Abstract
We have monitored the presence of bacteria belonging to the genus Legionella in the plumbing of buildings at the University of Perugia (Italy). More than 300 water samples were collected from 156 control-point taps in 41 buildings comprised in the eight campuses of the University. Legionella was absent in most samples, while it was found in only 12 buildings (29% of the total). Molecular analysis indicated the presence of L. pneumophila (serogroups 1, 8 and 6–10), L. taurinensis and L. anisa. In only three cases contamination levels were above the limit at which remedial actions are required, according to international guidelines. In two buildings, where the water temperature could be raised and maintained above 60 °C, thermal disinfection was effective in eradicating Legionella. Conversely, in buildings where contaminations were caused by heat exchangers that produced hot water at a maximum temperature of 50 °C, a chemical disinfection with silver hydrogen peroxide was carried out but was effective only in the short term. In this case study, Legionella contaminations and remediation effectiveness strongly depended on the network and heating-system characteristics, indicating how a multidisciplinary approach that integrates microbiological analysis with hydraulic surveys is necessary for an effective definition of Legionella prevention and control strategies.
1. Introduction
The occurrence of Legionella contaminations in a building’s tap water represents a serious health threat to end-users since it may infect human lungs, causing a form of atypical pneumonia called legionellosis or Legionnaires’ disease [1,2]. The last available report, related to 2015, shows that in Europe 30 countries reported more than 6,500 confirmed cases of legionellosis, corresponding to an overall rate of 14 per million inhabitants, with an increase over the 2011–2015 period [3]. Four countries (France, Germany, Italy and Spain) accounted for 69% of all notified cases in 2015, although their combined populations only represent approximately 50% of the total in Europe. Italy accounted for 1535 new cases, corresponding to an incidence rate of 26 cases per million.
The genus Legionella comprises more than 50 different bacterial species and at least 18 of them have been associated with human disease [1,3]. It is generally supposed that more than 90% of cases of legionellosis in Europe and the USA are caused by Legionella pneumophila, particularly L. pneumophila serogroup (sg) 1, but the real number of cases caused by other Legionella species is not well established, because they may also lead to Pontiac fever, an acute, self-limiting, flu-like illness that does not require healthcare and, thus, is likely to be largely under-diagnosed [1,3]. Culture and isolation, often followed by antibody-based identification assays, remain the “gold standard” for the diagnosis of Legionella in clinical specimens, despite being biased towards the detection of L. pneumophila [1]. In this respect, DNA-based molecular methods, such as the polymerase chain reaction (PCR), both conventional and quantitative (qPCR), have been increasingly standardized and implemented in the clinical laboratory for rapid and sensitive Legionella detection [1]. Furthermore, sequencing of Legionella-specific genes, such as macrophage infectivity potentiator (mip), is widely used for Legionella species identification [1,4].
Legionella is found naturally in freshwater environments, like lakes and streams, but can proliferate in human-made water systems such as a building’s plumbing and hot-water networks [2,5,6]. The growth of Legionella in water depends also on interactions, both positive and negative, with other microorganisms, such as heterotrophic Gram-negative bacteria (Enterobacteriaceae and Pseudomonadaceae, in particular), amoebae and protozoa living in the same environment [7,8]. Monitoring the presence of Legionella bacteria and preventing their colonization of the water distribution mains and the plumbing system of buildings are both technical and environmental challenges [5] and are therefore considered crucial according to international and national guidelines that regulate the quality of drinking water [9,10]. These guidelines provide technical guidance for those involved in the design, installation, commissioning, risk-assessment and management of building water systems that could produce aerosols of Legionella-contaminated water, and thus should be periodically monitored and disinfected if required. Guidelines also report the detailed method for routine microbiological monitoring of Legionella, based on plate culturing according to EN ISO 11731:2017. However, using culture has a number of limitations, including the time needed for confirmed results (10 to 14 days), poor sensitivity and recovery, and an inability to detect viable but non-culturable cells; consequently, qPCR has been proposed as a valuable alternative due to its speed (results can be achieved in less than 24 h), sensitivity, specificity, reproducibility and high-throughput potential [11]. Nevertheless, there are still knowledge gaps in interpreting qPCR results, which show scarce correlation with those using cultures [12], especially in respect of Italian and European guidelines that suggest the intervention needed for the prevention of Legionella contamination based solely on plate count values [9,10]. Revised guidelines for the control and prevention of legionellosis were issued in Italy in 2015 defining goals and standards [10]. These guidelines are quite specific for hospitals and hotels, but little if anything is said about educational institutions such as universities. Indeed, while literature data on environmental Legionella contamination (especially, in hot-water networks and air-conditioning systems) are available for hospitals, long-care facilities, accommodation sites, and private houses, the presence of Legionella in the water systems of university buildings has never been studied [13,14,15,16].
This paper reports a case study based on a one-year-long survey of the presence of Legionella in the plumbing system of the University of Perugia buildings. This University, one of the oldest and most accredited in Italy, is characterized by a sparse organization of its buildings which are distributed within eight different campuses and host more than 23,000 students and 4000 staff. Because of the high number of users and the scattered organization of the buildings, the implementation of an effective monitoring approach is the key to preventing and controlling Legionella contamination. In this framework, we aimed to test a multidisciplinary approach integrating both the microbiological analysis and the survey of building plumbing and water-heating systems. We have assessed the rate of Legionella colonization and carried out remedial actions in contaminated buildings according to Italian and European guidelines. Furthermore, we used the data collected to gain insights about the role of temperature regimes and the type of hot water production systems on the growth of Legionella in the network and on the effectiveness of different disinfection approaches used.
