The Role of Recent (1985–2014) Patterns of Land Abandonment and Environmental Factors in the Establishment and Growth of Secondary Forests in the Iberian Peninsula
Abstract
1. Introduction
2. Material and Methods
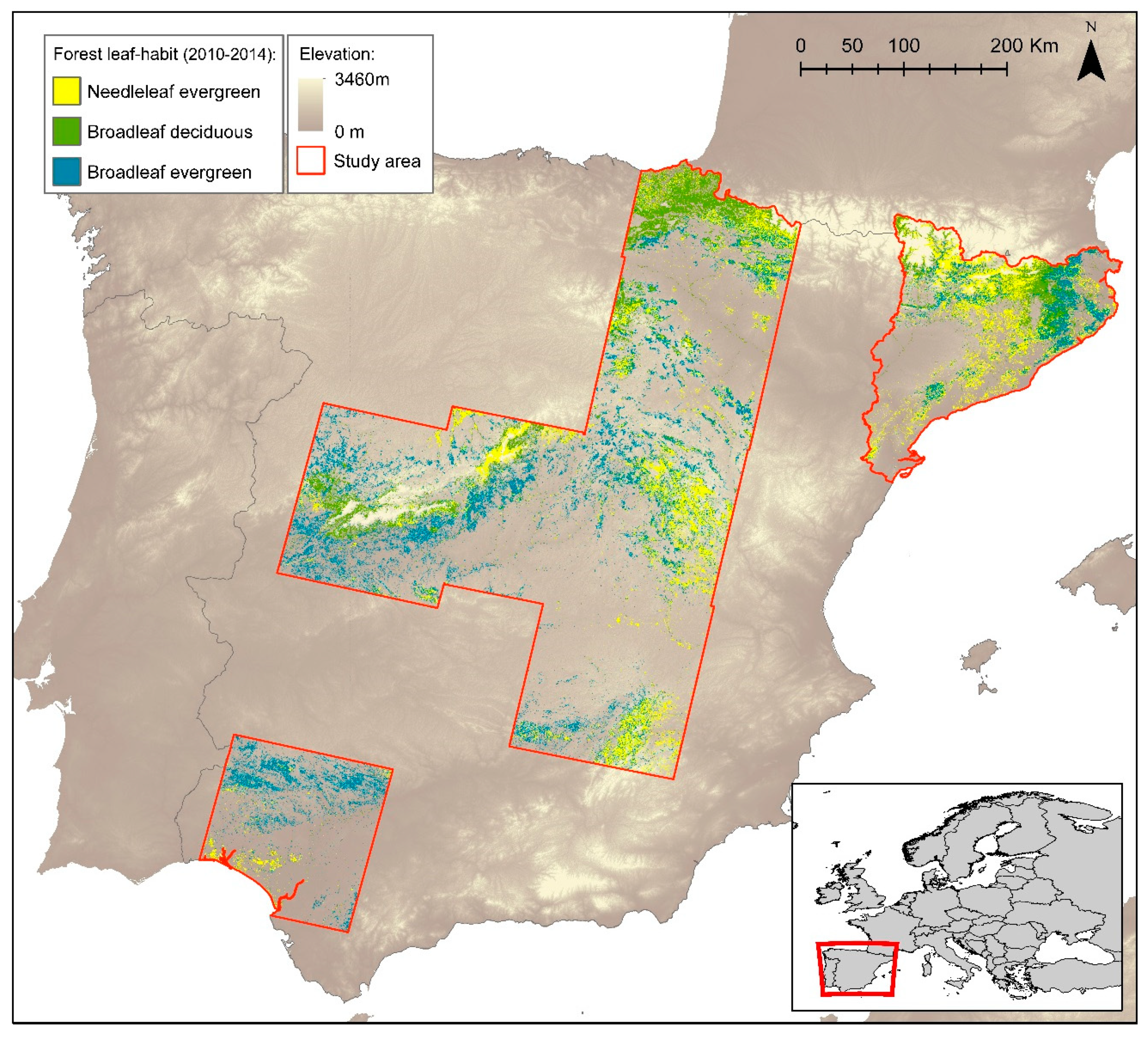
2.1. Study Area
2.2. Data Sources
2.3. Detecting Secondary Forest Establishment and Growth throughout the Study Period
2.4. Environmental Drivers of Secondary Forest Establishment and Growth
2.4.1. Climatic Variables
2.4.2. Topography
2.4.3. Forest Cover
2.5. Statistical Analyses
3. Results
3.1. Secondary Forest Establishment
3.2. Secondary Forest Growth
4. Discussion
4.1. Forest Expansion in the Iberian Peninsula
4.2. Patterns of Secondary Forest Establishment
4.3. Secondary Forest Growth
5. Conclusions and Future Implications
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, X.P.; Hansen, M.C.; Stehman, S.V.; Potapov, P.V.; Tyukavina, A.; Vermote, E.F.; Townshend, J.R. Global land change from 1982 to 2016. Nature 2018, 560, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.A.; DeFries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Global consequences of land use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef]
- Alkama, R.; Cescatti, A. Climate change: Biophysical climate impacts of recent changes in global forest cover. Science 2016, 351, 600–604. [Google Scholar] [CrossRef]
- FAO. Global Forest Resources Assessment 2020—Key Findings; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Meyfroidt, P.; Lambin, E.F. Global forest transition: Prospects for an end to deforestation. Annu. Rev. Environ. Resour. 2011, 36, 343–371. [Google Scholar] [CrossRef]
- Vilà-Cabrera, A.; Espelta, J.M.; Vayreda, J.; Pino, J. “New Forests” from the Twentieth Century are a Relevant Contribution for C Storage in the Iberian Peninsula. Ecosystems 2017, 20, 130–143. [Google Scholar] [CrossRef]
- Baśnou, C.; Álvarez, E.; Bagaria, G.; Guardiola, M.; Isern, R.; Vicente, P.; Pino, J. Spatial patterns of land use changes across a mediterranean metropolitan landscape: Implications for biodiversity management. Environ. Manag. 2013, 52, 971–980. [Google Scholar] [CrossRef]
- FAO. Forests and agriculture: Land-use challenges and opportunities. In State of the World’s Forests; FAO: Rome, Italy, 2016; Volume 45, pp. 811–922. [Google Scholar] [CrossRef]
- EEA. Environmental indicator. In EEA Report 30/2016; European Environment Agency: Copenhagen, Denmark, 2016; p. 60. [Google Scholar]
- Plieninger, T.; Gaertner, M.; Hui, C.; Huntsinger, L. Does land abandonment decrease species richness and abundance of plants and animals in Mediterranean pastures, arable lands and permanent croplands? Environ. Evid. 2013, 2, 1–7. [Google Scholar] [CrossRef]
- Regos, A.; Domínguez, J.; Gil-Tena, A.; Brotons, L.; Ninyerola, M.; Pons, X. Rural abandoned landscapes and bird assemblages: Winners and losers in the rewilding of a marginal mountain area (NW Spain). Reg. Environ. Chang. 2016, 16, 199–211. [Google Scholar] [CrossRef]
- Melero, Y.; Stefanescu, C.; Pino, J. General declines in Mediterranean butterflies over the last two decades are modulated by species traits. Biol. Conserv. 2016, 201, 336–342. [Google Scholar] [CrossRef]
