Restoration and Conservation of Priority Areas of Caatinga’s Semi-Arid Forest Remnants Can Support Connectivity within an Agricultural Landscape
Abstract
:1. Introduction
- (1)
- How have the cover and spatial configuration of Caatinga and agricultural habitat changed over the period of 1985–2018 in the São Francisco Valley landscape?
- (2)
- How would different patterns of future hypothetical continuing of agricultural expansion influence the connectivity of the landscape from the perspective of species with different levels of dispersal mobility?
- (3)
- If conserved or restored, which native vegetation areas preserve the connectivity of the landscape most effectively under agricultural expansion?
2. Materials and Methods
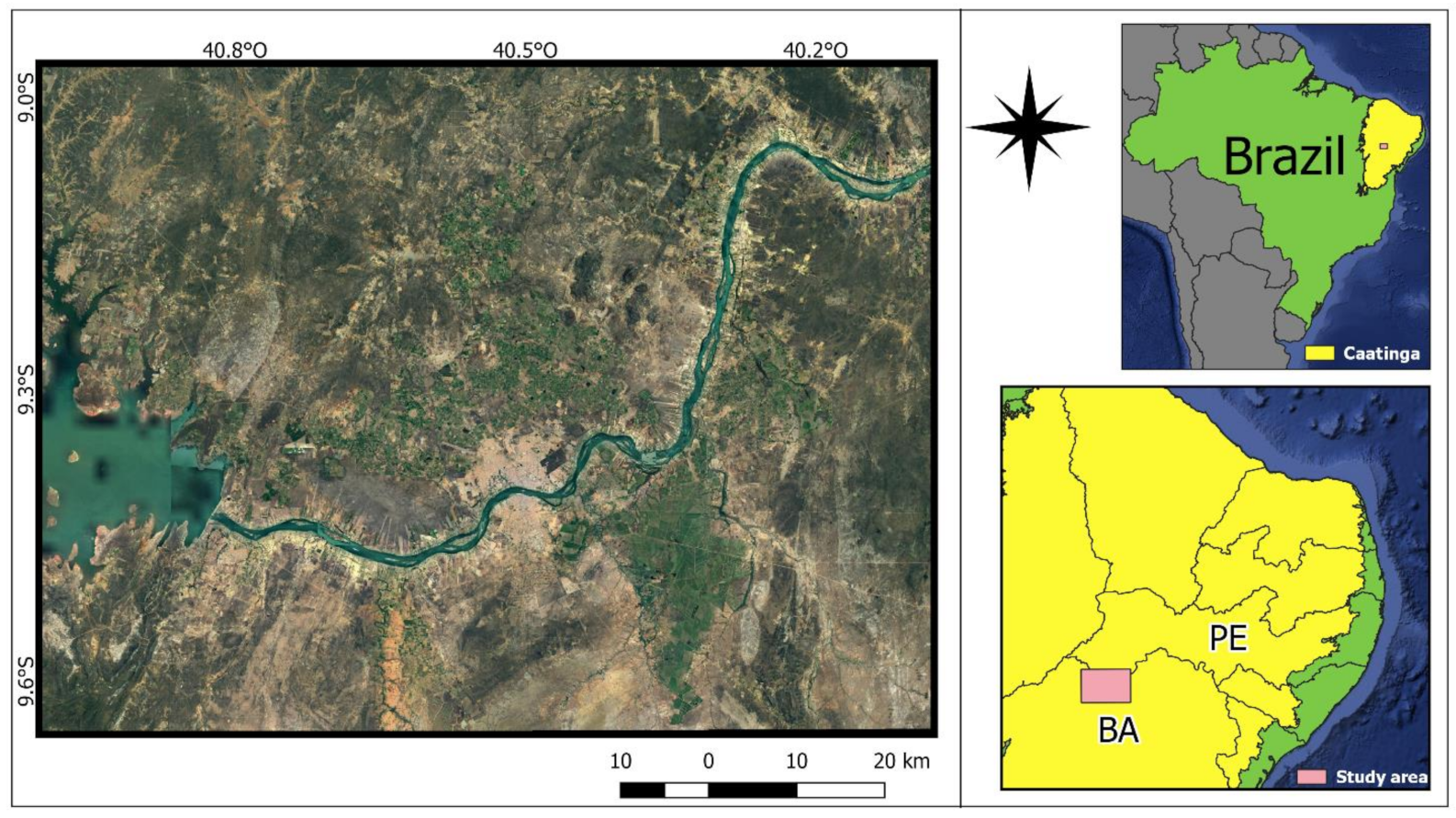
2.1. Study Area
2.2. Processing and Classification of Satellite Images
2.3. Landscape Composition and Configuration Analysis
2.4. Determining the Distance Threshold for Landscape Connectivity Analysis
2.5. Assessment of Hypothetical Agriculture Area Growth Projections
2.6. Prioritization of Caatinga Patches to Conserve and Restore
3. Results
3.1. Spatial–Temporal Change in Land Use and Land Cover
3.2. Fragmentation Process in Open and Dense Caatinga
3.3. Determining the Connectivity Distance Threshold
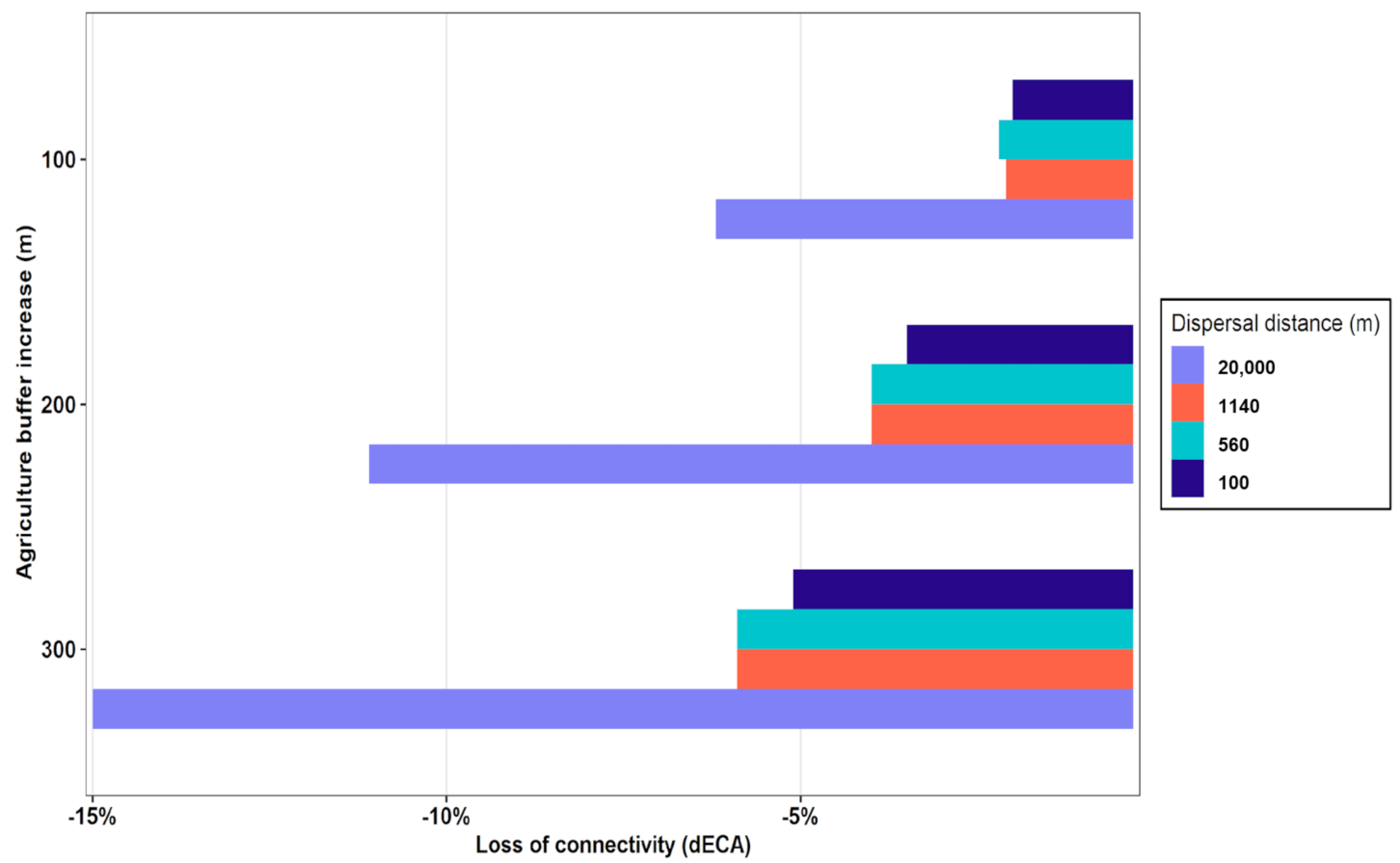
3.4. Changes in Connectivity under Projections of Agricultural Expansion
3.5. Identified Patches for Conservation and Restoration
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Confusion Matrix
| Class | Water | Urban | Pasture | Agriculture | Open Caatinga | Dense Caatinga | Total |
|---|---|---|---|---|---|---|---|
| Water | 47 | 0 | 1 | 0 | 0 | 2 | 50 |
| Urban | 0 | 35 | 13 | 2 | 0 | 0 | 50 |
| Pasture | 0 | 1 | 48 | 1 | 0 | 0 | 50 |
| Agriculture | 0 | 1 | 8 | 40 | 0 | 1 | 50 |
| Open Caatinga | 0 | 0 | 2 | 0 | 43 | 7 | 52 |
| Dense Caatinga | 0 | 0 | 0 | 0 | 0 | 50 | 50 |
| Total | 47 | 37 | 72 | 43 | 43 | 60 | 302 |
| Class | Water | Urban | Pasture | Agriculture | Open Caatinga | Dense Caatinga | Total |
|---|---|---|---|---|---|---|---|
| Water | 55 | 0 | 0 | 1 | 1 | 3 | 60 |
| Urban | 0 | 50 | 14 | 1 | 0 | 0 | 65 |
| Pasture | 0 | 0 | 53 | 0 | 4 | 0 | 57 |
| Agriculture | 1 | 0 | 6 | 82 | 0 | 0 | 89 |
| Open Caatinga | 0 | 0 | 1 | 1 | 47 | 1 | 50 |
| Dense Caatinga | 0 | 0 | 0 | 0 | 8 | 42 | 50 |
| Total | 56 | 50 | 74 | 85 | 60 | 46 | 371 |
| Class | Water | Urban | Pasture | Agriculture | Open Caatinga | Dense Caatinga | Total |
|---|---|---|---|---|---|---|---|
| Water | 175 | 0 | 0 | 4 | 0 | 0 | 179 |
| Urban | 0 | 83 | 12 | 0 | 1 | 0 | 96 |
| Pasture | 0 | 0 | 124 | 4 | 2 | 0 | 130 |
| Agriculture | 0 | 0 | 8 | 148 | 2 | 1 | 159 |
| Open Caatinga | 0 | 0 | 19 | 0 | 93 | 1 | 113 |
| Dense Caatinga | 0 | 0 | 0 | 0 | 26 | 120 | 146 |
| Total | 175 | 83 | 163 | 156 | 124 | 122 | 823 |
Appendix B
| Class | 1985 | 2000 | 2018 | Net Change (1985–2018) |
|---|---|---|---|---|
| ha | ha | ha | ha | |
| Water | 45,894 | 43,323 | 44,700 | −1193 |
| Urban | 2747 | 5924 | 17,439 | 14,692 |
| Pasture | 157,942 | 137,763 | 247,296 | 89,354 |
| Agriculture | 22,344 | 73,082 | 91,793 | 69,449 |
