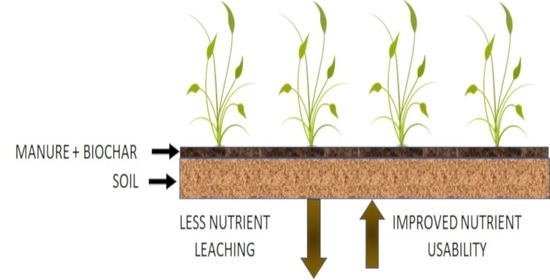
Comparing Biochar-Swine Manure Mixture to Conventional Manure Impact on Soil Nutrient Availability and Plant Uptake—A Greenhouse Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Collection, Biochar, Manure, and Manure-Biochar Incubation
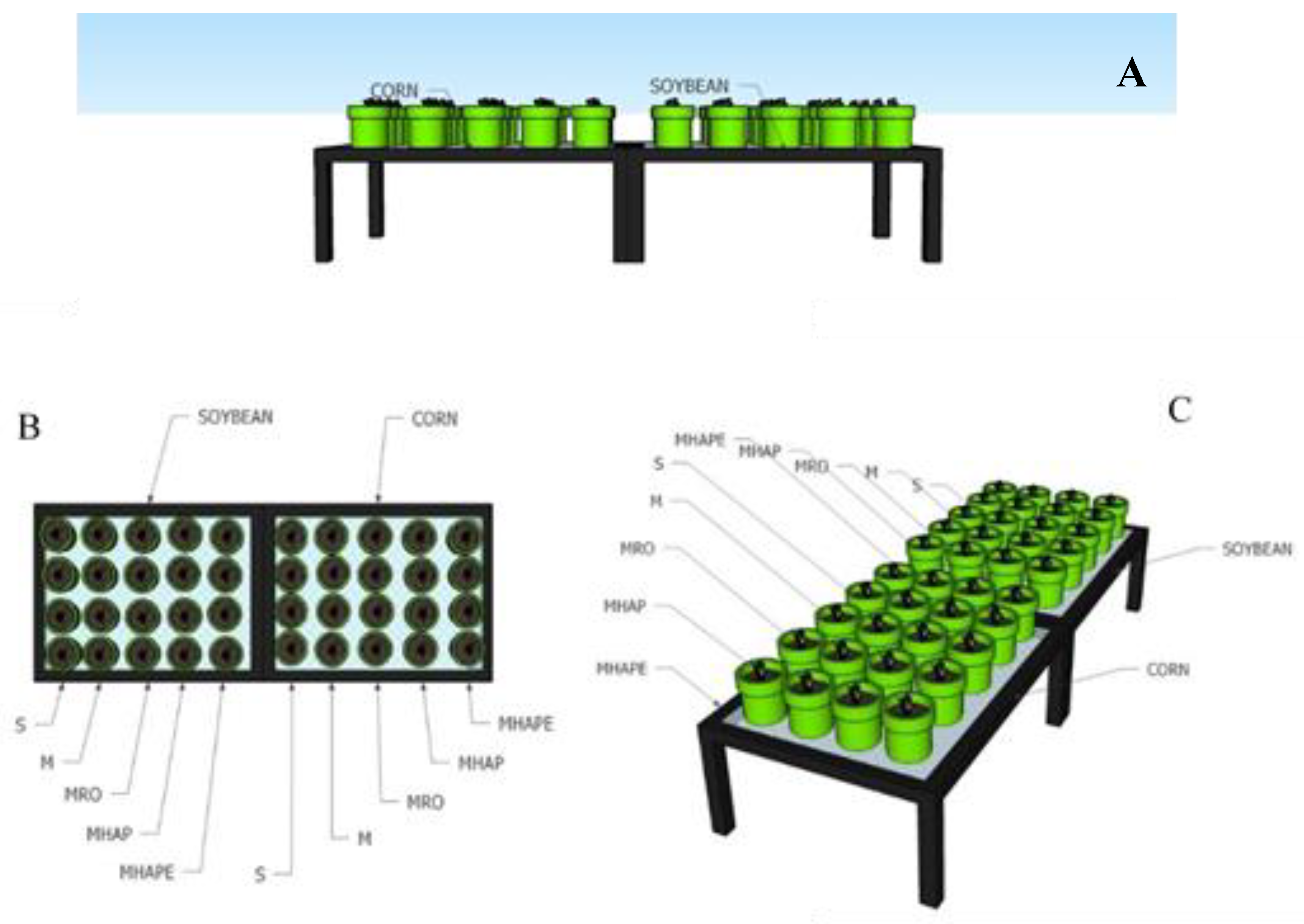
2.2. Soil Preparation, Greenhouse, and Pot Experiment
2.3. Soil and Biomass Analysis
2.4. Statistical Analysis
3. Results
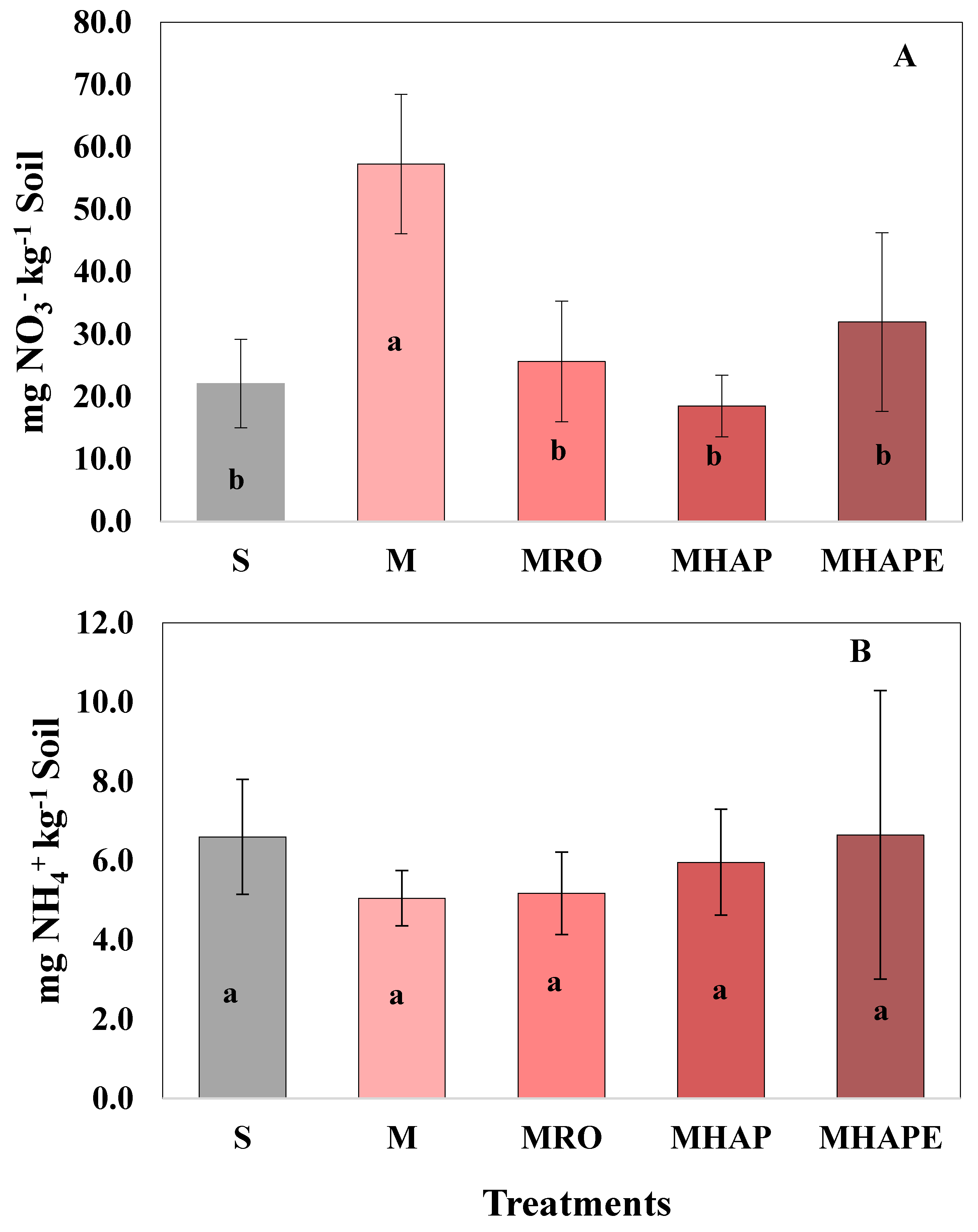
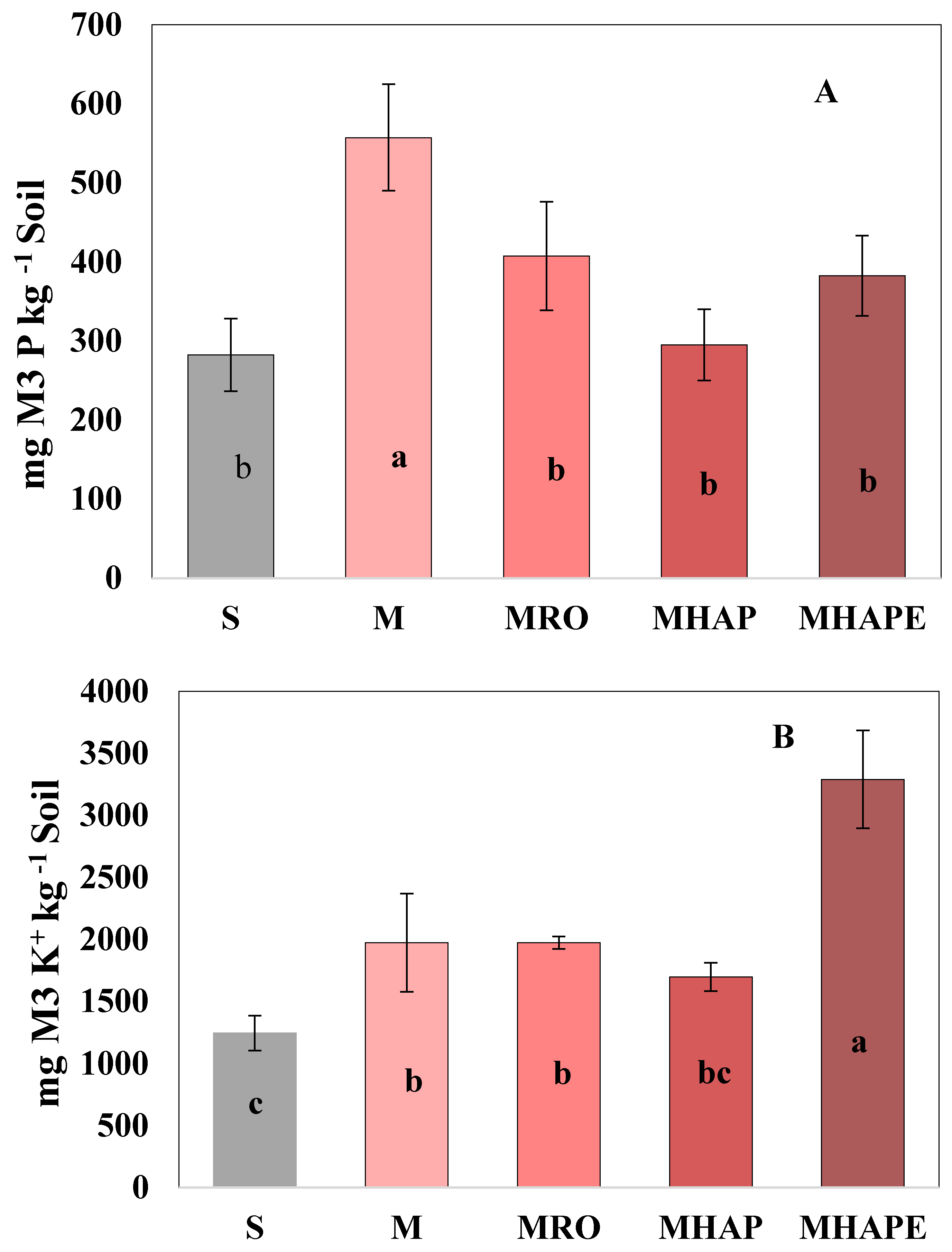
3.1. Impact of Treatments on Corn Planted Soil
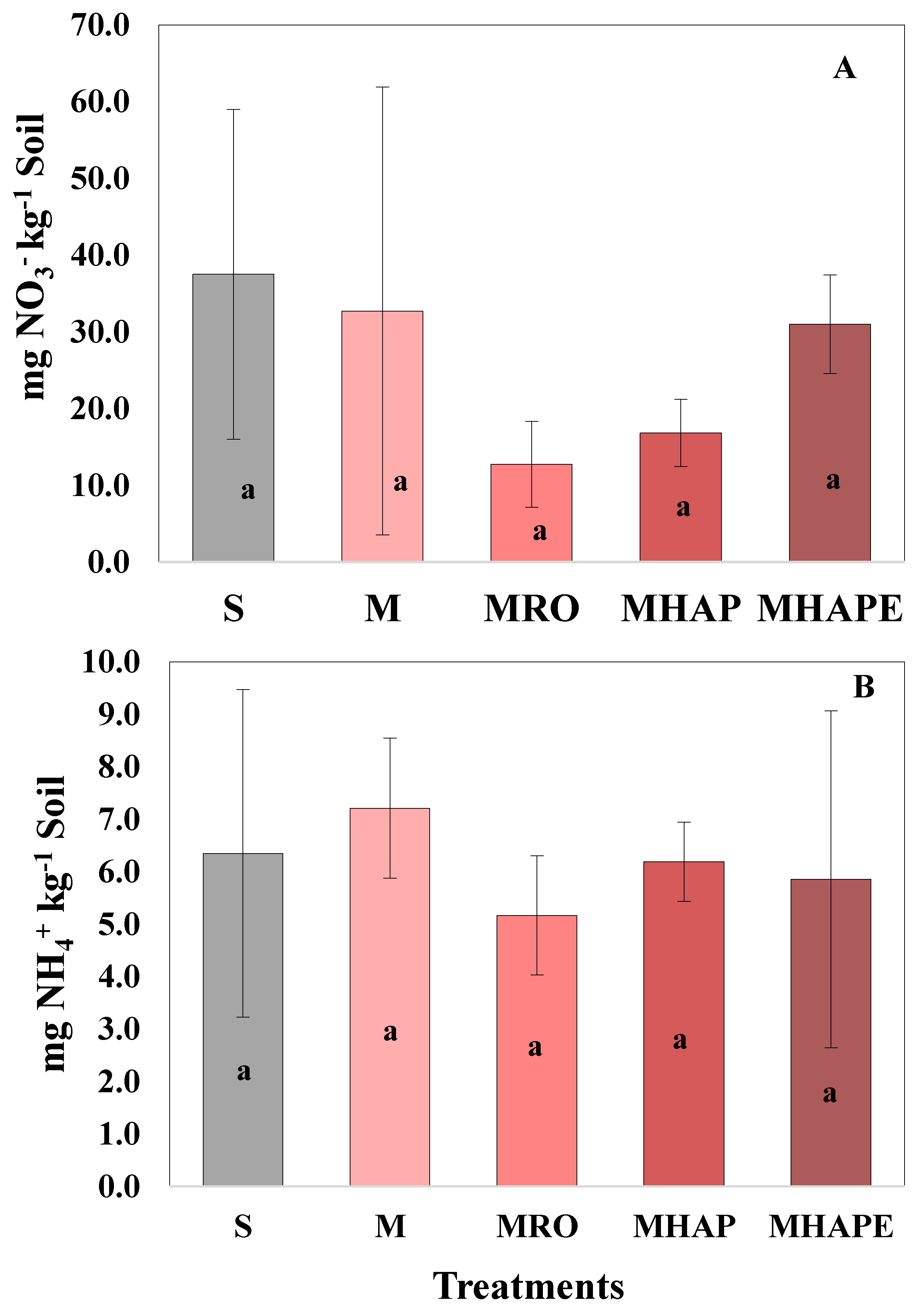
3.2. Impact of Treatments on Soybean Planted Soil
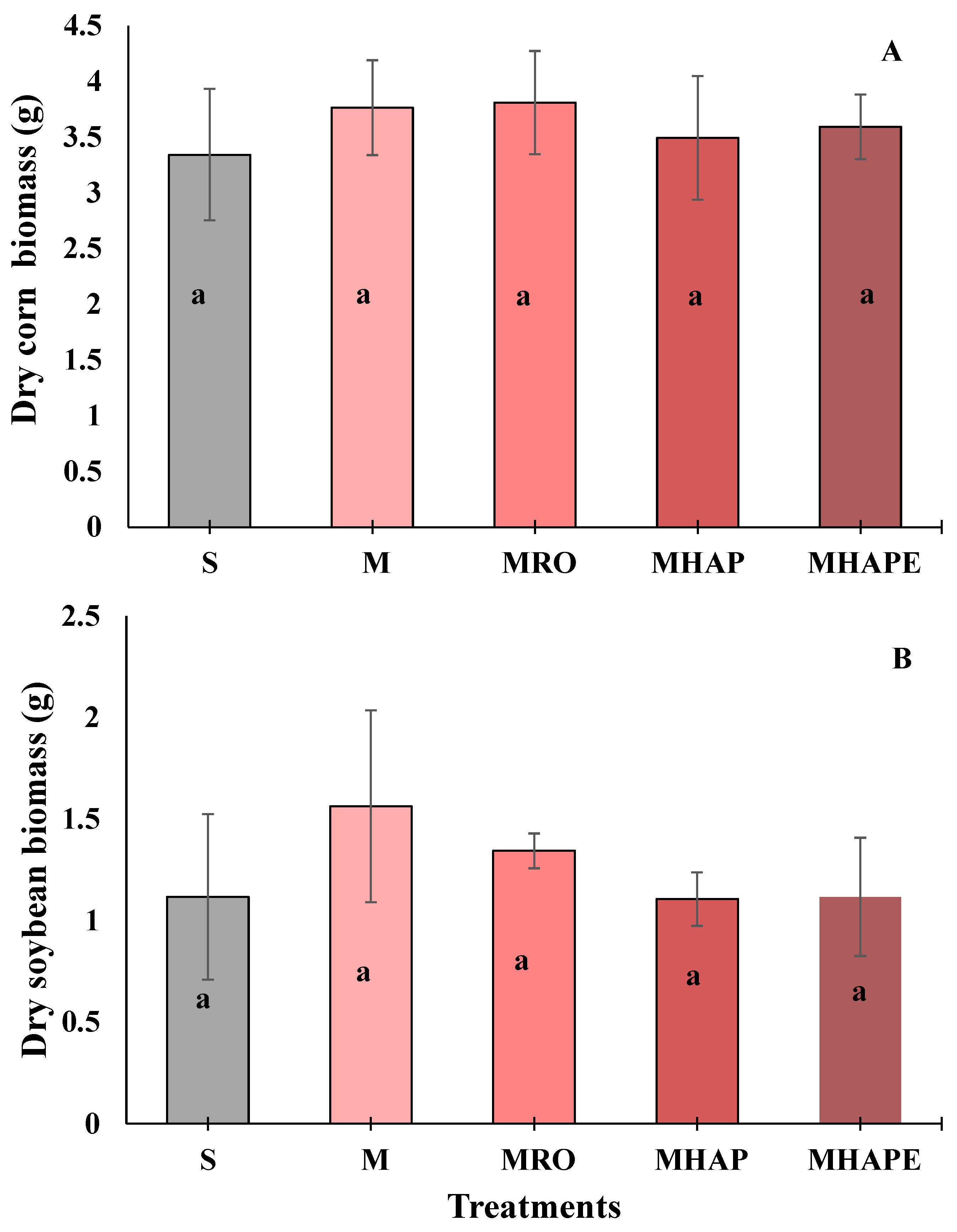
3.3. Impact of Treatments on Corn and Soybean Plant Growth and Biomass Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russell, A.E.; Laird, D.A.; Parkin, T.B.; Mallarini, A.P. Impact of Nitrogen Fertilization and Cropping System on Carbon Sequestration in Midwestern Mollisols. Soil Biol. Biochem. 2005, 69, 413–422. [Google Scholar] [CrossRef] [Green Version]
- Evanylo, G.; Sherony, C.; Spargo, J.; Starner, D.; Brosius, M.; Haering, K. Soil and water environmental effects of fertilizer-, manure-, and compost-based fertility practices in an organic vegetable cropping system. Agric. Ecosyst. Environ. 2008, 127, 50–58. [Google Scholar] [CrossRef]
- Wortman, S.E.; Holmes, A.A.; Miernicki, E.; Knoche, K.; Pittelkow, C.M. First-season crop yield response to organic soil amendments: A meta-analysis. Agron. J. 2017, 109, 1210–1217. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.I.; Mickelson, S.K.; Pederson, C.H.; Baker, J.L.; Kanwar, R.S.; Lorimor, J.C.; Webber, D. Swine manure rate, timing, and application method effects on post-harvest soil nutrients, crop yield, and potential water quality implications in a corn-soybean rotation. Trans. ASABE 2013, 56, 395–408. [Google Scholar] [CrossRef]
- Allen, B.L.; Mallarino, A.P. Effect of liquid swine manure rate, incorporation, and timing of rainfall on phosphorus loss with surface runoff. J. Environ. Qual. 2008, 37, 125–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, W.; O’Brien, P.L.; Hatfield, J.L. Crop Yield and Nitrous Oxide Emissions following Swine Manure Application: A Meta-Analysis. Agric. Environ. 2019, 4, 190024. [Google Scholar] [CrossRef]
- Lehmann, J.; Pereira da Silva, J.; Steiner, C. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: Fertilizer, manure and charcoal amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Laird, D.A.; Fleming, P.; Wang, B.; Horton, R.; Karlen, D. Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 2010, 158, 436–442. [Google Scholar] [CrossRef] [Green Version]
- Laird, D.A.; Fleming, P.D.; Davis, D.D.; Wang, B.; Horton, R.; Karlen, D.L. Impact of biochar amendments on the quality of a typical Midwestern agricultural soil. Geoderma 2010, 158, 443–449. [Google Scholar] [CrossRef] [Green Version]
- Rogovska, N.; Laird, D.A.; Rathke, S.J.; Karlen, D.L. Biochar impact on Midwestern Mollisols and maize nutrient availability. Geoderma 2014, 230–231, 340–347. [Google Scholar] [CrossRef] [Green Version]
- Zimmerman, A.R.; Gao, B.; Ahn, M. Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol. Biochem. 2011, 4, 1169–1179. [Google Scholar] [CrossRef]
