Kaleka Agroforest in Central Kalimantan (Indonesia): Soil Quality, Hydrological Protection of Adjacent Peatlands, and Sustainability
Abstract
:1. Introduction
- How does the groundwater level fluctuate in the various land uses (including Kaleka)?
- What are the soil’s physical, chemical, and biological characteristics and the existing soil quality indices in the various land uses?
- How does botanical composition and vegetation structure of the land uses relate to soil quality, water tables, and internal regeneration as sustainability indicator?
2. Methods
2.1. Location and Period of Study
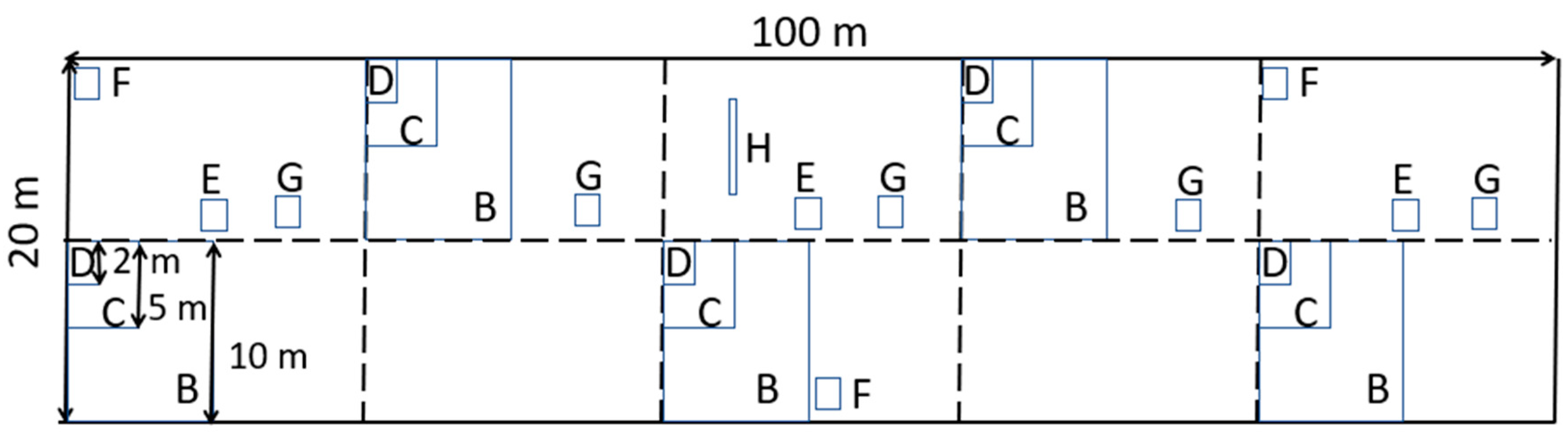
2.2. Survey Design for Kaleka as Local Land Use
2.3. Depth of Water Table
2.3.1. Measurements
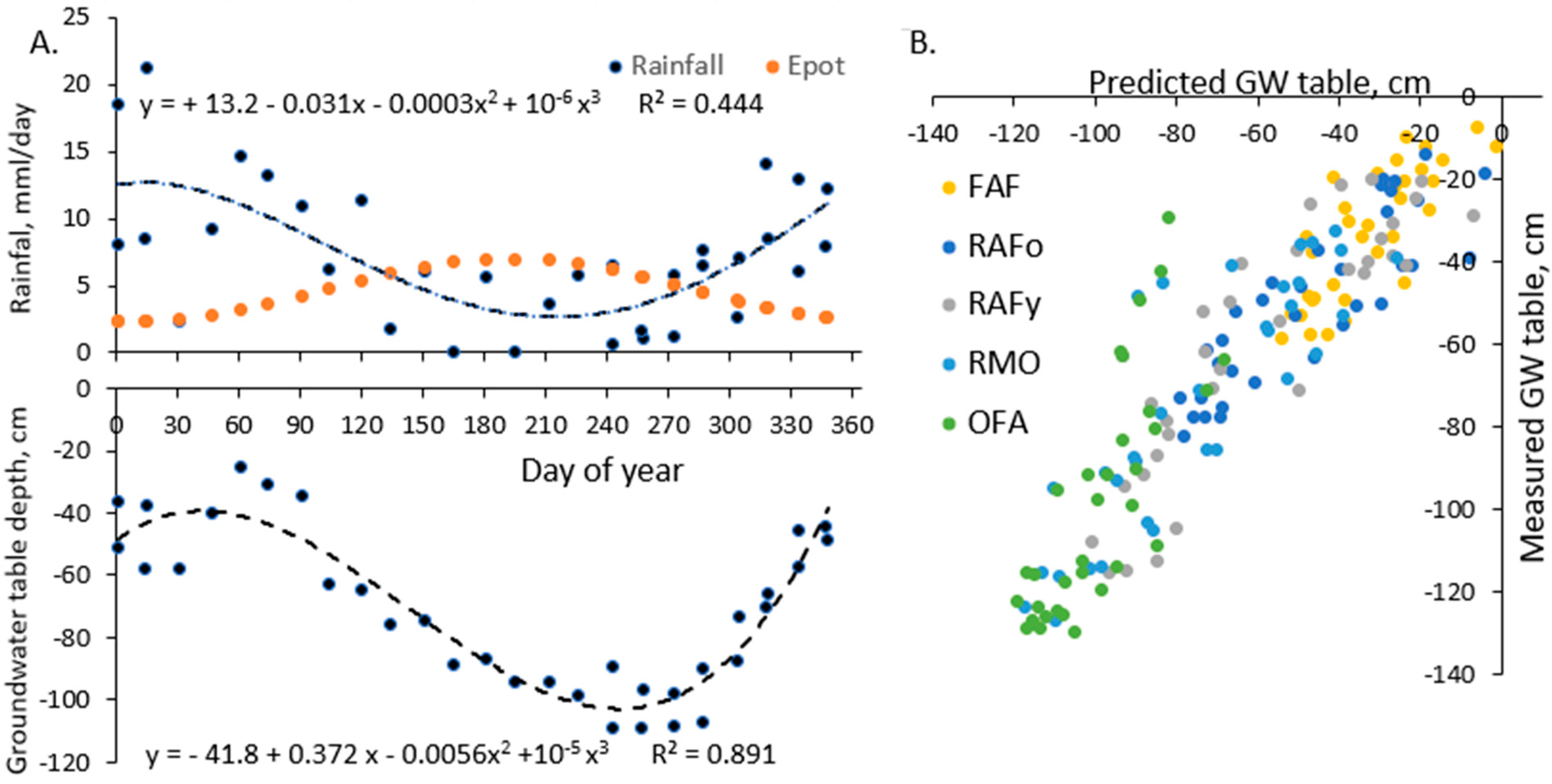
2.3.2. Simple Water Balance Model
2.4. Soil Characteristics
2.4.1. Samples for Physical and Chemical Analysis
2.4.2. Reference Level for Organic Carbon
2.4.3. Earthworm Populations
2.4.4. Soil Quality Index
2.5. Land Cover Characteristics
2.5.1. Plot Sampling
2.5.2. Plant Inventory
- ○
- Population density (individuals/ha) for the various size classes;
- ○
- Species richness = number of species observed in asset of samples;
- ○
- INP (%) = Importance index as the sum of KR + FR + DR, where KR is the relative density of a species, expressed as percentage, FR is the relative frequency of sub-plots containing the species, and DR is the relative dominance of the species in terms of basal area (based on DBH measurements);
- ○
- Shannon–Wiener diversity index H′ = −Σ[(ni/N)Ln(ni/N)], where ni is the number of individuals of a species i and N is the total across all species;
- ○
- Basal area = ∑ 0.25 π *(DBH2), m2/ha;
- ○
- Estimated aboveground biomass (Mg ha−1), AGBest = WD * exp (−1.499 + 2.148 ln(DBH) + 0.207 (ln(DBH))2 − 0.0281 (ln(DBH))3), where WD is the wood density (g cm−3) according to the ICRAF wood density database [69]. The allometric equation was derived from a large literature base for the humid tropics [70].
