Phenological Variation in Bluebunch Wheatgrass (Pseudoroegneria spicata): Implications for Seed Sourcing, Harvest, and Restoration
Abstract
:1. Introduction
2. Materials and Methods
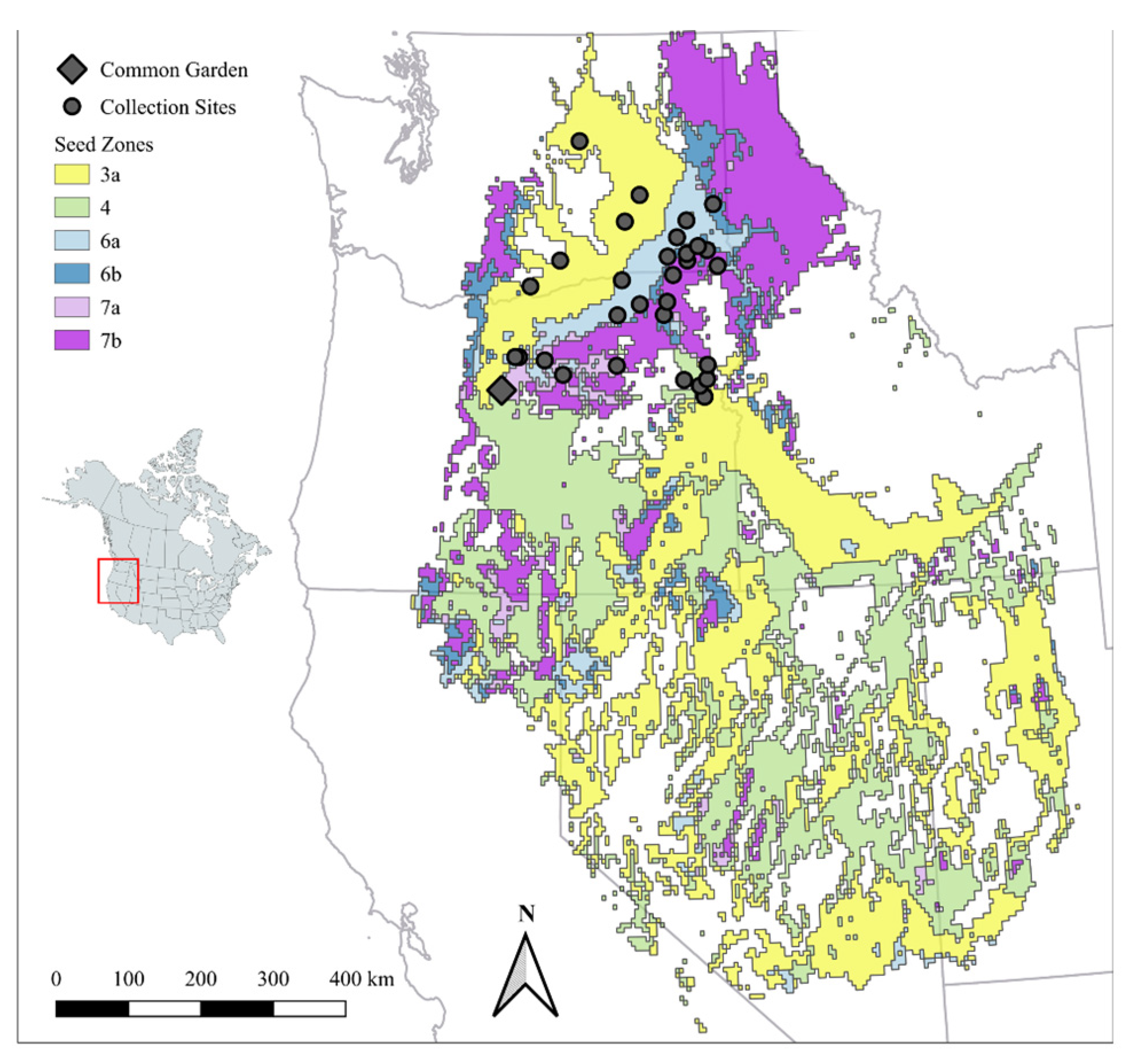
2.1. Experimental Species and Design
2.2. Data Collection
2.3. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pyke, D.A.; Wirth, T.A.; Beyers, J.L. Does Seeding After Wildfires in Rangelands Reduce Erosion or Invasive Species? Restor. Ecol. 2013, 21, 415–421. [Google Scholar] [CrossRef]
- Peppin, D.L.; Fulé, P.Z.; Sieg, C.H.; Beyers, J.L.; Hunter, M.E.; Robichaud, P.R. Recent trends in post-wildfire seeding in western US forests: Costs and seed mixes. Int. J. Wildland Fire 2011, 20, 702–708. [Google Scholar] [CrossRef] [Green Version]
- USDA. Increasing the Pace of Restoration and Job Creation on Our National Forests; Published Report; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 2012. [Google Scholar]
- Edwards, F.; Kulpa, S.M.; Kilkenny, F.F. Application of national seed strategy concepts. In Science Framework for Conservation and Restoration of the Sagebrush Biome: Linking the Department of the Interior’s Integrated Rangeland Fire Management Strategy to Long-Term Strategic Conservation Actions. Part 2. Management Applications; General Technical Report RMRS-GTR-389; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2019; Chapter 6; pp. 113–130. [Google Scholar]
- Johnson, G.; Sorensen, F.C.; Clair, J.B.S.; Cronn, R. Pacific Northwest Forest Tree Seed Zones: A Template for Native Plants? Nativ. Plants J. 2004, 5, 131–140. [Google Scholar] [CrossRef]
- Bucharova, A.; Michalski, S.; Hermann, J.-M.; Heveling, K.; Durka, W.; Hölzel, N.; Kollmann, J.; Bossdorf, O. Genetic differentiation and regional adaptation among seed origins used for grassland restoration: Lessons from a multispecies transplant experiment. J. Appl. Ecol. 2017, 54, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Jones, T.A. When local isn’t best. Evol. Appl. 2013, 6, 1109–1118. [Google Scholar] [CrossRef]
- Massatti, R.; Prendeville, H.R.; Larson, S.; Richardson, B.A.; Waldron, B.; Kilkenny, F. Population history provides foundational knowledge for utilizing and developing native plant restoration materials. Evol. Appl. 2018, 11, 2025–2039. [Google Scholar] [CrossRef]
- Richards, R.T.; Chambers, J.C.; Ross, C. Use of Native Plants on Federal Lands: Policy and Practice. J. Range Manag. 1998, 51, 625. [Google Scholar] [CrossRef]
- Broadhurst, L.M.; Lowe, A.; Coates, D.J.; Cunningham, S.; McDonald, M.; Vesk, P.A.; Yates, C. Seed supply for broadscale restoration: Maximizing evolutionary potential. Evol. Appl. 2008, 1, 587–597. [Google Scholar] [CrossRef]
- Rogers, D.L. Genetic Erosion: No Longer Just an Agricultural Issue. Nativ. Plants J. 2004, 5, 112–122. [Google Scholar] [CrossRef]