| Open Caatinga | 294,539 | 386,705 | 355,839 | 61,300 |
| Dense Caatinga | 323,650 | 200,319 | 90,637 | −233,012 |
| Stage | Agriculture Area (ha) | % Agriculture Area Compared to 2018 |
|---|---|---|
| Year 185 | 22,344 | −75.7 |
| Year 2000 | 73,082 | −20.4 |
| Year 2018 | 91,793 | - |
| Projection 1 | 97,466 | +6.2 |
| Projection 2 | 101,854 | +10.9 |
| Projection 3 | 105,145 | +14.5 |
Appendix C

References
- Newbold, T.; Hudson, L.N.; Hill, S.L.L.; Contu, S.; Lysenko, I.; Senior, R.A.; Börger, L.; Bennett, D.J.; Choimes, A.; Collen, B.; et al. Global effects of land use on local terrestrial biodiversity. Nature 2015, 520, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Song, X.-P.; Hansen, M.C.; Stehman, S.V.; Potapov, P.V.; Tyukavina, A.; Vermote, E.F.; Townshend, J.R. Global land change from 1982 to 2016. Nature 2018, 560, 639–643. [Google Scholar] [CrossRef]
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A. High-Resolution Global Maps. Science 2013, 134, 850–854. [Google Scholar] [CrossRef] [Green Version]
- Santos, C.A.G.; Nascimento, T.V.M.D.; Da Silva, R.M. Analysis of forest cover changes and trends in the Brazilian semiarid region between 2000 and 2018. Environ. Earth Sci. 2020, 79. [Google Scholar] [CrossRef]
- Laurance, W.F.; Sayer, J.; Cassman, K.G. Agricultural expansion and its impacts on tropical nature. Trends Ecol. Evol. 2014, 29, 107–116. [Google Scholar] [CrossRef]
- Maxwell, S.L.; Fuller, R.A.; Brooks, T.M.; Watson, J.E.M. Biodiversity: The ravages of guns, nets and bulldozers. Nature 2016, 536, 143–145. [Google Scholar] [CrossRef]
- Antongiovanni, M.; Venticinque, E.M.; Fonseca, C.R. Fragmentation patterns of the Caatinga drylands. Landsc. Ecol. 2018, 33, 1353–1367. [Google Scholar] [CrossRef]
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz, J.J.; Cayuela, L.; Echeverria, C.; Salas, J.; Benayas, J.M.R. Monitoring land cover change of the dryland forest landscape of Central Chile (1975–2008). Appl. Geogr. 2010, 30, 436–447. [Google Scholar] [CrossRef] [Green Version]
- Rudnick, D.; Ryan, S.J.; Beier, P.; Cushman, S.A.; Dieffenbach, F.; Epps, C.W.; Gerber, L.R.; Hartter, J.; Jenness, J.S.; Kintsch, J.; et al. The Role of Landscape Connectivity in Planning and Implementing Conservation and Restoration Priorities; Ecological Society of America: Washington, WA, USA, 2012. [Google Scholar]
- Crooks, K.R.; Sanjayan, M. Connectivity Conservation: Maintaining Connections for Nature; Cambridge University Press (CUP): Cambridge, UK, 2006. [Google Scholar]
- Hernández, A.; Miranda, M.; Arellano, E.C.; Saura, S.; Ovalle, C. Landscape dynamics and their effect on the functional connectivity of a Mediterranean landscape in Chile. Ecol. Indic. 2015, 48, 198–206. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Donner, S.D.; Maynard, J.A.; MacNeil, M.A.; Graham, N.A.J.; Maina, J.; Baker, A.C.; Alemu, J.B.; Beger, M.; Campbell, S.J.; et al. Prioritizing Key Resilience Indicators to Support Coral Reef Management in a Changing Climate. PLoS ONE 2012, 7, e0042884. [Google Scholar] [CrossRef]
- Saura, S.; Bodin, Ö.; Fortin, M.-J. Stepping stones are crucial for species’ long-distance dispersal and range expansion through habitat networks. J. Appl. Ecol. 2014, 51, 171–182. [Google Scholar] [CrossRef]