- Breed, M.F.; Ottewell, K.M.; Gardner, M.G.; Lowe, A.J. Clarifying climate change adaptation responses for scattered trees in modified landscapes. J. Appl. Ecol. 2011, 48, 637–641. [Google Scholar] [CrossRef]
- Bowen, M.E.; McAlpine, C.A.; House, A.P.N.; Smith, G.C. Regrowth forests on abandoned agricultural land: A review of their habitat values for recovering forest fauna. Biol. Conserv. 2007, 140, 273–296. [Google Scholar] [CrossRef]
- Hooker, T.D.; Compton, J.E. Forest ecosystem carbon and nitrogen accumulation during the first century after agricultural abandonment. Ecol. Appl. 2003, 13, 299–313. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, R.; Schulp, C.J.E.; Hengeveld, G.M.; Verburg, P.H.; Clevers, J.G.P.W.; Schelhaas, M.-J.; Herold, M. Assessing the influence of historic net and gross land changes on the carbon fluxes of Europe. Glob. Chang. Biol. 2015, 22, 2526–2539. [Google Scholar] [CrossRef]
- Espelta, J.M.; Cruz-Alonso, V.; Alfaro-Sánchez, R.; Hampe, A.; Messier, C.; Pino, J. Functional diversity enhances tree growth and reduces herbivory damage in secondary broadleaf forests, but does not influence resilience to drought. J. Appl. Ecol. 2020, 57, 2362–2372. [Google Scholar] [CrossRef]
- Compton, J.E.; Boone, R.D. Long-term impacts of agriculture on soil carbon and nitrogen in New England forests. Ecology 2000, 81, 2314–2330. [Google Scholar] [CrossRef]
- Fichtner, A.; von Oheimb, G.; Härdtle, W.; Wilken, C.; Gutknecht, J.L.M. Effects of anthropogenic disturbances on soil microbial communities in oak forests persist for more than 100 years. Soil Biol. Biochem. 2014, 70, 79–87. [Google Scholar] [CrossRef]
- Freschet, G.T.; Östlund, L.; Kichenin, E.; Wardle, D.A.; Freschet, G.T.; Östlund, L.; Kichenin, E.; Wardle, D.A. Aboveground and belowground legacies of native Sami land use on boreal forest in northern Sweden 100 years after abandonment. Ecology 2014, 95, 963–977. [Google Scholar] [CrossRef]
- Alfaro-Sánchez, R.; Jump, A.S.; Pino, J.; Díez-Nogales, O.; Espelta, J.M. Land use legacies drive higher growth, lower wood density and enhanced climatic sensitivity in recently established forests. Agric. For. Meteorol. 2019, 276–277, 107630. [Google Scholar] [CrossRef]
- Mausolf, K.; Leuschner, C.; Härdtle, W.; Hertel, D.; Fichtner, A.; von Oheimb, G.; Delory, B.M.; Jansen, K.; Temperton, V.M. Legacy effects of land-use modulate tree growth responses to climate extremes. Oecologia 2018, 187, 825–837. [Google Scholar] [CrossRef]
- Zhao, M.; Running, S.W. Drought-Induced Reduction in Global Terrestrial Net Primary Production from 2000 Through 2009. Science 2010, 329, 940–943. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, 1–55. [Google Scholar] [CrossRef]
- Vilà-Cabrera, A.; Rodrigo, A.; Martínez-Vilalta, J.; Retana, J. Lack of regeneration and climatic vulnerability to fire of Scots pine may induce vegetation shifts at the southern edge of its distribution. J. Biogeogr. 2012, 39, 488–496. [Google Scholar] [CrossRef]
- Schröter, D.; Cramer, W.; Leemans, R.; Prentice, I.C.; Araújo, M.B.; Arnell, N.W.; Bondeau, A.; Bugmann, H.; Carter, T.R.; Gracia, C.A.; et al. Ecology: Ecosystem service supply and vulnerability to global change in Europe. Science 2005, 310, 1333–1337. [Google Scholar] [CrossRef]