2. Materials and Methods
2.1. Case-Study Description
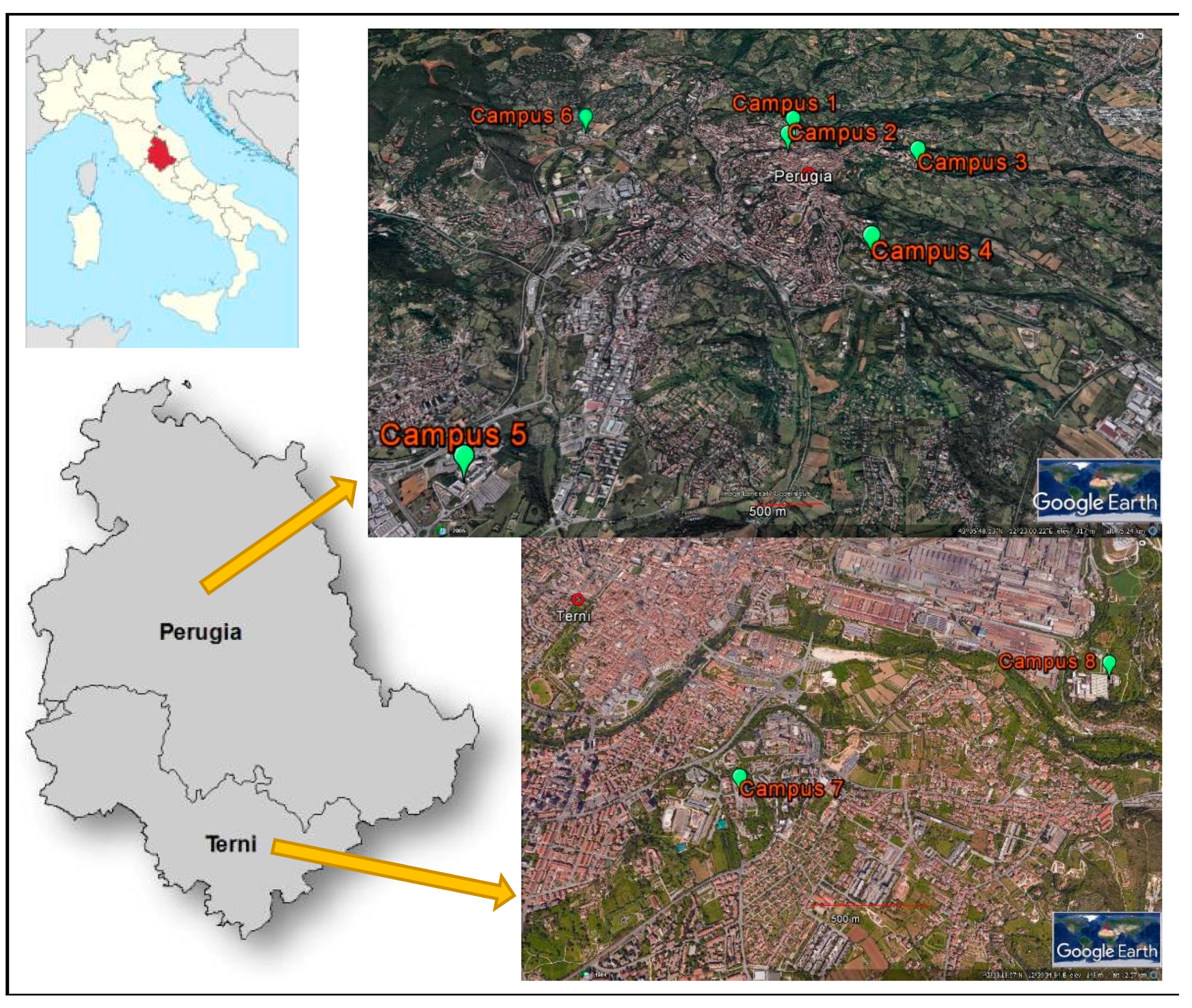
The University of Perugia was founded in 1308 and today offers a wide variety of courses in all fields of education as well as being involved in research and consultancy in various disciplines. The University is characterized by a sparse organization with its buildings distributed in eight different campuses located in the two main cities of Umbria, central Italy (Figure 1). Most campuses are in Perugia, where four (campuses Nos. 1–4) are located very close to the city centre and two (campuses Nos. 5 and 6) are in different suburbs of the city, while two campuses are in Terni (campuses Nos. 7 and 8). Each campus is composed of several buildings devoted to different academic activities, namely teaching, includes libraries and study halls, research and administration units, and hosts a total of more than 25,000 users. The largest campus is No. 1, with a total of 11 buildings and which also includes the administrative hub. With the exception of campus No. 2, all include scientific laboratories, including an animal facility in campus No. 3 and a teaching veterinary hospital in campus No. 4.
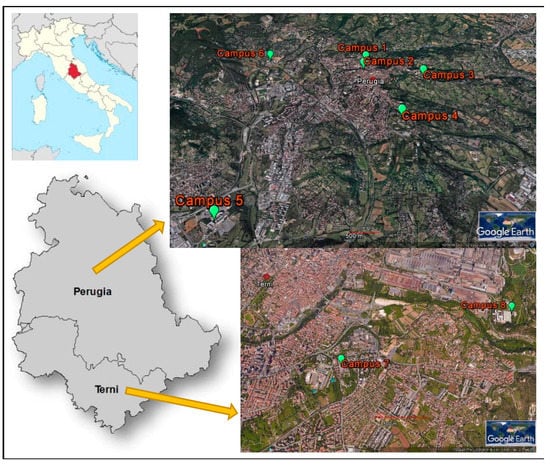
Figure 1.
Location of the eight campuses of the University of Perugia.
2.2. Sample Collection
From July 2015 to September 2016 water samples were collected from 156 control-point taps located in 41 University buildings. In the buildings where decontamination was necessary, follow-up monitoring was carried out according to Italian guidelines by sampling the positive sites 48 h, 1 and 3 months after the intervention [10]. One litre of water from both cold and hot water mains was taken in sterile containers with 0.1% sodium thiosulfate to neutralize residual chlorine. Sampling was done before and after flushing water for 5 min, the latter to account for possible contamination inside the plumbing system. Water temperature was measured during sampling with an electronic thermometer. Water distributed in the cities of Perugia and Terni is treated with chlorine dioxide and, in the examined period, the residual disinfectant concentrations ranged between 0.03 and 0.08 mg/L [17].
2.3. Enumeration of Legionella
The presence of Legionella was assessed by standard methods as indicated by Italian guidelines [10]. One-litre water samples were concentrated by filtration through 0.22 μm pore polycarbonate membrane (Merck Millipore, Billerica, MA, USA). After filtration, bacteria collected on the membranes were suspended in 10 mL of the original water sample, and 0.1 mL of the suspension was spread onto glycin vancomicin polymyxin cycloeximide agar plates (GVPC) (Thermo Fisher Scientific, Waltham, MA, USA) specific for the growth of Legionella bacteria. The inoculated plates were then incubated for 10 days at 36 ± 1 °C in a humid environment. A representative number of presumptive Legionella colonies were isolated by sub-culture on buffer cheracoll yeast extract agar plates with and without cysteine (BCYE, Thermo Fisher Scientific, Waltham, MA, USA) and incubated at 36 ± 1 °C for 2 days. Only colonies showing cysteine auxotrophy were enumerated as Legionella and results were reported as colony-forming units per litre of water sample (CFU/L). Chi-square statistics with Yates’ correction for continuity was used to detect significant differences in percentage contamination rates between samples from hot- and cold-water mains.
2.4. Identification of Legionella Isolates
The identification of Legionella isolates was performed by molecular analysis based on sequencing of the mip gene [4]. Genomic DNA was extracted from the isolates grown on BCYE medium using a thermal shock method, followed by PCR amplification of the mip gene with primers Legmip_f and Legmip_r [18]. PCR reactions were set up with 0.4 μL of DNA in a final volume of 30 μL and included a concentration of 200 μM deoxynucleotides (dNTPs) (Thermo Fisher Scientific, Waltham, MA, USA) and 0.4 μM of each primer (Macrogen, Seoul, Korea) and 1 U of Taq polymerase (Thermo Fisher Scientific, Waltham, MA, USA). PCR amplification consisted of 35 cycles of denaturation at 94 °C for 1 min, annealing for 2 min at 58 °C, and extension for 2 min at 72 °C. Amplicons were visualized by gel electrophoresis, purified with the Wizard SV Gel and PCR Clean-Up System (Promega, Madison, WI, USA) and sequenced by the Sanger method (Macrogen, Seoul, Korea). Electropherograms were analysed and edited with the Mega software (http://www.megasoftware.net) and the resulting sequences were searched for similar mip genes belonging to Legionella reference strains in the GenBank database with the Basic Local Alignment Search Tool (BLAST).