3. Results
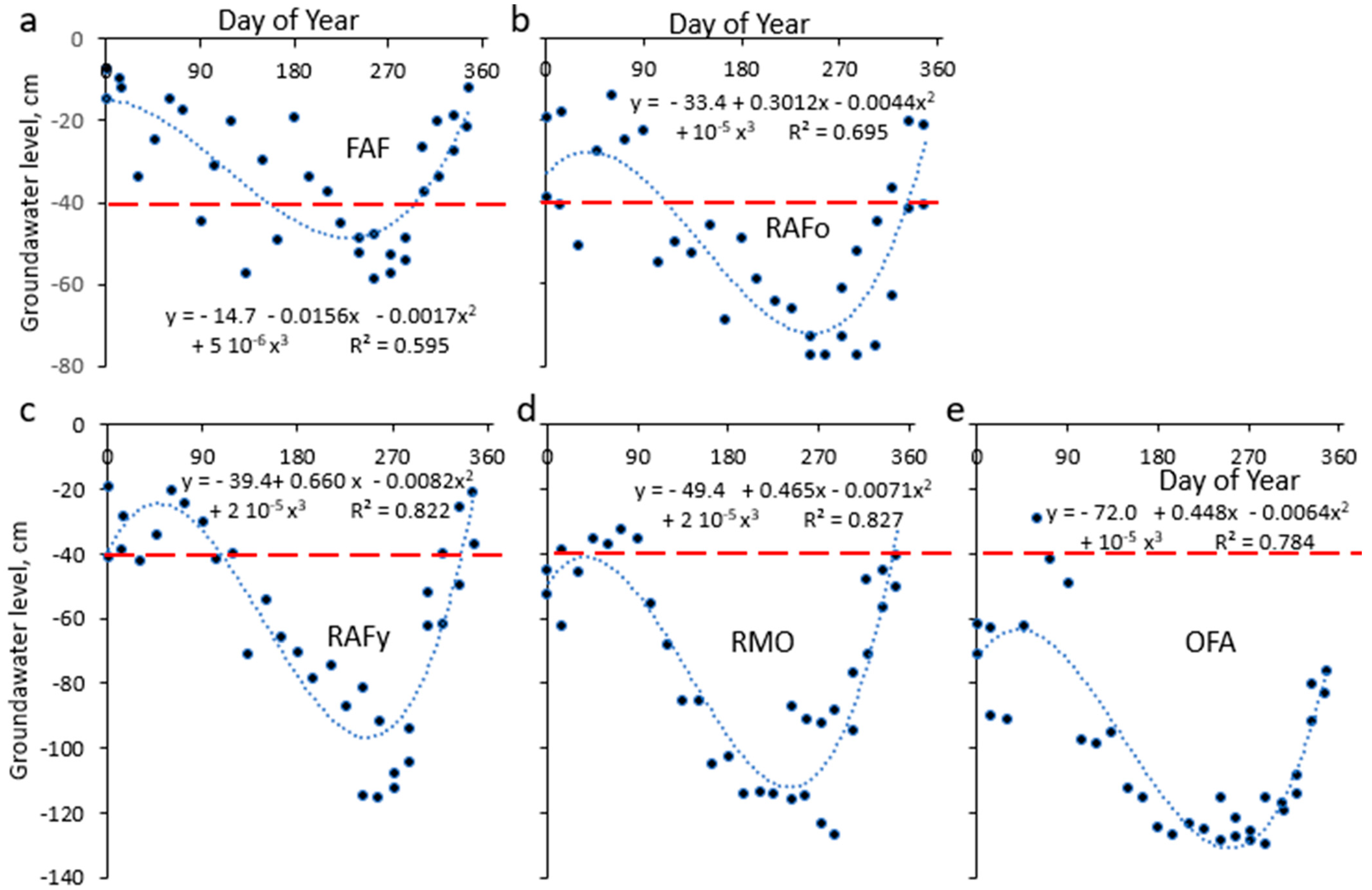
3.1. Groundwater Levels
3.2. Soil Characteristics
3.2.1. Soil Physical and Chemical Properties
3.2.2. Earthworms
3.2.3. Soil Quality Indicators
3.3. Vegetation
3.3.1. Tree Populations, Basal Area, and Tree Biomass
3.3.2. Species Composition
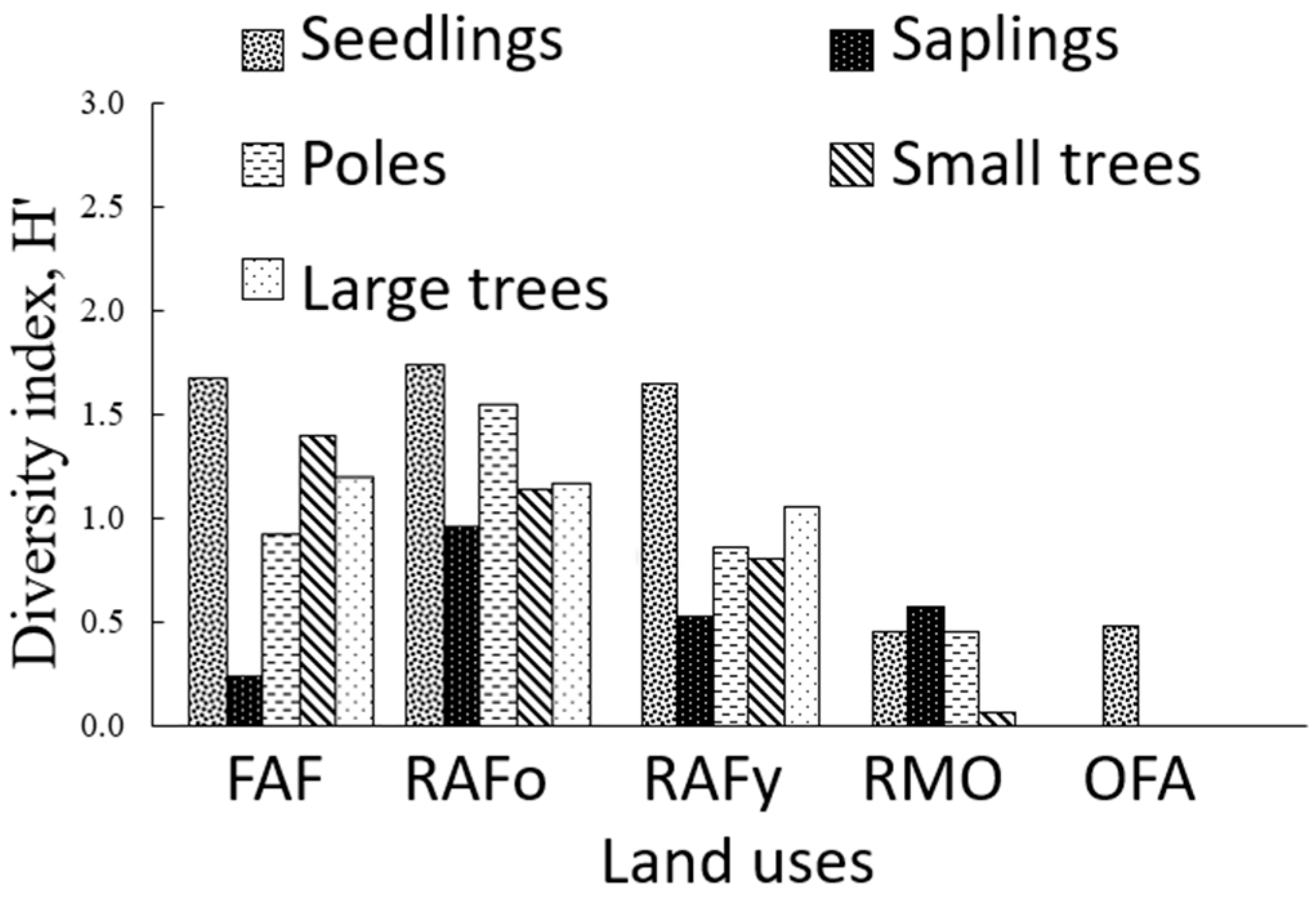
3.3.3. Diversity Indices
4. Discussion
4.1. Groundwater Level Fluctuations
4.2. Soil Quality
4.3. Botanical Composition and Vegetation Structure
4.4. Sustainability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A1. Simple Water Balance Model
Appendix A2. Relationship between Groundwater Levels and Preceding Rainfall
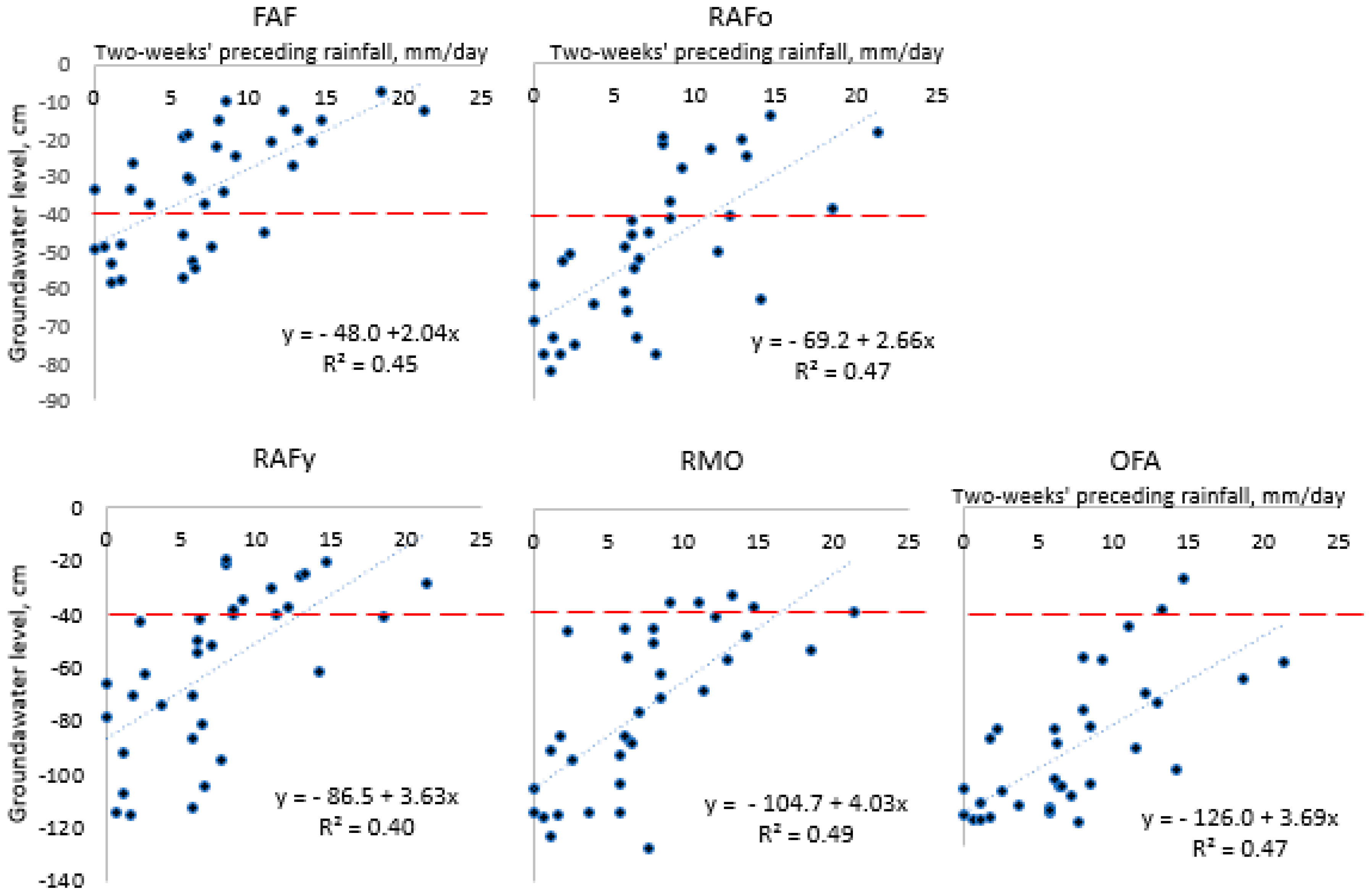
References
- Sanchez, P.A. Properties and Management of Soils in the Tropics, 2nd ed.; Cambridge University Press: Cambridge, UK, 2019; p. 666. [Google Scholar]
- Doran, J.W.; Zeiss, M.R. Soil health and sustainability: Managing the biotic component of soil quality. Appl. Soil Ecol. 2000, 15, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Van Noordwijk, M. Nutrient cycling in ecosystems versus nutrient budgets of agricultural systems. In Nutrient Disequilibria in Agro-Ecosystems: Concepts and Case Studies; Smaling, E.M.A., Oenema, O., Fresco, L.O., Eds.; CAB International: Wallingford, UK, 1999; pp. 1–26. [Google Scholar]
- Shepherd, K.D.; Shepherd, G.; Walsh, M.G. Land health surveillance and response: A framework for evidence-informed land management. Agric. Syst. 2015, 132, 93–106. [Google Scholar] [CrossRef]
- Power, A.G. Ecosystem services and agriculture: Tradeoffs and synergies. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2959–2971. [Google Scholar] [CrossRef]
- Van Noordwijk, M.; Poulsen, J.; Ericksen, P. Quantifying off-site effects of land use change: Filters, flows and fallacies. Agric. Ecosyst. Environ. 2004, 104, 19–34. [Google Scholar] [CrossRef] [Green Version]
- Dent, D.; Young, A. Soil Survey and Land Evaluation; George Allen & Unwin: London, UK, 1981. [Google Scholar]
- Mantel, S.; Wösten, H.; Verhagen, J. Biophysical Land Suitability for Oil Palm in Kalimantan, Indonesia; Report 2007/01; ISRIC—World Soil Information, Alterra, Plant Research International, Wageningen UR: Wageningen, The Netherlands, 2007. [Google Scholar]
- Joshi, L.; Shrestha, P.K.; Moss, C.; Sinclair, F.L. Locally derived knowledge of soil fertility and its emerging role in integrated natural resource management. In Below-Ground Interactions in Tropical Agro-Ecosystems: Concepts and Models with Multiple Plant Components; van Noordwijk, M., Ong, C.K., Cadisch, G., Eds.; CAB-International: Wallingford, UK, 2004; pp. 17–39. [Google Scholar]
- Barrios, E.; Coutinho, H.L.; Medeiros, C.A. InPaC-S: Participatory Knowledge Integration on Indicators of Soil Quality: Methodological Guide; World Agroforestry Centre (ICRAF): Nairobi, Kenya, 2012. [Google Scholar]
- Rousseau, G.X.; Deheuvels, O.; Arias, I.R.; Somarriba, E. Indicating soil quality in cacao-based agroforestry systems and old-growth forests: The potential of soil macrofauna assemblage. Ecol. Indic. 2012, 23, 535–543. [Google Scholar] [CrossRef]