- Trugman, A.T.; Anderegg, L.D.L.; Shaw, J.D.; Anderegg, W.R.L. Trait velocities reveal that mortality has driven widespread coordinated shifts in forest hydraulic trait composition. Proc. Natl. Acad. Sci. USA 2020, 117, 8532–8538. [Google Scholar] [CrossRef] [PubMed]
- García-Valdés, R.; Vayreda, J.; Retana, J.; Martínez-Vilalta, J. Low forest productivity associated with increasing drought-tolerant species is compensated by an increase in drought-tolerance richness. Glob. Chang. Biol. 2021, 27, 2113–2127. [Google Scholar] [CrossRef]
- Vayreda, J.; Martinez-Vilalta, J.; Gracia, M.; Canadell, J.G.; Retana, J. Anthropogenic-driven rapid shifts in tree distribution lead to increased dominance of broadleaf species. Glob. Chang. Biol. 2016, 22, 3984–3995. [Google Scholar] [CrossRef]
- Petersson, L.K.; Milberg, P.; Bergstedt, J.; Dahlgren, J.; Felton, A.M.; Götmark, F.; Salk, C.; Löf, M. Changing land use and increasing abundance of deer cause natural regeneration failure of oaks: Six decades of landscape-scale evidence. For. Ecol. Manag. 2019, 444, 299–307. [Google Scholar] [CrossRef]
- Lasanta, T.; Arnáez, J.; Pascual, N.; Ruiz-Flaño, P.; Errea, M.P.; Lana-Renault, N. Space-time process and drivers of land abandonment in Europe. Catena 2017, 149, 810–823. [Google Scholar] [CrossRef]
- Thompson, I.; Mackey, B.; McNulty, S.; Mosseler, A. Forest Resilience, Biodiversity, and Climate Change; Secretariat of the Convention on Biological Diversity: Montreal, QC, Canada, 2009; Volume 43, ISBN 9292251376. [Google Scholar]
- Tsujino, R.; Takafumi, H.; Agetsuma, N.; Yumoto, T. Variation in tree growth, mortality and recruitment among topographic positions in a warm temperate forest. J. Veg. Sci. 2006, 17, 281–290. [Google Scholar] [CrossRef]
- Ruiz-Benito, P.; Ratcliffe, S.; Jump, A.S.; Gómez-Aparicio, L.; Madrigal-González, J.; Wirth, C.; Kändler, G.; Lehtonen, A.; Dahlgren, J.; Kattge, J.; et al. Functional diversity underlies demographic responses to environmental variation in European forests. Glob. Ecol. Biogeogr. 2017, 26, 128–141. [Google Scholar] [CrossRef]
- Jucker, T.; Bouriaud, O.; Avacaritei, D.; Coomes, D.A. Stabilizing effects of diversity on aboveground wood production in forest ecosystems: Linking patterns and processes. Ecol. Lett. 2014, 17, 1560–1569. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Serrano, S.M.; Lopez-Moreno, J.I.; Beguería, S.; Lorenzo-Lacruz, J.; Sanchez-Lorenzo, A.; García-Ruiz, J.M.; Azorin-Molina, C.; Morán-Tejeda, E.; Revuelto, J.; Trigo, R.; et al. Evidence of increasing drought severity caused by temperature rise in southern Europe. Environ. Res. Lett. 2014, 9, 044001. [Google Scholar] [CrossRef]
- Domingo-Marimon, C. Contributions to the Knowledge of the Multitemporal Spatial Patterns of the Iberian Peninsula Droughts from a Geographic Information Science Perspective. Ph.D. Thesis, Universitat Autònoma de Barcelona, Bellaterra, Spain, 2016; pp. 163–165. [Google Scholar] [CrossRef]
- Vidal-Macua, J.J.; Zabala, A.; Ninyerola, M.; Pons, X. Developing spatially and thematically detailed backdated maps for land cover studies. Int. J. Digit. Earth 2017, 10, 175–206. [Google Scholar] [CrossRef]
- González-Guerrero, O.; Pons, X. The 2017 land use/land cover map of Catalonia based on sentinel-2 images and auxiliary data. Rev. Teledetec. 2020, 2020, 81–92. [Google Scholar] [CrossRef]