- Basey, A.C.; Fant, J.B.; Kramer, A.T. Producing native plant materials for restoration: 10 rules to collect and maintain genetic diversity. Nativ. Plants J. 2015, 16, 37–53. [Google Scholar] [CrossRef]
- Baughman, O.W.; Agneray, A.C.; Forister, M.L.; Kilkenny, F.F.; Espeland, E.K.; Fiegener, R.; Horning, M.E.; Johnson, R.C.; Kaye, T.N.; Ott, J.; et al. Strong patterns of intraspecific variation and local adaptation in Great Basin plants revealed through a review of 75 years of experiments. Ecol. Evol. 2019, 9, 6259–6275. [Google Scholar] [CrossRef] [Green Version]
- McKown, A.D.; Guy, R.D.; Klápště, J.; Geraldes, A.; Friedmann, M.; Cronk, Q.; El-Kassaby, Y.A.; Mansfield, S.; Douglas, C.J. Geographical and environmental gradients shape phenotypic trait variation and genetic structure in P opulus trichocarpa. New Phytol. 2014, 201, 1263–1276. [Google Scholar] [CrossRef]
- Williams, S.L. Reduced Genetic Diversity in Eelgrass Transplantations Affects Both Population Growth and Individual Fitness. Ecol. Appl. 2001, 11, 1472–1488. [Google Scholar] [CrossRef]
- Jump, A.S.; Marchant, R.; Penuelas, J. Environmental change and the option value of genetic diversity. Trends Plant Sci. 2009, 14, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Reusch, T.B.H.; Ehlers, A.; Hammerli, A.; Worm, B. Ecosystem recovery after climatic extremes enhanced by genotypic diversity. Proc. Natl. Acad. Sci. USA 2005, 102, 2826–2831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawford, K.M.; Whitney, K. Population genetic diversity influences colonization success. Mol. Ecol. 2010, 19, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Espeland, E.K.; Emery, N.C.; Mercer, K.L.; Woolbright, S.A.; Kettenring, K.; Gepts, P.; Etterson, J. Evolution of plant materials for ecological restoration: Insights from the applied and basic literature. J. Appl. Ecol. 2016, 54, 102–115. [Google Scholar] [CrossRef]
- Kilkenny, F.F. Genecological Approaches to Predicting the Effects of Climate Change on Plant Populations. Nat. Areas J. 2015, 35, 152–164. [Google Scholar] [CrossRef]
- Ying, C.C.; Yanchuk, A.D. The development of British Columbia’s tree seed transfer guidelines: Purpose, concept, methodology, and implementation. For. Ecol. Manag. 2006, 227, 1–13. [Google Scholar] [CrossRef]
- Johnson, R.C.; Erickson, V.J.; Mandel, N.L.; Clair, J.B.S.; Vance-Borland, K.W. Mapping genetic variation and seed zones for Bromus carinatus in the Blue Mountains of eastern Oregon. Botany 2010, 88, 725–736. [Google Scholar] [CrossRef]
- Clair, J.B.S.; Kilkenny, F.F.; Johnson, R.C.; Shaw, N.L.; Weaver, G. Genetic variation in adaptive traits and seed transfer zones for P seudoroegneria spicata (bluebunch wheatgrass) in the northwestern U nited S tates. Evol. Appl. 2013, 6, 933–948. [Google Scholar] [CrossRef]
- Hess, M.; Barralis, G.; Bleiholder, H.; Buhr, L.; Eggers, T.; Hack, H.; Stauss, R. Use of the extended BBCH scale—General for the descriptions of the growth stages of mono- and dicotyledonous weed species. Weed Res. 1997, 37, 433–441. [Google Scholar] [CrossRef]