- Dos Santos, R.C.; Lima, M.; Junior, C.A.D.S.; Battirola, L.D. Disordered conversion of vegetation committees connectivity between forest fragments in the Brazilian Legal Amazon. Appl. Geogr. 2019, 111, 102082. [Google Scholar] [CrossRef]
- De Fries, R.; Nagendra, H. Ecosystem management as a wicked problem. Science 2017, 356, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Kremen, C.; Merenlender, A.M. Landscapes that work for biodiversity and people. Science 2018, 362. [Google Scholar] [CrossRef] [Green Version]
- Garibaldi, L.A.; Oddi, F.J.; Miguez, F.E.; Bartomeus, I.; Orr, M.C.; Jobbágy, E.G.; Kremen, C.; Schulte, L.A.; Hughes, A.C.; Bagnato, C.; et al. Working landscapes need at least 20% native habitat. Conserv. Lett. 2021, 14, 1–10. [Google Scholar] [CrossRef]
- Urban, D.; Keitt, T. Landscape connectivity: A graph-theoretic perspective. Ecology 2001, 82, 1205–1218. [Google Scholar] [CrossRef]
- Pascual-Hortal, L.; Saura, S. Comparison and development of new graph-based landscape connectivity indices: Towards the priorization of habitat patches and corridors for conservation. Landsc. Ecol. 2006, 21, 959–967. [Google Scholar] [CrossRef]
- Qi, K.; Fan, Z.; Ng, C.N.; Wang, X.; Xie, Y. Functional analysis of landscape connectivity at the landscape, component, and patch levels: A case study of Minqing County, Fuzhou City, China. Appl. Geogr. 2017, 80, 64–77. [Google Scholar] [CrossRef]
- Volk, X.K.; Gattringer, J.P.; Otte, A.; Harvolk-Schöning, S. Connectivity analysis as a tool for assessing restoration success. Landsc. Ecol. 2018, 33, 371–387. [Google Scholar] [CrossRef]
- Leal, I.R.; Da Silva, J.M.C.; Tabarelli, M.; Lacher, T.E. Changing the Course of Biodiversity Conservation in the Caatinga of Northeastern Brazil. Conserv. Biol. 2005, 19, 701–706. [Google Scholar] [CrossRef]
- Velloso, A.L.; Sampaio, E.V.S.B.; Pareyn, F.G.C. Ecorregiões Propostas Para o Bioma Caatinga; The Nature Conservancy do Brasil: Recife, Brazil, 2002. [Google Scholar]
- Serviço Florestal Brasileiro (SFB). Florestas do Brasil em Resumo—2013: Dados de 2007–2012; Serviço Florestal Brasileiro: Santarém, Brasil, 2013. [Google Scholar]
- Ribeiro, E.M.S.; Arroyo-Rodríguez, V.; Santos, B.; Tabarelli, M.; Leal, I.R. Chronic anthropogenic disturbance drives the biological impoverishment of the Brazilian Caatinga vegetation. J. Appl. Ecol. 2015, 52, 611–620. [Google Scholar] [CrossRef]
- De Queiroz, L.P.; Cardoso, D.; Fernandes, M.F.; Moro, M.F. Diversity and Evolution of Flowering Plants of the Caatinga Domain. In Caatinga: The Largest Tropical Dry Forest Region in South America; Springer: Berlin/Heidelberg, Germany, 2017; pp. 23–63. [Google Scholar]
- Bond, W.J. Open Ecosystems: Ecology and Evolution Beyond the Forest Edge; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Leal, I.; Tabarelli, M.; Silva, J.M.C.D. Ecologia e Conservação da Caatinga; Universidade Federal de Pernambuco: Recife, Brazil, 2003. [Google Scholar]
- Koch, A.; Brierley, C.; Maslin, M.M.; Lewis, S.L. Earth system impacts of the European arrival and Great Dying in the Americas after 1492. Quat. Sci. Rev. 2019, 207, 13–36. [Google Scholar] [CrossRef]
