Study on Effects of Electron Donors on Phosphine Production from Anaerobic Activated Sludge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Instruments and Reagents
2.3. Apparatus and Methods
2.4. Analyses and Methods
2.5. Sampling Procedures
3. Results and Discussion
3.1. TP Removal and Phosphine Concentration During the Sludge Acclimation Process
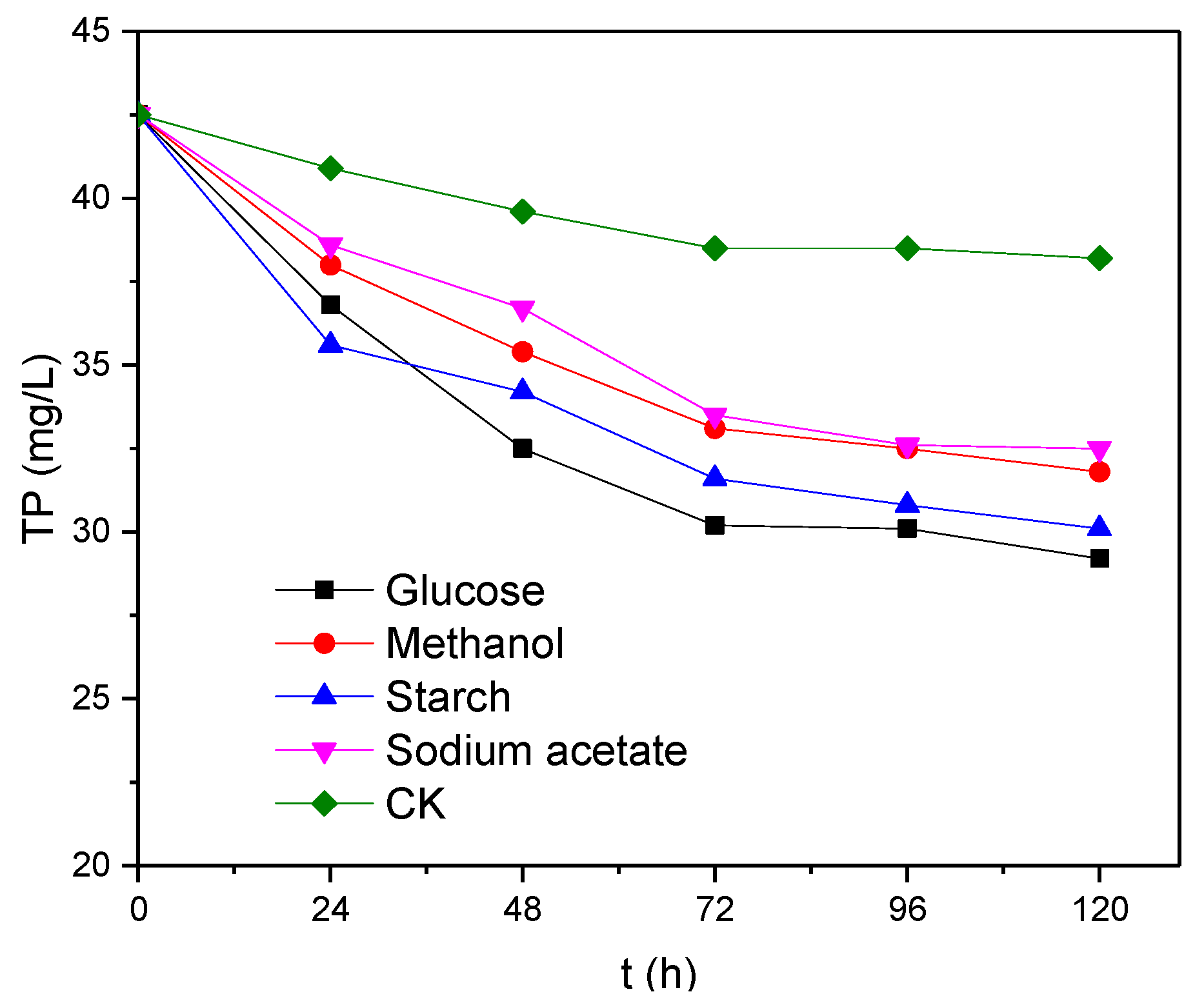
3.2. Effects of Electron Donor Types on the Removal of TP
3.3. Effect of Electron Donor Types on Phosphine Production
3.4. Effects of Electron Donor Concentration on Phosphine Production
3.5. Relationship between Methane Production and Phosphine Production
3.6. Discussion of the Origin and Fate of Phosphine
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Devai, I.; Felfoldy, L.; Wittner, I.; Plosz, S. Detection of phosphine: New aspects of the phosphorus cycle in the hydrosphere. Nature 1988, 6171, 343–345. [Google Scholar] [CrossRef]
- Devai, I.; Delaune, R.D. Evidence for phosphine production and emission from Louisiana and Florida marsh soils. Org. Geochem. 1995, 3, 277–279. [Google Scholar] [CrossRef]
- Liang, J.; Feng, C.T.; Zeng, G.M. Spatial distribution and source identification of heavy metals in surface soils in a typical coal mine city, Lianyuan, China. Environ. Pollut. 2017, 225, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Roels, J.; Van, L.H.; Verstraete, W. Determination of phosphine in biogas and sludge at ppt-levels with gas chromatography-thermionic specific detection. J. Chromatogr. 2002, 1, 229–237. [Google Scholar] [CrossRef]
- Roels, J.; Verstraete, W. Occurrence and origin of phosphine in landfill gas. Sci. Total Environ. 2004, 1, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Gassmann, G. Phosphine in the fluvial and marine hydrosphere. Mar. Chem. 1994, 3, 197–205. [Google Scholar] [CrossRef]
- Glindemann, D.; Bergmann, A.; Stottmeister, U.; Gassmann, G. Phosphine in the lower terrestrial troposphere. Sci. Nat. 1996, 83, 131–133. [Google Scholar] [CrossRef]
- Liu, J.A.; Kuschk, P.; Eismann, F.; Glindemann, D. Phosphine in the urban air of Beijing and its possible sources. Water Air Soil Pollut. 1999, 3–4, 597–604. [Google Scholar]
- Zhang, R.; Wu, M.; Wang, Q.; Geng, J.; Yang, X. The determination of atmospheric phosphine in Ny-Alesund. Chin. Sci. Bull. 2010, 55, 1662–1666. [Google Scholar] [CrossRef]
- Schink, B.; Friedrich, M. Bacterial metabolism: Phosphite oxidation by sulphate reduction. Nature 2000, 6791, 37. [Google Scholar] [CrossRef] [PubMed]
- Roels, J.; Verstraete, W. Biological formation of volatile phosphorus compounds. Bioresour. Technol. 2001, 3, 243–250. [Google Scholar] [CrossRef]