- Johnson, R.C.; Cashman, M.J.; Vance-Borland, K. Genecology and Seed Zones for Indian Ricegrass Collected in the Southwestern United States. Rangel. Ecol. Manag. 2012, 65, 523–532. [Google Scholar] [CrossRef]
- Reekie, E.G.; Bazzaz, F.A. Reproductive Effort in Plants. 1. Carbon Allocation to Reproduction. Am. Nat. 1987, 129, 876–896. [Google Scholar] [CrossRef]
- Kozlowski, J. Optimal allocation of resources to growth and reproduction: Implications for age and size at maturity. Trends Ecol. Evol. 1992, 7, 15–19. [Google Scholar] [CrossRef]
- Kulpa, S.M.; Leger, E.A. Strong natural selection during plant restoration favors an unexpected suite of plant traits. Evol. Appl. 2013, 6, 510–523. [Google Scholar] [CrossRef] [PubMed]
- Gross, R.S.; Werner, P.A. Relationships among Flowering Phenology, Insect Visitors, and Seed-Set of Individuals: Experimental Studies on Four Co-occurring Species of Goldenrod (Solidago: Compositae). Ecol. Monogr. 1983, 53, 95–117. [Google Scholar] [CrossRef]
- Elzinga, J.A.; Atlan, A.; Biere, A.; Gigord, L.; Weis, A.; Bernasconi, G. Time after time: Flowering phenology and biotic interactions. Trends Ecol. Evol. 2007, 22, 432–439. [Google Scholar] [CrossRef] [Green Version]
- Alatalo, J.; Totland, Ø. Response to simulated climatic change in an alpine and subarctic pollen-risk strategist, Silene acaulis. Glob. Chang. Biol. 1997, 3, 74–79. [Google Scholar] [CrossRef]
- Dyer, A.R.; Knapp, E.E.; Rice, K.J. Unintentional Selection and Genetic Changes in Native Perennial Grass Populations During Commercial Seed Production. Ecol. Restor. 2016, 34, 39–48. [Google Scholar] [CrossRef]
- Ogle, D. Bluebunch Wheatgrass; U.S. Department of Agriculture, Natural Resources Conservation Service, Idaho State Office: Boise, ID, USA, 2002; p. 3. [Google Scholar]
- Huber-Sannwald, E.; Pyke, D.A. Establishing Native Grasses in a Big Sagebrush-Dominated Site: An Intermediate Restoration Step. Restor. Ecol. 2005, 13, 292–301. [Google Scholar] [CrossRef]
- Falconer, D.S.; Mackay, T.F.C. Introduction to Quantitative Genetics; Subsequent edition; Benjamin-Cummings Pub. Co.: Harlow, UK, 1996; ISBN 978-0-582-24302-6. [Google Scholar]
- McMaster, G.S.; Wilhelm, W.W. Growing degree-days: One equation, two interpretations. Agric. For. Meteorol. 1997, 87, 291–300. [Google Scholar] [CrossRef] [Green Version]
- Frank, A.B.; Hofmann, L. Relationship among Grazing Management, Growing Degree-Days, and Morphological Development for Native Grasses on the Northern Great Plains. J. Range Manag. 1989, 42, 199. [Google Scholar] [CrossRef]
- Quinton, D.A.; McLean, A.; Stout, D.G. Vegetative and Reproductive Growth of Bluebunch Wheatgrass in Interior British Columbia. J. Range Manag. 1982, 35, 46. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. 2017. Available online: https://CRAN.R-project.org/package=DHARMa.
