohlke, J.K. A bacterial method for the nitrogen isotopic analysis of nitrate in seawater and freshwater. Anal. Chem. 2001, 73, 4145–4153. [Google Scholar] [CrossRef] [PubMed]
- Casciotti, K.L.; Sigman, D.M.; Hastings, M.G.; Bohlke, J.K.; Hilkert, A. Measurement of the oxygen isotopic composition of nitrate in seawater and freshwater using the denitrifier method. Anal. Chem. 2002, 74, 4905–4912. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; De Baets, B.; Botte, J.; Vermeulen, J.; Van Cleemput, O.; Boeckx, P. Comparison of the silver nitrate and bacterial denitrification methods for the determination of nitrogen and oxygen isotope ratios of nitrate in surface water. Rapid Commun. Mass Spectrom. 2010, 24, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Elliott, E.; Kendall, C.; Wankel, S.D.; Burns, D.; Boyer, E.; Harlin, K.; Bain, D.; Butler, T. Nitrogen isotopes as indicators of NOx source contributions to atmospheric nitrate deposition across the midwestern and northeastern united states. Environ. Sci. Technol. 2007, 41, 7661–7667. [Google Scholar] [CrossRef] [PubMed]
- Aravena, R.; Evans, M.; Cherry, J.A. Stable isotopes of oxygen and nitrogen in source identification of nitrate from septic systems. Ground Water 1993, 31, 180–186. [Google Scholar] [CrossRef]
- Wassenaar, L.I. Evaluation of the origin and fate of nitrate in the Abbotsford aquifer using the isotopes of 15N and 18O in NO3−. Appl. Geochem. 1995, 10, 391–405. [Google Scholar] [CrossRef]
- Fukada, T.; Hiscock, K.M.; Dennis, P.F.; Grischek, T. A dual isotope approach to identify denitrification in groundwater at a river-bank infiltration site. Water Res. 2003, 37, 3070–3078. [Google Scholar] [CrossRef]
- Moore, J.W.; Semmens, B.X. Incorporating uncertainty and prior information into stable isotope mixing models. Ecol. Lett. 2008, 11, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Whitman, T.; Lehmann, J. A dual-isotope approach to allow conclusive partitioning between three sources. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Parnell, A.C.; Phillips, D.L.; Bearhop, S.; Semmens, B.X.; Ward, E.J.; Moore, J.W.; Jackson, A.L.; Grey, J.; Kelly, D.J.; Inger, R. Bayesian stable isotope mixing models. Environmetrics 2013, 24, 387–399. [Google Scholar] [CrossRef]
- Jin, J.; Yuan, C.; Jiang, S.; Xu, H. Assessment of drought resistance ability for pond and retaining dam irrigated area of Jianghuai hilly area based on water supply and demand balance analysis. J. Hydraul. Eng. 2013, 44, 534–541. (In Chinese) [Google Scholar]
- Widory, D.; Kloppmann, W.; Chery, L.; Bonnin, J.; Rochdi, H.; Guinamant, J.-L. Nitrate in groundwater: An isotopic multi-tracer approach. J. Contam. Hydrol. 2004, 72, 165–188. [Google Scholar] [CrossRef] [PubMed]
- Chinese Environmental Protection Agency. Water Quality-Determination of Nitrate-Nitrogen-Ultraviolet Spectrophotometry (HJ/T 346–2007); China Environmental Science Press: Beijing, China, 2007. (In Chinese) [Google Scholar]
- Massoudieh, A.; Kayhanian, M. Bayesian chemical mass balance method for surface water contaminant source apportionment. J. Environ. Eng. 2012, 139, 250–260. [Google Scholar] [CrossRef]
- Yang, L.; Han, J.; Xue, J.; Zeng, L.; Shi, J.; Wu, L.; Jiang, Y. Nitrate source apportionment in a subtropical watershed using Bayesian model. Sci. Total Environ. 2013, 463, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Parnell, A.C.; Inger, R.; Bearhop, S.; Jackson, A.L. Source partitioning using stable isotopes: Coping with too much variation. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinese Environmental Protection Agency. National Surface Water Environmental Quality Standards of China (GB3838-2002); China Standards Press: Beijing, China, 2002. (In Chinese) [Google Scholar]
- Panno, S.; Kelly, W.; Martinsek, A.; Hackley, K.C. Estimating background and threshold nitrate concentrations using probability graphs. Ground Water 2006, 44, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Peterson, E.W.; Benning, C. Factors influencing nitrate within a low-gradient agricultural stream. Environ. Earth Sci. 2013, 68, 1233–1245. [Google Scholar] [CrossRef]