- Alves, R.R.; Mendonça, L.E.; Confessor, M.V.; Vieira, W.L.; Lopez, L.C. Hunting strategies used in the semi-arid region of northeastern Brazil. J. Ethnobiol. Ethnomed. 2009, 5, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, T.R.; Da Silva, B.B.; De Moura, M.S.B.; Verhoef, A.; Nóbrega, R.L. The use of remote sensing for reliable estimation of net radiation and its components: A case study for contrasting land covers in an agricultural hotspot of the Brazilian semiarid region. Agric. For. Meteorol. 2020, 291, 108052. [Google Scholar] [CrossRef]
- Marinho, F.P.; Mazzochini, G.G.; Manhães, A.P.; Weisser, W.W.; Ganade, G. Effects of past and present land use on vegetation cover and regeneration in a tropical dryland forest. J. Arid Environ. 2016, 132, 26–33. [Google Scholar] [CrossRef]
- Da Silva, J.M.C.; Leal, I.R.; Tabarelli, M. Caatinga: The Largest Tropical Dry Forest Region in South America; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Antongiovanni, M.; Venticinque, E.M.; Matsumoto, M.; Fonseca, C.R. Chronic anthropogenic disturbance on Caatinga dry forest fragments. J. Appl. Ecol. 2020, 57, 2064–2074. [Google Scholar] [CrossRef]
- De Espindola, G.M.; Figueredo, E.D.S.; Júnior, P.P.; Filho, A.A.D.R. Cropland expansion as a driver of land-use change: The case of Cerrado-Caatinga transition zone in Brazil. Environ. Dev. Sustain. 2021. [Google Scholar] [CrossRef]
- Teixeira, A.H.D.C.; Leivas, J.F.; Andrade, R.G.; Hernandez, F.B.T.; Momesso, F.R.A. Modelling radiation and energy balances with Landsat 8 images under different thermohydrological conditions in the Brazilian semi-arid region. Remote Sens. Agric. Ecosystems. Hydrol. 2015, XVII, 96370U. [Google Scholar] [CrossRef] [Green Version]
- Correia, M.F.; Dias, M.A.F.D.S.; Aragão, M.R.D.S. Soil occupation and atmospheric variations over Sobradinho Lake area. Part two: A regional modeling study. Theor. Appl. Clim. 2006, 94, 115–128. [Google Scholar] [CrossRef]
- Selwyn, B. Globalized Horticulture: The Formation and Global Integration of Export Grape Production in North East Brazil. J. Agrar. Chang. 2010, 10, 537–563. [Google Scholar] [CrossRef]
- Pearson, D. Key Roles for Landscape Ecology in Transformative Agriculture Using Aotearoa—New Zealand as a Case Example. Land 2020, 9, 146. [Google Scholar] [CrossRef]
- Project MapBiomas—Collection 4.1 of Brazilian Land Cover & Use Map Series. Available online: https://mapbiomas.org (accessed on 5 November 2020).
- Roman, P. The São Francisco interbasin water transfer in Brazil: Tribulations of a megaproject through constraints and con-troversy. Water Altern. 2017, 10, 395–419. [Google Scholar]
- Correia, M.F.; Dias, M.A.F.D.S.; Aragão, M.R.D.S. Soil occupation and atmospheric variations over Sobradinho Lake area. Part one: An observational analysis. Theor. Appl. Clim. 2006, 94, 103–113. [Google Scholar] [CrossRef]
- De Jesus, J.B.; Kuplich, T.M.; Barreto, Í.D.D.C.; Da Rosa, C.N.; Hillebrand, F.L. Temporal and phenological profiles of open and dense Caatinga using remote sensing: Response to precipitation and its irregularities. J. For. Res. 2021, 32, 1067–1076. [Google Scholar] [CrossRef]