3. Results and Discussion
3.1. Rate of Legionella Colonization
Table 1 reports the Legionella contamination rates, expressed as the ratio of contaminated buildings to the total number of buildings comprised in each campus, together with the number of taps per contamination severity level. In this respect, we have classified each sampling point in three levels of contamination: (i) absence of contamination, when Legionella counts were below the detection limit of 102 CFU/L; (ii) low contamination, when counts comprised between 102 and 104 CFU/L; and (iii) high contamination, when counts were over 104 CFU/L (Table 1). The severity level was determined by the maximum value detected among all the samples taken from each control point (i.e., hot and cold water).

Table 1.
Rate of Legionella contamination in the building plumbing system of University campuses.
Although only three campuses were completely free of Legionella, the contamination rates were quite low since these bacteria were detected in only 12 buildings among the 41 monitored (29%). With respect to the control points, the rate of contamination was even lower, with only 23 contaminated taps among the total of 156 analyzed (14.7%). Furthermore, high-level contaminations were found in only 9 of these taps (5.8% of the total) located in 5 buildings on only 2 campuses (Nos. 5 and 6). Importantly, these buildings are used by less than 20% of the total University users and mostly by students, who tend to have sporadic attendance at the premises. This implies a low frequency of exposure and, consequently, a limited risk of infection for users [19].
A recent meta-analysis indicated the frequent occurrence of legionellosis in occupational settings, with 27.3% of cases in office buildings [19]. Nevertheless, only a few papers have investigated the presence of Legionella in this kind of system, and none in university buildings. Our data indicated that the contamination rate recorded in the University of Perugia buildings was lower than that found in offices or schools in other countries, ranging from more than 30% in Germany [16] to 60% in Japan and Hungary [14,15], and even lower than the 30.5% rate recorded in private apartments in Italy [13]. Moreover, even if many of the buildings at the University of Perugia are very ancient (ranging in age from the 1960s back to the 13th century) and it has been previously shown that the rate of positive samples increases with the age of the building [15], we found that only 1 of the 12 contaminated buildings was older than one century, while all the others were very recent (i.e., less than 30 years-old). It is important to underline that the age of a building’s water distribution system is often difficult to assess because pipelines may undergo partial or complete reconstruction. In any case, it was reported that new plumbing systems were significantly less likely to be contaminated by Legionella only when they were less than 9 years old [15].
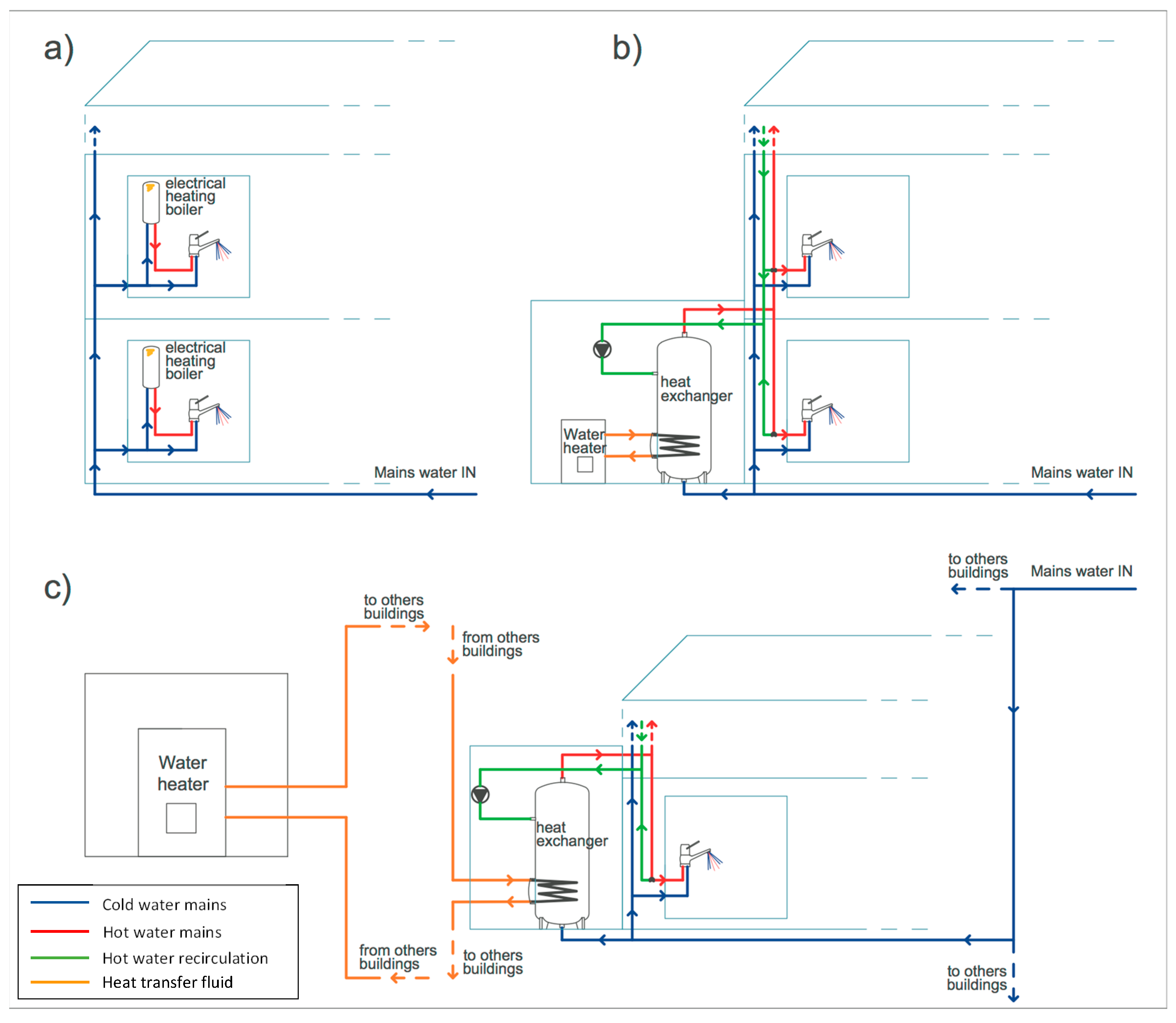
It is well established that the type of hot-water production system has a strong influence on the potential threat of microbial contaminations in plumbing [15,20]. In this respect, the buildings of the University of Perugia, even within the same campus, were very heterogeneous (Table 1). Some buildings, namely those located in campuses Nos. 1, 2, 3, 4 and 8, did not have hot-water production and, indeed, no contaminated taps were found therein. On the contrary, all the contaminations were found in buildings where a hot-water production system was in place, either based on single-point electrical boilers with hot-water storage located in individual premises or on a centralized facility with recirculating hot-water network. Buildings that had only a limited number of single-point heaters in a few premises showed either an absence of Legionella (those in campuses Nos. 1, 2 and 3) or low concentrations (those in campus No. 4). Most of the contaminated taps were found in the buildings of campuses Nos. 3, 5, 6 and 7, which were characterized by either the centralized production of hot water or the presence of single-point heaters in all the premises. Nevertheless, only 9 of these taps, those in campuses Nos. 5 and 6, showed high levels of contamination (above 104 CFU/L). While all the control points of campus No. 6, where a centralized hot-water production system was present, were found contaminated, campus No. 5, characterized by hot water produced in each premise by electrical boilers, presented a more heterogeneous situation with only 9 taps, out of the 40 monitored, found to be contaminated by Legionella. This corresponded to a 22% contamination rate, with 12% and 10% of low and high levels of contamination, respectively. It is well known that Legionella is more likely to be found in hot-water mains and, furthermore, the production of hot water by centralized systems has been often associated with higher rates of contamination [13,14,15,21]. Stagnation, low circulation efficiency, and temperature drop within the hot-water network are also considered typical risk factors of large buildings with extensive plumbing systems [15]. Nevertheless, in the largest buildings of the University, namely those of campus No. 6, no significant decrease of the hot-water temperature at distal sampling points was observed (data not shown).
