Changes in Soil Features and Phytomass during Vegetation Succession in Sandy Areas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
Plot Design
2.2. Determination of Phytomass
2.3. Soil Investigation
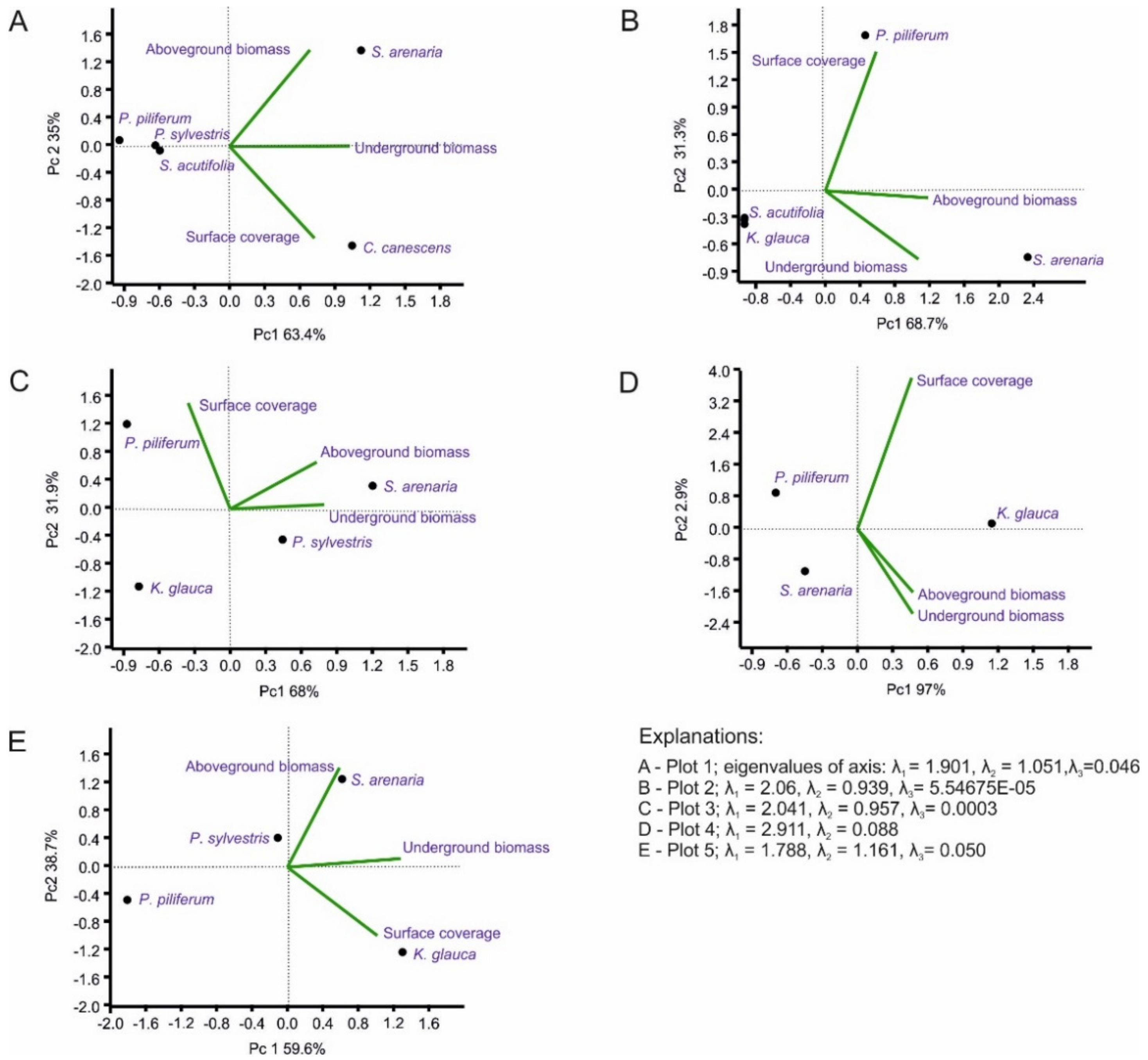
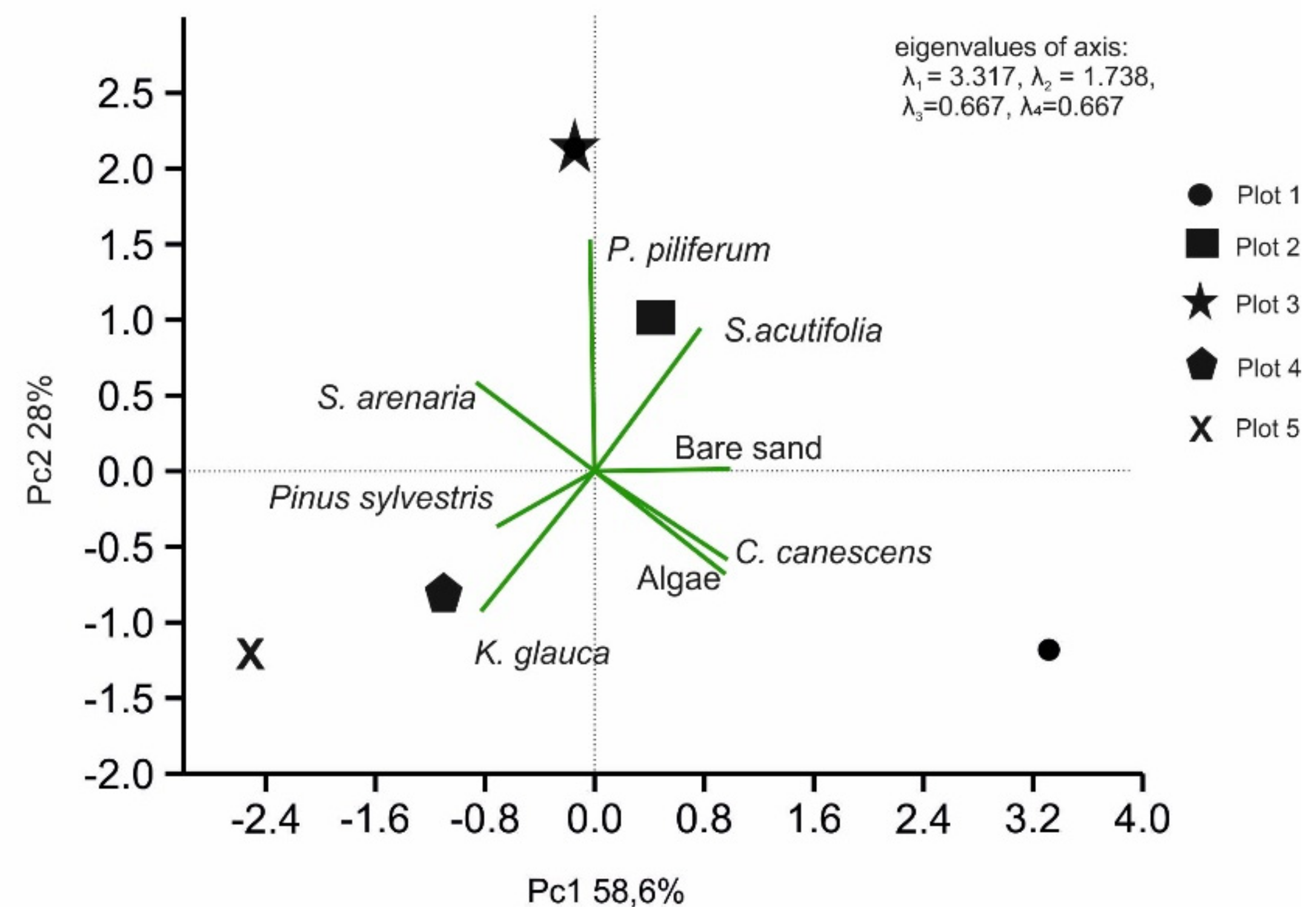
2.4. Statistical Analysis
3. Results
3.1. Vegetation Differentiation on the Plots
3.1.1. Plot I
3.1.2. Plot II
3.1.3. Plot III
3.1.4. Plot IV
3.1.5. Plot V
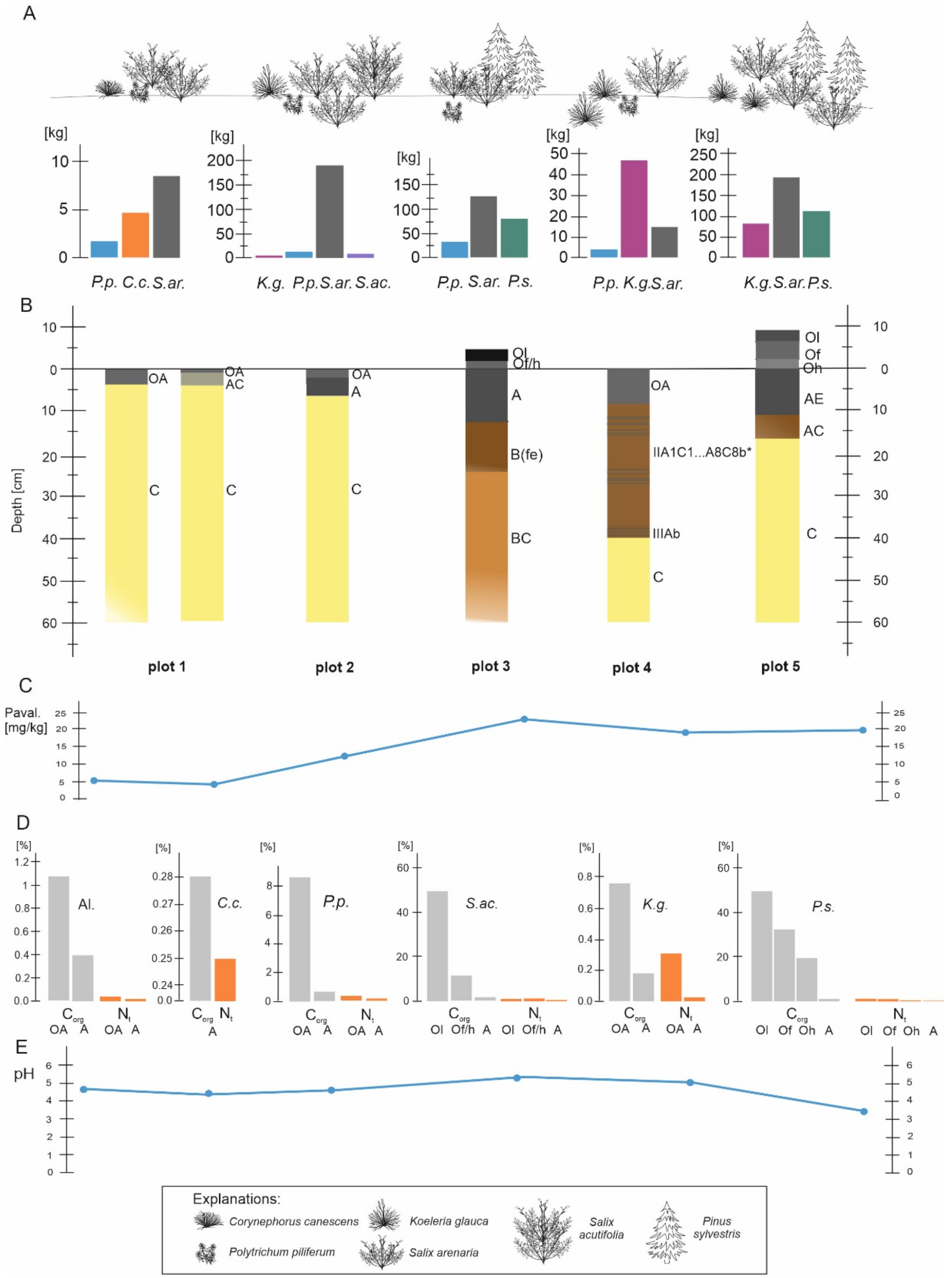
3.2. Soil Morphology
3.3. Chemical Properties of the Soils in the Plots
3.4. Differentiation of Phytomass in the Plots
3.5. Contents of Selected Macroelements
- Algal crust-OA: K > Na > Al > Mg > Ca > Fe > P > Zn
- Corynephorus canescens-A: Al > Fe > Na > Ca > P > K > Mg > Zn
- Corynephorus canescens-C: Al > Fe > Na > Ca > K > Mg > P > Zn
- Koeleria glauca-A: Al > Fe > K > Na > Ca > Mg > P > Zn
- Salix arenaria-A: Al > Fe > K > Na > Ca > P > Mg > Zn
- Salix acutifolia-A: Al > Fe > K > Ca > Na > Mg > Zn > P
- Pinus sylvestris-A: Al > K > Ca > Na > Zn > Fe > Mg > P
- Pinus sylvestris-AC: Al > K > Fe > Na > Ca > Mg > Zn > P
4. Discussion
Soil Formation Processes and Features in the Plots
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Titlyanova, A.A.; Bazilevich, N.I.; Shmakova, E.I.; Snytko, V.A.; Dubynina, S.S.; Magomedova, L.N.; Nefedyeva, L.G.; Semenyuk, N.V.; Tishkov, A.A. Biological Productivity of Grasslands. In Geographical Regularities and Ecological Features, 2nd ed.; ISSA SB RAS: Novosibirsk, Russia, 2018; p. 110. [Google Scholar]
- Dickerman, J.A.; Stewart, A.J.; Wentzel, R.G. Estimates of net annual aboveground production: Sensitivity to sampling frequency. Ecology 1986, 67, 650–659. [Google Scholar] [CrossRef]
- Schenk, H.J.; Jackson, R.B. The global biogeography of roots. Ecol. Monogr. 2002, 72, 311–328. [Google Scholar] [CrossRef]
- Bazilevič, N.I.; Titlânova, A.A. Biotičeskij Krugovorot na Pâti Kontinentah: Azot i Zolʹnye Élementy v Prirodnyh Nazemnyh ékosistemah; Izdatel‘stvo SO RAN: Novosibirsk, Russia, 2008; pp. 1–381. [Google Scholar]
- Rodin, L.E.; Smirnov, N.N. Resursy Biosfery (Itogi Sovetskih Issledovanij po Meždunarodnoj Biologičeskoj Programme); Nauka: Sankt Peterburg, Russia, 1975; pp. 1–287. [Google Scholar]
- Kazanceva, T.I. Produktivnost’ Zonal’nyh Rastitel’nyh Soobŝestv Stepej i Pustyn’ Gobijskoj Časti Mongolii; Nauka: Moskva, Russia, 2009; pp. 1–336. [Google Scholar]
- Alaback, P.B. Biomass regression equations for understory plants in coastal Alaska: Effects of species and sampling design on estimates. Northwest Sci. 1986, 60, 90–103. [Google Scholar]
- Cairns, M.A.; Brown, S.; Helmer, E.H.; Baumgardner, G.A. Root biomass allocation in the world’s upland forests. Oecologia 1997, 111, 1–11. [Google Scholar] [CrossRef]
- Chiarucci, A.; Wilson, J.B.; Anderson, B.J.; de Dominicis, V. Cover versus biomass as an estimate of species abundance: Does it make a difference to the conclusions? J. Veg. Sci. 1999, 10, 35–42. [Google Scholar] [CrossRef]
- Schultze, E.D.; Lloyd, J.; Kelliher, F.M.; Wirth, C.; Rebmann, C.; Luehker, B.; Mund, M.; Knohi, A.; Milyukova, I.; Schulze, W.; et al. Productivity of forest in the Euro-Siberian boreal region and their potential to act as a carbon sink—A synthesis. Glob. Chang. Biol. 1999, 5, 703–722. [Google Scholar] [CrossRef]
- Titlyanova, A.A.; Romanova, I.P.; Kosych, N.P.; Mironycheva-Tokareva, N.P. Pattern and processes in above-ground and below-ground components of herbsland ecosystems. J. Veg. Sci. 1999, 10, 307–320. [Google Scholar] [CrossRef]
- Usol’cev, V.A. Fitomassa Lesov Severnoj Evrazii: Baza Dannyh i Geografiâ; UrO RAN: Ekaterinburg, Russia, 2001; pp. 1–708. [Google Scholar]