- White, J.C.; Wulder, M.A.; Hobart, G.; Luther, J.; Hermosilla, T.; Griffiths, P.; Coops, N.; Hall, R.J.; Hostert, P.; Dyk, A.; et al. Pixel-Based Image Compositing for Large-Area Dense Time Series Applications and Science. Can. J. Remote Sens. 2014, 40, 192–212. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, J.L.B.; Bezerra, A.C.; Silva, T.T.F.; Batista, P.H.D.; Moura, G.B.D.A.; Lopes, P.M.O. Quantification Caatinga vegetable coverage and water availability by remote sensing in the Brazilian semiarid. J. Hyperspectral Remote Sens. 2019, 9, 166–176. [Google Scholar] [CrossRef]
- Da Silveira, H.L.F.; Galvão, L.S.; Sanches, I.D.; De Sá, I.B.; Taura, T.A. Use of MSI/Sentinel-2 and airborne LiDAR data for mapping vegetation and studying the relationships with soil attributes in the Brazilian semi-arid region. Int. J. Appl. Earth Obs. Geoinf. 2018, 73, 179–190. [Google Scholar] [CrossRef]
- The United States Geological Survey (USGS). Landsat 8 Surface Reflectance Code (LaSRC) Product; The United States Geological Survey: Reston, VG, USA, 2018. [Google Scholar]
- The United States Geological Survey (USGS). Product Guide: Landsat Climate Data Record (CDR) Surface Reflectance; The United States Geological Survey: Reston, VG, USA, 2013. [Google Scholar]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Parente, L.; Ferreira, L.; Faria, A.; Nogueira, S.; Araújo, F.; Teixeira, L.; Hagen, S. Monitoring the brazilian pasturelands: A new mapping approach based on the landsat 8 spectral and temporal domains. Int. J. Appl. Earth Obs. Geoinf. 2017, 62, 135–143. [Google Scholar] [CrossRef]
- Reynolds, J.; Wesson, K.; Desbiez, A.L.J.; Ochoa-Quintero, J.M.; Leimgruber, P. Using Remote Sensing and Random Forest to Assess the Conservation Status of Critical Cerrado Habitats in Mato Grosso do Sul, Brazil. Land 2016, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Andrade, E.M.; Valbrun, W.; De Almeida, A.M.M.; Rosa, G.; Da Silva, A.G.R. Land-Use Effect on Soil Carbon and Nitrogen Stock in a Seasonally Dry Tropical Forest. Agronomy 2020, 10, 158. [Google Scholar] [CrossRef] [Green Version]
- Echeverria, C.; Coomes, D.; Salas, J.; Rey-Benayas, J.M.; Lara, A.; Newton, A. Rapid deforestation and fragmentation of Chilean Temperate Forests. Biol. Conserv. 2006, 130. [Google Scholar] [CrossRef]
- Chuvieco, E. Teledetección Ambiental: La Observación de la Tierra Desde el Espacio; Ariel Ciencia: Barcelona, Spain, 2002. [Google Scholar]
- Hernández, A.; Miranda, M.D.; Arellano, E.C.; Dobbs, C. Landscape trajectories and their effect on fragmentation for a Mediterranean semi-arid ecosystem in Central Chile. J. Arid Environ. 2016, 127, 74–81. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Hesselbarth, M.H.K.; Sciaini, M.; With, K.A.; Wiegand, K.; Nowosad, J. Landscapemetrics: An open-source R tool to calculate landscape metrics. Ecography 2019, 42, 1648–1657. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Xiao, R.; Zhang, M.; Wang, C.; Ma, Z.; Xiu, Y.; Wang, Q.; Guo, Y. Hydrological connectivity dynamics and conservation priorities for surface-water patches in the Yellow River Delta National Nature Reserve, China. Ecohydrol. Hydrobiol. 2020, 20. [Google Scholar] [CrossRef]