- Lentz, R.D.; Ippolito, J.A. Biochar and manure affects calcareous soil and corn silage nutrient concentrations and uptake. J. Environ. Qual. 2012, 41, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Giesler, R.; Andersson, T.; Lövgren, L.; Persson, P. Phosphate sorption in aluminum- and iron-rich humus soils. Soil Sci. Soc. Am. J. 2005, 69, 77–86. [Google Scholar] [CrossRef]
- Torrent, J.; Schwertmann, U.; Barrón, V. Fast and slow phosphate sorption by goethite-rich natural materials. Clays Clay Miner. 1992, 40, 14–21. [Google Scholar] [CrossRef]
- Lyngsie, G.; Katika, K.; Fabricius, I.L.; Hansen, H.C.B.; Borggaard, O.K. Phosphate removal by iron oxide-coated diatomite: Laboratory test of a new method for cleaning drainage water. Chemosphere 2019, 222, 884–890. [Google Scholar] [CrossRef]
- Bakshi, S.; Laird, D.A.; Smith, R.G.; Brown, R.C. Capture and Release of Orthophosphate by Fe-Modified Biochars: Mechanisms and Environmental Applications. Sustain. Chem. Eng. 2021, in press. [Google Scholar] [CrossRef]
- Cai Ru Wang, X.; Ji, X.; Peng, B.; Tan, C.; Huang, X. Phosphate reclaim from simulated and real eutrophic water by magnetic biochar derived from water hyacinth. J. Environ. Manag. 2017, 187, 212–219. [Google Scholar] [CrossRef]
- Jing, R.; Nan, L.; Lei, L.; Jing-Kun, A.; Lin, Z.; Nan-Qi, R. Granulation and ferric oxides loading enable biochar derived from cotton stalk to remove phosphate from water. Bioresour. Technol. 2015, 178, 119–125. [Google Scholar]