- Heinrichs, S.; Bernhardt-Römermann, M.; Schmidt, W. The estimation of aboveground biomass and nutrient pools of understory plants in closed Norway spruce forests and on clear cuts. Eur. J. Forest Res. 2010, 129, 613. [Google Scholar] [CrossRef] [Green Version]
- Aerts, R.; Berendse, F. Aboveground nutrient turnover and net primary production of an evergreen and a deciduous species in a heathland ecosystem. J. Ecol. 1989, 77, 343–356. [Google Scholar] [CrossRef]
- Bobbink, R.; Dubbelden, K.D.; Willems, J.H. Seasonal dynamics of phytomass and nutrients in chalk grassland. Oikos 1989, 55, 216–224. [Google Scholar] [CrossRef]
- Van Rheenen, J.W.; Werger, M.J.A.; Bobbink, R.; Daniels, F.J.A.; Mulders, W.H.M. Short-term accumulation of organic matter and nutrient contents in two dry sand ecosystems. Vegetatio 1995, 120, 161–171. [Google Scholar] [CrossRef]
- Martίnez, F.; Merino, O.; Martin, A.; Garcıa Martin, D.; Merino, J. Belowground structure and production in a Mediterranean sand dune shrub community. Plant. Soil 1998, 201. [Google Scholar] [CrossRef]
- Van der Heijden, E.W.; de Vries, F.W.; Kuyper, T.W. Mycorrhizal associations of Salix repens L. communities in succession of dune ecosystems. I. Above-ground and below-ground views of ectomycorrhizal fungi in relation to soil chemistry. Can. J. Bot. 1999, 77, 1821–1832. [Google Scholar] [CrossRef]
- Khan, D.; Faheemuddin, M.; Shaukat, S.S.; Alam, M.M. Seasonal variation in structure, composition, phytomass, and net primary production in a Lasiurus scindicus Henr., and Cenchrus setigerus Vahl., dominated dry sandy desert site of Karachi. Pak. J. Bot. 2000, 32, 171–210. [Google Scholar]
- Xiao, C.W.; Yuste, J.C.; Janssens, I.A.; Roskams, P.; Nachtergale, L.; Carrara, A.; Sanchez, B.Y.; Ceulemans, R. Above- and belowground biomass and net primary production in a 73-year-old Scots pine forest. Tree Physiol. 2003, 23, 505–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storm, C.; Süss, K. Are low-productive plant communites responsive to nutrient addition evidence from sand pioneer grassland. J. Veg. Sci. 2008, 19, 343–354. [Google Scholar] [CrossRef]
- Yu, Z.; Zeng, D.; Jiang, F.; Zhao, Q. Responses of biomass to the addition of water, nitrogen and phosphorus in Keerqin sandy grassland, Inner Mongolia, China. J. For. Res. 2009, 20, 23–26. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Wardle, D.A. Aboveground-Belowground Linkages: Biotic Interactions, Ecosystem Processes, and Global Change Austral. Ecology; Oxford University Press: Oxford, UK, 2010; pp. 1–320. [Google Scholar]
- Prach, K. Primary Forest Succession in Sand Dune Areas; Research Institute for Forestry and Landscape Planning: Wageningen, The Netherlands, 1989; p. 117. [Google Scholar]
- Elgersma, A.M. Primary forest succession on poor sandy soil related to site factors. Biodivers. Conserv. 1998, 7, 193–206. [Google Scholar] [CrossRef]
- Walker, L.R. Ecosystems of Disturbed Ground; Elsevier: Amsterdam, The Netherlands, 1999; Volume 16, pp. 1–868. [Google Scholar]
- Jentsch, A.A.; Beyschlag, W. Vegetation ecology of dry acidic grasslands in the lowland area of central Europe. Flora 2003, 198, 3–25. [Google Scholar] [CrossRef]
- Faliński, J.B. Long-term studies on vegetation dynamics: Some notes on concepts, fundamentals and conditions. Community Ecol. 2003, 4. [Google Scholar] [CrossRef]
- Hršak, V. Vegetation succession and soil gradients on inland sand dunes. Ekol. Bratisl. 2004, 22, 24–39. [Google Scholar]
- Rahmonov, O.; Oleś, W. Vegetation succession over an area of a medieval ecological disaster. The case of the Błędów Desert, Poland. Erdkunde 2010, 64. [Google Scholar] [CrossRef]
