Global Climate Change Effects on Soil Microbial Biomass Stoichiometry in Alpine Ecosystems
Abstract
1. Introduction
2. Stoichiometry Characteristics of Soil Microbial Biomass in Alpine Ecosystems
3. Impact of Global Change on Soil Microbial Biomass Stoichiometry in Alpine Ecosystems
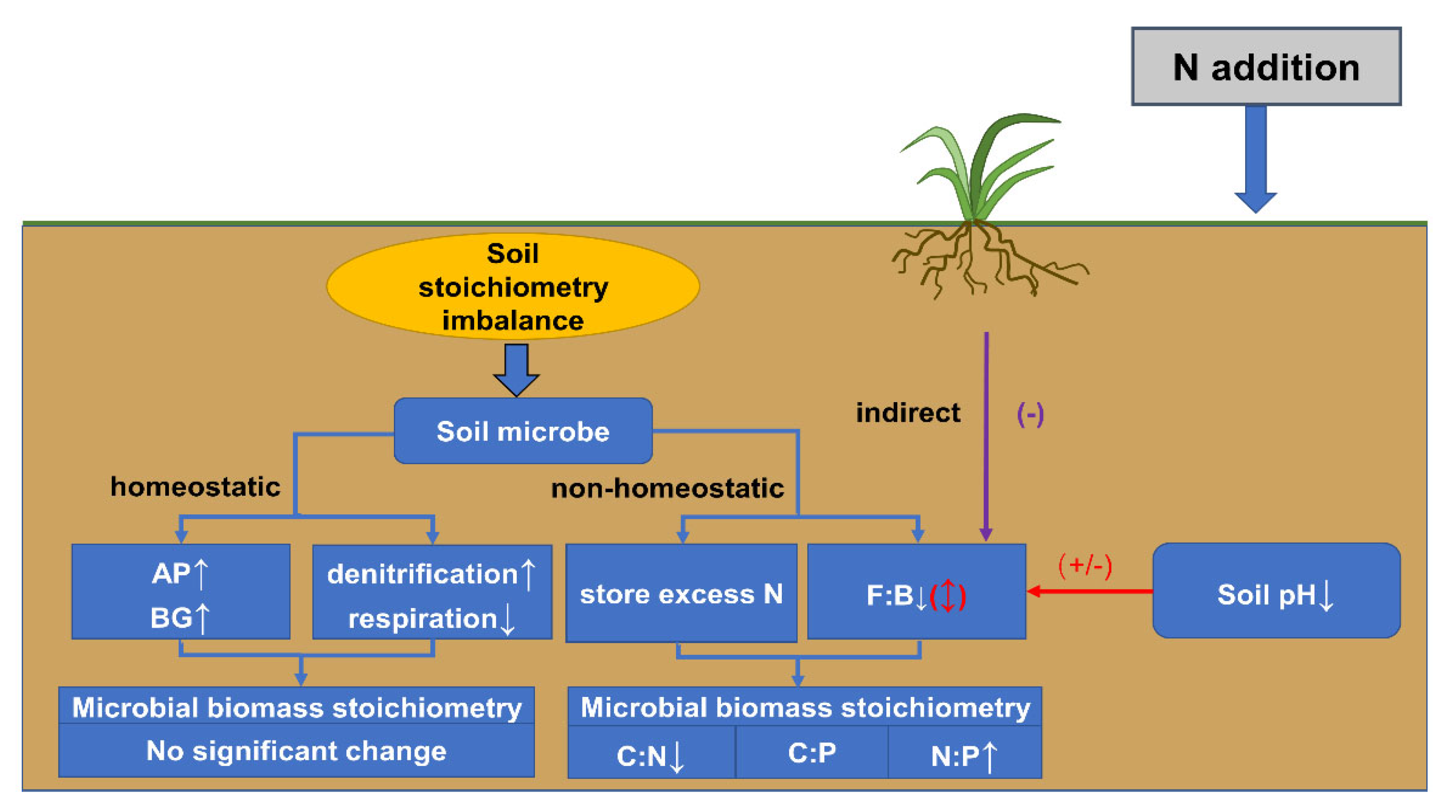
3.1. Impact of Increased N Deposition
3.2. Impact of Global Warming
3.3. Impacts of Changing Precipitation Patterns
3.4. Impact of Elevated Atmospheric CO2 Concentration
4. Future Perspectives
- (1)
- Global climate change is often a combination of multiple factors. Different global change drivers may have cumulative or opposite effects on the environment and microbes, e.g., elevated CO2 increases soil moisture, but warming decreases soil water. Thus, future studies should use integrated experiments to investigate how different climate change drivers affect alpine ecosystem stoichiometry to build better models and to explore the microbial stoichiometric response under natural conditions.