- Li, R.; Wang, J.J.; Gaston, L.A.; Zhou, B.; Li, M.; Xiao, R.; Wang, Q.; Zhang, Z.; Huang, H.; Liang, W.; et al. An overview of carbothermal synthesis of metal–biochar composites for the removal of oxyanion contaminants from aqueous solution. Carbon 2018, 129, 674–687. [Google Scholar] [CrossRef]
- Banik, C.; Koziel, J.; De, M.; Bonds, D.; Chen, B.; Singh, A.; Licht, M. Soil Nutrients and Carbon Dynamics in the Presence of Biochar-swine Manure Mixture Under Controlled Leaching Experiment Using a Midwestern USA Mollisolls. Front. Environ. Sci. 2021, in press. [Google Scholar] [CrossRef]
- Maurer, D.L.; Koziel, J.A.; Kalus, K.; Anderson, D.S.; Opalinski, S. Pilot-Scale Testing of Non-Activated Biochar for Swine Manure Treatment and Mitigation of Ammonia, Hydrogen Sulfide, Odorous Volatile Organic Compounds (VOCs) and Greenhouse Gas Emissions. Sustainability 2017, 9, 929. [Google Scholar] [CrossRef] [Green Version]
- Meiirkhanuly, Z.; Koziel, J.A.; Białowiec, A.; Banik, C.; Brown, R.C. The-Proof-of-Concept of Biochar Floating Cover Influence on Water pH. Water 2019, 11, 1802. [Google Scholar] [CrossRef] [Green Version]
- Meiirkhanuly, Z.; Koziel, J.A.; Chen, B.; Białowiec, A.; Lee, M.; Wi, J.; Banik, C.; Brown, R.C.; Bakshi, S. Mitigation of Gaseous Emissions from Swine Manure with the Surficial Application of Biochars. Atmosphere 2020, 11, 1179. [Google Scholar] [CrossRef]
- Meiirkhanuly, Z.; Koziel, J.A.; Białowiec, A.; Banik, C.; Brown, R.C. The proof-of-the concept of biochar floating cover influence on swine manure pH: Implications for mitigation of gaseous emissions from area sources. Front. Chem. 2020, 8, 656. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Koziel, J.A.; Białowiec, A.; Lee, M.; Ma, H.; Li, P.; Meiirkhanuly, Z.; Brown, R.C. The Impact of Surficial Biochar Treatment on Acute H2S Emissions during Swine Manure Agitation before Pump-Out: Proof-of-the-Concept. Catalysts 2020, 10, 940. [Google Scholar] [CrossRef]