- Agakhanyantz, O.E.; Lopatin, I.K. Main characteristics of the ecosystems of the pamirs, USSR. Arct. Alp. Res. 1978, 10, 397–407. [Google Scholar] [CrossRef]
- Ma, Q.; Cui, L.; Song, H.; Gao, C.; Hao, Y.; Luan, J.; Wang, Y.; Li, W. Aboveground and belowground biomass relationships in the zoige peatland, eastern qinghai—tibetan plateau. Wetlands 2017, 37. [Google Scholar] [CrossRef]
- Sun, J.; Niu, S.; Wang, J. Divergent biomass partitioning to aboveground and belowground across forests in China. J. Plant. Ecol. 2018, 11, 484–492. [Google Scholar] [CrossRef]
- Bazilevič, N.I. Biologičeskaâ Produktivnost’ Ékosistem Severnoj Evrazii; Nauka: Moscow, Russia, 1993; pp. 1–293. [Google Scholar]
- Jackson, R.B.; Schenk, H.J.; Jobbágy, E.G.; Canadell, J.; Colello, G.D.; Dickinson, R.E.; Field, C.B.; Friedlingstein, P.; Heimann, M.; Hibbard, K.; et al. Belowground consequences of vegetation change and their treatment in models. Ecol. Appl. 2000, 10. [Google Scholar] [CrossRef]
- Archer, N.A.L.; Quinton, J.N.; Hess, T.M. Below-ground relationships of soil texture, roots and hydraulic conductivity in two-phase mosaic vegetation in south-east Spain. J. Arid Environ. 2002, 52, 535–553. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Peng, S.; Fang, J. Biomass distribution of natural grasslands and it response to climate change in north China. Arid Zone Res. 2008, 25. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, J.; Ji, C.; Han, W. Above- and belowground biomass allocation in Tibetan grasslands. J. Veg. Sci. 2009, 20. [Google Scholar] [CrossRef]
- Chen, G.; Yang, Y.; Robinson, D. Allocation of gross primary production in forest ecosystems: Allometric constraints and environmental responses. New Phytol. 2013, 200, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, K.; Xu, X.; Song, T.; Xu, Y.; Zeng, F. Biogeographical patterns of biomass allocation in leaves, stems, and roots in China’s forests. Sci. Rep. 2015, 3, 159–197. [Google Scholar] [CrossRef] [Green Version]
- Bonkowski, M. Protozoa and plant growth: The microbial loop in soil revisited. New Phytol. 2004, 162, 617–631. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Bowman, W.D.; Kaufmann, R.; Schmidt, S.K. A temporal approach to linking aboveground and belowground ecology. Trends Ecol. Evol. 2005, 20. [Google Scholar] [CrossRef] [PubMed]
- Van der Putten, W.H.; Vet, L.E.M.; Harvey, J.A.; Wäckers, F.L. Linking above- and belowground multitrophic interactions of plants, herbivores, pathogens, and their antagonists. Trends Ecol. Evol. 2001, 16. [Google Scholar] [CrossRef]
- Van der Putten, W.H.; Bardgett, R.D.; de Ruiter, P.C.; Hol, W.H.G.; Meyer, K.M.; Bezemer, T.M.; Bradford, M.A.; Christensen, S.; Eppinga, M.B.; Fukami, T.; et al. Empirical and theoretical challenges in aboveground—Belowground ecology. Oecologia 2009, 161. [Google Scholar] [CrossRef] [PubMed]
- Wardle, D.A. Communities and Ecosystems: Linking the Aboveground and the Belowground Components. In Monographs in Population Biology; Princeton University Press: Princeton, NJ, USA, 2002; pp. 1–408. [Google Scholar]
- Grime, J.P. Plant Strategies, Vegetation Processes, and Ecosystem Properties, 2nd ed.; Wiley: New York, NY, USA, 2001; pp. 1–456. [Google Scholar]
- Walker, L.R.; del Moral, R. Primary Succession and Ecosystem Rehabilitation, 1st ed.; Cambridge University Press: Cambridge, UK, 2003; pp. 1–456. [Google Scholar]
- Rose, S.L. Above and belowground community development in a marine sand dune ecosystem. Plant Soil 1988, 109, 215–226. [Google Scholar] [CrossRef]
- Rahmonov, O.; Piątek, J. Sand colonization and initiation of soil development by cyanobacteria and algae. Ekol. Bratisl. 2007, 26, 51–62. [Google Scholar]