- Santoro, M.; Cartus, O. Dataset record: ESA Biomass Climate Change Initiative (Biomass_cci): Global datasets of forest above-ground biomass for the year 2017, v1. Cent. Environ. Data Anal. 2019. [Google Scholar] [CrossRef]
- ESA CCI Biomass Project. ESA CCI Biomass Product User Guide Version 1.0. 2019, pp. 1–35. Available online: http://cci.esa.int/biomass (accessed on 10 November 2020).
- FRA. Forest Resources Assessment: Terms and Definition FRA 2020. Working Paper 188; FAO: Rome, Italy, 2018. [Google Scholar]
- Palmero-Iniesta, M.; Pino, J.; Pesquer, L.; Espelta, J.M. Recent forest area increase in Europe: Expanding and regenerating forests differ in their regional patterns, drivers and productivity trends. Eur. J. For. Res. 2021, 140, 793–805. [Google Scholar] [CrossRef]
- Gerard, F.; Petit, S.; Smith, G.; Thomson, A.; Brown, N.; Manchester, S.; Wadsworth, R.; Bugar, G.; Halada, L.; Bezák, P.; et al. Land cover change in Europe between 1950 and 2000 determined employing aerial photography. Prog. Phys. Geogr. 2010, 34, 183–205. [Google Scholar] [CrossRef]
- Geri, F.; Rocchini, D.; Chiarucci, A. Landscape metrics and topographical determinants of large-scale forest dynamics in a Mediterranean landscape. Landsc. Urban Plan. 2010, 95, 46–53. [Google Scholar] [CrossRef]
- Vidal-Macua, J.; Ninyerola, M.; Zabala, A.; Domingo-Marimon, C.; Pons, X. Factors affecting forest dynamics in the Iberian Peninsula from 1987 to 2012. The role of topography and drought. For. Ecol. Manag. 2017, 406, 290–306. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Palmero-Iniesta, M.; Espelta, J.M.; Gordillo, J.; Pino, J. Changes in forest landscape patterns resulting from recent afforestation in Europe (1990–2012): Pre-existing forest defragmentation versus new patch proliferation. Ann. For. Sci. 2020, 77, 1–15. [Google Scholar] [CrossRef]
- Vanderwel, M.C.; Lyutsarev, V.S.; Purves, D.W. Climate-related variation in mortality and recruitment determine regional forest-type distributions. Glob. Ecol. Biogeogr. 2013, 22, 1192–1203. [Google Scholar] [CrossRef]
- Bonan, G. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, T.; Zhao, X.; Huang, K.; Gao, S.; Wu, H.; Luo, H. Assessments of drought impacts on vegetation in China with the optimal time scales of the climatic drought index. Int. J. Environ. Res. Public Health 2015, 12, 7615–7634. [Google Scholar] [CrossRef]
- Ivits, E.; Horion, S.; Fensholt, R.; Cherlet, M. Drought footprint on European ecosystems between 1999 and 2010 assessed by remotely sensed vegetation phenology and productivity. Glob. Chang. Biol. 2014, 20, 581–593. [Google Scholar] [CrossRef]
- Zargar, A.; Sadiq, R.; Naser, B.; Khan, F.I. A review of drought indices. Environ. Rev. 2011, 19, 333–349. [Google Scholar] [CrossRef]
- Agnew, C.T. Using the SPI to Identify Drought. Drought Netw. News 2000, 12, 5–12. [Google Scholar]
- Mishra, A.K.; Singh, V.P. A review of drought concepts. J. Hydrol. 2010, 391, 202–216. [Google Scholar] [CrossRef]
- Reuter, H.I.; Nelson, A.; Jarvis, A. An evaluation of void-filling interpolation methods for SRTM data. Int. J. Geogr. Inf. Sci. 2007, 21, 983–1008. [Google Scholar] [CrossRef]
- Barton, K.; Barton, M.K. Package “MuMIn.”. In Multi-Model Inference; Version 1(6); Available online: cran.r-project.org (accessed on 8 March 2019).