- Estrada, E.; Bodin, Ö. Using network centrality measures to manage landscape connectivity. Ecol. Appl. 2008, 18, 1810–1825. [Google Scholar] [CrossRef] [Green Version]
- Decout, S.; Manel, S.; Miaud, C.; Luque, S. Integrative approach for landscape-based graph connectivity analysis: A case study with the common frog (Rana temporaria) in human-dominated landscapes. Landsc. Ecol. 2012, 27, 267–279. [Google Scholar] [CrossRef]
- Ayram, C.A.C.; Mendoza, M.E.; Salicrup, D.R.P.; Granados, E.L. Identifying potential conservation areas in the Cuitzeo Lake basin, Mexico by multitemporal analysis of landscape connectivity. J. Nat. Conserv. 2014, 22, 424–435. [Google Scholar] [CrossRef]
- Chaves, I.D.B.; Lopes, V.; Ffolliott, P.; Paes-Silva, A.P. Uma Classificação Morfo-Estrutural Para Descrição E Avaliação da Biomassa da Vegetação da Caatinga a Morpho-Structural Classification for Description and Evaluation of the Biomass of the Caatinga Vegetation. Rev. Caatinga 2008, 12, 204–213. [Google Scholar]
- Herrera, L.P.; Sabatino, M.C.; Jaimes, F.R.; Saura, S. Landscape connectivity and the role of small habitat patches as stepping stones: An assessment of the grassland biome in South America. Biodivers. Conserv. 2017, 26, 3465–3479. [Google Scholar] [CrossRef]
- Stevens, V.M.; Trochet, A.; Blanchet, S.; Moulherat, S.; Clobert, J.; Baguette, M. Dispersal syndromes and the use of life-histories to predict dispersal. Evol. Appl. 2013, 6, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Thomson, F.J.; Moles, A.T.; Auld, T.D.; Kingsford, R. Seed dispersal distance is more strongly correlated with plant height than with seed mass. J. Ecol. 2011, 99, 1299–1307. [Google Scholar] [CrossRef]
- Melo, G.C.; Pinheiro, L.T.; Passos, D.C.; Galdino, C.A.B. Spatial organisation of the neotropical lizard tropidurus hispidus (Squamata: Tropiduridae). Salamandra 2017, 53, 435–438. [Google Scholar]
- Macdonald, D.W. Dwindling resources and the social behaviour of Capybaras, (Hydrochoerus hydrochaeris) (Mammalia). J. Zool. 1981, 194, 371–391. [Google Scholar] [CrossRef]
- Arispe, R.; Venegas, C.; Rumiz, D. Abundancia y patrones de actividad del mapache (Procyon cancrivorus) en un bosque chiquitano de Bolivia. Mastozool. Neotrop. 2008, 15, 323–333. [Google Scholar]
- Michalski, F.; Peres, C.A. Anthropogenic determinants of primate and carnivore local extinctions in a fragmented forest landscape of southern Amazonia. Biol. Conserv. 2005, 124, 383–396. [Google Scholar] [CrossRef]
- Bou, N.; Cuyckens, G.; González, E.; Meneghel, M. Conservation planning in Uruguay based on small felids (Leopardus spp.) as umbrella species. Stud. Neotrop. Fauna Environ. 2019, 54, 169–180. [Google Scholar] [CrossRef]
- Giordano, A.J. Ecology and status of the jaguarundi P uma yagouaroundi: A synthesis of existing knowledge. Mammal Rev. 2016, 46, 30–43. [Google Scholar] [CrossRef]
- Clauzel, C.; Foltête, J.; Girardet, X.; Vuidel, G. Graphab 2.4. User Manual. 2019. Available online: https://sourcesup.renater.fr/www/graphab/download/manual-2.4-en.pdf (accessed on 9 June 2020).
