1. Introduction
Mammalian carnivores tend to have large home ranges, low densities, and slow growth rates, making them especially vulnerable to extinction [
1,
2,
3]. Because of the lack of protection, habitat loss, and human action; most wild carnivores have undergone significant decreases in their abundance and diversity [
1,
2,
4,
5,
6,
7,
8]. The conflict with humans is the leading cause of the decline in carnivore populations [
9,
10]. These conflicts happen mainly because of suspected predation on livestock and on some wild species with trophy hunting interests [
11]. These human carnivore conflicts are a worldwide problem [
10,
12] with plenty of examples of carnivores killing livestock or even attacking humans. Carnivores have an essential role in the community of which they are part of, primarily by regulating it through trophic cascades. Their effects can be produced by consumption or by behavior [
13]. The consumption function is also called lethal and can directly regulate prey population size [
8,
14] and mesopredators (in the case of apex predators) [
15,
16]; or indirectly by providing carrion [
17,
18], promoting higher biodiversity levels [
19], or even influencing soil composition [
20]. Their effects by behavior can be direct and indirect as well: Directly by influencing prey behavior and habitat use [
21,
22], prey pack size [
23], reproductive physiology [
24], and natural selection [
25]. Indirectly by modulating prey population dynamics [
26,
27], limiting herbivory, or maintaining plant diversity [
28,
29]. Therefore, carnivore protection is one of the priorities in biological conservation using the top-down approach [
10].
Most ecological ecosystems are human-modified environments [
4,
30] due to urban development or exploitation of natural resources. Carnivores are affected by human activities in many different ways: by habitat fragmentation, physical barriers limiting gene flow, road death tolls, behavioral changes, dispersal, disease spreading, and exposure to poisons [
3,
31,
32].
An essential aspect in carnivore conservation and management is based on their interactions in sympatry. It is crucial to understand the structure of the ecological community in which they are inserted [
33], as it may influence the distribution, activity patterns, and or diet of the carnivores involved. The competitive exclusion principle proposes that two species with identical niches cannot coexist indefinitely; therefore, some degree of partitioning must materialize in the realized niche of coexisting species [
33,
34,
35,
36]. Such partitioning is commonly observed across time, space, and trophic axes. In addition, the particular association of coexistence established between apex predators and mesopredators should be considered. The latter being defined as those at intermediate trophic levels, where the former control the populations of the latter [
15,
16,
33,
37].
The present study is focused on the apparent occurrences, relative abundance indexes (RAI) [
38], and connections of an apex predator, the puma (
Puma concolor) and one mesopredator, the South American grey fox (
Lycalopex griseus), considering ecological variables such as the abundance of others wild and exotic carnivores, human intervention, farmland effect, prey availability, and habitat quality. Consideration of dogs is also important as well because we observed abundant free-roaming individuals were observed, which may influence both native species (by predation, competition, disease transmission) [
39,
40,
41,
42], and livestock [
43,
44]. The chosen study locations are relevant, because they are threatened areas with different levels of human intervention. These features offer the ideal setting for studying of how different carnivore species respond to human presence and activities. The primary hypothesis was that the apparent occurrence and RAI of puma and fox would be positively associated with RAI of prey and livestock and negatively related to human intervention. On the other hand, the secondary hypotheses were related to the interactions between puma and fox faced with different degrees of human intervention.
In this work, the puma and fox apparent occurrence and RAI between three contrasting landscapes were compared, characterized by considerable differences in human population and intervention. Under this central hypothesis, lower RAI and apparent occurrence of both predators in the pre-mountain landscape are expected, which was more anthropized. Besides this, the authors were interested in assessing several secondary hypotheses that might explain the variability observed between localities. They included a negative relationship between puma and fox, positive effects of prey and livestock apparent abundance, and negative effects of humans and free-roaming dogs on the apparent occurrence and RAI of both carnivores. Nonetheless, the large collinearity between most of these explanatory variables and the small number of localities where they were tested precluded proper isolation of their effects, leading to shape the current assessment as an exploratory analysis.