- Chen, B.; Koziel, J.A.; Białowiec, A.; Lee, M.; Ma, H.; O’Brien, S.; Li, P.; Meiirkhanuly, Z.; Brown, R.C. Mitigation of Acute Ammonia Emissions During Swine Manure Agitation Before Pump-Out with Biochar: Proof-of-the-Concept. Front. Environ. Sci. 2021, in press. [Google Scholar] [CrossRef]
- Chen, B.; Koziel, J.A.; Banik, C.; Ma, H.; Lee, M.; O’Brien, S.C.; Li, P.; Andersen, D.; Białowiec, A.; Brown, R.C. Mitigation of gaseous emissions from stored swine manure: Effect of biochar dose and reapplication on a pilot-scale. Atmosphere 2021, 12, 96. [Google Scholar] [CrossRef]
- Chen, B.; Koziel, J.A.; Banik, C.; Ma, H.; Lee, M.; Wi, J.; Meiirkhanuly, Z.; O’Brien, S.C.; Li, P.; Andersen, D.S.; et al. Mitigation of odor, NH3, H2S, GHG, and VOC emissions with current products for use in deep-pit swine manure storage structures. Front. Environ. Sci. 2020, 8. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter. In Methods of Soil Analysis: Part 3 Chemical Methods, 3rd ed.; Bartels, J.M., Ed.; ASA and SSSA Book Series 5; Soil Science Society of America: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar] [CrossRef]
- McGeehan, S.L.; Naylor, D.V. Automated instrumental analysis of carbon and nitrogen in plant and soil samples. Commun. Soil Sci. Plant Anal. 1988, 19, 493–505. [Google Scholar] [CrossRef]