- Saura, S.; Estreguil, C.; Mouton, C.; Rodríguez-Freire, M. Network analysis to assess landscape connectivity trends: Application to European forests (1990–2000). Ecol. Indic. 2011, 11, 407–416. [Google Scholar] [CrossRef]
- Arnan, X.; Arcoverde, G.B.; Pie, M.R.; Ribeiro-Neto, J.D.; Leal, I.R. Increased anthropogenic disturbance and aridity reduce phylogenetic and functional diversity of ant communities in Caatinga dry forest. Sci. Total Environ. 2018, 631–632, 429–438. [Google Scholar] [CrossRef]
- Halinski, R.; Garibaldi, L.A.; Dos Santos, C.F.; Acosta, A.L.; Guidi, D.D.; Blochtein, B. Forest fragments and natural vegetation patches within crop fields contribute to higher oilseed rape yields in Brazil. Agric. Syst. 2020, 180, 102768. [Google Scholar] [CrossRef]
- Bodin, Ö.; Saura, S. Ranking individual habitat patches as connectivity providers: Integrating network analysis and patch removal experiments. Ecol. Model. 2010, 221, 2393–2405. [Google Scholar] [CrossRef]
- Huang, I.B.; Keisler, J.; Linkov, I. Multi-criteria decision analysis in environmental sciences: Ten years of applications and trends. Sci. Total Environ. 2011, 409, 3578–3594. [Google Scholar] [CrossRef]
- Talukdar, S.; Singha, P.; Mahato, S.; Shahfahad; Pal, S.; Liou, Y.-A.; Rahman, A. Land-Use Land-Cover Classification by Machine Learning Classifiers for Satellite Observations—A Review. Remote. Sens. 2020, 12, 1135. [Google Scholar] [CrossRef] [Green Version]
- Beuchle, R.; Grecchi, R.C.; Shimabukuro, Y.E.; Seliger, R.; Eva, H.D.; Sano, E.; Achard, F. Land cover changes in the Brazilian Cerrado and Caatinga biomes from 1990 to 2010 based on a systematic remote sensing sampling approach. Appl. Geogr. 2015, 58, 116–127. [Google Scholar] [CrossRef]
- Cunha, A.P.M.A.; Alvalá, R.C.S.; Kubota, P.Y.; Vieira, R.M.S.P. Impacts of land use and land cover changes on the climate over Northeast Brazil. Atmos. Sci. Lett. 2015, 16, 219–227. [Google Scholar] [CrossRef]
- Fernandes, M.R.D.M.; Matricardi, E.; De Almeida, A.Q.; Fernandes, M.M. Mudanças do Uso e de Cobertura da Terra na Região Semiárida de Sergipe. Floresta Ambient. 2015, 22, 472–482. [Google Scholar] [CrossRef] [Green Version]
- Hepcan, C.C. Quantifying landscape pattern and connectivity in a Mediterranean coastal settlement: The case of the Urla district, Turkey. Environ. Monit. Assess. 2013, 185, 143–155. [Google Scholar] [CrossRef]
- Cayuela, L.; Benayas, J.M.R.; Echeverría, C. Clearance and fragmentation of tropical montane forests in the Highlands of Chiapas, Mexico (1975–2000). For. Ecol. Manag. 2006, 226, 208–218. [Google Scholar] [CrossRef]
- Hirayama, H.; Tomita, M.; Hara, K. Quantitative monitoring of changes in forest habitat connectivity following the great eastern Japan earthquake and tsunami. Landsc. Ecol. 2020, 35, 1519–1530. [Google Scholar] [CrossRef]
- Pfeifer, M.; Lefebvre, V.; Peres, C.A.; Banks-Leite, C.; Wearn, O.R.; Marsh, C.; Butchart, S.H.M.; Arroyo-Rodríguez, V.; Barlow, J.; Cerezo, A.; et al. Creation of forest edges has a global impact on forest vertebrates. Nature 2017, 551, 187–191. [Google Scholar] [CrossRef]
- Ashrafzadeh, M.R.; Khosravi, R.; Adibi, M.A.; Taktehrani, A.; Wan, H.Y.; Cushman, S.A. A multi-scale, multi-species approach for assessing effectiveness of habitat and connectivity conservation for endangered felids. Biol. Conserv. 2020, 245, 108523. [Google Scholar] [CrossRef]
- Mendenhall, C.D.; Karp, D.S.; Meyer, C.F.J.; Hadly, E.A.; Daily, G.C. Predicting biodiversity change and averting collapse in agricultural landscapes. Nature 2014, 509, 213–217. [Google Scholar] [CrossRef]
- Lyra-Jorge, M.C.; Ciocheti, G.; Pivello, V.R. Carnivore mammals in a fragmented landscape in northeast of São Paulo State, Brazil. Biodivers. Conserv. 2008, 17, 1573–1580. [Google Scholar] [CrossRef]
- Akçakaya, H.R.; Mills, G.; Doncaster, C.P. The role of metapopulation conservation. In Key Topics in Conservation Biology; Macdonald, D.W., Service, K., Eds.; Blackwell Publishing: Oxford, UK, 2007; pp. 64–84. [Google Scholar]
- Laurance, W.F. Do edge effects occur over large spatial scales? Trends Ecol. Evol. 2000, 15, 134–135. [Google Scholar] [CrossRef]
- Brasil Congresso Nacional do Brasil. Protecão da Vegetacão Nativa (Ley N° 12651). Available online: https://www2.camara.leg.br/legin/fed/lei/2012/lei-12651-25-maio-2012-613076-veto-136200-pl.html (accessed on 25 March 2021).