- Rahmonov, O.; Cabała, J.; Bednarek, R.; Rożek, D.; Florkiewicz, A. Role of soil algae on the initial stages of soil formation in sandy polluted areas. Ecol. Chem. Eng. S 2015, 22, 675–690. [Google Scholar] [CrossRef] [Green Version]
- Malam, I.O.; Le Bissonnais, Y.; Défarge, C.A.; Trichet, J. Role of a cyanobacterial cover on structural stability of sandy soils in the Sahelian part of western Niger. Geoderma 2001, 101, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Rahmonov, O.; Kowalski, W.J.; Bednarek, R. Characterization of the Soil organic matter and plant tissues in an initial stage of plant succession and Soil development by means of Curie-point pyrolysis coupled with GC-MS. Eurasian Soil Sci. 2010, 43. [Google Scholar] [CrossRef]
- Marynowski, L.; Rahmonov, O.; Smolarek-Lach, J.; Rybicki, M.; Simoneit, B.R.T. Origin and significance of saccharides during initial pedogenesis in a temperate climate region. Geoderma 2020, 361, 114064. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Richter, A.; Bol, R.; Garnett, M.H.; Baumler, R.; Xu, X.L.; Lopez-Capel, E.; Manning, D.A.C.; Hobbs, P.J.; Hartley, I.R.; et al. Heterotrophic microbial communities use ancient carbon following glacial retreat. Biol. Lett. 2007, 3. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.K.; Reed, S.C.; Nemergut, D.R.; Grandy, A.S.; Cleveland, C.C.; Weintraub, M.N.; Hill, A.W.; Costello, E.K.; Meyer, A.F.; Neff, J.C.; et al. The earliest stages of ecosystem succession in high-elevation (5000 metres above sea level), recently deglaciated soils. Proc. Biol. Sci. 2008, 22, 2793–2802. [Google Scholar] [CrossRef] [Green Version]
- Bardgett, R.D.; Walker, L.R. Impact of coloniser plant species on the development of decomposer microbial communities following deglaciation. Soil Biol. Biochem. 2004, 36, 555–559. [Google Scholar] [CrossRef]
- Neutel, A.M.; Heesterbeek, J.A.P.; van de Koppel, J.; Hoenderboom, G.; Vos, A.; Kaldeway, C.; Berendse, F.; de Ruiter, P.C. Reconciling complexity with stability in naturally assembling food webs. Nature 2007, 449, 599–602. [Google Scholar] [CrossRef] [Green Version]
- Goleusov, P.V.; Lisetskii, F.N. Soil development in anthropogenically disturbed forest-steppe landscapes. Eurasian Soil Sc. 2008, 41, 1480–1486. [Google Scholar] [CrossRef]
- De Vries, W.; Wamelink, W.; van Dobben, H.; Kros, J.; Reinds, G.J.; Mol-Dijkstra, J.P.; Smart, S.M.; Evans, C.D.; Rowe, E.C.; Belyazid, S.; et al. Use of dynamic soil-vegetation models to assess impacts of nitrogen deposition on plant species composition: An overview. Ecol. Appl. 2010, 20, 60–79. [Google Scholar] [CrossRef] [Green Version]
- Waring, B.G.; Álvarez-Cansino, L.; Barry, K.E.; Becklund, K.K.; Dale, S.; Gei, M.G.; Keller, A.B.; Lopez, O.R.; Markesteijn, L.; Mangan, S.; et al. Pervasive and strong effects of plants on soil chemistry: A meta-analysis of individual plant “Zinke” effects. Proc. Biol. Sci. 2015, 7. [Google Scholar] [CrossRef]
- Veblen, K.E. Season- and herbivore-dependent competition and facilitation in a semiarid savanna. Ecology 2008, 89, 1532–1540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okuniewska-Nowaczyk, I.; Rahmonov, O.; Szczypek, T. Palynological record of the history of vegetation in the sandy areas of southern Poland. Geogr. Nat. Resour. 2018, 39, 396–402. [Google Scholar] [CrossRef]
- Rahmonov, O.; Snytko, V.A.; Szczypek, T. Formation of phytogenic hillocks in Southern Poland. Geogr. Nat. Resour. 2009, 30. [Google Scholar] [CrossRef]
- Geoportal of Poland. Available online: https://mapy.geoportal.gov.pl (accessed on 21 January 2021).