- Likens, G.E.; Buso, D.C. Variation in stream water chemistry throughout the hubbard brook valley. Biogeochemistry 2006, 78, 1–30. [Google Scholar] [CrossRef]
- Choi, W.-J.; Han, G.-H.; Lee, S.-M.; Lee, G.-T.; Yoon, K.-S.; Choi, S.-M.; Ro, H.-M. Impact of land-use types on nitrate concentration and δ15N in unconfined groundwater in rural areas of Korea. Agric. Ecosyst. Environ. 2007, 120, 259–268. [Google Scholar] [CrossRef]
- Bateman, A.S.; Kelly, S.D. Fertilizer nitrogen isotope signatures. Isot. Environ. Health Stud. 2007, 43, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Toor, G.S. δ15N and δ18O reveal the sources of nitrate-nitrogen in urban residential storm water runoff. Environ. Sci. Technol. 2016, 50, 2881–2889. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Q.; Wang, X.K.; Sun, F.X.; Sun, J.C.; Liu, J.T.; Ouyang, Z.Y. Assessment of temporal and spatial differences of source apportionment of nitrate in an urban river in China, usingδ15N and δ18O values and an isotope mixing model. Environ. Sci. Pollut. Res. 2015, 22, 20226–20233. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Liu, W.G. Using dual isotopes to identify sources and transformations of nitrogen in water catchments with different land uses, Loess Plateau of China. Environ. Sci. Pollut. Res. 2016, 23, 388–401. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Cheng, H.G.; Pu, X.; Liu, X.L.; Cheng, Q.D. Nitrate behaviors and source apportionment in an aquatic system from a watershed with intensive agricultural activities. Environ. Sci. Process. Impacts 2015, 17, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Xi, B.; Gao, R.; He, L.; Liu, H.; Dai, X.; Yu, Y. Identifying diffused nitrate sources in a stream in an agricultural field using a dual isotopic approach. Sci. Total Environ. 2014, 484, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; De Baets, B.; Van Cleemput, O.; Hennessy, C.; Berglund, M.; Boeckx, P. Use of a Bayesian isotope mixing model to estimate proportional contributions of multiple nitrate sources in surface water. Environ. Pollut. 2012, 161, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Yun, S.T.; Mayer, B.; Lee, J.H.; Kim, T.S.; Kim, H.K. Quantification of nitrate sources in groundwater using hydrochemical and dual isotopic data combined with a Bayesian mixing model. Agric. Ecosyst. Environ. 2015, 199, 369–381. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Zhou, A.G.; Zhou, J.W.; Liu, C.F.; Cai, H.S.; Liu, Y.D.; Xu, W. Evaluating the sources and fate of nitrate in the alluvial aquifers in the shijiazhuang rural and suburban area, China: Hydrochemical and multi-isotopic approaches. Water 2015, 7, 1515–1537. [Google Scholar] [CrossRef]
- Hastings, M.G.; Steig, E.; Sigman, D. Seasonal variations in N and O isotopes of nitrate in snow at summit, Greenland: Implications for the study of nitrate in snow and ice cores. J. Geophys. Res. Atmos. 2004, 109. [Google Scholar] [CrossRef]
- Hollocher, T.C. Source of the oxygen atoms of nitrate in the oxidation of nitrite by nitrobacter agilis and evidence against a P-O-N anhydride mechanism in oxidative phosphorylation. Arch. Biochem. Biophys. 1984, 233, 721–727. [Google Scholar] [CrossRef]
- Williard, K.W.; DeWalle, D.R.; Edwards, P.J.; Sharpe, W.E. 18O isotopic separation of stream nitrate sources in mid-appalachian forested watersheds. J. Hydrol. 2001, 252, 174–188. [Google Scholar] [CrossRef]
- Mayer, B.; Bollwerk, S.M.; Mansfeldt, T.; Hütter, B.; Veizer, J. The oxygen isotope composition of nitrate generated by nitrification in acid forest floors. Geochim. Cosmochim. Acta 2001, 65, 2743–2756. [Google Scholar] [CrossRef]
- Chesapeake Bay Program. Dissolved Oxygen. Available online: http://www.chesapeakebay.net/discover/bayecosystem/dissolvedoxygen (accessed on 5 April 2016).