- Plaas, E.; Meyer-Wolfarth, F.; Banse, M.; Bengtsson, J.; Bergmann, H.; Faber, J.; Taylor, A. Towards valuation of biodiversity in agricultural soils: A case for earthworms. Ecol. Econ. 2019, 159, 291–300. [Google Scholar] [CrossRef]
- Hossain, M.F.; Chen, W.; Zhang, Y. Bulk density of mineral and organic soils in the Canada’s arctic and sub-arctic. Inf. Process. Agric. 2015, 2, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Jim, C.Y.; Ng, Y.Y. Porosity of roadside soil as indicator of edaphic quality for tree planting. Ecol. Eng. 2018, 120, 364–374. [Google Scholar] [CrossRef]
- Fu, Y.; Tian, Z.; Amoozegar, A.; Heitman, J. Measuring dynamic changes of soil porosity during compaction. Soil Tillage Res. 2019, 193, 114–121. [Google Scholar] [CrossRef]
- Jaenicke, J.; Wösten, H.; Budiman, A.; Siegert, F. Planning hydrological restoration of peatlands in Indonesia to mitigate carbon dioxide emissions. Mitig. Adapt. Strateg. Glob. Chang. 2010, 15, 223–239. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, W.; Hirano, T.; Roswintiarti, O. Estimation Model of Ground Water Table at Peatland in Central Kalimantan, Indonesia. In Tropical Peatland Ecosystems; Springer: Tokyo, Japan, 2016; pp. 445–453. [Google Scholar]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Huang, B.; Sun, W.; Zhao, Y.; Zhu, J.; Yang, R.; Zou, Z.; Su, J. Temporal and spatial variability of soil organic matter and total nitrogen in an agricultural ecosystem as affected by farming practices. Geoderma 2007, 139, 336–345. [Google Scholar] [CrossRef]
- Lal, R. Sequestering carbon in soils of agro-ecosystems. Food Policy 2011, 36, S33–S39. [Google Scholar] [CrossRef]
- Hairiah, K.; Utami, S.R.; Suprayogo, D.; Sunaryo, S.; Sitompul, S.M.; Lusiana, B.; Mulia, R.; van Noordwijk, M.; Cadisch, G. Pengelolaan Tanah Masam Secara Biologi. Refleksi Pengalaman Dari Lampung Utara; International Centre for Research in Agroforestry: Bogor, Indonesia, 2000. [Google Scholar]
- Chan, K.Y.; Baker, G.H.; Conyers, M.K.; Scott, B.; Munro, K. Complementary ability of three European earthworms (Lumbricidae) to bury lime and increase pasture production in acidic soils of south-eastern Australia. Appl. Soil Ecol. 2004, 26, 257–271. [Google Scholar] [CrossRef]
- Velásquez, E.; Lavelle, P.; Andrade, M. GISQ, a multifunctional indicator of soil quality. Soil Biol. Biochem. 2007, 39, 3066–3080. [Google Scholar] [CrossRef]
- Fründ, H.C.; Graefe, U.; Tischer, S. Earthworms as bioindicators of soil quality. In Biology of Earthworms; Springer: Berlin/Heidelberg, Germany, 2011; pp. 261–278. [Google Scholar]
- Jongmans, A.G.; Pulleman, M.M.; Balabane, M.; Van Oort, F.; Marinissen, J.C.Y. Soil structure and characteristics of organic matter in two orchards differing in earthworm activity. Appl. Soil Ecol. 2003, 24, 219–232. [Google Scholar] [CrossRef]
- Jouquet, P.; Blanchart, E.; Capowiez, Y. Utilization of earthworms and termites for the restoration of ecosystem functioning. Appl. Soil Ecol. 2014, 73, 34–40. [Google Scholar] [CrossRef]