- Crawley, M.J. The R Book: Mixed-Effects Models; Wiley Publishing: Silwood Park, UK, 2007; pp. 627–666. ISBN 978-0-470-51024-7. [Google Scholar]
- Gittleman, J.L.; Kot, M. Adaptation: Statistics and a null model for estimating phylogenetic effects. Syst. Zool. 1990, 39, 227–241. [Google Scholar] [CrossRef]
- Dormann, C.F.; McPherson, J.M.; Araújo, M.B.; Bivand, R.; Bolliger, J.; Carl, G.; Davies, R.G.; Hirzel, A.; Jetz, W.; Kissling, W.D.; et al. Methods to account for spatial autocorrelation in the analysis of species distributional data: A review. Ecography 2007, 30, 609–628. [Google Scholar] [CrossRef]
- Keenan, R.J.; Reams, G.A.; Achard, F.; de Freitas, J.V.; Grainger, A.; Lindquist, E. Dynamics of global forest area: Results from the FAO Global Forest Resources Assessment 2015. For. Ecol. Manag. 2015, 352, 9–20. [Google Scholar] [CrossRef]
- Gold, S.; Korotkov, A.; Sasse, V. The development of European forest resources, 1950 to 2000. For. Policy Econ. 2006, 8, 183–192. [Google Scholar] [CrossRef]
- Fuchs, R.; Herold, M.; Verburg, P.H.; Clevers, J.G.P.W.; Eberle, J. Gross changes in reconstructions of historic land cover/use for Europe between 1900 and 2010. Glob. Chang. Biol. 2015, 21, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Regos, A.; Ninyerola, M.; Moré, G.; Pons, X. Linking land cover dynamics with driving forces in mountain landscape of the Northwestern Iberian Peninsula. Int. J. Appl. Earth Obs. Geoinf. 2015, 38, 1–14. [Google Scholar] [CrossRef]
- Terres, J.M.; Scacchiafichi, L.N.; Wania, A.; Ambar, M.; Anguiano, E.; Buckwell, A.; Coppola, A.; Gocht, A.; Källström, H.N.; Pointereau, P.; et al. Farmland abandonment in Europe: Identification of drivers and indicators, and development of a composite indicator of risk. Land Use Policy 2015, 49, 20–34. [Google Scholar] [CrossRef]
- Kuemmerle, T.; Levers, C.; Erb, K.; Estel, S.; Jepsen, M.R.; Müller, D.; Plutzar, C.; Stürck, J.; Verkerk, P.J.; Verburg, P.H.; et al. Hotspots of land use change in Europe. Environ. Res. Lett. 2016, 11, 064020. [Google Scholar] [CrossRef]
- Leal Filho, W.; Mandel, M.; Al-Amin, A.Q.; Feher, A.; Chiappetta Jabbour, C.J. An assessment of the causes and consequences of agricultural land abandonment in Europe. Int. J. Sustain. Dev. World Ecol. 2017, 24, 554–560. [Google Scholar] [CrossRef]
- Fuchs, R.; Herold, M.; Verburg, P.H.; Clevers, J.G.P.W. A high-resolution and harmonized model approach for reconstructing and analysing historic land changes in Europe. Biogeosciences 2013, 10, 1543–1559. [Google Scholar] [CrossRef]
- Vadell, E.; De Miguel, S.; Fernández Centeno, G.; Robla, E.; Lerner, M.; Pemán García, J. La forestación de tierras agrícolas: Balance de un instrumento de política forestal para el cambio del uso de la tierra. Cuad. Soc. Española Cienc. For. 2019, 45, 1–20. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M. Differences in spatial patterns of drought on different time scales: An analysis of the Iberian Peninsula. Water Resour. Manag. 2006, 20, 37–60. [Google Scholar] [CrossRef]
- Páscoa, P.; Gouveia, C.M.; Russo, A.; Trigo, R.M. Drought trends in the Iberian Peninsula over the last 112 years. Adv. Meteorol. 2017, 4653126. [Google Scholar] [CrossRef]
- García-Valdecasas Ojeda, M.; Gámiz-Fortis, S.R.; Romero-Jiménez, E.; Rosa-Cánovas, J.J.; Yeste, P.; Castro-Díez, Y.; Esteban-Parra, M.J. Projected changes in the Iberian Peninsula drought characteristics. Sci. Total Environ. 2021, 757, 143702. [Google Scholar] [CrossRef]