- Rivett, M.O.; Buss, S.R.; Morgan, P.; Smith, J.W.; Bemment, C.D. Nitrate attenuation in groundwater: A review of biogeochemical controlling processes. Water Res. 2008, 42, 4215–4232. [Google Scholar] [CrossRef] [PubMed]
- Barnes, R.T.; Raymond, P.A. Land-use controls on sources and processing of nitrate in small watersheds: Insights from dual isotopic analysis. Ecol. Appl. 2010, 20, 1961–1978. [Google Scholar] [CrossRef] [PubMed]
- Burns, D.A.; Boyer, E.W.; Elliott, E.M.; Kendall, C. Sources and transformations of nitrate from streams draining varying land uses: Evidence from dual isotope analysis. J. Environ. Qual. 2009, 38, 1149–1159. [Google Scholar] [CrossRef] [PubMed]





| Watersheds | Land Use Types | ||||||
|---|---|---|---|---|---|---|---|
| Forest | Crop Land | Residential | Bare Land | Water Body | Sum | ||
| ESW | Area (km2) | 7.04 | 1.06 | 0.01 | 0.21 | 0.5 | 8.82 |
| Proportion (%) | 79.82 | 12.02 | 0.11 | 2.38 | 5.67 | 100 | |
| MSW | Area (km2) | 32.97 | 12.34 | 0.93 | 3.04 | 1.19 | 50.47 |
| Proportion (%) | 65.33 | 24.45 | 1.84 | 6.02 | 2.36 | 100 | |
| WSW | Area (km2) | 13.14 | 3.82 | 0.13 | 0.57 | 0.29 | 17.95 |
| Proportion (%) | 73.2 | 21.28 | 0.72 | 3.18 | 1.62 | 100 | |
| ZSW | Area (km2) | 1.3 | 1.04 | 0.01 | 0.1 | 0.19 | 2.64 |
| Proportion (%) | 49.24 | 39.39 | 0.38 | 3.79 | 7.2 | 100 | |
| WW | Area (km2) | 54.75 | 18.16 | 1.06 | 3.89 | 2.28 | 80.13 |
| Proportion (%) | 68.33 | 22.66 | 1.32 | 4.85 | 2.84 | 100 | |
| Watersheds | NH4+/TN (%) | NO3−/TN (%) | NO2−/TN (%) | DIN/TN (%) | DON/TN (%) |
|---|---|---|---|---|---|
| ESW | 12.14 | 52.97 | 2.09 | 67.2 | 32.8 |
| MSW | 10.58 | 45.97 | 1.82 | 58.37 | 41.63 |
| WSW | 8.47 | 68.22 | 1.36 | 78.05 | 21.95 |
| ZSW | 16.41 | 42.39 | 2.48 | 61.28 | 38.72 |
| Watersheds | Sites | Long. (°) | Lat. (°) | TN (mg/L) | NO3− (mg N /L) | NH4+ (mg/L) | δ15N-NO3− (‰) | δ18O-NO3− (‰) | δD-H2O (‰) | δ18O-H2O (‰) |
|---|---|---|---|---|---|---|---|---|---|---|
| ESW | ER | 118.23 | 32.28 | 1.48 ± 0.15 | 0.17 ± 0.10 | 0.10 ± 0.12 | 6.5 ± 0.9 | −0.4 ± 3.7 | −40.0 ± 7.1 | −5.5 ± 1.4 |