- Guo, X.; Du, W.; Wang, X.; Yang, Z. Degradation and structure change of humic acids corresponding to water decline in Zoige peatland, Qinghai-Tibet Plateau. Sci. Total Environ. 2013, 445, 231–236. [Google Scholar] [CrossRef]
- Wösten, J.H.M.; Clymans, E.; Page, S.E.; Rieley, J.O.; Limin, S.H. Peat–water interrelationships in a tropical peatland ecosystem in Southeast Asia. Catena 2008, 73, 212–224. [Google Scholar] [CrossRef]
- Ritzema, H.; Limin, S.; Kusin, K.; Jauhiainen, J.; Wösten, H. Canal blocking strategies for hydrological restoration of degraded tropical peatlands in Central Kalimantan, Indonesia. Catena 2014, 114, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Dohong, A.; Tanika, L. Hydrological Management Practices. In Tropical Peatland Eco-Management; Springer: Singapore, 2021; pp. 567–593. [Google Scholar]
- Nuroniah, H.S.; Tata, H.L.; Mawazin, M.; Martini, E.; Dewi, S. Assessment on the suitability of planting non-native peatlands species Falcataria moluccana (Miq.) Barneby & Grimes in rewetted peatlands. Sustainability 2021, 13, 70152021. [Google Scholar] [CrossRef]
- Page, S.; Hosciło, A.; Wösten, H.; Jauhiainen, J.; Silvius, M.; Rieley, J.; Ritzema, H.; Tansey, K.; Graham, L.; Vasander, H.; et al. Restoration ecology of lowland tropical peatlands in Southeast Asia: Current knowledge and future research directions. Ecosystems 2009, 12, 888–905. [Google Scholar] [CrossRef]
- Cattau, M.E.; Harrison, M.E.; Shinyo, I.; Tungau, S.; Uriarte, M.; DeFries, R. Sources of anthropogenic fire ignitions on the peat-swamp landscape in Kalimantan, Indonesia. Glob. Environ. Chang. 2016, 39, 205–219. [Google Scholar] [CrossRef]
- Dohong, A.; Aziz, A.A.; Dargusch, P. A review of the drivers of tropical peatland degradation in South-East Asia. Land Use Policy 2017, 69, 349–360. [Google Scholar] [CrossRef]
- Huijnen, V.; Wooster, M.J.; Kaiser, J.W.; Gaveau, D.L.; Flemming, J.; Parrington, M.; Van Weele, M. Fire carbon emissions over maritime southeast Asia in 2015 largest since 1997. Sci. Rep. 2016, 6, 268862016. [Google Scholar] [CrossRef] [Green Version]
- Miettinen, J.; Shi, C.; Liew, S.C. Fire distribution in Peninsular Malaysia, Sumatra and Borneo in 2015 with special emphasis on peatland fires. Environ. Manag. 2017, 60, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Khakim, M.Y.N.; Bama, A.A.; Yustian, I.; Poerwono, P.; Tsuji, T.; Matsuoka, T. Peatland subsidence and vegetation cover degradation as impacts of the 2015 El niño event revealed by Sentinel-1A SAR data. Int. J. Appl. Earth Obs. Geoinf. 2020, 84, 1019532020. [Google Scholar] [CrossRef]
- Silvianingsih, Y.A.; Hairiah, K.; Suprayogo, D.; van Noordwijk, M. Agroforests swiddening and livelihoods between restored peat domes and river: Effects of the 2015 fire ban in Central Kalimantan (Indonesia). Int. For. Rev. 2020, 22, 382–396. [Google Scholar] [CrossRef]
- Galudra, G.; Van Noordwijk, M.; Suyanto, S.; Sardi, I.; Pradhan, U.; Catacutan, D. Hot spots of confusion: Contested policies and competing carbon claims in the peatlands of Central Kalimantan, Indonesia. Int. For. Rev. 2011, 13, 431–441. [Google Scholar] [CrossRef]