- Gavilán, R.G.; Vilches, B.; Gutiérrez-Girón, A.; Blanquer, J.M.; Escudero, A. Sclerophyllous versus deciduous forests in the Iberian Peninsula: A standard case of Mediterranean climatic vegetation distribution. In Geographical Changes in Vegetation and Plant Functional Types; Springer: Berlin, Germany, 2018; pp. 101–116. [Google Scholar]
- Terradas, J. Holm oak and holm oak forests: An introduction. In Ecology of Mediterranean Evergreen Oak Forests; Springer: Berlin, Germany, 1999; pp. 3–14. [Google Scholar]
- Pasho, E.; Camarero, J.J.; de Luis, M.; Vicente-Serrano, S.M. Impacts of drought at different time scales on forest growth across a wide climatic gradient in north-eastern Spain. Agric. For. Meteorol. 2011, 151, 1800–1811. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M. Evaluating the impact of drought using remote sensing in a Mediterranean, Semi-arid Region. Nat. Hazards 2007, 40, 173–208. [Google Scholar] [CrossRef]
- Blanco, E.; Casado, M.A.; Costa, M.; Escribano, R.; García, M.; Génova, M.; Gómez, A.; Gómez, F.; Moreno, J.C.; Morla, C.; et al. Los bosques ibéricos. Una Interpreación. Geobotánica; Planeta: Barcelona, Spain, 1997; pp. 120–134. [Google Scholar]
- Sardans, J.; Rodà, F.; Peñuelas, J. Phosphorus limitation and competitive capacities of Pinus halepensis and Quercus ilex subsp. rotundifolia on different soils. Plant Ecol. 2004, 174, 307–319. [Google Scholar] [CrossRef]
- Gómez-Aparicio, L.; García-Valdés, R.; Ruíz-Benito, P.; Zavala, M.A. Disentangling the relative importance of climate, size and competition on tree growth in Iberian forests: Implications for forest management under global change. Glob. Chang. Biol. 2011, 17, 2400–2414. [Google Scholar] [CrossRef]
- Coll, M.; Peñuelas, J.; Ninyerola, M.; Pons, X.; Carnicer, J. Multivariate effect gradients driving forest demographic responses in the Iberian Peninsula. For. Ecol. Manag. 2013, 303, 195–209. [Google Scholar] [CrossRef]
- Carnicer, J.; Barbeta, A.; Sperlich, D.; Coll, M.; Penuelas, J. Contrasting trait syndromes in angiosperms and conifers are associated with different responses of tree growth to temperature on a large scale. Front. Plant Sci. 2013, 4, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Bartletta, M.K.; Klein, T.; Jansen, S.; Choat, B.; Sack, L. The correlations and sequence of plant stomatal, hydraulic, and wilting responses to drought. Proc. Natl. Acad. Sci. USA 2016, 113, 13098–13103. [Google Scholar] [CrossRef] [PubMed]
- Klein, T. The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric and anisohydric behaviours. Funct. Ecol. 2014, 28, 1313–1320. [Google Scholar] [CrossRef]
- Hacke, U.G.; Sperry, J.S.; Pockman, W.T.; Davis, S.D.; McCulloh, K.A. Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 2001, 126, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.; Shpringer, I.; Fikler, B.; Elbaz, G.; Cohen, S.; Yakir, D. Relationships between stomatal regulation, water-use, and water-use efficiency of two coexisting key Mediterranean tree species. For. Ecol. Manag. 2013, 302, 34–42. [Google Scholar] [CrossRef]
- Ferrio, J.P.; Florit, A.; Vega, A.; Serrano, L.; Voltas, J. Δ13C and tree-ring width reflect different drought responses in Quercus ilex and Pinus halepensis. Oecologia 2003, 137, 512–518. [Google Scholar] [CrossRef]
- Laamrani, A.; Valeria, O.; Bergeron, Y.; Fenton, N.; Cheng, L.Z.; Anyomi, K. Effects of topography and thickness of organic layer on productivity of black spruce boreal forests of the canadian clay belt region. For. Ecol. Manag. 2014, 330, 144–157. [Google Scholar] [CrossRef]