- Hanrahan, G.; Salmassi, T.M.; Khachikian, C.S.; Foster, K.L. Reduced inorganic phosphorus in the natural environment: Significance, speciation and determination. Talanta 2005, 2, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Roels, J.; Huyghe, G.; Verstraete, W. Microbially mediated phosphine emission. Sci. Total Environ. 2005, 3, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Rutishauser, B.V.; Bachofen, R. Phosphine formation from sewage sludge cultures. Anaerobe 1999, 5, 525–531. [Google Scholar] [CrossRef]
- Jenkins, R.O.; Morris, T.A.; Craig, P.J.; Ritchie, A.W.; Ostah, N. Phosphine generation by mixed- and monoseptic-cultures of anaerobic bacteria. Sci. Total Environ. 2000, 1–3, 73–81. [Google Scholar] [CrossRef]
- Prezhdo, O.V.; Gawdzik, B.; Zubkova, V.V.; Prezhdo, V.V. Molecular structure and electrical properties of some phosphonates, phosphine-oxides and phosphates. J. Mol. Struct. 2009, 1–3, 146–153. [Google Scholar] [CrossRef]
- Zhang, P.L.; Rong, H.W.; Zhang, K.F.; Liu, T.; Cao, Y.F. Effect of Diverse Mud and Phosphorus Sources on Total Phosphorus Removal Efficiencies. Guangdong Chem. Ind. 2011, 38, 118–124. (In Chinese) [Google Scholar]
- Yang, Z.; Zhou, J.; Li, J.; Han, Y.; He, O. Pre-processing of raw wastewater in a septic tank leads to phosphorus removal by phosphine production in a sequencing batch biofilm reactor (SBBR). Desalin. Water Treat. 2016, 2, 810–818. [Google Scholar] [CrossRef]
- Luo, Y.; Bao, J.G.; Li, S.M.; Hu, X.Y.; Li, N.; Zeng, W.D. Experimental Study on the Release of Phosphine to Improve the Removal Effect of Phosphorus from Sewage. Saf. Environ. Eng. 2014, 5, 94–103. (In Chinese) [Google Scholar]
- Wang, J.F.; Niu, X.J.; Ma, J.L.; Lu, M.Q. Conversion of phosphorus to phosphine by microbial deoxidization under anaerobic conditions. Microbiol. China 2015, 1, 34–41. [Google Scholar]
- Chen, Y.; Zhou, J.; Gan, C.J.; Li, J.J. Effects of DO and aeration mode on the process ofphosphorus removal by phosphate reduction. Ind. Water Treat. 2011, 10, 31–34. (In Chinese) [Google Scholar]
- Chen, Y.; Zhou, J.; Gan, C.J.; Li, J.J. Effect of initial pH value on phosphorus removal of phosphate reduction. Chin. Environ. Eng. 2011, 5, 2428–2431. (In Chinese) [Google Scholar]
- Wan, J.B.; Deng, M.; He, H.Y.; Tang, A.P. Factors Influencing Release of Phosphine in Piggery Wastewater. China Water Wastewater 2013, 23, 117–120. (In Chinese) [Google Scholar]
- Niu, X.J.; Wei, A.S.; Li, Y.D.; Mi, L.N.; Yang, Z.Q.; Song, X.F. Phosphine in paddy fields and the effects of environmental factors. Chemosphere 2013, 9, 1942–1947. [Google Scholar] [CrossRef] [PubMed]
- Eismann, F.; Glindemann, D.; Bergmann, A.; Kuschk, P. Balancing phosphine in manure fermentation. J. Environ. Sci. Health Part B 1997, 6, 955–968. [Google Scholar] [CrossRef] [PubMed]
- Glindemann, D.; Eismann, F.; Bergmann, A. Phosphine by bio-corrosion of phosphide-rich iron. Environ. Sci. Pollut. Res. 1998, 5, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Glindemann, D.; Edwards, M.; Schrems, O. Phosphine and methylphosphine production by simulated lightning—A study for the volatile phosphorus cycle and cloud formation in the earth atmosphere. Atmos. Environ. 2004, 38, 6867–6874. [Google Scholar] [CrossRef]