3.2. Effects of Water Heating
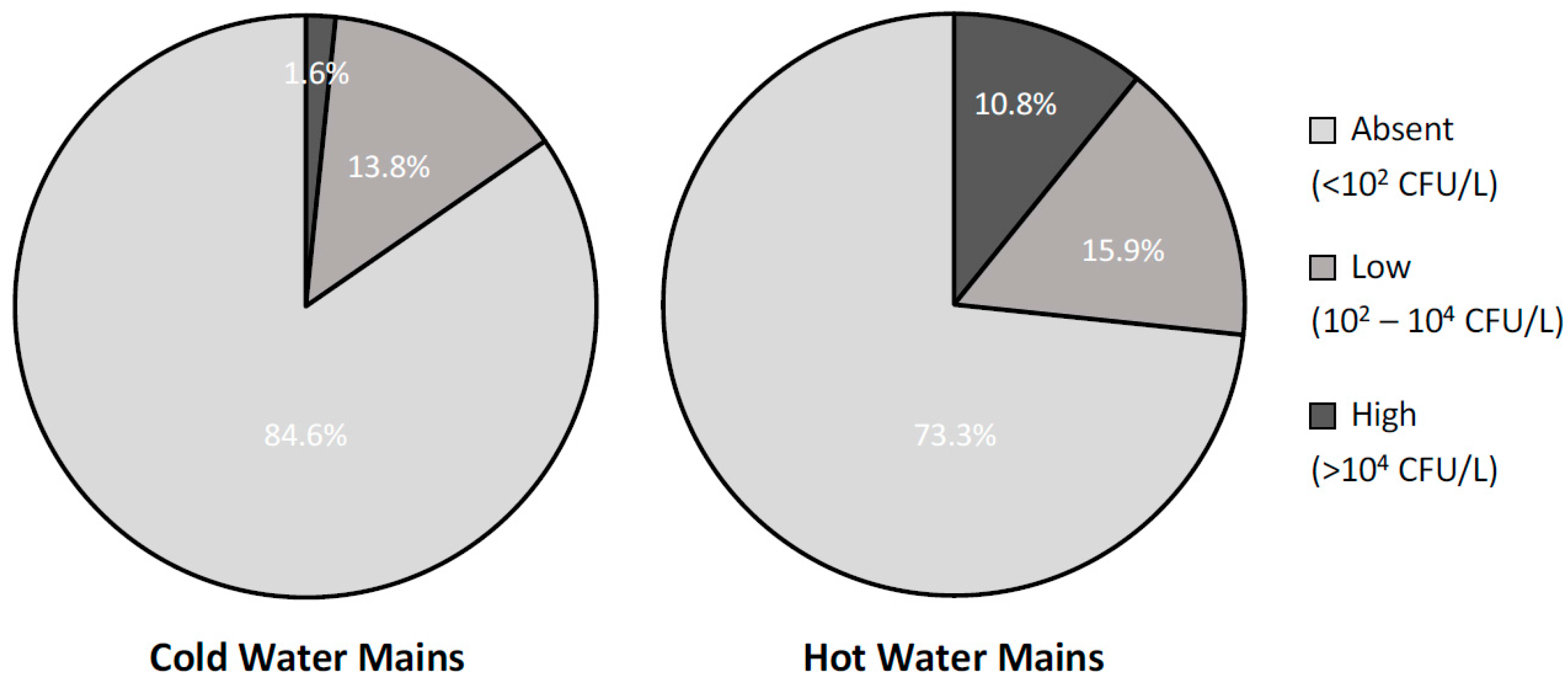
To further investigate the effects of water heating on the presence of Legionella in building plumbing, Figure 2 reports the contamination rates, divided by severity levels, found in samples collected from the cold- and hot-water distribution networks. As expected, hot-water mains showed a statistically significant higher rate of contamination than the cold-water ones (26.7% and 15.4%, respectively; X2 = 5, p = 0.02) and, in particular, a much higher rate of high-severity level contaminations (10.8% and 1.6%, respectively; X2 = 10, p = 0.001). Interestingly, all the contaminated samples from cold-water mains were taken in buildings where a hot-water production system was also in place and where water samples from the hot-water mains were contaminated as well. Furthermore, if only post-flush samples, i.e., those representing the status of the entire network rather than a single tap, were considered, the rate of contaminated samples from the cold-water mains dropped to 8.1% with no high-level contaminations present. This evidence suggests that the presence of Legionella in the cold-water mains might be due, at least in part, to possible cross-contaminations with the hot-water ones occurring in the terminal part of the plumbing.
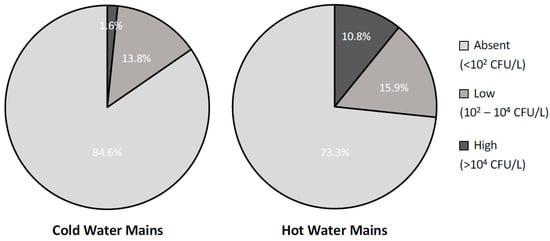
Figure 2.
Percentage distribution of the analysed water samples (n = 302) by level of contamination severity in samples collected from cold- and hot-water mains (n = 182 and 120, respectively).