- Ming, Q.; Guo, S.; Jiao, Y. High gradient effects of forest biomass energy in mountainous region—A case of meili snow mountain. Procedia Earth Planet. Sci. 2011, 2, 315–320. [Google Scholar] [CrossRef][Green Version]
- Tateno, R.; Hishi, T.; Takeda, H. Above-and belowground biomass and net primary production in a cool-temperate deciduous forest in relation to topographical changes in soil nitrogen. For. Ecol. Manag. 2004, 193, 297–306. [Google Scholar] [CrossRef]
- Helman, D.; Osem, Y.; Yakir, D.; Lensky, I.M. Relationships between climate, topography, water use and productivity in two key Mediterranean forest types with different water-use strategies. Agric. For. Meteorol. 2017, 232, 319–330. [Google Scholar] [CrossRef]
- Vidal-Macua, J.J.; Ninyerola, M.; Zabala, A.; Domingo-Marimon, C.; Gonzalez-Guerrero, O.; Pons, X. Environmental and socioeconomic factors of abandonment of rainfed and irrigated crops in northeast Spain. Appl. Geogr. 2018, 90, 155–174. [Google Scholar] [CrossRef]
- Nainggolan, D.; de Vente, J.; Boix-Fayos, C.; Termansen, M.; Hubacek, K.; Reed, M.S. Afforestation, agricultural abandonment and intensification: Competing trajectories in semi-arid Mediterranean agro-ecosystems. Agric. Ecosyst. Environ. 2012, 159, 90–104. [Google Scholar] [CrossRef]
- Poorter, L.; Lianes, E.; Moreno-de las Heras, M.; Zavala, M.A. Architecture of Iberian canopy tree species in relation to wood density, shade tolerance and climate. Plant Ecol. 2012, 213, 707–722. [Google Scholar] [CrossRef]
- Montoya, D.; Zavala, M.A.; Rodríguez, M.A.; Purves, D.W. Animal versus wind dispersal and the robustness of tree species to deforestation. Science 2008, 320, 1502–1504. [Google Scholar] [CrossRef]
- Paula, S.; Arianoutsou, M.; Kazanis, D.; Tavsanoglu, Ç.; Lloret, F.; Buhk, C.; Ojeda, F.; Luna, B.; Moreno, J.M.; Rodrigo, A.; et al. Fire-related traits for plant species of the Mediterranean Basin. Ecology 2009, 90, 1420. [Google Scholar] [CrossRef]
- Fernandes, M.R.; Aguiar, F.C.; Ferreira, M.T. Assessing riparian vegetation structure and the influence of land use using landscape metrics and geostatistical tools. Landsc. Urban Plan. 2011, 99, 166–177. [Google Scholar] [CrossRef]
- Zhang, T.; Niinemets, Ü.; Sheffield, J.; Lichstein, J.W. Shifts in tree functional composition amplify the response of forest biomass to climate. Nature 2018, 556, 99–102. [Google Scholar] [CrossRef]
- Kankaanpää, S.; Carter, T.R. An Overview of Forest Policies Affecting Land Use in Europe; The Finnish Environment Institute: Helsinki, Finland, 2004; ISBN 9521117389. [Google Scholar]
- Metzger, M.J.; Bunce, R.G.H.; Jongman, R.H.G.; Mücher, C.A.; Watkins, J.W. A climatic stratification of the environment of Europe. Glob. Ecol. Biogeogr. 2005, 14, 549–563. [Google Scholar] [CrossRef]
- Doblas-Miranda, E.; Alonso, R.; Arnan, X.; Bermejo, V.; Brotons, L.; de las Heras, J.; Estiarte, M.; Hódar, J.A.; Llorens, P.; Lloret, F.; et al. A review of the combination among global change factors in forests, shrublands and pastures of the Mediterranean Region: Beyond drought effects. Glob. Planet. Chang. 2017, 148, 42–54. [Google Scholar] [CrossRef]
- Duveneck, M.J.; Scheller, R.M.; White, M.A. Effects of alternative forest management on biomass and species diversity in the face of climate change in the northern Great Lakes region (USA). Can. J. For. Res. 2014, 44, 700–710. [Google Scholar] [CrossRef]
- Vilà, M.; Vayreda, J.; Comas, L.; Ibáñez, J.J.; Mata, T.; Obón, B. Species richness and wood production: A positive association in Mediterranean forests. Ecol. Lett. 2007, 10, 241–250. [Google Scholar] [CrossRef]