- Rahu, A.A.; Hidayat, K.; Ariyadi, M.; Hakim, L. Ethnoecology of Kaleka: Dayak’s agroforestry in Kapuas, Central Kalimantan Indonesia. Res. J. Agric. For. Sci. 2013, 1, 5–12. [Google Scholar]
- Suyanto, S.; Khususiyah, N.; Sardi, I.; Buana, Y.; van Noordwijk, M. Analysis of Local Livelihoods from Past to Present in the Central Kalimantan Ex-Mega Rice Project Area. Working Paper 94; World Agroforestry Centre: Bogor, Indonesia, 2009; 70p, Available online: http://apps.worldagroforestry.org/sea/Publications/files/workingpaper/WP0124-10.pdf (accessed on 25 July 2021).
- Lubis, Z.B. Social Mapping of Access to Peat Swamp Forest and Peatland Resources. Working Paper; Indonesia-Australia Forest Carbon Partnership: Jakarta, Indonesia, 2013; Available online: http://simlit.puspijak.org/files/buku/Social_Mapping_of_Access_to_Peat_Swamp_Forest-Eng_PP3.pdf (accessed on 28 June 2021).
- Jemi, R.; Joni, H.; Toni, H.; Aguswan, J.; Triyadi, A.; Putir, P.E.; Lusiana, H. Kaleka Lapetan Agroforestry Masyarakat Dayak di Desa Bahu Palawa Kabupaten Pulang Pisau. In Proceedings of the conference SEMIRAT Bidang Ilmu Pertanian BKS PTN Barat, Palangka Raya, Indonesia, 14–15 November 2015. [Google Scholar]
- Rahu, A.A.; Hidayat, K.; Ariyadi, M.; Hakim, L. Management of Kaleka (Traditional Garden) Dayak community in Kapuas, Central Kalimantan. Int. J. Sci. Res. 2014, 3, 205–210. [Google Scholar]
- Medrilzam, M.; Dargusch, P.; Herbohn, J.; Smith, C. The socio-ecological drivers of forest degradation in part of the tropical peatlands of Central Kalimantan, Indonesia. Forestry 2014, 87, 335–345. [Google Scholar] [CrossRef]
- Jewitt, S.L.; Nasir, D.; Page, S.E.; Rieley, J.O.; Khanal, K. Indonesia’s contested domains. Deforestation, rehabilitation and conservation-with-development in Central Kalimantan’s tropical peatlands. Int. For. Rev. 2014, 16, 405–420. [Google Scholar] [CrossRef] [Green Version]
- Mulyoutami, E.; Rismawan, R.; Joshi, L. Local knowledge and management of simpukng (forest gardens) among the Dayak people in East Kalimantan, Indonesia. For. Ecol. Manag. 2009, 257, 2054–2061. [Google Scholar] [CrossRef]
- Torquebiau, E. Man-made dipterocarp forest in Sumatra. Agrofor. Syst. 1984, 2, 103–127. [Google Scholar] [CrossRef]
- Van Noordwijk, M.; Tata, H.L.; Xu, J.; Dewi, S.; Minang, P.A. Segregate or integrate for multifunctionality and sustained change through rubber-based agroforestry in Indonesia and China. In Agroforestry—The Future of Global Land Use; Springer: Dordrecht, The Netherlands, 2012; pp. 69–104. [Google Scholar]
- Lawrence, D.C. Trade-offs between rubber production and maintenance of diversity: The structure of rubber gardens in West Kalimantan, Indonesia. Agrofor. Syst. 1996, 34, 83–100. [Google Scholar] [CrossRef]
- BMKG: Badan Meteorologi, Klimatologi dan Geofisika. Available online: Dataonline.bmkg.go.id/data_iklim (accessed on 15 August 2021).
- MacKinnon, K.; Hatta, G.; Mangalik, A.; Halim, H. The Ecology of Kalimantan; Periplus Editions: Hong Kong, China, 1996. [Google Scholar]
- BBSDLP. Atlas Peta Tanah Semi Detail Skala 1:50,000; Ministry of Agriculture: Jakarta, Indonesia, 2016. Available online: www.litbang.pertanian.go.id (accessed on 25 July 2021).