- (2)
- Microbes respond to changes in substrate stoichiometry with two completely different strategies: (i) maintaining stoichiometric homeostasis and (ii) maintaining non-stoichiometric homeostasis. However, the processes and mechanisms involved are complex and are currently not well understood. In the future, a combination of experimental approaches should be adopted to further explore the mechanisms of microbial communities in response to changes in substrate stoichiometry at different experimental scales, from macroscopic to microscopic (e.g., molecular studies of sugars, amino acids, proteins, RNA) features.
- (3)
- P is a critical limiting factor in nature, and elevated CO2 and increased N deposition alleviate C and N limitation in ecosystems to some extent, while possibly leading to increased MBC:MBP and MBN:MBP. Current research on microbial biomass stoichiometry in alpine ecosystems is mainly focused on MBC:MBN. In future studies, research on microbial biomass stoichiometry related to microbial P should be increased to explore the effects of P limitation on alpine microbial biomass stoichiometry in the context of global climate change factors.
- (4)
- Plant and microbial interdependence and competition in alpine ecosystems. In the context of global change, plants and microbes respond individually, while there exists a strong interaction between the two. However, in the current study, it is relatively rare to consider plant-litter-soil as a complete system. To address the above issues, the linkage with plant and litter stoichiometry can be explored under the premise of studying soil microbial stoichiometry to better understand the nutrient cycling in alpine ecosystems in the context of global climate change factors.
- (5)
- In alpine ecosystems that respond rapidly to global climate change, applying microbial stoichiometry models can extend the available data to explore the role of microbial stoichiometry in biogeochemical cycling in this rapidly changing habitat. Currently, there is no universal chemometric model suitable for alpine ecosystems, and the role of chemometrics in biogeochemical models should be emphasized in the future, thus enabling the development and improvement of coupled plant-soil-microbial stoichiometry models for alpine ecosystems.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Donhauser, J.; Frey, B. Alpine Soil Microbial Ecology in a Changing World. FEMS Microbiol. Ecol. 2018, 94, fiy099. [Google Scholar] [CrossRef] [PubMed]
- Bing, H.; Wu, Y.; Zhou, J.; Sun, H.; Luo, J.; Wang, J.; Yu, D. Stoichiometric Variation of Carbon, Nitrogen, and Phosphorus in Soils and Its Implication for Nutrient Limitation in Alpine Ecosystem of Eastern Tibetan Plateau. J. Soils Sediments 2016, 16, 405–416. [Google Scholar] [CrossRef]
- Seddon, A.W.R.; Macias-Fauria, M.; Long, P.R.; Benz, D.; Willis, K.J. Sensitivity of Global Terrestrial Ecosystems to Climate Variability. Nature 2016, 531, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Stefanidis, S.P. Ability of Different Spatial Resolution Regional Climate Model to Simulate Air Temperature in a Forest Ecosystem of Central Greece. J. Environ. Prot. Ecol. 2021, 22, 1488–1495. [Google Scholar]
- Tolika, K.; Anagnostopoulou, C.; Velikou, K.; Vagenas, C. A Comparison of the Updated Very High Resolution Model RegCM3_10km with the Previous Version RegCM3_25km over the Complex Terrain of Greece: Present and Future Projections. Theor. Appl. Climatol. 2016, 126, 715–726. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, Q.; Peng, C.; Wu, N.; Wang, Y.; Fang, X.; Gao, Y.; Zhu, D.; Yang, G.; Tian, J.; et al. The Impacts of Climate Change and Human Activities on Biogeochemical Cycles on the Qinghai-Tibetan Plateau. Glob. Change Biol. 2013, 19, 2940–2955. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, S.; Niu, B.; Chen, Q.; Wang, J.; Zhao, J.; Luo, T.; Zhang, G. Effect of Increasing Precipitation and Warming on Microbial Community in Tibetan Alpine Steppe. Environ. Res. 2020, 189, 109917. [Google Scholar] [CrossRef]