- Database of Institute of Meteorology and Water Management—National Research Institute. Available online: https://danepubliczne.imgw.pl (accessed on 21 January 2021).
- Steshenko, A.P. Osobennosti stroyeniya podzemnykh organov rasteniy predel’nykh vysot proizrastaniya na Pamire. Akademiya Nauk SSSR 1960, 2, 284. [Google Scholar]
- World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; Food And Agriculture Organization of the United Nations: Rome, Italy, 2015.
- Bednarek, R.; Dziadowiec, H.; Pokojska, U.; Prusinkiewicz, Z. Badania Ekologiczno-Gleboznawcze; PWN: Warszawa, Poland, 2004; pp. 1–344. [Google Scholar]
- Munsell, A.H. Munsell Soil Color Charts; GretagMacbeth: New York, NY, USA, 2000. [Google Scholar]
- Morrison, D.F. Multivariate Statistical Methods, 3rd ed.; McGraw-Hill: New York, NY, USA, 1976; pp. 1–85. [Google Scholar]
- Cattell, R.B. The Scree Test for the Number of Factors. Multivar. Behav. Res. 1966, 1, 245–276. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.; Field, C.B.; Berry, J.A. Allometric growth and allocation in forests: A perspective from fluxnet. Ecol. Appl. 2011, 21, 1546–1556. [Google Scholar] [CrossRef] [PubMed]
- Belnap, J. Factors Influencing Nitrogen Fixation and Nitrogen Release in Biological Soil Crusts. In Biological Soil Crusts: Structure, Function, and Management; Springer: Berlin/Heidelberg, Germany, 2003; Volume 150, p. 241. [Google Scholar]
- Marshall, J.K. Corynephorus canescens (L.) P. Beauv. Biological flora of the British Isles. J. Ecol. 1967, 55, 207–220. [Google Scholar] [CrossRef]
- Marshall, J.K. Factors limiting the survival of Corynephorus canescens (L.) Beauv. in Great Britain at the northern edge of Its distribution. Oikos 1968, 19, 206–216. [Google Scholar] [CrossRef]
- Rahmonov, O.; Różkowski, J.; Szymczyk, A. Relationship between compositions of grey hair-grass (Corynephorus canescens (L.) P. Beauv.) tissues and soil properties during primary vegetation succession. In Proceedings of the IOP Conference Series: Earth Environmental Science, Prague, Czech Republic, 9–13 September 2019. [Google Scholar]
- Drew, M.C. Root Development and Activities. In Arid-Land Ecosystems; Goodall, D.W., Perry, R.A., Eds.; Cambridge University Press: London, UK, 1979; pp. 573–598. [Google Scholar]
- Curt, T.; Prévosto, B. Root biomass and rooting profile of naturally regenerated beech in mid-elevation Scots pine woodlands. Plant. Ecol. 2003, 167, 269–282. [Google Scholar] [CrossRef]
- Rahmonov, O.; Snytko, V.A.; Szczypek, T. Phytogenic hillocks as an effect of indirect human activity. Z. Geomorphol. 2009, 53. [Google Scholar] [CrossRef]
- Sewerniak, P.; Jankowski, M. Deforestation increases differences in morphology and properties of dune soils located on contrasting slope aspects in the Toruń military area (N Poland). Ecol. Quest 2015, 21, 61–63. [Google Scholar] [CrossRef] [Green Version]
- Sewerniak, P.; Jankowski, M. Topographically-controlled site conditions drive vegetation pattern on inland dunes in Poland. Acta Oecologica 2017, 82. [Google Scholar] [CrossRef]
- Skvortsov, A.K. Willows of the USSR—A Systematic Review; Nauka: Moscow, Russia, 1968; pp. 1–135. [Google Scholar]
- Rahmonov, O. The role of Salix acutifolia as an ecological engineer during the primary forest succession. In Proceedings of the 17th International Symposium on Landscape Ecology-Landscape and Landscape Ecology, Bratislava, Slovakia, 25–27 May 2016; pp. 312–318. [Google Scholar]
- Rahmonov, O.; Krzysztofik, R.; Środek, D.; Smolarek-Lach, J. Vegetation- and environmental changes on non-reclaimed spoil heaps in southern Poland. Biology 2020, 9, 164. [Google Scholar] [CrossRef] [PubMed]
- Ponomareva, V.V. Teoriya Podzoloobrazovatel’nogo Prozessa; Izdatelstvo Nauka: Moscow, Leningrad, USSR, 1964; pp. 1–381. [Google Scholar]
- Ponomareva, V.V.; Plotnikova, T.A. Humus and Soil Formation; Izdatelstvo Nauka: Leningrad, USSR, 1980; pp. 1–223. [Google Scholar]
- Aleksandrova, L.N. Soil Organic Matter and the Processes of its Transformation; Izdatelstvo Nauka: Leningrad, USSR, 1980; pp. 1–288. [Google Scholar]
- Rodin, L.E.; Bazilevich, N.I. Production and Mineral Cycling in Terrestial Vegetation; Oliver and Boyd: London, UK, 1965; p. 288. (In Russian) [Google Scholar]
- Zinke, P.J. The pattern of influence of individual forest trees on soil properties. Ecology 1962, 43, 130–133. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Pilmanis, A.M. Plant-soil interactions in deserts. Biogeochemistry 1998, 42, 169–187. [Google Scholar] [CrossRef]
- Rahmonov, O.; Rzetala, M.; Rahmonov, M.; Kozyreva, E.; Jagus, A.; Rzetala, M. The formation of soil chemistry and the development of fertility islands under plant canopies in sandy areas. Res. J. Chem. Environ. 2011, 15, 823–829. [Google Scholar]
- Aleksandrowicz, Z. Piaski i formy wydmowe Pustyni Błędowskiej. Ochrona Przyrody 1962, 28, 227–253. [Google Scholar]