- Notohadiprawiro, Y. Mega-project of Central Kalimantan Wetland Development Project for Food Crop Production: Belief and Truth. 1998. Available online: http://faperta.ugm.ac.id/download/publikasi_dosen/tejoyuwono/1991/1998%20mega.pdf (accessed on 25 July 2021).
- Badan Pusat Statistik (BPS). Kecamatan Jabiren Raya Dalam Angka; National Bureau of Statistics: Jakarta, Indonesia, 2018. [Google Scholar]
- Genstat 64-Bit Release 19.1; (PC/Windows 8), Copyright 2018; VSN International Ltd.,; University of Brawijaya: Malang, Indonesia, 2018.
- Walkley, A.; Black, I.A. An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid tritation method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Anderson, J.M.; Ingram, J.S.I. Tropical Soil Biology and Fertility. A Handbook of Methods, 2nd ed.; CAB International: Wallingford, UK, 1993. [Google Scholar]
- Van Noordwijk, M.; Woomer, P.L.; Cerri, C.; Bernoux, M.; Nugroho, K. Soil carbon dynamics in the humid tropical forest zone. Geoderma 1997, 79, 187–225. [Google Scholar] [CrossRef]
- Hairiah, K.; van Noordwijk, M.; Sari, R.R.; Saputra, D.D.; Widianto, W.; Suprayogo, D.; Kurniawan, S.; Prayogo, C.; Gusli, S. Soil carbon stocks in Indonesian (agro)forest transitions: Compaction conceals lower carbon concentrations in standard accounting. Agric. Ecosyst. Environ. 2020, 294, 106879. [Google Scholar] [CrossRef]
- Baretta, D.; Brown, G.G.; James, S.W.; Cardoso, E.J.B.N. Earthworm populations sampled using collection methods in Atlantic forests with Araucaria angustifolia. Sci. Agric. 2007, 64, 384–392. [Google Scholar] [CrossRef]
- Vasu, D.; Singh, S.K.; Ray, S.K.; Duraisami, V.P.; Tiwary, P.; Chandran, P.; Anantwar, S.G. Soil quality index (SQI) as a tool to evaluate crop productivity in semi-arid Deccan plateau, India. Geoderma 2016, 282, 70–79. [Google Scholar] [CrossRef]
- Rahmanipour, F.; Marzaioli, R.; Bahrami, H.A.; Fereidouni, Z.; Bandarabadi, S.R. Assessment of soil quality indices in agricultural lands of Qazvin Province, Iran. Ecol. Indic. 2014, 40, 19–26. [Google Scholar] [CrossRef]
- Biswas, S.; Hazra, G.C.; Purakayastha, T.J.; Saha, N.; Mitran, T.; Roy, S.S.; Mandal, B. Establishment of critical limits of indicators and indices of soil quality in rice-rice cropping systems under different soil orders. Geoderma 2017, 292, 34–48. [Google Scholar] [CrossRef]
- Nusantara, R.W.; Aspan, A.; Alhaddad, A.M.; Suryadi, U.E.; Makhrawie, M.; Fitria, I.; Rezekikasari, R. Peat soil quality index and its determinants as influenced by land use changes in Kubu Raya District, West Kalimantan, Indonesia. Biodiversitas J. Biol. Divers. 2018, 19, 535–540. [Google Scholar] [CrossRef]
- Sharma, K.L.; Mandal, U.K.; Srinivas, K.; Vittal, K.P.R.; Mandal, B.; Grace, J.K.; Ramesh, V. Long-term soil management effects on crop yields and soil quality in a dryland Alfisol. Soil Tillage Res. 2005, 83, 246–259. [Google Scholar] [CrossRef]
- Kusmana, C. Metode Survey Vegetasi; Insitut Pertanian Bogor Press: Bogor, Indonesia, 1997. [Google Scholar]
- Hairiah, K.; Ekadinata, A.; Sari, R.R.; Rahayu, S. Pengukuran Cadangan Carbon: Dari Tingkat Lahan ke Bentang Lahan. Petunjuk Praktis, 2nd ed.; World Agroforestry Centre, ICRAF: Bogor, Indonesia, 2011. [Google Scholar]
- ICRAF. Wood Density Database. Available online: http://db.worldagroforestry.org/ (accessed on 25 June 2021).
- Chave, J.; Andalo, C.; Brown, S.; Cairns, M.A.; Chambers, J.Q.; Eamus, D.; Folster, H.; Fromard, F.; Higuchi, N.; Kira, T.; et al. Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 2005, 145, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Nortcliff, S. Standardisation of soil quality attributes. Agric. Ecosyst. Environ. 2002, 88, 161–168. [Google Scholar] [CrossRef]
- Eriksen-Hamel, N.S.; Speratti, A.B.; Whalen, J.K.; Légère, A.; Madramootoo, C.A. Earthworm populations and growth rates related to long-term crop residue and tillage management. Soil Tillage Res. 2009, 104, 311–316. [Google Scholar] [CrossRef]
- Feijoo, A.; Carvajal, A.F.; Zúñiga, M.C.; Quintero, H.; Fragoso, C. Diversity and abundance of earthworms in land use systems in central-western Colombia. Pedobiologia 2011, 54, S69–S75. [Google Scholar] [CrossRef]
- Mulia, R.; Hoang, S.V.; Dinh, V.M.; Duong, N.B.T.; Nguyen, A.D.; Lam, D.H.; Thi Hoang, D.T.; van Noordwijk, M. Earthworm diversity, forest conversion and agroforestry in Quang Nam province, Vietnam. Land 2021, 10, 36. [Google Scholar] [CrossRef]
- Topoliantz, S.; Ponge, J.F. Burrowing activity of the geophagous earthworm Pontoscolex corethrurus (Oligochaeta: Glossoscolecidae) in the presence of charcoal. Appl. Soil Ecol. 2003, 23, 267–271. [Google Scholar] [CrossRef] [Green Version]
- Wijaya Adhi, I.P.G.; Subiksa, I.G.M.; Kasdi, S.D.; Ardi, S. Pengelolaan Tanah dan Air Lahan Rawa: Suatu Tinjauan Hasil Penelitian Proyek Swamps II. In Review Hasil–Hasil Penelitian Proyek Swamps II di Bogor, 19–20 February 1993; Badan Penelitian dan Pengembangan Pertanian; Departemen Pertanian: Bogor, Indonesia, 1993; p. 22. [Google Scholar]