- Solomon, S.; Qin, D.; Manning, M.; Marquis, M.; Averyt, K.; Tignor, M.M.B.; Miller, J. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2007. [Google Scholar]
- Lawrence, D.M.; Slater, A.G. A Projection of Severe Near-Surface Permafrost Degradation during the 21st Century. Geophys. Res. Lett. 2005, 32, 1–5. [Google Scholar] [CrossRef]
- Bowman, W.D.; Nemergut, D.R.; McKnight, D.M.; Miller, M.P.; Williams, M.W. A Slide down a Slippery Slope—Alpine Ecosystem Responses to Nitrogen Deposition. Plant Ecol. Divers. 2015, 8, 727–738. [Google Scholar] [CrossRef]
- Han, Y.; Dong, S.; Zhao, Z.; Sha, W.; Li, S.; Shen, H.; Xiao, J.; Zhang, J.; Wu, X.; Jiang, X.; et al. Response of Soil Nutrients and Stoichiometry to Elevated Nitrogen Deposition in Alpine Grassland on the Qinghai-Tibetan Plateau. Geoderma 2019, 343, 263–268. [Google Scholar] [CrossRef]
- Xu, Z.; Wan, C.; Xiong, P.; Tang, Z.; Hu, R.; Cao, G.; Liu, Q. Initial Responses of Soil CO2 Efflux and C, N Pools to Experimental Warming in Two Contrasting Forest Ecosystems, Eastern Tibetan Plateau, China. Plant Soil 2010, 336, 183–195. [Google Scholar] [CrossRef]
- Matson, P.A.; McDowell, W.H.; Townsend, A.R.; Vitousek, P.M. The Globalization of N Deposition: Ecosystem Consequences in Tropical Environments. Biogeochemistry 1999, 46, 67–83. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, Y.; Lu, M.; Schädel, C.; Han, W. Terrestrial C:N Stoichiometry in Response to Elevated CO2 and N Addition: A Synthesis of Two Meta-Analyses. Plant Soil 2011, 343, 393–400. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, S.; Gao, Q.; Liu, S.; Zhou, H.; Ganjurjav, H.; Wang, X. Climate Change and Human Activities Altered the Diversity and Composition of Soil Microbial Community in Alpine Grasslands of the Qinghai-Tibetan Plateau. Sci. Total Environ. 2016, 562, 353–363. [Google Scholar] [CrossRef]
- Weedon, J.T.; Aerts, R.; Kowalchuk, G.A.; van Bodegom, P.M. Enzymology under Global Change: Organic Nitrogen Turnover in Alpine and Sub-Arctic Soils. Biochem. Soc. Trans. 2011, 39, 309–314. [Google Scholar] [CrossRef]
- Mooshammer, M.; Wanek, W.; Zechmeister-Boltenstern, S.; Richter, A. Stoichiometric Imbalances between Terrestrial Decomposer Communities and Their Resources: Mechanisms and Implications of Microbial Adaptations to Their Resources. Front. Microbiol. 2014, 5, 22. [Google Scholar] [CrossRef]
- Sterner, R.; Elser, J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2002. [Google Scholar]
- Finzi, A.C.; Cole, J.J.; Doney, S.C.; Holland, E.A.; Jackson, R.B. Research Frontiers in the Analysis of Coupled Biogeochemical Cycles. Front. Ecol. Environ. 2011, 9, 74–80. [Google Scholar] [CrossRef]
- Elser, J.J.; Fagan, W.F.; Denno, R.F.; Dobberfuhl, D.R.; Folarin, A.; Huberty, A.; Interlandi, S.; Kilham, S.S.; McCauley, E.; Schulz, K.L.; et al. Nutritional Constraints in Terrestrial and Freshwater Food Webs. Nature 2000, 408, 578–580. [Google Scholar] [CrossRef]
- Allison, S.D.; Vitousek, P.M. Responses of Extracellular Enzymes to Simple and Complex Nutrient Inputs. Soil Biol. Biochem. 2005, 37, 937–944. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Liptzin, D. C:N:P Stoichiometry in Soil: Is There a “Redfield Ratio” for the Microbial Biomass? Biogeochemistry 2007, 85, 235–252. [Google Scholar] [CrossRef]