- Damptey, F.G.; Birkhofer, K.; Nsiah, P.K.; de la Riva, E.G. Soil properties and biomass attributes in a former gravel mine area after two decades of forest restoration. Land 2020, 9, 209. [Google Scholar] [CrossRef]






| Numbers of Plots | General Description |
|---|---|
| Plot I | Located on a flattened surface (deflation field) between two low (relative elevations 1–1.6 m) hillocks composed of aeolian sands. Unconsolidated and loose sand with rare vegetation (Figure 1: I, Table 2 *), with active aeolian processes. Soil: initial loose soils (Leptosols). |
| Plot II | Located on a gentle slope of a dune rise, with a relative height of 80–150 cm above the flattened surface. Loose sand partly stabilised by Polytrichum piliferum (Figure 1: II). Low intensity of aeolian processes. Soil: initial loose soils (Leptosols). |
| Plot III | Located on a flattened surface. Weakly expressed nanodepressions. An accumulation of plant detritus was observed, collected by rainwater in small ridges; this was associated with areas characterised by well-developed moss and algae. Stabilised by the turf of P. piliferum and clumps of Salix arenaria (Figure 1: III). Soil: initial loose soils (Leptosols) where there are no plants and soils weakly developed from loose materials (Arenosols). |
| Plot IV | Located on the flattened top of the aeolian ridge and its slope. The surfaces are stable and grassed by Koeleria gluca (Figure 1: IV). There are no destructive aeolian processes. Soil: weakly developed from loose sandy materials (Arenosols). |
| Plot V | Located on the upper part of the slope of the aeolian ridge of the eastern exposition. This surface is the most stable, and the soil was divided into different subhorizons (especially organic horizon) under Pinus sylvestris (Figure 1: V). Soil: weakly developed from loose materials (Arenosols). |
| Vegetation and Other Elements | Research Plots [%] | ||||
|---|---|---|---|---|---|
| I | II | III | IV | V | |
| Corynephorus canescens (L.) P.Beauv. | 9.83 | 1.94 | - | - | - |
| Koeleria glauca (Schrad.) DC. | - | 1.55 | 0.21 | 65.66 | 68.4 |
| Polytrichum piliferum Hedw. | 2.11 | 38.71 | 65.6 | 15.95 | 1.79 |
| Salix arenaria L. | 2.38 | 13.8 | 12.61 | 8.42 | 16.43 |
| Salix acutifolia Willd. | 1.25 | 0.84 | 1.47 | - | - |
| Pinus sylvestris L. | 0.21 | 0.29 | 2.23 | - | 9.05 |
| Juniperus communis L. | - | - | 0.05 | - | 0.13 |
| Betula pendula Roth. | - | - | 0.03 | - | - |
| Algae | 48.15 | 7.52 | - | 5.18 | - |
| accumulation of organic matter | - | 7.90 | 6.42 | - | - |
| Bare sand | 36.07 | 27.45 | 11.38 | 4.79 | 4.20 |
| Total | 100 | 100 | 100 | 100 | 100 |
| Plant Organs | Vegetation Communities and Their Area in m2 | Total in Phytocenosis kg/400 m2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C. canescens [3.32 m2] | P. piliferum [8.44 m2] | Salix arenaria [9.54 m2] | Salix acutifolia [5.03 m2] | Pinus sylvestris [0.85 m2] | |||||||
| [g/m2] | [kg]- in Community | [g/m2] | [kg]- in Community | [g/m2] | [kg]- in Community | [g/m2] | [kg]- in Community | [g/m2] | [kg]- in Community | ||
| Aboveground biomass: | 10.40 | 0.41 | 76.6 * | 0.65 * | 428.9 | 4.09 | 20.1 | 0.10 | 22.71 | 0.019 | 5.269 |
| leaves | - ** | - | - | - | 24.30 | 0.23 | - | - | - | - | |
| needls | - | - | - | - | - | - | - | - | 0.3 | 0.00011 | |
| living branches | - | - | - | - | 367.6 | 3.50 | - | - | 19.70 | 0.017 | |
| dead branches | - | - | - | - | - | - | 20.1 | 0.10 | - | - | |
| annual increment | - | - | - | - | 37.0 | 0.35 | - | - | 2.88 | 0.002 | |
| Underground biomass: | 108.3 | 4.26 | - | - | 466.7 | 4.46 | 200.7 | 1.00 | 17.53 | 0.014 | 9.734 |
| Roots diameter: <1 mm | 108.3 | 4.26 | - | - | 25.8 | 0.25 | - | - | 6.4 | 0.005 | |
| 1–10 mm | - | - | - | - | 320.5 | 3.06 | 6.4 | 0.03 | 11.13 | 0.009 | |
| >10 mm | - | - | - | - | 120.4 | 1.15 | 194.3 | 0.97 | - | - | |
| TOTAL: | 118.7 | 4.67 | 76.6 | 0.65 | 895.6 | 8.55 | 220.8 | 1.1 | 40.24 | 0.033 | 15.003 |
| Plant Organs | Vegetation Communities and Their Area in m2 | Total in Phytocenosis kg/400 m2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C. canescens [7.79 m2] | Koeleria glauca [6.2 m2] | Polytrichum piliferum ([54.83 m2] | Salix arenaria [55.26 m2] | Salix acutifolia [3.36 m2] | |||||||
| [g/m2] | [kg]- in Community | [g/m2] | [kg]- in Community | [g/m2] | [kg]- in Community | [g/m2] | [kg]- in Community | [g/m2] | [kg]- in Community | ||
| Aboveground biomass: | 10.4 | 0.08 | 29.0 | 0.18 | 84.3 | 13.05 | 610.2 | 33.72 | 101.3 | 0.34 | 47.37 |
| leaves | - | - | - | - | 179.7 | 9.93 | 43 | 0.14 | |||
| living branches | - | - | - | - | - | - | 388.1 | 21.45 | 19.70 | 0.07 | |
| dead branches | - | - | - | - | - | - | - | - | 38.6 | 0.13 | |
| annual increment | - | - | - | - | - | - | 42.4 | 2.34 | - | - | |
| Underground biomass: | 108.3 | 0.84 | 163.0 | 1.01 | - | - | 2813.2 | 155.46 | 588.3 | 1.98 | 159.29 |