| ES | 118.22 | 32.28 | 1.17 ± 0.65 | 0.68 ± 0.53 | 0.14 ± 0.16 | 6.2 ± 1.4 | 3.6 ± 4.3 | −37.3 ± 12.3 | −5.2 ± 2.3 | |
| MSW | MR1 | 118.25 | 32.26 | 1.42 ± 0.36 | 0.35 ± 0.26 | 0.19 ± 0.22 | 4.5 ± 1.8 | 1.2 ± 3.9 | −41.1 ± 8.0 | −5.4 ± 1.5 |
| MS1 | 118.22 | 32.25 | 0.85 ± 0.20 | 0.56 ± 0.45 | 0.06 ± 0.01 | 4.9 ± 1.3 | 5.0 ± 5.7 | −41.1 ± 12.8 | −5.2 ± 2.0 | |
| MR2 | 118.19 | 32.23 | 1.38 ± 0.78 | 0.72 ± 0.75 | 0.06 ± 0.04 | 4.7 ± 1.9 | −1.2 ± 2.6 | −41.2 ± 8.4 | −5.0 ± 2.0 | |
| MS2 | 118.20 | 32.25 | 0.93 ± 0.83 | 0.15 ± 0.21 | 0.05 ± 0.02 | 3.2 ± 5.6 | 2.2 ± 5.5 | −34.8 ± 15.7 | −3.9 ± 2.4 | |
| MS3 | 118.20 | 32.26 | 0.79 ± 0.02 | 0.08 ± 0.07 | 0.07 ± 0.06 | 3.9 ± 2.2 | 1.8 ± 4.7 | −38.4 ± 12.0 | −4.47 ± 2.1 | |
| MS4 | 118.22 | 32.27 | 1.19 ± 0.83 | 0.40 ± 0.19 | 0.24 ± 0.47 | 6.1 ± 4.1 | 3.2 ± 8.7 | −35.8 ± 12.3 | −4.5 ± 2.1 | |
| MS5 | 118.21 | 32.27 | 1.02 ± 0.52 | 0.53 ± 0.33 | 0.12 ± 0.13 | 4.6 ± 1.2 | 5.1 ± 6.9 | −37.2 ± 10.4 | −4.9 ± 1.7 | |
| PLF | 118.25 | 32.26 | 2.07 ± 1.68 | 0.60 ± 0.56 | 0.81 ± 0.67 | −67.5 ± 25.7 | −10.0 ± 3.4 | |||
| PCL | 118.19 | 32.25 | 2.54 ± 1.56 | 0.70 ± 0.46 | 1.37 ± 1.12 | −61.4 ± 23.5 | −9.0 ± 2.9 | |||
| WSW | WS1 | 118.14 | 32.26 | 7.43 ± 1.60 | 6.71 ± 1.48 | 0.08 ± 0.02 | ||||
| WS2 | 118.15 | 32.26 | 5.26 ± 1.75 | 4.49 ± 1.62 | 0.09 ± 0.12 | |||||
| WS3 | 118.15 | 32.27 | 4.44 ± 1.84 | 3.81 ± 1.61 | 0.07 ± 0.07 | |||||
| WS4 | 118.15 | 32.27 | 2.86 ± 1.42 | 2.45 ± 1.39 | 0.10 ± 0.18 | 2.9 ± 1.8 | 4.7 ± 3.6 | −48.2 ± 14.5 | −6.9 ± 1.8 | |
| WR | 118.16 | 32.27 | 2.53 ± 1.55 | 1.90 ± 1.35 | 0.14 ± 0.25 | |||||
| WS5 | 118.17 | 32.27 | 2.12 ± 1.10 | 1.69 ± 1.09 | 0.07 ± 0.03 | |||||
| WS6 | 118.18 | 32.28 | 1.35 ± 0.68 | 0.85 ± 0.58 | 0.13 ± 0.12 | 6.4 ± 3.0 | 2.9 ± 2.6 | −41.3 ± 7.2 | −5.5 ± 1.9 | |
| WS7 | 118.20 | 32.28 | 1.34 ± 0.71 | 0.70 ± 0.54 | 0.20 ± 0.23 | 5.5 ± 1.4 | 2.1 ± 4.3 | −45.2 ± 11.9 | −6.2 ± 2.5 | |
| PWY | 118.16 | 32.27 | 2.73 ± 1.27 | 0.69 ± 0.57 | 1.58 ± 0.77 | −60.1 ± 18.5 | −8.8 ± 2.3 | |||