- Schulte, E.E.; Hopkins, B.G. Estimation of Soil Organic Matter by Weight Loss-On Ignition. In Soil Organic Matter: Analysis and Interpretation; Magdoff, F.R., Tabatabai, M.A., Hanlon, E.A., Jr., Eds.; Special Publication No. 46; Soil Science Society of America: Madison, WI, USA, 1996; pp. 21–32. [Google Scholar] [CrossRef]
- De, M.; Riopel, J.A.; Cihacek, L.J.; Lawrinenko, M.; Baldwin-Kordick, R.; Hall, S.J.; McDaniel, M.D. Soil health recovery after grassland reestablishment on cropland: The effects of time and topographic position. Soil Sci. Soc. Am. J. 2019, 84, 568–586. [Google Scholar] [CrossRef]
- Doane, T.A.; Horwath, W.R. Spectrophotometric determination of nitrate with a single reagent. Anal. Lett. 2003, 36, 2713–2722. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich-3 soil test extractant: A modification of Mehlich-2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Suarez, D.L. Beryllium, Magnesium, Calcium, Strontium, and Barium. In Methods of Soil Analysis Part 3: Chemical Methods; Sparks, D.L., Ed.; ASA Book Series 5; Soil Science Society of America: Madison, WI, USA, 1996; pp. 575–602. [Google Scholar] [CrossRef]
- Linsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Gavlak, R.G.; Horneck, D.A.; Miller, R.O.; Kotuby-Amacher, J. Soil, Plant, and Water Reference Methods for the Western Regions, 2nd ed.; WREP-125; WCC-103 Publication: Fort Collins, CO, USA, 2003. [Google Scholar]
- Abendroth, L.J.; Elmore, R.W.; Boyer, M.J.; Marlay, S.K. Corn Growth and Development, PMR-1009; Iowa State University: Ames, IA, USA, 2011; Available online: https://store.extension.iastate.edu/Product/Corn-Growth-and-Development (accessed on 6 January 2021).