| Roots diameter: <1 mm | 108.3 | 0.84 | 163.0 | 1.01 | - | - | 141.6 | 7.82 | - | - | |
| 1–10 mm | - | - | - | - | - | - | 2130.1 | 117.71 | 12.5 | 0.04 | |
| >10 mm | - | - | - | - | - | - | 541.5 | 29.92 | 575.8 | 1.93 | |
| TOTAL: | 118.7 | 0.92 | 192.0 | 1.19 | 84.3 | 13.05 | 3423.4 | 188.97 | 689.6 | 2.32 | 206.66 |
| Plant Organs | Vegetation Communities and Their Area in m2 | Total in Phytocenosis kg/400 m2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Koeleria glauca [0.84 m2] | Polytrichum piliferum [262.5 m2] | Salix arenaria [50.44 m2] | Pinus sylvestris [8.94 m2] | ||||||
| [g/m2] | [kg]- in Community | [g/m2] | [kg]- in Community | [g/m2] | [kg]- in Community | [g/m2] | [kg]- in Community | ||
| Aboveground biomass: | 20.0 | 0.02 | 112.8 | 29.61 | 1715.1 | 86.51 | 5500.1 | 49.17 | 165.31 |
| leaves | - | - | - | - | 53.9 | 2.72 | - | - | |
| needls | - | - | - | - | - | - | 504.2 | 4,51 | |
| living branches | - | - | - | - | 1649.6 | 83.21 | 1785.2 | 15.96 | |
| trunk | - | - | - | - | - | - | 2485.6 | 22.22 | |
| annual increment | - | - | - | - | 11.6 | 0.58 | 211.2 | 1.89 | |
| Plant litter | - | - | - | - | - | - | 513.9 | 4,59 | |
| Underground biomass: | 116.0 | 0.10 | - | - | 732.2 | 36.93 | 2585.5 | 23.11 | 60.14 |
| Roots diameter: <1 mm | 116.0 | 0.10 | - | - | 70.8 | 3.57 | 123.5 | 1.10 | |
| 1–10 mm | - | - | - | - | 305.4 | 15.40 | 870.2 | 7.78 | |
| >10 mm | - | - | - | - | 356.0 | 17.96 | 1591.8 | 14.23 | |
| TOTAL: | 136.0 | 0.12 | 112.8 | 29.61 | 2447.3 | 123.44 | 8085.6 | 72.28 | 225.45 |
| Plant Organs | Vegetation Communities and Their Area in m2 | Total in Phytocenosis kg/400 m2 | |||||
|---|---|---|---|---|---|---|---|
| Koeleria glauca [262.67 m2] | Polytrichum piliferum [63.82 m2] | Salix arenaria [33.65 m2] | |||||
| [g/m2] | [kg]- in Community | [g/m2] | [kg]- in Community | [g/m2] | [kg]- in Community | ||
| Aboveground biomass: | 86.9 | 22.83 | 63.7 | 4.07 | 252.4 | 8.49 | 35.39 |
| leaves | - | - | - | - | 44.6 | 1.50 | |
| living branches | - | - | - | - | 183.3 | 6.17 | |
| Annual increment | - | - | - | - | 14.9 | 0.50 | |
| Plant litter | - | - | - | - | 9.6 | 0.32 | |
| Underground biomass: | 87.2 | 22.90 | - | - | 182.5 | 6.14 | 29.04 |
| Roots diameter: <1 mm | 87.2 | 22.90 | - | - | 17.9 | 0.60 | |
| 1–10 mm | - | - | - | - | 101.6 | 3.42 | |
| >10 mm | - | - | - | - | 63.0 | 2.12 | |
| TOTAL: | 174.1 | 45.73 | 63.7 | 4.07 | 434.9 | 14.63 | 64.43 |
| Plant Organs | Vegetation Communities and Their Area in m2 | Total in Phytocenosis kg/400 m2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Koeleria glauca [273.8 m2] | Polytrichum piliferum [7.88 m2] | Salix arenaria [65.73 m2] | Pinus sylvestris [36.2 m2] | ||||||
| [g/m2] | [kg]- in Community | [g/m2] | [Kg]- In Community | [g/m2] | [kg]- in Community | [g/m2] | [kg]- in Community | ||
| Aboveground biomass: | 98.8 | 27.05 | 116.1 | 0.91 | 2258.2 | 148.43 | 2007.8 | 72.69 | 249.08 |
| leaves | - | - | - | - | 599.5 | 39.41 | - | - | |
| needls | - | - | - | - | - | - | 122.1 | 4.42 | |
| trunk | - | - | - | - | - | - | 1245.5 | 45.09 | |
| living branches | - | - | - | - | 1543.1 | 101.43 | 378.6 | 13.71 | |
| Dead branches | - | - | - | - | - | - | - | - | |
| Annual increment | - | - | - | - | 101.7 | 6.68 | 10.7 | 0.39 | |
| Plant litter | - | - | - | - | 13.9 | 0.91 | 250.9 | 9.08 | |
| Underground biomass: | 215.9 | 59.11 | - | - | 732.2 | 48.12 | 1147.0 | 41.53 | 148.76 |
| Roots diameter: <1 mm | 215.9 | 59.11 | - | - | 70.8 | 4.65 | 221.7 | 8.03 | |
| 1–10 mm | - | - | - | - | 305.4 | 20.07 | 485.1 | 17.56 | |
| >10 mm | - | - | - | - | 356.0 | 23.40 | 440.2 | 15.94 | |
| TOTAL: | 314.7 | 86.16 | 116.1 | 0.91 | 2990.4 | 196.55 | 3154.8 | 114.22 | 397.84 |
| Vegetation/Genetic Horizons | Deepth [cm] | Color According to Munsell | Texture [mm] | |||||
|---|---|---|---|---|---|---|---|---|
| Dry | Wet | >1 | 1.0–0.5 | 0.5–0.25 | 0.25–0.1 | <0.1 | ||
| very coarse | coarse | medium | fine | very fine | ||||
| Algal crust | ||||||||
| OA | 0–1 | 10YR 4/1 | 10YR 2/1 | 0.1 | 31.7 | 49.5 | 15.0 | 3.7 |
| AC | 1–5 | 10YR 6/6 | 10YR 5/6 | 0.1 | 11.0 | 63.4 | 24.7 | 0.8 |
| C | 5–60 | 10YR 7/3 | 10YR 6/4 | 0.7 | 27.0 | 57.6 | 14.5 | 0.2 |
| Polytrichum piliferum | ||||||||
| A | 3–7 | 10YR 4/2 | 10YR 3/1 | 0.0 | 14.8 | 60.2 | 22.2 | 0.8 |
| Salix arenaria (16 y.) | ||||||||
| A | 0–7 | 10YR 3/2 | 10YR 2/1 | 0.0 | 23.0 | 54.7 | 20.0 | 2.3 |
| AC | 7–22 | 10YR 5/4 | 10YR 4/5 | 0.1 | 17.3 | 61.9 | 20.0 | 0.7 |
| C | 27–60 | 10YR 7/3 | 10YR 6/4 | 0.1 | 16.2 | 58.1 | 24.4 | 1.2 |
| Salix acutifolia (21 y.) | ||||||||
| A | 0–13 | 10YR 4/2 | 10YR 3/1 | 0.1 | 20.8 | 55.2 | 21.5 | 2.4 |
| B(fe) | 13–27 | 10YR 5/4 | 10YR 4/3 | 0.4 | 20.3 | 58.7 | 20.2 | 0.4 |
| BC | 27–60 | 10YR 6/4 | 10YR 5/4 | 0.4 | 27.4 | 59.3 | 12.7 | 0.2 |
| Pinus sylvestris (26 y.) | ||||||||
| AE | 0–11 | 10YR 5/2 | 10YR 3/2 | 0.0 | 25.1 | 58.9 | 14.2 | 1.8 |