4. Discussion
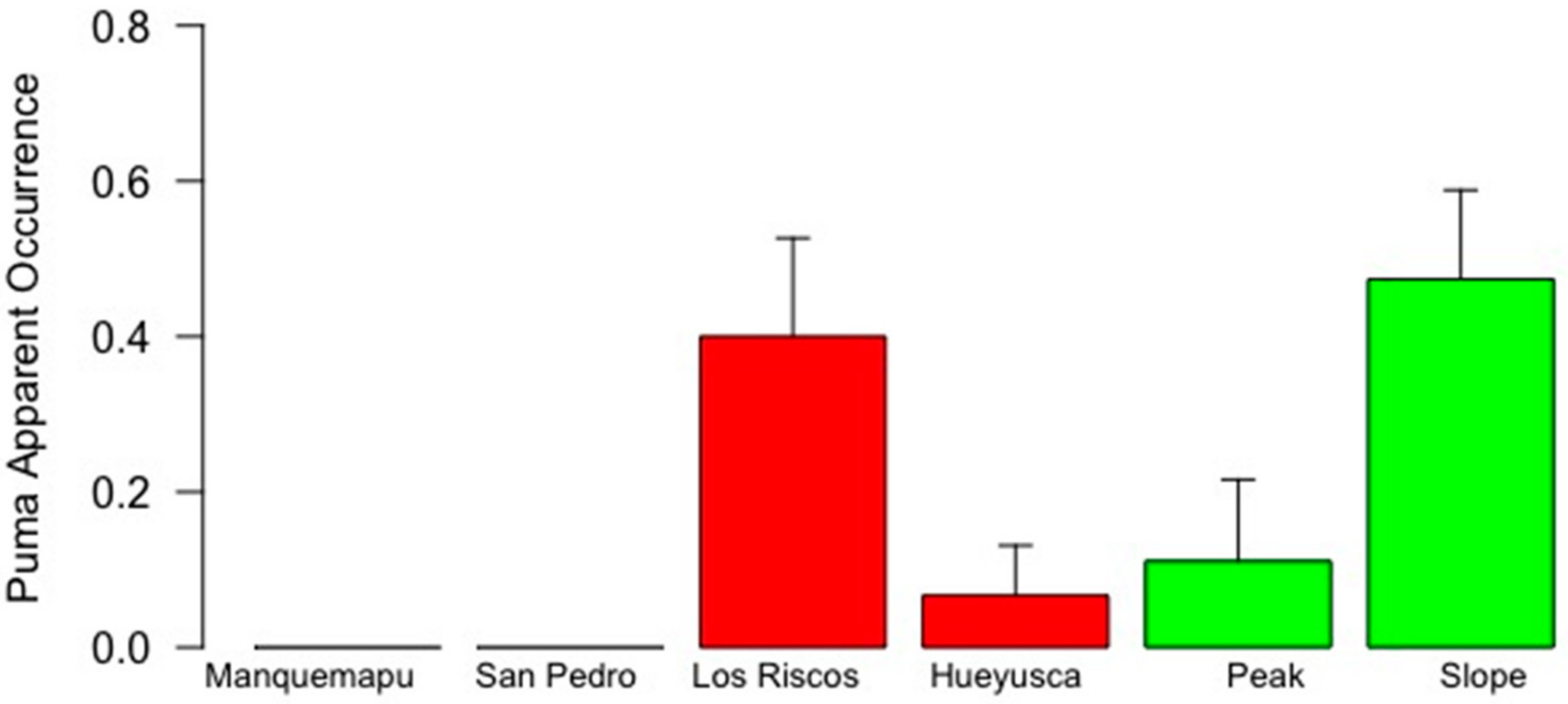
The absence of puma and fox records obtained in the two locations from the coast landscape (Manquemapu and San Pedro Bay) was surprising. Nonetheless, it matched results from a parallel study conducted by us, which showed that carnivore feces were scarce in these locations. These sites are isolated, weakly intervened, and with small settlements of fishermen and wood handcrafters (INRA values of 4.167 and 12.500, respectively). When the study was designed, it was assumed that the mountain range would act as a biological corridor [
83], but the current situation probably is the opposite, acting as a barrier and limiting dispersal from the coast landscape. In the past, the entire mountain range suffered from several big fires [
57]; some people think they came about by natural causes and others that they were man-made to acquire the burned wood from Patagonian cypress, which is protected as a natural monument and can only be exploited when burnt (independently of cause). Currently, the Patagonian cypress forest at the peak is quite open, full of dead trees, a few survivors, and some recruits (F. García-Solís, personal observation). Unfortunately, Patagonian cypress trees take longer to grow, living up to 3600 years [
84]. All this renders the peak location of the mountain range a harsh environment, with almost no shelter for herbivores, thus limiting carnivore presence.
Camera trapping of unmarked species can be challenging, as it is difficult to use capture-recapture methods when assessing their relative abundances and could have biased inference estimating abundances [
85,
86]. In our study, the two carnivore species were unmarked, thus we assumed equal detectability and potential bias, as camera traps cannot record all animal presences in an area [
87]. Their camera records were considered as independent events when consecutive images that contained the same species were recognizable as different individuals, a method used in several studies [
60,
64,
68]. The use of lures is a widespread method in camera trapping, but optimizing the detectability of a target species can produce bias in calculating abundances, as the species behavior may be altered, or some species may be attracted whereas others may be repelled [
88,
89,
90,
91].
4.1. Relative Integrated Anthropization Index (INRA)
The working hypothesis about habitat quality was related to the fact that the locations from the pre-mountain range would have the highest INRA levels, followed by coast and then by mountain range. Our results supported this mostly, except for the Manquemapu and peak locations. The former had lower INRA, affording better habitat quality than the latter. This lower INRA may be accounted for by the operation of the Manquemapu Management Plan, regulated by its Mapuche Huilliche community. This plan considers zoning areas of human use, dead Patagonian cypress recovery harvest, sustainable management, and collection of marine resources [
92]. Although the peak has low human intervention, its forest may offer lower habitat quality owing to its past fire history. The current landscape is a very open forest, full of burned trunks which some of which show small brunches with leaves, this harsh environment could be a barrier to the dispersal of carnivores.