- Cao, H.; Liu, J.A.; Zhuang, Y. Emission sources of atmospheric phosphine and simulation of phosphine formation. Sci. China Ser. B: Chem. 2000, 43, 162–168. [Google Scholar] [CrossRef]
- Pasek, M.A.; Sampson, J.M.; Atlas, Z. Redox chemistry in the phosphorus biogeochemical cycle. Proc. Nat. Acad. Sci. USA 2014, 111, 15468–15473. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H.; Zhuang, Y.H.; Liu, J.A. Phosphorus cycling through phosphine in paddy fields. Sci. Total Environ. 2000, 258, 195–203. [Google Scholar] [CrossRef]
- Eismann, F.; Glindemann, D.; Bergmannt, A. Soils as source and sink of phosphine. Chemosphere 1997, 35, 523–533. [Google Scholar] [CrossRef]
- Renbin, Z.; Deming, K.; Liguang, S. Tropospheric Phosphine and Its Sources in Coastal Antarctica. Environ. Sci. Technol. 2006, 40, 7656. [Google Scholar]
- Zhu, Y.; Ding, L.; Ren, H. Fate of matrix-bound phosphine during acidification with anaerobic bacteria. Environ. Sci. 2005, 26, 139–142. (In Chinese) [Google Scholar]





| Element | Dosage (mg/L) | Compound Form | Dosage of Compound (mg/L) |
|---|---|---|---|
| Mg | 12.0 | MgCl2·6H2O | 101.5 |
| Ca | 20.2 | CaCl2 | 55.5 |
| Fe | 4.0 | FeCl2·4H2O | 14.22 |
| Co | 0.1 | CoCl2·6H2O | 0.4 |
| Ni | 0.2 | NiCl2·6H2O | 0.8 |
| Mo | 0.1 | MoCl2·4H2O | 0.23 |
| Zn | 0.1 | ZnCl2 | 0.32 |
| Mn | 0.1 | MnCl2·4H2O | 0.36 |
| Electron Donors | PH3 Concentration in Biogas (ng/m3) | Volume of Biogas (mL) | Quantity of PH3 (ng) | MBP Concentration (ng/kg) (Dry Weight) | Quantity of MBP (ng) | MBP/PH3 |
|---|---|---|---|---|---|---|
| glucose | 920 | 275 | 0.25 | 5763 | 61.09 | 241 |
| starch | 1250 | 201 | 0.25 | 5056 | 53.59 | 213 |
| methanol | 955 | 239 | 0.23 | 3789 | 40.16 | 176 |
| sodium acetate | 950 | 231 | 0.22 | 3508 | 37.18 | 169 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.; Zhang, C.; Rong, H.; Zhao, M.; Wei, W.; Zhao, L. Study on Effects of Electron Donors on Phosphine Production from Anaerobic Activated Sludge. Water 2017, 9, 563. https://doi.org/10.3390/w9080563
Cao J, Zhang C, Rong H, Zhao M, Wei W, Zhao L. Study on Effects of Electron Donors on Phosphine Production from Anaerobic Activated Sludge. Water. 2017; 9(8):563. https://doi.org/10.3390/w9080563
Chicago/Turabian StyleCao, Jianping, Chaosheng Zhang, Hongwei Rong, Meihua Zhao, Wei Wei, and Limin Zhao. 2017. "Study on Effects of Electron Donors on Phosphine Production from Anaerobic Activated Sludge" Water 9, no. 8: 563. https://doi.org/10.3390/w9080563
APA StyleCao, J., Zhang, C., Rong, H., Zhao, M., Wei, W., & Zhao, L. (2017). Study on Effects of Electron Donors on Phosphine Production from Anaerobic Activated Sludge. Water, 9(8), 563. https://doi.org/10.3390/w9080563