- Khasanah, N.M.; van Noordwijk, M. Subsidence and carbon dioxide emissions in a smallholder peatland mosaic in Sumatra, Indonesia. Mitig. Adapt. Strateg. Glob. Chang. 2019, 24, 147–163. [Google Scholar] [CrossRef] [Green Version]
- Tata, H.L. Mixed farming systems on peatlands in Jambi and Central Kalimantan provinces, Indonesia: Should they be described as paludiculture? Mires Peat 2019, 25, 1–17. [Google Scholar]
- Kaya, M.; Kammesheidt, L.; Weidelt, H.J. The forest garden system of Saparua island Central Maluku, Indonesia, and its role in maintaining tree species diversity. Agrofor. Syst. 2002, 54, 225–234. [Google Scholar] [CrossRef]
- Tata, M.H.L.; Pradjadinata, S. Regenerasi alami hutan rawa gambut terbakar dan lahan gambut terbakar di tumbang nusa, kalimantan tengah dan implikasinya terhadap konservasi. J. Penelit. Hutan Dan Konserv. Alam 2013, 10, 327–342. [Google Scholar] [CrossRef] [Green Version]
- Budiharta, S. Floristic composition at biodiversity protection area in Lubuk Kakap, District of Ketapang, West Kalimantan. Biodiversitas J. Biol. Divers. 2010, 11, 3. [Google Scholar] [CrossRef]
- Brearley, F.Q.; Prajadinata, S.; Kidd, P.S.; Proctor, J. Structure and floristics of an old secondary rain forest in Central Kalimantan, Indonesia, and a comparison with adjacent primary forest. For. Ecol. Manag. 2004, 195, 385–397. [Google Scholar] [CrossRef]
- Sofiah, S.; Metusala, D.; Trimanto, T.; Nurfadilah, S. Flora diversity, composition and ecology in Besiq Bermai tropical forest of Damai District, East Kalimantan. BIOTROPIA Southeast Asian J. Trop. Biol. 2018, 25, 85–94. [Google Scholar]
- Sari, R.R.; Saputra, D.D.; Hairiah, K.; Rozendaal, D.; Roshetko, J.M.; van Noordwijk, M. Gendered species preferences link tree diversity and carbon stocks in Cacao agroforest in Southeast Sulawesi, Indonesia. Land 2020, 9, 108. [Google Scholar] [CrossRef] [Green Version]
- Noor, M. Lahan Gambut: Pengembangan, Konservasi, dan Perubahan Iklim; Gadjah Mada University Press: Yogyakarta, Indonesia, 2010. [Google Scholar]
- Van Noordwijk, M.; Bizard, V.; Wangpakapattanawong, P.; Tata, H.L.; Villamor, G.B.; Leimona, B. Tree cover transitions and food security in Southeast Asia. Glob. Food Secur. 2014, 3, 200–208. [Google Scholar] [CrossRef] [Green Version]
- Van Noordwijk, M.; Martikainen, P.; Bottner, P.; Cuevas, E.; Rouland, C.; Dhillion, S.S. Global change and root function. Glob. Chang. Biol. 1998, 4, 759–772. [Google Scholar] [CrossRef]
- Van Noordwijk, M. Agroforestry-Based ecosystem services: Reconciling values of humans and nature in sustainable development. Land 2021, 10, 699. [Google Scholar] [CrossRef]



| FAF | RAFo | RAFy | RMO | OFA | |
|---|---|---|---|---|---|
| Measured: | |||||
| Average HGW | −33.0 | −49.6 | −59.9 | −75.1 | −98.9 |
| Standard Deviation | 15.9 | 20.3 | 30.1 | 30.3 | 28.2 |
| Fraction HGW < −40 | 64.7% | 29.4% | 29.4% | 14.7% | 2.9% |
| Parameters adjusted per land use: | |||||
| Soil porosity [vol/vol] | 0.569 | 0.556 | 0.608 | 0.602 | 0.541 |
| Hd [cm] | −40 | −40 | −40 | −60 | −110 |
| Hupt [cm] | −14.3 | −30 | −47.5 | −57 | −33 |
| Model performance: | |||||
| Slope Predicted: Observed | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| SE slope | 0.05 | 0.04 | 0.04 | 0.03 | 0.03 |
| Fraction of variance accounted for | 0.93 | 0.96 | 0.95 | 0.97 | 0.97 |
| Estimated water balance (2018): | |||||
| Drain Fraction | 68.2% | 55.1% | 51.8% | 53.7% | 76.7% |
| Evapotranspiration fraction | 30.1% | 43.2% | 50.8% | 49.8% | 21.8% |
| Delta ground water storage | 1.6% | 1.6% | −4.5% | −4.0% | 1.6% |
| Bulk Density | Particle Density | Porosity | Clay | Silt | Sand | Corg | Cref | Corg/Cref | pH (H2O) | pH (KCl) | Alexch cmol kg−1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g cm−3 | % | |||||||||||
| Land uses * | ||||||||||||
| FAF | 0.95 b | 2.21 c | 56.9 a | 76.7 a | 23.1 a | 0 | 2.04 a | 2.58 b | 0.72 a | 3.63 | 3.53 | 7.70 a |
| RAFo | 0.97 b | 2.18 c | 55.6 a | 68.6 a | 32.2 ab | 0.1 | 2.76 a | 2.55 b | 1.03 a | 3.64 | 3.54 | 8.24 ab |
| RAFy | 0.85 a | 2.17 c | 60.8 b | 60.9 ab | 39.0 bc | 0 | 6.11 b | 2.44 a | 2.48 b | 3.71 | 3.61 | 9.79 c |
| RMO | 0.83 a | 2.10 b | 60.2 b | 50.2 bc | 49.6 d | 0.2 | 5.43 b | 2.36 a | 2.21 b | 3.66 | 3.61 | 10.04 c |
| OFA | 0.94 b | 2.04 a | 54.1 a | 51.1c | 48.8 cd | 0 | 5.13 b | 2.36 a | 2.26 b | 3.73 | 3.61 | 8.92 b |
| s.e.d | 0.04 | 0.03 | 1.66 | 5.07 | 4.9 | 0.1 | 1.06 | 0.04 | 0.44 | 0.06 | 0.04 | 0.38 |
| Soil depth (cm) | ||||||||||||
| 0–10 | 0.77 a | 2.08 a | 62.8 c | 58.7 | 41.1 | 0.1 | 6.20 b | 3.41 c | 1.84 a | 3.67 | 3.57 | 8.47 a |
| 10–20 | 0.93 a | 2.15 b | 56.8 a | 58.9 | 40.9 | 0.1 | 4.01 a | 2.16 b | 1.88 a | 3.69 | 3.61 | 8.84 a |
| 20–30 | 1.03 c | 2.19 b | 53.0 a | 66.9 | 33.5 | 0 | 2.68 a | 1.81 a | 1.49 a | 3.67 | 3.57 | 9.50 b |
| s.e.d | 0.03 | 0.02 | 1.29 | 3.93 | 3.79 | 0.1 | 0.82 | 0.03 | 0.34 | 0.04 | 0.03 | 0.23 |
| Depth\Land Use * | FAF | RAFo | RAFy | RMO | OFA | s.e.d. |
|---|---|---|---|---|---|---|
| Population (individuals m−2) | ||||||
| 0–10 cm | 64.4 g | 54.7 g | 35.1 f | 18.2 cde | 14.7 bcd | 5.07 |
| 10–20 cm | 9.8 abcd | 28.0 ef | 19.1 de | 2.2 a | 11.1 abcd | |
| 20–30 cm | 0.9 a | 10.7 abcd | 8.9 abcd | 7.1 abc | 4.0 ab | |
| Biomass (g m−2) | ||||||