| ZSW | ZR | 118.19 | 32.30 | 2.18 ± 1.42 | 0.18 ± 0.10 | 0.36 ± 0.56 | 6.1 ± 3.5 | −2.8 ± 2.0 | −35.0 ± 11.9 | −2.9 ± 1.9 |
| ZS | 118.20 | 32.29 | 2.13 ± 2.67 | 0.67 ± 0.38 | 0.46 ± 0.81 | 5.0 ± 5.9 | 0.9 ± 4.3 | −43.4 ± 11.6 | −5.6 ± 2.6 | |
| PZJ | 118.21 | 32.29 | 2.69 ± 2.41 | 0.85 ± 0.80 | 1.24 ± 0.94 | −1.9 ± 4.9 | 59.0 ± 6.58 | −46.2 ± 31.1 | −6.82 ± 3.8 | |
| WW | WW | 118.21 | 32.29 | 1.48 ± 0.92 | 0.72 ± 0.45 | 0.21 ± 0.21 | 5.8 ± 3.6 | 2.2 ± 3.8 | −46.2 ± 31.1 | −6.82 ± 3.8 |
| Sources | ESW | MSW | WSW | ZSW | WW | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry | |
| NP | 0.18 | 0.12 | 0.15 | 0.08 | 0.17 | 0.09 | 0.11 | 0.04 | 0.15 | 0.09 |
| M & S | 0.21 | 0.29 | 0.31 | 0.42 | 0.26 | 0.37 | 0.25 | 0.32 | 0.28 | 0.36 |
| NS | 0.36 | 0.31 | 0.20 | 0.19 | 0.29 | 0.25 | 0.17 | 0.24 | 0.24 | 0.27 |
| NF | 0.11 | 0.16 | 0.21 | 0.17 | 0.14 | 0.16 | 0.27 | 0.23 | 0.19 | 0.16 |
| NFA | 0.14 | 0.11 | 0.13 | 0.14 | 0.14 | 0.12 | 0.20 | 0.17 | 0.14 | 0.11 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Lu, B.; Wang, J.; Zhang, H.; Guo, L.; Lin, H. Using Dual Isotopes and a Bayesian Isotope Mixing Model to Evaluate Nitrate Sources of Surface Water in a Drinking Water Source Watershed, East China. Water 2016, 8, 355. https://doi.org/10.3390/w8080355
Wang M, Lu B, Wang J, Zhang H, Guo L, Lin H. Using Dual Isotopes and a Bayesian Isotope Mixing Model to Evaluate Nitrate Sources of Surface Water in a Drinking Water Source Watershed, East China. Water. 2016; 8(8):355. https://doi.org/10.3390/w8080355
Chicago/Turabian StyleWang, Meng, Baohong Lu, Jianqun Wang, Hanwen Zhang, Li Guo, and Henry Lin. 2016. "Using Dual Isotopes and a Bayesian Isotope Mixing Model to Evaluate Nitrate Sources of Surface Water in a Drinking Water Source Watershed, East China" Water 8, no. 8: 355. https://doi.org/10.3390/w8080355
APA StyleWang, M., Lu, B., Wang, J., Zhang, H., Guo, L., & Lin, H. (2016). Using Dual Isotopes and a Bayesian Isotope Mixing Model to Evaluate Nitrate Sources of Surface Water in a Drinking Water Source Watershed, East China. Water, 8(8), 355. https://doi.org/10.3390/w8080355