- Fehr, W.R.; Caviness, C.F.; Burmood, D.T.; Pennington, J.S. Stage of development descriptions for soybeans, Glycine max (L.). Merrill. Crop Sci. 1971, 11, 929–931. [Google Scholar] [CrossRef]
- Angers, D.A.; Chantigny, M.H.; MacDonald, J.D.; Rochette, P.; Cote, D. Differential retention of carbon, nitrogen, and phosphorus in grassland soil profiles with long-term manure application. Nutr. Cycl. Agroecosyst. 2009, 86, 225–229. [Google Scholar] [CrossRef]
- Chastain, J.P.; Camberato, J.J.; Albrecht, J.E.; Adam, J. Swine Manure Production and Nutrient Content; South Carolina Confined Animal Manure Managers Certification Program; Clemson University: Clemson, SC, USA, 1999; Chapter 3; pp. 1–17. [Google Scholar]
- Lv, Y.; Francis, C.; Wu, P.; Chen, X.; Zhao, X. Maize–soybean intercropping interactions above and below ground. Crop Sci. 2014, 54, 914–922. [Google Scholar] [CrossRef]


| Control | Biochar Only | Biochar + Manure Mixture | |||
|---|---|---|---|---|---|
| Term | Definition | Term | Definition | Term | Definition |
| S | Soil only | RO | Fast pyrolysis red oak biochar | MRO | Manure + RO biochar |
| M | Manure only | HAP | Fast pyrolysis, highly alkaline porous corn stover biochar | MHAP | Manure + HAP biochar |
| - | - | HAPE | Fast pyrolysis, highly porous autothermal corn stover biochar engineered with iron | MHAPE | Manure + HAPE biochar |
| Treatment | Total-N | K | Ca | Mg | Fe | Cu | Mn | Zn |
|---|---|---|---|---|---|---|---|---|
| M | b | d | d | c | c | a | c | a |
| MRO | d | c | c | c | c | c | c | d |
| MHAP | a | a | a | a | b | b | b | b |
| MHAPE | c | b | b | b | a | b | a | c |
| Treatments | pH | OM (%) | Total C (%) | Total N (%) | Total C:N Ratio | Ca mg kg−1 | Mg mg kg−1 | Fe mg kg−1 | Mn mg kg−1 | Cu mg kg−1 | Zn mg kg−1 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S (control) | 7.7 ± 0.27 | 2.92 ± 0.15 | 1.79 ± 0.09 | 0.17 ± 0.001 | 10.51 ± 0.32 | 1875 ± 104 | 530.0 ± 33.9 | 128.3 ± 14.3 | 154.7 ± 5.91 | 2.74 ± 0.20 | 5.16 ± 0.59 |
| M (control) | 7.7 ± 0.23 | 2.98 ± 0.09 | 2.01 ± 0.13 | 0.20 ± 0.01 | 10.04 ± 0.31 | 1849 ± 74 | 555.0 ± 26.2 | 119.5 ± 6.57 | 141.8 ± 2.86 | 8.45 ± 0.58 | 14.2 ± 1.24 |
| MRO | 7.6 ± 0.07 | 3.30 ± 0.11 | 2.43 ± 0.28 | 0.18 ± 0.02 | 13.15 ± 1.10 (p = 0.0001) | 1658 ± 90 | 479.0 ± 25.8 (p = 0.02) | 107.8 ± 3.27 | 139.0 ± 4.84 | 3.07 ± 0.26 (p <0.0001) | 6.96 ± 0.87 (p <0.0001) |
| MHAP | 7.6 ± 0.08 | 3.36 ± 0.16 (p = 0.02) | 2.35 ± 0.28 | 0.19 ± 0.02 | 12.36 ± 0.37 (p = 0.002) | 1655 ± 113 | 463.0 ± 31.0 (p = 0.006) | 105.7 ± 3.96 | 141.2 ± 6.17 | 2.55 ± 0.28 (p <0.0001) | 6.10 ± 1.08 (p <0.0001) |
| MHAPE | 7.3 ± 0.11 | 3.31 ± 0.12 (p = 0.04) | 2.11 ± 0.18 | 0.19 ± 0.02 | 11.40 ± 0.50 (p = 0.02) | 1888 ± 165 | 520.8 ± 11.0 | 167.5 ± 11.5 (p <0.0001) | 132.5 ± 2.5 | 2.84 ± 0.12 (p <0.0001) | 6.04 ± 0.45 (p <0.0001) |
| Treatments | pH | OM (%) | Total C (%) | Total N (%) | Total C/N Ratio | Ca mg kg−1 | Mg mg kg−1 | Fe mg kg−1 | Mn mg kg−1 | Cu mg kg−1 | Zn mg kg−1 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S (control) | 7.65 ± 0.29 | 3.16 ± 0.25 | 1.82 ± 0.07 | 0.18 ± 0.01 | 10.14 ± 0.39 | 1962 ±194 | 574.8 ± 39.6 | 121.5 ± 15.9 | 156.3 ± 11.5 | 2.62 ± 0.27 | 4.40 ± 0.422 |
| M (control) | 7.55 ± 0.22 | 3.13 ± 0.08 | 1.91 ± 0.06 | 0.19 ± 0.01 | 10.07 ± 0.22 | 1732 ± 231 | 485.0 ± 41.9 | 95.0 ± 10.6 | 127.0 ± 8.9 | 4.31 ± 0.61 | 8.33 ± 1.15 |