- Phillips, R.P.; Ibáñez, I.; D’Orangeville, L.; Hanson, P.J.; Ryan, M.G.; McDowell, N.G. A belowground perspective on the drought sensitivity of forests: Towards improved understanding and simulation. For. Ecol. Manag. 2016, 380, 309–320. [Google Scholar] [CrossRef]
- Vayreda, J.; Martinez-Vilalta, J.; Gracia, M.; Retana, J. Recent climate changes interact with stand structure and management to determine changes in tree carbon stocks in Spanish forests. Glob. Chang. Biol. 2012, 18, 1028–1041. [Google Scholar] [CrossRef]
- Cameron, A.D. Importance of early selective thinning in the development of long-term stand stability and improved log quality: A review. Forestry 2002, 75, 25–35. [Google Scholar] [CrossRef]




| Secondary Forest Environment | ||||||
|---|---|---|---|---|---|---|
| Elevation (m) | Slope (%) | Forest cover (%) | ||||
| All | 667 ± 0.8 | 6.17 ± 0.01 | 30.3 ± 0.05 | |||
| BD | 681 ± 1.5 | a | 8.26 ± 0.02 | a | 36.7 ± 0.10 | a |
| BE | 666 ± 1.1 | b | 5.25 ± 0.02 | b | 27.6 ± 0.06 | b |
| NE | 684 ± 1.8 | a | 6.05 ± 0.03 | c | 29.2 ± 0.11 | b |
| Mean annual temperature (°C) | Annual precipitation (mm) | Drought frequency (event/year) | ||||
| All | 13.6 ± 0.01 | 710 ± 0.49 | 0.46 ± 0.00 | |||
| BD | 12.5 ± 0.01 | a | 895 ± 0.87 | a | 0.46 ± 0.00 | a |
| BE | 14.0 ± 0.01 | b | 654 ± 0.59 | b | 0.46 ± 0.00 | a |
| NE | 13.7 ± 0.01 | c | 623 ± 1.00 | c | 0.48 ± 0.00 | b |
| Drought duration (months) | Drought severity | Drought intensity | ||||
| All | 4.74 ± 0.00 | −6.91 ± 0.00 | −1.30 ± 0.00 | |||
| BD | 4.72 ± 0.00 | a | −6.88 ± 0.00 | a | −1.29 ± 0.00 | a |
| BE | 4.79 ± 0.00 | b | −6.98 ± 0.00 | b | −1.31 ± 0.00 | b |
| NE | 4.60 ± 0.00 | c | −6.74 ± 0.00 | c | −1.30 ± 0.00 | c |
| SPEI | Longitude | Latitude | ||||
| All | 0.004 ± 0.000 | 435,901 ± 440 | 4,453,824 ± 369 | |||
| 0 | ||||||
| BD | 0.005 ± 0.000 | a | 552,225 ± 786 | a | 4,604,454 ± 642 | a |
| BE | 0.005 ± 0.000 | b | 349,954 ± 535 | b | 4,401,588 ± 436 | b |
| NE | 0.004 ± 0.000 | c | 526,596 ± 899 | c | 4,404,518 ± 733 | c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmero-Iniesta, M.; Espelta, J.M.; Padial-Iglesias, M.; Gonzàlez-Guerrero, Ò.; Pesquer, L.; Domingo-Marimon, C.; Ninyerola, M.; Pons, X.; Pino, J. The Role of Recent (1985–2014) Patterns of Land Abandonment and Environmental Factors in the Establishment and Growth of Secondary Forests in the Iberian Peninsula. Land 2021, 10, 817. https://doi.org/10.3390/land10080817
Palmero-Iniesta M, Espelta JM, Padial-Iglesias M, Gonzàlez-Guerrero Ò, Pesquer L, Domingo-Marimon C, Ninyerola M, Pons X, Pino J. The Role of Recent (1985–2014) Patterns of Land Abandonment and Environmental Factors in the Establishment and Growth of Secondary Forests in the Iberian Peninsula. Land. 2021; 10(8):817. https://doi.org/10.3390/land10080817
Chicago/Turabian StylePalmero-Iniesta, Marina, Josep Maria Espelta, Mario Padial-Iglesias, Òscar Gonzàlez-Guerrero, Lluís Pesquer, Cristina Domingo-Marimon, Miquel Ninyerola, Xavier Pons, and Joan Pino. 2021. "The Role of Recent (1985–2014) Patterns of Land Abandonment and Environmental Factors in the Establishment and Growth of Secondary Forests in the Iberian Peninsula" Land 10, no. 8: 817. https://doi.org/10.3390/land10080817
APA StylePalmero-Iniesta, M., Espelta, J. M., Padial-Iglesias, M., Gonzàlez-Guerrero, Ò., Pesquer, L., Domingo-Marimon, C., Ninyerola, M., Pons, X., & Pino, J. (2021). The Role of Recent (1985–2014) Patterns of Land Abandonment and Environmental Factors in the Establishment and Growth of Secondary Forests in the Iberian Peninsula. Land, 10(8), 817. https://doi.org/10.3390/land10080817