- Zabel, F.; Delzeit, R.; Schneider, J.M.; Seppelt, R.; Mauser, W.; Václavík, T. Global impacts of future cropland expansion and intensification on agricultural markets and biodiversity. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz, K.; Guschal, M.; Kowarik, I.; De Almeida-Cortez, J.S.; Sampaio, E.V.D.S.B.; Cierjacks, A. Grazing reduces plant species diversity of Caatinga dry forests in northeastern Brazil. Appl. Veg. Sci. 2019, 22, 348–359. [Google Scholar] [CrossRef]
- Wittman, H.; Chappell, M.J.; Abson, D.J.; Kerr, R.B.; Blesh, J.; Hanspach, J.; Perfecto, I.; Fischer, J. A social–ecological perspective on harmonizing food security and biodiversity conservation. Reg. Environ. Chang. 2017, 17, 1291–1301. [Google Scholar] [CrossRef] [Green Version]
- Bucher, E.H. Chaco and Caatinga—South American Arid Savannas, Woodlands and Thickets BT-Ecology of Tropical Savannas; Huntley, B.J., Walker, B.H., Eds.; Springer: Berlin/Heidelberg, Germany, 1982; pp. 48–79. [Google Scholar]
- Maneta, M.; Torres, M.; Wallender, W.; Vosti, S.; Kirby, M.; Bassoi, L.; Rodrigues, L. Water demand and flows in the São Francisco River Basin (Brazil) with increased irrigation. Agric. Water Manag. 2009, 96, 1191–1200. [Google Scholar] [CrossRef] [Green Version]
- Tamburini, G.; Bommarco, R.; Wanger, T.C.; Kremen, C.; Van Der Heijden, M.G.A.; Liebman, M.; Hallin, S. Agricultural diversification promotes multiple ecosystem services without compromising yield. Sci. Adv. 2020, 6, eaba1715. [Google Scholar] [CrossRef] [PubMed]
- De Araujo, H.F.; Machado, C.C.; Pareyn, F.G.; Nascimento, N.F.D.; Araújo, L.D.A.; Borges, L.A.D.A.; Santos, B.A.; Beirigo, R.M.; Vasconcellos, A.; Dias, B.D.O.; et al. A sustainable agricultural landscape model for tropical drylands. Land Use Policy 2021, 100, 104913. [Google Scholar] [CrossRef]
- Schulz, C.; Koch, R.; Cierjacks, A.; Kleinschmit, B. Land change and loss of landscape diversity at the Caatinga phytogeographical domain—Analysis of pattern-process relationships with MODIS land cover products (2001–2012). J. Arid Environ. 2017, 136, 54–74. [Google Scholar] [CrossRef]





| Dense Caatinga | Open Caatinga | |||||||
|---|---|---|---|---|---|---|---|---|
| Landscape Indices | 1985 | 2000 | 2018 | % Change (1985–2018) | 1985 | 2000 | 2018 | % Change (1985–2018) |
| Number of patches | 7670 | 11,263 | 14,208 | 85.2% | 11,309 | 9895 | 14,546 | 28.6% |
| Largest patch index (%) | 16.1 | 6.1 | 1.2 | −14.9% | 10.4 | 18.9 | 15.7 | 5.3% |
| Mean patch area (ha) | 42.2 | 17.8 | 6.4 | −84.8% | 26.1 | 39.1 | 24.5 | −6.1% |
| Mean Euclidean nearest neighbor distance (m) | 128.6 | 130.6 | 148.9 | 15.8% | 114.9 | 102.6 | 106.6 | −7.2% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar, A.A.; Arellano, E.C.; Muñoz-Sáez, A.; Miranda, M.D.; Oliveira da Silva, F.; Zielonka, N.B.; Crowther, L.P.; Silva-Ferreira, V.; Oliveira-Reboucas, P.; Dicks, L.V. Restoration and Conservation of Priority Areas of Caatinga’s Semi-Arid Forest Remnants Can Support Connectivity within an Agricultural Landscape. Land 2021, 10, 550. https://doi.org/10.3390/land10060550
Salazar AA, Arellano EC, Muñoz-Sáez A, Miranda MD, Oliveira da Silva F, Zielonka NB, Crowther LP, Silva-Ferreira V, Oliveira-Reboucas P, Dicks LV. Restoration and Conservation of Priority Areas of Caatinga’s Semi-Arid Forest Remnants Can Support Connectivity within an Agricultural Landscape. Land. 2021; 10(6):550. https://doi.org/10.3390/land10060550
Chicago/Turabian StyleSalazar, Andrés A., Eduardo C. Arellano, Andrés Muñoz-Sáez, Marcelo D. Miranda, Fabiana Oliveira da Silva, Natalia B. Zielonka, Liam P. Crowther, Vinina Silva-Ferreira, Patricia Oliveira-Reboucas, and Lynn V. Dicks. 2021. "Restoration and Conservation of Priority Areas of Caatinga’s Semi-Arid Forest Remnants Can Support Connectivity within an Agricultural Landscape" Land 10, no. 6: 550. https://doi.org/10.3390/land10060550
APA StyleSalazar, A. A., Arellano, E. C., Muñoz-Sáez, A., Miranda, M. D., Oliveira da Silva, F., Zielonka, N. B., Crowther, L. P., Silva-Ferreira, V., Oliveira-Reboucas, P., & Dicks, L. V. (2021). Restoration and Conservation of Priority Areas of Caatinga’s Semi-Arid Forest Remnants Can Support Connectivity within an Agricultural Landscape. Land, 10(6), 550. https://doi.org/10.3390/land10060550