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. arXiv 2015, arXiv:1406.5823. [Google Scholar]
- McGraw, K.O.; Wong, S.P. Forming inferences about some intraclass correlation coefficients. Psychol. Methods 1996, 1, 30–46. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Lüdecke, D. Sjstats: Statistical Functions for Regression Models; Zenodo: Geneva, Switzerland, 2017. [Google Scholar]
- Johnson, R.; Stritch, L.; Olwell, P.; Lambert, S.; Horning, M.E.; Cronn, R. What are the best seed sources for ecosystem restoration on BLM and USFS lands? Nativ. Plants J. 2010, 11, 117–131. [Google Scholar] [CrossRef]
- Bower, A.D.; Clair, J.B.S.; Erickson, V. Generalized provisional seed zones for native plants. Ecol. Appl. 2014, 24, 913–919. [Google Scholar] [CrossRef]
- Kramer, A.T.; Larkin, D.J.; Fant, J.B. Assessing Potential Seed Transfer Zones for Five Forb Species from the Great Basin Floristic Region, USA. Nat. Areas J. 2015, 35, 174–188. [Google Scholar] [CrossRef]
- Ferdinandez, Y.S.N.; Coulman, B.E.; Fu, Y.-B. Detecting Genetic Changes over Two Generations of Seed Increase in an Awned Slender Wheatgrass Population Using AFLP Markers. Crop. Sci. 2005, 45, 1064–1068. [Google Scholar] [CrossRef]
| Zone | Stage | Chi Sq | DF | p-Value |
|---|---|---|---|---|
| 3a | Anthesis | 3.48 | 3 | 0.32 |
| Ripening | 2.27 | 3 | 0.52 | |
| Dispersal | 1.50 | 3 | 0.68 | |
| 4 | Anthesis | 1.63 | 4 | 0.80 |
| Ripening | 5.39 | 4 | 0.25 | |
| Dispersal | 7.40 | 4 | 0.17 | |
| 6a | Anthesis | 0.11 | 3 | 0.99 |
| Ripening | 4.54 | 3 | 0.21 | |
| Dispersal | 2.03 | 3 | 0.56 | |
| 6b | Anthesis | 5.42 | 4 | 0.25 |
| Ripening | 23.97 | 4 | 0.00 | |
| Dispersal | 37.27 | 4 | 0.00 | |
| 7a | Anthesis | 2.87 | 4 | 0.58 |
| Ripening | 3.01 | 4 | 0.56 | |
| Dispersal | 8.96 | 4 | 0.06 | |
| 7b | Anthesis | 9.61 | 4 | 0.047 |
| Ripening | 6.06 | 4 | 0.20 | |
| Dispersal | 4.97 | 4 | 0.29 |
| Stage | Zone | Population | Block | Plant | Block: Zone | Block: Pop | Unexplained |
|---|---|---|---|---|---|---|---|
| Anthesis | 9.0% | 1.4% | 0.0% | 5.2% | 2.5% | 0.5% | 81.5% |
| Ripening | 10.3% | 11.4% | 2.1% | 52.7% | 5.8% | 0.0% | 17.7% |
| Dispersal | 4.2% | 16.1% | 0.4% | 21.0% | 3.0% | 0.0% | 55.4% |
| Zone | n Plants | Harvest Date | n Ripe | n Lost | % Lost |
|---|---|---|---|---|---|
| 3a | 43 | 18-Jul | 35 | 8 | 19 |
| 4 | 61 | 10-Jul | 51 | 10 | 16 |
| 6a | 66 | 10-Jul | 56 | 10 | 15 |
| 6b | 78 | 10-Jul | 57 | 21 | 27 |
| 7a | 78 | 10-Jul | 70 | 8 | 10 |
| 7b | 65 | 18-Jul | 48 | 17 | 26 |
| All Zones | 391 | 10-Jul | 296 | 95 | 24 |
| n = 43 | CD | 2-Jun | 9-Jun | 12-Jun | 16-Jun | 21-Jun | 24-Jun | 28-Jun | 2-Jul | 6-Jul | 10-Jul | 18-Jul | 22-Jul | 26-Jul | 30-Jul | 3-Aug |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Score | GDD | 596 | 703 | 723 | 745 | 782 | 812 | 867 | 926 | 973 | 1016 | 1098 | 1138 | 1202 | 1265 | 1309 |
| 6.1 | Anthesis | 5 | 4 | 0 | 4 | 9 | 0 | 3 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| 6.2 | 0 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 6.3 | 0 | 2 | 0 | 0 | 2 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 6.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 6.5 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 6.6 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | |
| 6.7 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 6.8 | 0 | 0 | 1 | 2 | 2 | 2 | 5 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | |
| Sum | 5 | 9 | 1 | 7 | 19 | 4 | 12 | 5 | 1 | 1 | 1 | 2 | 1 | 0 | 0 | |
| 6.9 | Development | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7.0 | 0 | 0 | 1 | 1 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | |
| 7.1 | 0 | 2 | 11 | 10 | 3 | 13 | 5 | 1 | 4 | 4 | 0 | 0 | 2 | 2 | 0 | |
| 7.2 | 0 | 0 | 0 | 0 | 2 | 2 | 3 | 2 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | |
| 7.3 | 0 | 0 | 0 | 0 | 3 | 7 | 8 | 7 | 4 | 3 | 1 | 2 | 0 | 0 | 1 | |
| Sum | 0 | 2 | 12 | 11 | 8 | 24 | 19 | 12 | 9 | 7 | 1 | 4 | 3 | 2 | 1 | |
| 7.4 | Harvest | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 4 | 2 | 0 | 1 | 1 | 0 | 0 |
| 7.5 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 12 | 4 | 11 | 4 | 3 | 2 | 0 | 0 | |
| 7.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 2 | 1 | 1 | 1 | 0 | |
| 7.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 5 | 4 | 3 | 1 | 0 | 1 | 0 | |
| 7.8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 7 | 14 | 7 | 5 | 5 | 3 | 0 | |
| 7.9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 6 | 0 | 2 | 1 | 1 | |
| 8.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 5 | 3 | 0 | 1 | 1 | |
| 8.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 8 | 3 | 2 | 3 | 4 | |
| Sum | 0 | 0 | 0 | 0 | 0 | 2 | 4 | 17 | 25 | 33 | 35 | 17 | 13 | 10 | 6 | |
| 8.2 | Dispersal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 4 |
| 8.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | |
| 8.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 4 | 3 | 0 | 0 | |
| 8.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 2 | 3 | 0 | |
| 8.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | |
| 8.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 4 | 5 | 10 | |
| 8.8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 2 | |
| 8.9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 14 | 17 | 18 | |
| Sum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 19 | 25 | 30 | 35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prive, K.; Orr, M.R.; Kilkenny, F.F.; Reuter, R.J.; Prendeville, H.R. Phenological Variation in Bluebunch Wheatgrass (Pseudoroegneria spicata): Implications for Seed Sourcing, Harvest, and Restoration. Land 2021, 10, 1064. https://doi.org/10.3390/land10101064
Prive K, Orr MR, Kilkenny FF, Reuter RJ, Prendeville HR. Phenological Variation in Bluebunch Wheatgrass (Pseudoroegneria spicata): Implications for Seed Sourcing, Harvest, and Restoration. Land. 2021; 10(10):1064. https://doi.org/10.3390/land10101064
Chicago/Turabian StylePrive, Kathryn, Matthew R. Orr, Francis F. Kilkenny, Ronald J. Reuter, and Holly R. Prendeville. 2021. "Phenological Variation in Bluebunch Wheatgrass (Pseudoroegneria spicata): Implications for Seed Sourcing, Harvest, and Restoration" Land 10, no. 10: 1064. https://doi.org/10.3390/land10101064
APA StylePrive, K., Orr, M. R., Kilkenny, F. F., Reuter, R. J., & Prendeville, H. R. (2021). Phenological Variation in Bluebunch Wheatgrass (Pseudoroegneria spicata): Implications for Seed Sourcing, Harvest, and Restoration. Land, 10(10), 1064. https://doi.org/10.3390/land10101064