| MRO | 7.63 ± 0.08 | 3.33 ± 0.14 | 2.27 ± 0.14 | 0.19 ± 0.01 | 11.81 ± 0.32 (p = 0.002) | 1764 ± 97.5 | 496.5 ± 22.5 | 93.8 ± 0.43 | 136.7 ± 2.86 | 2.75 ± 0.20 (p = 0.001) | 6.08 ± 0.72 (p = 0.02) |
| MHAP | 7.70 ± 0.10 | 3.41 ± 0.11 | 2.43 ± 0.22 (p = 0.006) | 0.20 ± 0.01 | 11.93 ± 0.89 (p = 0.005) | 1732 ± 58.9 | 487.5 ± 15.4 | 87.7 ± 3.56 | 128.7 ± 2.28 | 2.44 ± 0.09 (p =0.0002) | 5.01 ± 0.65 (p = 0.001) |
| MHAPE | 7.33 ± 0.13 | 3.74 ± 0.10 (p = 0.001) | 2.39 ± 0.20 (p = 0.01) | 0.21 ± 0.01 | 11.37 ± 0.29 (p = 0.02) | 2382 ± 308 (p = 0.009) | 684.5 ± 63.9 (p = 0.0002) | 187.0 ± 29.43 (p <0.0001) | 159.5 ± 15.4 (p = 0.006) | 3.76 ± 0.49 | 6.76 ± 0.92 (p = 0.04) |
| Crop | Treatments | Total C | Total N | P | K | Ca | Mg | Fe | Mn | Cu | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|
| % | mg kg−1 | ||||||||||
| Corn | S (control) | 43.2 ± 0.36 | 1.6 ± 0.30 | 0.14 ± 0.01 | 2.4 ± 0.08 b | 0.5 ± 0.05 | 0.4 ± 0.03 | 48.7 ± 7.0 | 42.6 ± 4.7 ab | 3.1 ± 0.14 | 12.7 ± 1.2 |
| M (control) | 43.2 ± 0.19 | 1.6 ± 0.13 | 0.15 ± 0.02 | 2.7 ± 0.16 bc | 0.4 ± 0.02 | 0.4 ± 0.01 | 41.4 ± 2.1 | 49.5 ± 6.7 a | 2.9 ± 0.18 | 12.5 ± 1.7 | |
| MRO | 43.2 ± 0.21 | 1.7 ± 0.22 | 0.14 ± 0.01 | 2.9 ± 0.08 bc | 0.4 ± 0.04 | 0.4 ± 0.03 | 42.9 ± 5.3 | 36.2 ±3.3 b | 3.0 ± 0.29 | 12.2 ± 1.0 | |
| MHAP | 43.1 ± 0.12 | 1.7 ± 0.17 | 0.13 ± 0.01 | 3.0 ± 0.22 bc | 0.5 ± 0.04 | 0.4 ± 0.03 | 42.4 ± 1.8 | 36.9 ±4.8 b | 2.9 ± 0.16 | 12.8 ± 0.92 | |
| MHAPE | 42.6 ± 0.22 | 1.9 ± 0.15 | 0.12 ± 0.01 | 3.5 ±0.13 a | 0.5 ± 0.05 | 0.4 ± 0.03 | 47.5 ± 6.7 | 37.1 ± 2.9 ab | 2.9 ± 0.19 | 14.3 ± 1.1 | |
| Soybean | S (control) | 42.7 ± 0.23 | 4.2 ± 0.67 a | 0.26 ± 0.04 a | 2.4 ± 0.08 | 4.2 ± 5.06 | 0.57 ± 0.03 | 70.8 ± 5.6 | 97.8 ± 7.1 ab | 4.8 ± 0.61 | 25.6 ± 2.7 |
| M (control) | 42.7 ± 0.32 | 2.3 ± 0.33 b | 0.19 ± 0.003 ab | 2.2 ± 0.07 | 1.3 ± 0.004 | 0.54 ± 0.03 | 60.3 ± 5.3 | 111.6 ± 22 a | 3.8 ± 0.19 | 24.0 ± 2.5 | |
| MRO | 42.6 ± 0.15 | 2.9 ± 0.36 b | 0.19 ± 0.02 ab | 2.3 ± 0.14 | 1.2 ± 0.05 | 0.53 ± 0.02 | 59.2 ± 3.7 | 69.9 ±6.4 c | 4.1 ± 0.47 | 21.6 ± 2.1 | |
| MHAP | 42.8 ± 0.41 | 3.4 ± 0.27 ab | 0.22 ± 0.04 ab | 2.5 ± 0.14 | 1.3 ± 0.05 | 0.55 ± 0.02 | 65.2 ± 1.46 | 72.3 ±3.9 bc | 4.4 ± 0.55 | 23.3 ± 3.8 | |
| MHAPE | 43.3 ± 0.19 | 3.5 ± 0.21 ab | 0.16 ± 0.01 b | 2.4 ± 0.13 | 1.3 ± 0.09 | 0.52 ± 0.01 | 59.2 ± 6.3 | 64.9 ±4.9 c | 3.9 ± 0.22 | 20.9 ± 0.99 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banik, C.; Koziel, J.A.; Bonds, D.; Singh, A.K.; Licht, M.A. Comparing Biochar-Swine Manure Mixture to Conventional Manure Impact on Soil Nutrient Availability and Plant Uptake—A Greenhouse Study. Land 2021, 10, 372. https://doi.org/10.3390/land10040372
Banik C, Koziel JA, Bonds D, Singh AK, Licht MA. Comparing Biochar-Swine Manure Mixture to Conventional Manure Impact on Soil Nutrient Availability and Plant Uptake—A Greenhouse Study. Land. 2021; 10(4):372. https://doi.org/10.3390/land10040372
Chicago/Turabian StyleBanik, Chumki, Jacek A. Koziel, Darcy Bonds, Asheesh K. Singh, and Mark A. Licht. 2021. "Comparing Biochar-Swine Manure Mixture to Conventional Manure Impact on Soil Nutrient Availability and Plant Uptake—A Greenhouse Study" Land 10, no. 4: 372. https://doi.org/10.3390/land10040372
APA StyleBanik, C., Koziel, J. A., Bonds, D., Singh, A. K., & Licht, M. A. (2021). Comparing Biochar-Swine Manure Mixture to Conventional Manure Impact on Soil Nutrient Availability and Plant Uptake—A Greenhouse Study. Land, 10(4), 372. https://doi.org/10.3390/land10040372