4.2. Predator Apparent Occurrence
Since the use of occupancy or co-occurrence models, was not supported by the data, the use of the apparent occurrence was selected. Los Riscos (pre-mountain range) is characterized by the presence of exotic plantations of eucalyptus (
Eucalyptus nitens and
Eucalytus globulus), which they are not native forests still afford a habitat for the puma [
53], providing shelter from humans in the surroundings, and probably also food, by being populated by hare and pudu. Further, in that particular landscape, there is a vital remnant of native forest [
53,
93,
94], which provides habitat for the puma’s prey. In addition, there is the presence of livestock, which pumas may perceive as a potential food resource. The slope location from the mountain range landscape is characterized by scarce human presence and low activity and preserves most of its native vegetation, rendering it relatively unaltered by humans, which may explain the high puma apparent occurrence. Our results from the marginal likelihood ratio test showed that there was not a significant effect of human activity on puma, pumas may tolerate human presence better than expected. In addition, the effect from prey could not show to be influential either, this could be explained for the prey cannot be detected by the cameras since carnivorous attractant was used. In parallel, the models from the exploratory analysis showed some positive relations for RAI of fox and livestock, both being a potential food resource for puma.
Apparent fox occurrence was influenced by RAI of puma, livestock and INRA. These values were higher in the pre-mountain than in the mountain range. The mesopredator release hypothesis [
16] may explain the higher fox presence in the former landscape because the higher human activity may interfere with apparent puma occurrence. A complementary explanation is that foxes, being mesopredators with smaller size may tolerate environments with higher human activity [
95]. This parallels the positive effect of INRA on fox apparent occurrence. Alternatively, the presence of puma can facilitate the presence of foxes since the puma behavior of burying its prey after eating to store it for later; this buried prey being subsequently scavenged by foxes [
96,
97,
98], explaining the positive correlation between them. The exploratory analysis supported the positive effects of INRA and puma on fox. Additionally, South American grey foxes are known to visit exotic plantations due to potential prey such as rodents and hares [
99].
4.3. Predator Relative Abundance Indexes
Relative Abundance indices are not necessarily the most informative about abundance species and can have some weaknesses such as: be biased due to the different detection among species, especially in elusive ones; species with extensive home range are more detected, increasing RAI values; and bias due to the different responses to the camera setup among the species [
100]. Puma RAI was influenced by the variables of location and fox RAI. Los Riscos and slope present higher values, probably due to low levels of human activity and restricted pass policy, in addition to the presence of livestock as potential prey (our data showed a positive but not so strong correlation. The positive and relevant correlation between RAI of fox and puma could be explained by intraguild predation, which is an extreme form of interspecific competition when species that act as competitors also function as predators [
101,
102,
103]. In this case, the puma is a potential predator of foxes, the latter’s RAI may improve an increase in that of puma. The exploratory analysis showed a negative relation with INRA, and one model showed a negative relation also with human activity, which could be explained by the sensitivity of puma to habitat quality.
Despite large differences in fox RAI between landscapes and locations, our analysis suggests that puma RAI and INRA were positively associated with fox RAI. For instance, Hueyusca showed both the highest fox RAI and high levels of human presence and activity (INRA = 29.167), suggesting once more that foxes may flourish in such anthropized situation. The exploratory analysis supported the survivorship of foxes in human-intervened environments and showed a positive relation of foxes with their prey and the presence of livestock.
It was noteworthy that, even though the cameras traps registered numerous dogs, they were not identified as important ecological variables in any of the models depicting RAI and the apparent occurrence of puma and fox. This result was unexpected, as the impact of free-roaming dogs over wildlife by predation, activity alteration (fear-related), hybridization, and spreading of diseases is well known [
42,
104]. The present data show that dog numbers were larger in the pre-mountain range whereas those of puma were so in the mountain range. Thus, these two carnivores were segregated over the spatial axis so that dogs may not have an important effect over pumas. Nevertheless, dogs and foxes are abundant in the pre-mountain range, but even if they share space, they are segregated over time, foxes being more active during the night and dogs during the day [
8,
104].
It can be suggested that eucalyptus plantations in the pre-mountain range could offer an adequate habitat for the puma and the fox due to the presence of shelter from humans from the surroundings and prey availability such as hare, pudu, rodents, and potentially livestock. This was not the case of the coastal range, where we obtained almost no animal records, so it is possible that the mountain range could be acting as a biological barrier rather than a biological corridor. Due to the nature of the present data, it was no possible to detect any relevant effect between the two coexisting carnivores, between their respective prey, or the very abundant presence of dogs. Consequently, we recommend further studies in this specific area and habitats, improving the sampling efforts by implementing a considerable number of cameras and for more extended periods to obtain better data and clearer the relations and conclusions.