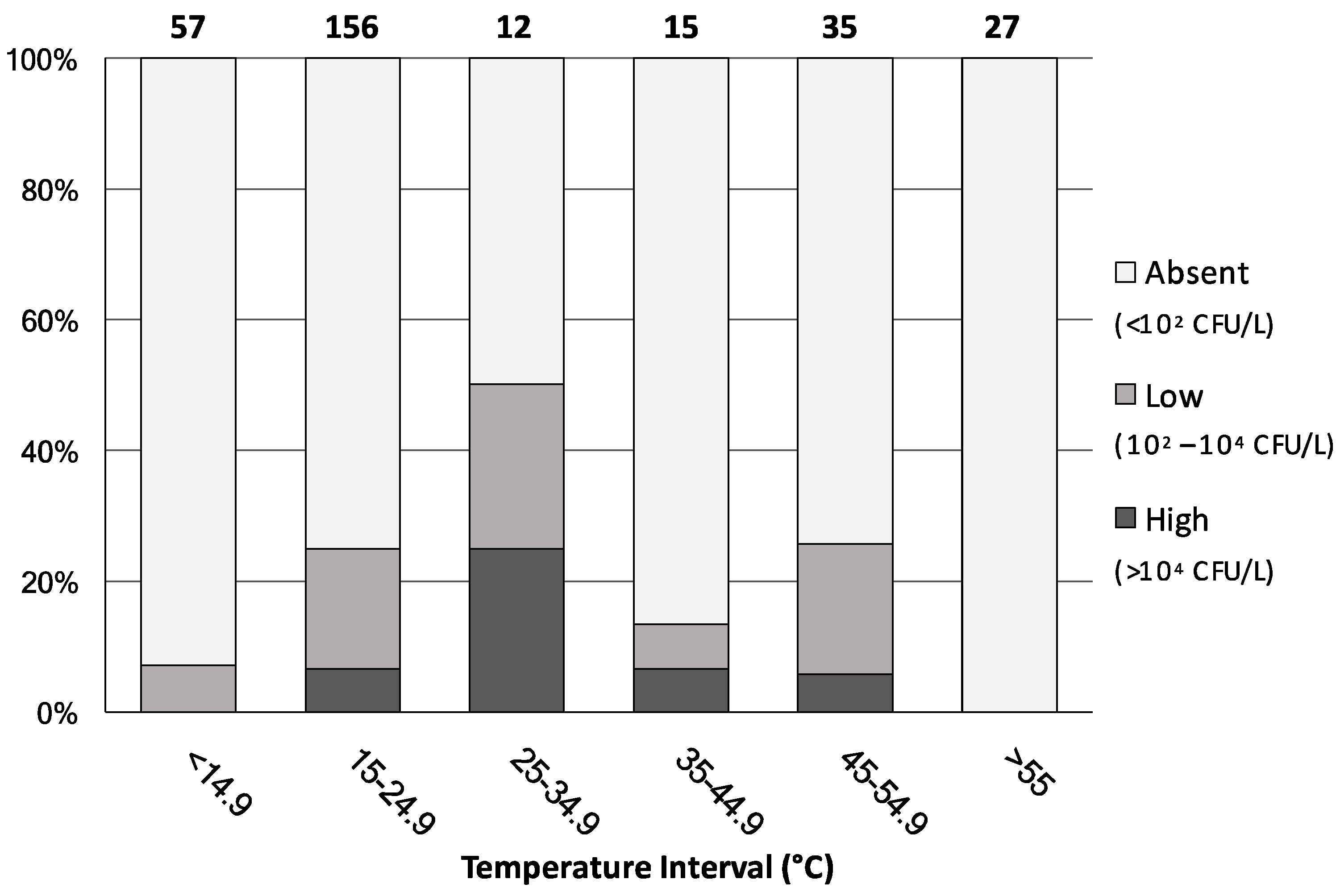
Figure 3 shows the percentage distribution of all 302 water samples, irrespective of their water-network origin, analysed by level of contamination severity and water-temperature interval.
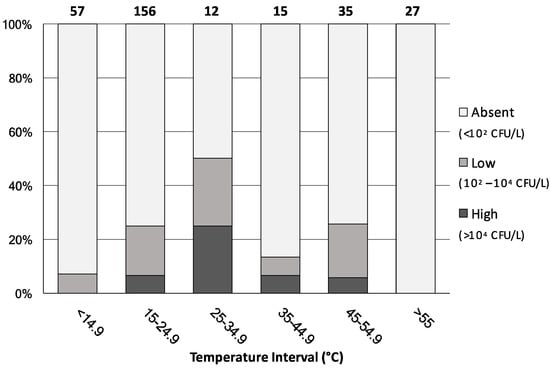
Figure 3.
Percentage distribution of the analysed water samples (n = 302) by level of contamination severity and by water-temperature intervals. Numbers above bars indicate the number of samples in each interval.
When water temperature was below 14.9 °C or above 55 °C, the rate of Legionella colonization was either minimal or totally absent, respectively. On the contrary, the highest proportion of the Legionella positive samples was mainly observed in the 25–55 °C temperature ranges, where almost the totality of the 62 samples came from hot-water mains. Interestingly, among the 156 samples showing a water temperature of between 15 and 24.9 °C, 39 (25% of the total) were found contaminated with Legionella. These samples originated either from the cold-water mains or from the hot-water network where the hot-water production system was shut-off or not working properly. These data confirm that water temperature represents a key risk factor for Legionella colonization and further support the international recommended value of 55 °C for hot-water temperature [9]. Nevertheless, in public buildings it often happens that the temperature of hot water is found below 50 °C, because of energy-saving measures, to prevent the risk of burns, or due to malfunctions in the water-heating and distribution systems [15,21]. Our results also indicate the occurrence of this microorganism in cold-water samples. Indeed, although the growth of Legionella is believed to be restricted to temperatures between 25 and 42 °C, it has been isolated from natural freshwater environments at temperatures below 15 °C [22] and, recently, detected in public and private cold-water taps across the United States [23].