- Heuck, C.; Weig, A.; Spohn, M. Soil Microbial Biomass C:N:P Stoichiometry and Microbial Use of Organic Phosphorus. Soil Biol. Biochem. 2015, 85, 119–129. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, M.; Kou, Y.; Liu, D.; Liu, Q.; Zhang, Z.; Jiang, Z.; Yin, H. Differential Effects of N Addition on the Stoichiometry of Microbes and Extracellular Enzymes in the Rhizosphere and Bulk Soils of an Alpine Shrubland. Plant Soil 2020, 449, 285–301. [Google Scholar] [CrossRef]
- Maslov, M.N.; Maslova, O.A. Nitrogen Limitation of Microbial Activity in Alpine Tundra Soils along an Environmental Gradient: Intra-Seasonal Variations and Effect of Rising Temperature. Soil Biol. Biochem. 2021, 156, 108234. [Google Scholar] [CrossRef]
- Liu, X.; Lamb, E.G.; Zhang, S. Nitrogen Addition Impacts on Soil Microbial Stoichiometry Are Driven by Changes in Plant Resource Stoichiometry Not by the Composition of Main Microbial Groups in an Alpine Meadow. Biol. Fertil. Soils 2020, 56, 261–271. [Google Scholar] [CrossRef]
- Yuan, X.; Qin, W.; Xu, H.; Zhang, Z.; Zhou, H.; Zhu, B. Sensitivity of Soil Carbon Dynamics to Nitrogen and Phosphorus Enrichment in an Alpine Meadow. Soil Biol. Biochem. 2020, 150, 107984. [Google Scholar] [CrossRef]
- He, Y.; Xu, X.; Kueffer, C.; Zhang, X.; Shi, P. Leaf Litter of a Dominant Cushion Plant Shifts Nitrogen Mineralization to Immobilization at High but Not Low Temperature in an Alpine Meadow. Plant Soil 2014, 383, 415–426. [Google Scholar] [CrossRef]
- Ma, Z.; Zhao, W.; Zhao, C.; Wang, D.; Liu, M.; Li, D.; Liu, Q. Plants Regulate the Effects of Experimental Warming on the Soil Microbial Community in an Alpine Scrub Ecosystem. PLoS ONE 2018, 13, e0195079. [Google Scholar] [CrossRef]
- Fu, G.; Shen, Z.; Zhang, X.; Zhou, Y. Response of Soil Microbial Biomass to Short-Term Experimental Warming in Alpine Meadow on the Tibetan Plateau. Appl. Soil Ecol. 2012, 61, 158–160. [Google Scholar] [CrossRef]
- Xu, W.; Yuan, W. Responses of Microbial Biomass Carbon and Nitrogen to Experimental Warming: A Meta-Analysis. Soil Biol. Biochem. 2017, 115, 265–274. [Google Scholar] [CrossRef]
- Yue, K.; Fornara, D.A.; Yang, W.; Peng, Y.; Li, Z.; Wu, F.; Peng, C. Effects of Three Global Change Drivers on Terrestrial C:N:P Stoichiometry: A Global Synthesis. Glob. Change Biol. 2017, 23, 2450–2463. [Google Scholar] [CrossRef]
- Xu, X.; Ouyang, H.; Richter, A.; Wanek, W.; Cao, G.; Kuzyakov, Y. Spatio-Temporal Variations Determine Plant-Microbe Competition for Inorganic Nitrogen in an Alpine Meadow. J. Ecol. 2011, 99, 563–571. [Google Scholar] [CrossRef]
- Hamilton, E.W.; Frank, D.A. Can Plants Stimulate Soil Microbes and Their Own Nutrient Supply? Evidence from a Grazing Tolerant Grass. Ecology 2001, 82, 2397–2402. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, J.; Xu, X.; Qin, X. Stoichiometry of Soil Microbial Biomass Carbon and Microbial Biomass Nitrogen in China’s Temperate and Alpine Grasslands. Eur. J. Soil Biol. 2017, 83, 1–8. [Google Scholar] [CrossRef]
- Fanin, N.; Fromin, N.; Buatois, B.; Hättenschwiler, S. An Experimental Test of the Hypothesis of Non-Homeostatic Consumer Stoichiometry in a Plant Litter-Microbe System. Ecol. Lett. 2013, 16, 764–772. [Google Scholar] [CrossRef] [PubMed]
- D’Alo, F.; Odriozola, I.; Baldrian, P.; Zucconi, L.; Ripa, C.; Cannone, N.; Malfasi, F.; Brancaleoni, L.; Onofri, S. Microbial Activity in Alpine Soils under Climate Change. Sci. Total Environ. 2021, 783, 147012. [Google Scholar] [CrossRef] [PubMed]
- Margesin, R.; Jud, M.; Tscherko, D.; Schinner, F. Microbial Communities and Activities in Alpine and Subalpine Soils: Communities and Activities in Alpine and Subalpine Soils. FEMS Microbiol. Ecol. 2009, 67, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.H.; Wang, C.K. Reviews and Syntheses: Soil Resources and Climate Jointly Drive Variations in Microbial Biomass Carbon and Nitrogen in China’s Forest Ecosystems. Biogeosciences 2015, 12, 6751–6760. [Google Scholar] [CrossRef]