| AC | 11–17 | 10YR 5/3 | 10YR 4/3 | 0.5 | 27.6 | 56.4 | 14.2 | 1.3 |
| C | 17–30 | 10YR 6/4 | 10YR 5/4 | 0.4 | 29.6 | 53.2 | 16.2 | 0.6 |
| Koeleria glauca | ||||||||
| OA | 0–8 | 10YR 5/2 | 10YR 4/1 | 0.0 | 6.4 | 50.1 | 41.3 | 2.2 |
| IIA1/C1…A8/C8b | 8–37 | 10YR 6/2 | 10YR 5/2 | 0.0 | 6.9 | 56.0 | 36.7 | 0.4 |
| III Ab | 37–40 | 10YR 6/2 | 10YR 4/2 | 0.0 | 0.3 | 51.1 | 47.5 | 1.1 |
| C | 4o-60 | 10YR 6/2 | 10YR 5/2 | 0.0 | 5.5 | 71.6 | 22.8 | 0.1 |
| Vegetation | Horyzont | Depth [cm] | Loss Ignition [%] | Corg [%] | Nt [%] | C/N | Pavial. [mg/kg] | Hh cmol(+)*kg−1 | pH | |
|---|---|---|---|---|---|---|---|---|---|---|
| H2O | KCl | |||||||||
| Algal crust | OA | 0–1 | 2.87 | 1.10 | 0.060 | 18 | 10.41 | 1.59 | 5.1 | 4.5 |
| A | 1–5 | 1.29 | 0.41 | 0.025 | 16 | 3.81 | 0.94 | 5.9 | 4.9 | |
| C | 5–60 | 0.33 | 0.19 | 0.010 | 19 | 2.22 | 0.29 | 5.9 | 5.1 | |
| Polytrichum piliferum | OA | 0–2 | 14.80 | 8.49 | 0.292 | 29 | 47.44 | 5.49 | 4.9 | 4.1 |
| A | 2–7 | 1.39 | 0.62 | 0.029 | 21 | 16.12 | 0.96 | 5.5 | 4.7 | |
| C | 7–60 | 0.62 | 0.22 | 0.013 | 17 | 2.13 | 0.39 | 4.7 | 4.5 | |
| Corynephorus canescens | OA | 0–4 | 0.61 | 0.28 | 0.021 | 13 | 4.32 | 1.52 | 4.9 | 4.2 |
| C | 4–60 | 0.28 | 0.18 | 0.009 | 20 | 4.30 | 0.24 | 6.3 | 5.4 | |
| Koeleria glauca | OA | 0–8 | 1.02 | 0.75 | 0.031 | 24 | 24.10 | 0.90 | 5.9 | 4.9 |
| IIA1C1...A8C8b * | 8–37 | 0.34 | 0.18 | 0.017 | 11 | 17.12 | 0.38 | 6.2 | 5.8 | |
| IIIAb | 37–40 | 0.33 | 0.25 | 0.014 | 18 | 19.18 | 0.38 | 6.3 | 5.8 | |
| C | 40–60 | 0.28 | 0.18 | 0.011 | 16 | 15.16 | 0.38 | 6.1 | 5.8 | |
| Salix arenaria | O (Ol/f/h) | 2–0 | 24.00 | 12.30 | 0.492 | 25 | 36.21 | 6.91 | 5.6 | 4.9 |
| A | 0–7 | 1.98 | 1.42 | 0.072 | 20 | 14.29 | 1.81 | 5.6 | 4.6 | |
| AC | 7–22 | 0.48 | 0.33 | 0.015 | 22 | 12.42 | 0.64 | 5.5 | 4.8 | |
| C | 22–60 | 0.33 | 0.17 | 0.006 | 28 | 27.12 | 0.39 | 5.8 | 5.0 | |
| Salix acutifolia | Ol | 4–2 | 82.50 | 49.20 | 1.560 | 32 | 6.45 | 26.43 | 5.5 | 5.1 |
| Of/h | 2–0 | 21.90 | 11.50 | 0.558 | 20 | 6.89 | 7.05 | 5.8 | 5.2 | |
| A | 0–13 | 1.45 | 0.59 | 0.040 | 15 | 2.69 | 0.83 | 6.1 | 5.4 | |
| B(fe) | 13–27 | 1.01 | 0.34 | 0.022 | 15 | 19.13 | 0.92 | 5.9 | 5.2 | |
| BC | 27–60 | 0.58 | 0.17 | 0.007 | 24 | 25.11 | 0.53 | 5.8 | 5.2 | |
| Pinus sylvestris | Ol | 9–6 | 96.00 | 49.40 | 0.903 | 54 | 5.32 | 50.92 | 3.9 | 3.2 |
| Of | 5–2 | 62.10 | 32.12 | 0.615 | 52 | 6.49 | 16.38 | 4.4 | 3.7 | |
| Oh | 2–1 | 44.20 | 19.51 | 0.508 | 38 | 65.23 | 5.12 | 4.3 | 3.4 | |
| AE | 0–11 | 0.77 | 0.43 | 0.015 | 29 | 31.09 | 1.07 | 5.5 | 4.8 | |
| AC | 11–17 | 0.63 | 0.2‘7 | 0.008 | 34 | 5.21 | 0.69 | 5.4 | 4.7 | |
| C | 17–60 | 0.63 | 0.22 | 0.005 | 44 | 3.44 | 0.53 | 5.4 | 4.8 | |
| Vegetation | Horyzont | Depth [cm] | Total Content [mg/kg] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ca | Mg | K | Na | P | Fe | Al | Zn | |||
| Algae | OA | 0–4 | 310 | 515 | 5113 | 1766 | 180 | 286 | 1090 | 97 |
| Polytrichum piliferum | A | 3–7 | 450 | 280 | 2700 | 660 | 110 | 7750 | 10,400 | 6 |
| C | 7–60 | 220 | 180 | 600 | 450 | 89 | 4700 | 14,800 | 180 | |
| Corynephorus canescens | OA | 0–4 | 340 | 145 | 210 | 490 | 220 | 3800 | 11,200 | 9 |
| C | 4–60 | 330 | 130 | 240 | 620 | 110 | 1335 | 9800 | 6 | |
| Koeleria glauca | OA | 0–8 | 330 | 230 | 2500 | 650 | 70 | 4000 | 11,300 | 65 |
| IIA1C1...A8C8b | 8–37 | 280 | 100 | 220 | 610 | 120 | 2300 | 9200 | 40 | |
| IIIAb | 37–40 | 540 | 290 | 2900 | 850 | 160 | 4400 | 1900 | 27 | |
| C | 40–60 | 400 | 150 | 3700 | 790 | 70 | 1900 | 12,300 | 70 | |
| Salix arenaria | A | 0–7 | 310 | 140 | 2100 | 490 | 220 | 3800 | 14,000 | 9 |
| C | 22–60 | 340 | 130 | 2400 | 620 | 110 | 1300 | 12,200 | 6 | |
| Salix acutifolia | A | 0–13 | 840 | 290 | 2800 | 680 | 60 | 3800 | 14,100 | 290 |
| B(fe) | 13–27 | 290 | 130 | 2600 | 620 | 30 | 1500 | 13,100 | 9 | |
| BC | 27–60 | 210 | 120 | 2500 | 590 | 40 | 1500 | 9800 | 9 | |
| Pinus sylvestris | AE | 0–11 | 750 | 240 | 2750 | 740 | 70 | 250 | 14,000 | 650 |
| AC | 11–17 | 310 | 140 | 2600 | 770 | 50 | 1500 | 13,000 | 70 | |
| C | 17–60 | 360 | 150 | 2200 | 630 | 0,00 | 1100 | 8800 | 20 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahmonov, O.; Skreczko, S.; Rahmonov, M. Changes in Soil Features and Phytomass during Vegetation Succession in Sandy Areas. Land 2021, 10, 265. https://doi.org/10.3390/land10030265
Rahmonov O, Skreczko S, Rahmonov M. Changes in Soil Features and Phytomass during Vegetation Succession in Sandy Areas. Land. 2021; 10(3):265. https://doi.org/10.3390/land10030265
Chicago/Turabian StyleRahmonov, Oimahmad, Sylwia Skreczko, and Małgorzata Rahmonov. 2021. "Changes in Soil Features and Phytomass during Vegetation Succession in Sandy Areas" Land 10, no. 3: 265. https://doi.org/10.3390/land10030265
APA StyleRahmonov, O., Skreczko, S., & Rahmonov, M. (2021). Changes in Soil Features and Phytomass during Vegetation Succession in Sandy Areas. Land, 10(3), 265. https://doi.org/10.3390/land10030265