3.3. Phylogenetic Analysis
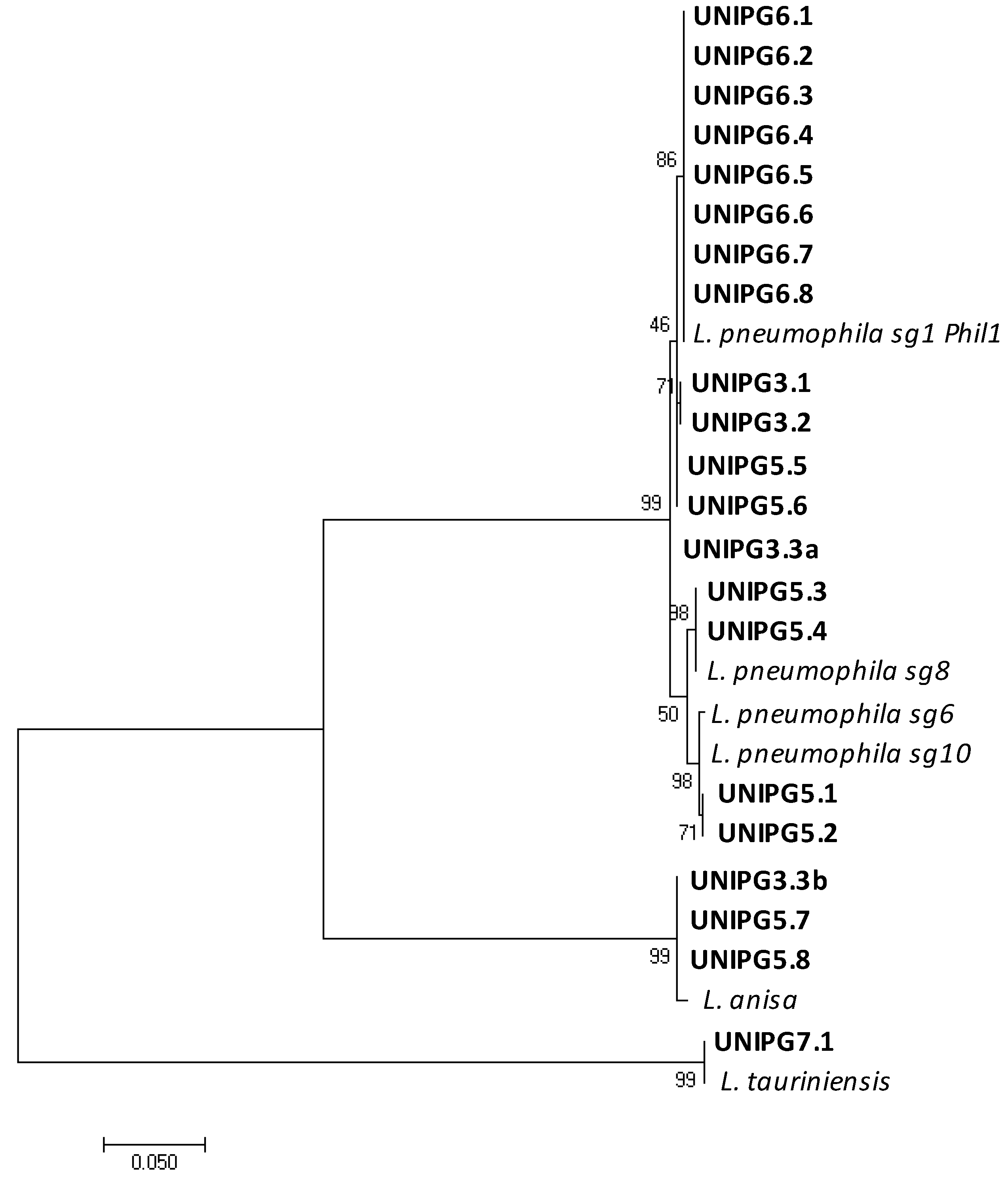
Considering that several species of Legionella have been identified but only few of them have been associated with human disease [1], we used a molecular analysis based on mip gene sequencing to identify the Legionella species and to differentiate the serogroups of L. pneumophila within the strains retrieved from the University buildings’ plumbing (Table 1 and Figure 4).
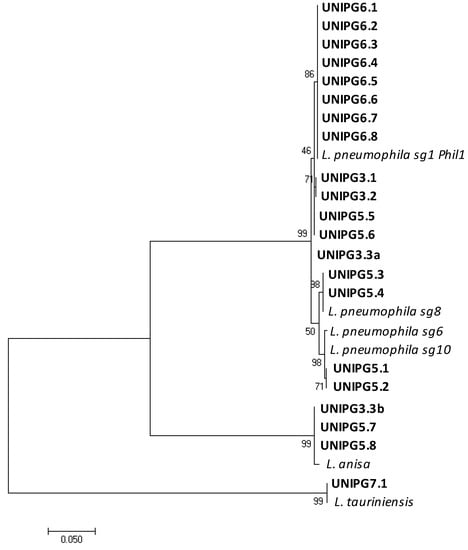
Figure 4.
Maximum likelihood phylogenetic tree showing similarities between mip gene sequences of isolates found in this study (in bold) and those of closely related reference strains of Legionella retrieved in the GenBank database. Only one representative isolate for each group of identical sequences from the same control point is reported.
Since several isolates were cultured from each water sample, all the sequences were aligned, and a representative one was chosen from each group of isolates obtained from the same control-point tap and identified as the same Legionella species. The phylogenetic tree reported in Figure 4 shows the genetic relationships among the mip gene sequences of the 21 isolates found in this study and those of closely related reference strains of Legionella. The presence of L. pneumophila was shown in 17 taps, with sg 1 being the most represented (13 taps located in campuses Nos. 3, 5 and 6), while sg 8 and sg 6–10 were found only in 4 contaminated taps of campus No. 5. Other distant related Legionella species were also found, namely L. anisa (in 1 tap of campus No. 3 and 2 taps of campus No. 5) and L. tauriniensis in 1 tap of campus No. 7. Interestingly, only a single species of Legionella was found in each contaminated tap, with the only exception of one tap in a building of campus No. 3 where two different species were recovered, namely L. pneumophila sg 1, and L. anisa. As pointed out for the contamination rates, campus No. 5 showed a high heterogeneity also in respect of the Legionella species found in the hot water from the different electrical boilers (L. pneumophila sg 1, sg 8, sg 6–10 and L. anisa). On the contrary, in all the control points of campus No. 6, characterized by a centralized hot-water production system, only L. pneumophila sg1 was found. This evidence further supports the hypothesis that Legionella growth and proliferation may likely occur inside the hot-water production system and from there spread to the different taps. Our data are largely in agreement with other works indicating that strains of L. pneumophila, the species mostly associated to legionellosis, are those mostly often found to contaminate taps of private apartments, hotels and hospitals in Italy [13] as well as buildings in Hungary, Germany, the USA and Japan [14,15,16,23]. Nevertheless, we also recovered several isolates of L. anisa, a species already found in building plumbing [14] and known to be associated with human infections as one of the pathogens causing Pontiac fever [1]; as well as one isolate identified as L. tauriniensis, a species recently isolated from a hospital humidifier in Turin, Italy [24] but never linked to human disease.
3.4. Remedial Actions in the Contaminated Buildings
Italian and international guidelines suggest the intervention needed for the prevention of Legionella contamination in the plumbing of premises based both on Legionella concentration and positive sample rate [9,10]. While international guidelines usually define 103 CFU/L as the limit of the Legionella count for public health concerns, the Italian ones consider a system completely under control only when Legionella is absent in all samples. With counts up to 104 CFU/L, carrying out disinfection of the water-distribution system is suggested when 20% or 50% of the collected samples per building are positive, according to Italian and international guidelines respectively. However, immediate action is generally recommended in the case of Legionella counts over 104 CFU/L. In the case study reported here, Legionella was found in five campuses but in only two cases, namely campuses Nos. 5 and 6, the severity levels of contamination were above the limit at which a remedial action is required (Table 2). In addition, the building of the laboratory animal facility in campus No. 3 required disinfection because, despite a contamination classified as a low level of severity, Legionella was present in most of the control points assessed (Table 2). In the two remaining cases, namely campuses Nos. 4 and 7, no immediate decontamination was necessary because of the low levels of severity and rates recorded. Furthermore, in the case of campus No. 7 the contamination was ascribed to a strain of L. tauriniensis, a species never associated with clinical cases.

Table 2.
Rate of Legionella contamination in the building plumbing system of the University campuses.
The type of remedial action implemented was based on the characteristics of the hot-water production and distribution systems of each campus (Figure 5) and on the indications of treatment methods for both emergency and long-term measures provided by Italian and European Guidelines [9,10]. To confirm the effectiveness of the decontamination interventions, follow-up monitoring of the presence of Legionella was carried out according to Italian guidelines by repeating the water sampling of the positive taps 48 h, 1 and 3 months after the intervention [10] and the results are reported in Table 2.