| 0–10 cm | 30.8 f | 23.8 e | 11.3 d | 7.1 cd | 3.6 abc | 2.58 |
| 10–20 cm | 3.6 abc | 11.4 d | 6.6 bcd | 1.1 abc | 3.3 abc | |
| 20–30 cm | 0.1 a | 5.2 abc | 3.0 abc | 2.5 abc | 0.9 ab | |
| Size (g/individual) | ||||||
| 0–10 cm | 0.49 f | 0.42 ef | 0.34 def | 0.27 bcde | 0.20 abcd | 0.08 |
| 10–20 cm | 0.12 abc | 0.43 ef | 0.31 cdef | 0.11 ab | 0.20 abcd | |
| 20–30 cm | 0.02 a | 0.37 def | 0.29 bcde | 0.13 abc | 0.10 ab | |
| Soil Indicator | SQI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FAF | RAFo | RAFy | RMO | OFA | ||||||
| Bulk density | 0.35 | L | 0.39 | L | 0.35 | L | 0.49 | M | 0.56 | M |
| Particle density | 0.12 | VL | 0.10 | VL | 0.23 | VL | 0.25 | L | 0.13 | VL |
| Corg | 0.06 | VL | 0.11 | VL | 0.31 | L | 0.27 | L | 0.25 | L |
| Clay + silt | 0.13 | VL | 0.14 | VL | 0.13 | VL | 0.13 | VL | 0.13 | VL |
| pH | 0.13 | VL | 0.13 | VL | 0.15 | VL | 0.13 | VL | 0.15 | VL |
| Porosity | 0.12 | VL | 0.10 | VL | 0.19 | VL | 0.18 | VL | 0.07 | VL |
| Water table | 0.56 | M | 0.46 | M | 0.39 | M | 0.29 | L | 0.15 | VL |
| Alexch | 0.44 | M | 0.38 | L | 0.23 | L | 0.20 | L | 0.31 | M |
| Earthworms | 0.37 | L | 0.47 | M | 0.31 | L | 0.12 | VL | 0.13 | VL |
| Total | 2.28 | 2.28 | 2.27 | 2.05 | 1.87 | |||||
| Average | 0.25 | L | 0.25 | L | 0.25 | L | 0.23 | L | 0.21 | L |
| Land Use * | Understory | Saplings | Poles | Medium Trees | Large Trees | Basal Area | Aboveground Tree Biomass |
|---|---|---|---|---|---|---|---|
| Individuals ha−1 | m2 ha−1 | Mg ha−1 | |||||
| FAF | 24,167 a | 107 | 320 b | 142 b | 87 b | 26.8 bc | 299 c |
| RAFo | 28,000 a | 533 | 347 b | 127 b | 102 b | 34.4 c | 281 c |
| RAFy | 32,833 a | 373 | 527 c | 132 b | 28 a | 20.2 b | 144 b |
| RMO | 37,000 a | 293 | 320 b | 217 c | 40 a | 20.8 b | 148 b |
| OFA | 79,667 b | 0 | 0 a | 0 a | 0 a | 0.0 a | 0 a |
| s.e.d | 13,671 | 202 | 76 | 27.75 | 19.08 | 3.71 | 42.7 |
| Scientific Name | Local Name | Under-Story | LCs | Saplings | LCs | Poles | LCs | Medium Trees | LCs | Large Trees | LCs |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hevea brasiliensis | Karet | 132.7 | 2,3,4 | 300 | 1,2,3,4 | 226 | 1,2,3,4 | 287 | 2,3,4 | 300 | 2,3,4 |
| Artocarpus integer | Mangkahai | 0 | 0 | 194 | 4 | 82 | 1,3,4 | 133 | 1,3 | 158 | 1,3 |
| Sandoricum koetjape | Katapi | 37.4 | 1 | 0 | 0 | 0 | 0 | 31 | 3 | 164 | 3 |
| Durio sp. | Dahuyan | 0 | 0 | 101 | 4 | 0 | 0 | 0 | 0 | 70 | 1 |
| Mangifera caesia | Binjai | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 59 | 2 |
| Baccaurea mottleyana | Rambai | 0 | 0 | 62 | 2 | 41 | 2 | 0 | 0 | 49 | 1 |
| Artocarpus nitidus | Tampang | 0 | 0 | 0 | 0 | 0 | 0 | 70 | 2 | 0 | 0 |
| Lansium domesticum | Langsat | 0 | 0 | 0 | 0 | 0 | 0 | 65 | 1 | 0 | 0 |
| Nephelium lappaceum | Rambutan | 30.6 | 4 | 0 | 0 | 240 | 1 | 47 | 1,4 | 0 | 0 |
| Peronema canescens | Sungkai | 0 | 0 | 0 | 0 | 0 | 0 | 41 | 2 | 0 | 0 |
| Calophyllum hosei | Jinjit | 42.6 | 1,2 | 0 | 0 | 38 | 2 | 0 | 0 | 0 | 0 |
| Garcinia sp. | Manggis | 0 | 0 | 300 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vitex pubescens | Kalapapa | 0 | 0 | 300 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Parkia speciosa | Petai | 0 | 0 | 168 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mezzettia parviflora | Kambalitan | 0 | 0 | 55 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mangifera odorata | Kweni | 0 | 0 | 49 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dillenia sp. | Simpur | 79.5 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pteris sp. | Hawuk | 67.2 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Guazuma ulmifolia | Kalanduyung | 44.7 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Stenochlaena palustris | Kalakai | 43.1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Calamus sp. | Rotan | 36.4 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Citrullus lanatus | Semangka | 36.1 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Coffea canephora | Kopi | 36.1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silvianingsih, Y.A.; Hairiah, K.; Suprayogo, D.; van Noordwijk, M. Kaleka Agroforest in Central Kalimantan (Indonesia): Soil Quality, Hydrological Protection of Adjacent Peatlands, and Sustainability. Land 2021, 10, 856. https://doi.org/10.3390/land10080856
Silvianingsih YA, Hairiah K, Suprayogo D, van Noordwijk M. Kaleka Agroforest in Central Kalimantan (Indonesia): Soil Quality, Hydrological Protection of Adjacent Peatlands, and Sustainability. Land. 2021; 10(8):856. https://doi.org/10.3390/land10080856
Chicago/Turabian StyleSilvianingsih, Yosefin Ari, Kurniatun Hairiah, Didik Suprayogo, and Meine van Noordwijk. 2021. "Kaleka Agroforest in Central Kalimantan (Indonesia): Soil Quality, Hydrological Protection of Adjacent Peatlands, and Sustainability" Land 10, no. 8: 856. https://doi.org/10.3390/land10080856
APA StyleSilvianingsih, Y. A., Hairiah, K., Suprayogo, D., & van Noordwijk, M. (2021). Kaleka Agroforest in Central Kalimantan (Indonesia): Soil Quality, Hydrological Protection of Adjacent Peatlands, and Sustainability. Land, 10(8), 856. https://doi.org/10.3390/land10080856