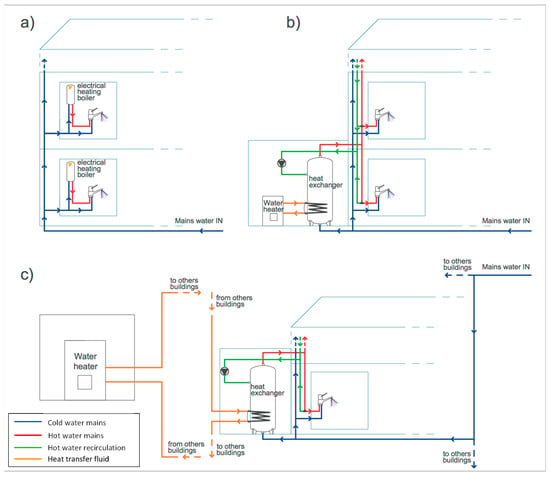
Figure 5.
Hot-water production and distribution systems of campus (a) No. 5, (b) No. 3, and (c) No. 6.
Campus No. 5 is composed of six new buildings where each premise has its own single-point electrical boiler with hot-water storage (Figure 5a). As already underlined, due to the system of hot-water production, these buildings presented the highest heterogeneity of Legionella contamination, both in terms of severity level and Legionella species recovered. Indeed, to monitor the Legionella presence in this campus properly, a considerable sampling effort, based on the analysis of water samples from 40 different control points, was undertaken. All the taps served by electrical boilers set at a temperature higher than 55 °C were free of Legionella, while contaminations were found only within the boilers that were shut-off or set below 45 °C, with the only exception of one tap where the hot-water temperature was 53 °C. Nevertheless, the electrical boilers allowed higher hot-water temperatures to be achieved and maintained. A thermal disinfection of the contaminated boilers was thus implemented, raising and maintaining the water temperature above the level at which Legionella cells do not survive (>60 °C) [2,9]. This approach proved to be effective as Legionella was absent in the water samples taken in the following monitoring period (Table 2). The effectiveness of constant maintenance of the hot-water temperature above 60 °C has been largely demonstrated in hospitals, hotels and households, particularly those with small hot-water production systems, such as single-point electrical boilers [15,16]. Indeed, provided there is sufficient heating capacity, this approach is relatively easy to implement and monitor, although increasing energy consumption and risk of scalding for users may be possible disadvantages [9,15].
The building hosting the laboratory animal facility was the only one of campus No. 3 showing Legionella contamination. This building featured a centralized hot-water production system, where water is stored in a tank, heated via a coil (heat exchanger) from a boiler and then distributed to the premises (Figure 5b). In this case, a combination of hyperchlorination (75 mg/L of sodium hypochlorite for 3 h) and thermal shock (70 °C for 90 min) was implemented [9,25]. Furthermore, the thermal treatment was repeated every week. This intervention was proven to be successful in the follow-up monitoring as no recolonization of the plumbing system by Legionella was observed (Table 2). Chlorine is often used in the treatment of hot-water systems, although consistent effective levels, especially at distal points, may be difficult to achieve in large plumbing networks and in the presence of hot water or above pH 7 [9,25].
Campus No. 6 features the most complicated hot-water network, with centralized systems where hot water is produced and stored by three heat exchangers, one for each of the three buildings, using the room heating system coming from a centralized facility (Figure 5c). High levels of L. pneumophila sg 1 were found in the samples taken from the hot-water taps and in the heat exchangers, despite the hot-water production system being off at the time of sampling. In fact, sampling was performed in the summer period when room heating was not necessary and, consequently, hot water was not produced. In this period, the daily average ambient temperature ranged from 20 to 32 °C, and, in fact, the temperature of the water samples analyzed ranged from 23 to 25 °C, a level that does not limit Legionella proliferation. Legionella was not found in cold-water samples taken after flushing, as well as in the common part of the water distribution system (network inlet, reservoir tank, water softener), indicating that the contamination was limited to the hot-water mains and originated inside the heat exchanger, where water stagnation and presence of loose deposits may have favored the growth of Legionella [6]. After turning on the hot-water production system, water temperature ranged, during the day, between 22 and 48 °C. This was due to the hot-water production system, based on heat exchange with the heat-transfer fluid, not enabling values high enough for a thermal disinfection to be achieved [9,10,25,26]. Consequently, a chemical treatment with silver hydrogen peroxide was carried out by adding 6 kg/m3 of disinfectant directly to the heat exchanger, filling the hot-water mains, and allowing this to remain in contact for 12 h. One week after disinfection, Legionella was absent in all samples, while after one month hot-water samples were contaminated again, though at low levels of severity (Table 2). After four months, the contamination overreached the levels of before disinfection (Table 2), suggesting that the re-growth of Legionella was favored by the conditions inside the heat exchanger, probably because of the presence of loose deposits and a permissive temperature for cell growth. Since the treatment with silver hydrogen peroxide was shown to be effective in controlling Legionella growth only in the short-term, it was decided to close down the independent hot-water production and distribution systems (Figure 5c) to prevent infections. Despite treatment with a stable concentrated solution of hydrogen peroxide and silver being able to exploit the bactericidal action of the two components and their synergy, this approach has been, to date, used in only a limited way for Legionella decontamination, especially as an emergency measure. It should, therefore, be further validated in real-case scenarios to ensure its effectiveness [9,25].
4. Conclusions
A one-year-long survey of the presence of Legionella bacteria in the plumbing of premises within the sparse campuses of the University of Perugia indicated that contaminations were limited to a few buildings and mainly ascribed to L. pneumophila. The contamination rate of the University buildings was much lower than that reported for office or school plumbing systems in other countries, namely Germany, Hungary and Japan, and even lower than that recorded in private apartments in Italy. Contaminations were related to the type of hot-water production systems, with centralized ones showing the highest rates. Disinfections were effective in those cases where the hot-water production systems enabled maintenance of the water temperature above the level at which Legionella cells do not survive (>60 °C). On the contrary, in a campus where the contamination originated in the heat exchanger, a chemical disinfection with silver hydrogen peroxide was carried out but proved to be effective only in the short-term.
The results obtained in this case study, indicate how a multidisciplinary approach that integrates microbiological analysis with the survey of buildings’ plumbing and water-heating systems is necessary for a useful monitoring of Legionella in complex occupational settings, such as academic buildings. Indeed, while international and Italian guidelines provide general indicators for the control of the infection risk, other parameters, such as the identification of the Legionella strains present in the plumbing, the characteristics of the hot-water production system, and the potential exposure routes should be considered for the implementation of an effective strategy to prevent Legionella survival and diffusion.
Acknowledgments
This research was funded by the University of Perugia, the “Fondo Ricerca di Base 2015” program of the Dept. of Chemistry, Biology and Biotechnology, Fondazione Cassa Risparmio Perugia, under the project “Hydraulic and Microbiological Combined Approach Towards Water Quality Control (No. 2015.0383.021)”, and the Italian Ministry of Education, University and Research (MIUR) under the projects of relevant national interest (PRIN): “Advanced Analysis Tools for the Management of Water Losses in Urban Aqueducts” and “Tools and Procedures for an Advanced and Sustainable Management of Water Distribution Systems”.
Author Contributions
Ermanno Federici and Silvia Meniconi conceived and designed the experiments; Elisa Ceci, Elisa Mazzetti, Chiara Casagrande, Elena Montalbani and Stefania Businelli performed the experiments; Ermanno Federici, Elisa Ceci and Elisa Mazzetti analyzed the data; Stefania Businelli, Tatiana Mariani and Paolo Mugnaioli contributed reagents/materials/analysis tools; Ermanno Federici, Silvia Meniconi, Giovanni Cenci and Bruno Brunone wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mercante, J.W.; Winchell, J.M. Current and emerging Legionella diagnostics for laboratory and outbreak investigations. Clin. Microbiol. Rev. 2015, 28, 95–133. [Google Scholar] [CrossRef] [PubMed]
- Berjeaud, J.M.; Chevalier, S.; Schlusselhuber, M.; Portier, E.; Loiseau, C.; Aucher, W.; Lesouhaitier, O.; Verdon, J. Legionella pneumophila: The paradox of a highly sensitive opportunistic waterborne pathogen able to persist in the environment. Front. Microbiol. 2016, 7, 486. [Google Scholar] [CrossRef] [PubMed]
- Legionnaire’s Disease-Annual Epidemiological Report for 2015; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2017.
- Ratcliff, R.M. Sequence-based identification of Legionella. In Legionella: Methods and Protocols; Springer Science + Business Media: Berlin, Germany, 2012; pp. 57–72. [Google Scholar]
- Liu, G.; Verberk, J.Q.; Van Dijk, J.C. Bacteriology of drinking water distribution systems: An integral and multidimensional review. Appl. Microbiol. Biotechnol. 2013, 97, 9265–9276. [Google Scholar] [CrossRef] [PubMed]
- Prest, E.I.; Hammes, F.; van Loosdrecht, M.C.; Vrouwenvelder, J.S. Biological Stability of Drinking Water: Controlling Factors, Methods, and Challenges. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Stojek, N.M.; Dutkiewicz, J. Co-existence of Legionella and other Gram-negative bacteria in potable water from various rural and urban sources. Ann. Agric. Environ. Med. 2011, 18, 330–334. [Google Scholar] [PubMed]
- Laganà, P.; Caruso, G.; Piccione, D.; Gioffrè, M.E.; Pino, R.; Delia, S. Legionella spp., amoebae and not-fermenting Gram negative bacteria in an Italian university hospital water system. Ann. Agric. Environ. Med. 2014, 21, 489–493. [Google Scholar] [CrossRef] [PubMed]
- European Technical Guidelines for the Prevention, Control and Investigation of Infections Caused by Legionella Species; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2017.
- Linee Guida per la Prevenzione ed il Controllo Della Legionellosi; Ministero della Salute: Rome, Italy, 2015. (In Italian)
- Collins, S.; Stevenson, D.; Walker, J.; Bennett, A. Evaluation of Legionella real-time PCR against traditional culture for routine and public health testing of water samples. J. Appl. Microbiol. 2017, 122, 1692–1703. [Google Scholar] [CrossRef] [PubMed]
- Whiley, H.; Taylor, M. Legionella detection by culture and qPCR: Comparing apples and oranges. Crit. Rev. Microbiol. 2016, 42, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Leoni, E.; De Luca, G.; Legnani, P.P.; Sacchetti, R.; Stampi, S.; Zanetti, F. Legionella waterline colonization: Detection of Legionella species in domestic, hotel and hospital hot water systems. J. Appl. Microbiol. 2005, 98, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Edagawa, A.; Kimura, A.; Tanaka, H.; Tomioka, K.; Sakabe, K.; Nakajima, C.; Suzuki, Y. Detection of culturable and nonculturable Legionella species from hot water systems of public buildings in Japan. J. Appl. Microbiol. 2008, 105, 2104–2114. [Google Scholar] [CrossRef] [PubMed]
- Barna, Z.; Kádár, M.; Kálmán, E.; Szax, A.S.; Vargha, M. Prevalence of Legionella in premise plumbing in Hungary. Water Res. 2016, 90, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Kruse, E.B.; Wehner, A.; Wisplinghoff, H. Prevalence and distribution of Legionella spp. in potable water systems in Germany, risk factors associated with contamination, and effectiveness of thermal disinfection. Am. J. Infect. Control 2016, 44, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Ferramosche Betti, R.; Umbra Acque S.p.A., Perugia, Italy. Personal communication, 2017.
- Ratcliff, R.M.; Lanser, J.A.; Manning, P.A.; Heuzenroeder, M.W. Sequence-Based Classification Scheme for the Genus Legionella Targeting the mip Gene. J. Clin. Microbiol. 1998, 36, 1560–1567. [Google Scholar] [PubMed]
- Principe, L.; Tomao, P.; Visca, P. Legionellosis in the occupational setting. Environ. Res. 2017, 152, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Falkinham, J.O.; Hilborn, E.D.; Arduino, M.J.; Pruden, A.; Edwards, M.A. Epidemiology and ecology of opportunistic premise plumbing pathogens: Legionella pneumophila, Mycobacterium avium, and Pseudomonas aeruginosa. Environ. Health. Perspect. 2015, 123, 749. [Google Scholar] [CrossRef] [PubMed]
- Toyosada, K.; Otani, T.; Shimizu, Y.; Managi, S. Water quality study on the hot and cold water supply systems at Vietnamese Hotels. Water 2017, 9, 251. [Google Scholar] [CrossRef]
- Wullings, B.A.; van der Kooij, D. Occurrence and genetic diversity of uncultured Legionella spp. in drinking water treated at temperatures below 15 °C. Appl. Environ. Microbial. 2006, 72, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Donohue, M.J.; O’Connell, K.; Vesper, S.J.; Mistry, J.H.; King, D.; Kostich, M.; Pfaller, S. Widespread molecular detection of Legionella pneumophila serogroup 1 in cold water taps across the United States. Environ. Sci. Technol. 2014, 48, 3145–3152. [Google Scholar] [CrossRef] [PubMed]
- Lo Presti, F.; Riffard, S.; Meugnier, H.; Reyrolle, M.; Lasne, Y.; Grimont, P.A.; Grimont, F.; Vandenesch, F.; Etienne, J.; Fleurette, J.; et al. Legionella taurinensis sp. nov., a new species antigenically similar to Legionella spiritensis. Int. J. Syst. Bacteriol. 1999, 49, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.E.; Stout, J.E.; Victor, L.Y. Controlling Legionella in hospital drinking water: An evidence-based review of disinfection methods. Infect. Control Hosp. Epidemiol. 2011, 32, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Meier, T.; Bendinger, B. Survival of pathogens in drinking water plumbing systems: Impact factors and sanitation options. Water Sci. Technol. Water Supply 2016, 16, 931–941. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).