- Nie, X.-Q.; Wang, D.; Zhou, G.-Y.; Xiong, F.; Du, Y.-G. Soil Microbial Biomass Carbon, Nitrogen, Phosphorus and Their Stoichiometric Characteristics in Alpine Wetlands in the Three Rivers Sources Region. Chin. J. Plant Ecol. 2021, 45, 996–1005. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Chen, L.-Y.; Peng, Y.-F.; Ding, J.-Z.; Li, F.; Yang, G.-B.; Kou, D.; Liu, L.; Fang, K.; Zhang, B.-B.; et al. Linking Microbial C:N:P Stoichiometry to Microbial Community and Abiotic Factors along a 3500-Km Grassland Transect on the Tibetan Plateau. Glob. Ecol. Biogeogr. 2016, 25, 1416–1427. [Google Scholar] [CrossRef]
- Wu, J. Change in Soil Microbial Biomass and Regulating Factors in an Alpine Meadow Site on the Qinghai-Tibetan Plateau. Soil Sci. Plant Nutr. 2020, 66, 177–194. [Google Scholar] [CrossRef]
- Beermann, F.; Teltewskoi, A.; Fiencke, C.; Pfeiffer, E.-M.; Kutzbach, L. Stoichiometric Analysis of Nutrient Availability (N, P, K) within Soils of Polygonal Tundra. Biogeochemistry 2015, 122, 211–227. [Google Scholar] [CrossRef]
- Chu, H.; Grogan, P. Soil Microbial Biomass, Nutrient Availability and Nitrogen Mineralization Potential among Vegetation-Types in a Low Arctic Tundra Landscape. Plant Soil 2010, 329, 411–420. [Google Scholar] [CrossRef]
- Rinnan, R.; Michelsen, A.; Bååth, E.; Jonasson, S. Mineralization and Carbon Turnover in Subarctic Heath Soil as Affected by Warming and Additional Litter. Soil Biol. Biochem. 2007, 39, 3014–3023. [Google Scholar] [CrossRef]
- Schmidt, S.K.; Lipson, D.A.; Ley, R.E.; Fisk, M.C.; West, A.E. Impacts of Chronic Nitrogen Additions Vary Seasonally and by Microbial Functional Group in Tundra Soils. Biogeochemistry 2004, 69, 1–17. [Google Scholar] [CrossRef]
- Xu, X.; Thornton, P.E.; Post, W.M. A Global Analysis of Soil Microbial Biomass Carbon, Nitrogen and Phosphorus in Terrestrial Ecosystems: Global Soil Microbial Biomass C, N and P. Glob. Ecol. Biogeogr. 2013, 22, 737–749. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, L.; Lu, X.; Yan, Y. Characteristics of Soil Nutrients and Their Ecological Stoichiometry in Different Land Use Types in the Nianchu River Basin. Land 2022, 11, 1001. [Google Scholar] [CrossRef]
- Sistla, S.A.; Asao, S.; Schimel, J.P. Detecting Microbial N-Limitation in Tussock Tundra Soil: Implications for Arctic Soil Organic Carbon Cycling. Soil Biol. Biochem. 2012, 55, 78–84. [Google Scholar] [CrossRef]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the Nitrogen Cycle: Recent Trends, Questions, and Potential Solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef]
- Basto, S.; Thompson, K.; Phoenix, G.; Sloan, V.; Leake, J.; Rees, M. Long-Term Nitrogen Deposition Depletes Grassland Seed Banks. Nat. Commun. 2015, 6, 6185. [Google Scholar] [CrossRef]
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.A.; Holland, E.A.; et al. Nitrogen Cycles: Past, Present, and Future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Jin, Y. Stoichiometric Responses of Soil Microflora to Nutrient Additions for Two Temperate Forest Soils. Biol. Fertil. Soils 2017, 53, 397–406. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, H.; Wu, Y.; Wang, J.; Zhao, Z.; Li, Y.; Qiao, L.; Chen, K.; Liu, G.; Xue, S. Direct and Indirect Influences of Long-Term Fertilization on Microbial Carbon and Nitrogen Cycles in an Alpine Grassland. Soil Biol. Biochem. 2020, 149, 107922. [Google Scholar] [CrossRef]
- Raynaud, X.; Lata, J.-C.; Leadley, P.W. Soil Microbial Loop and Nutrient Uptake by Plants: A Test Using a Coupled C:N Model of Plant–Microbial Interactions. Plant Soil 2006, 287, 95–116. [Google Scholar] [CrossRef]
- Zheng-Hu, Z.; Chuan-Kuan, W. Center for Ecological Research, Northeast Forestry University, Harbin 150040, China Responses and Regulation Mechanisms of Microbial Decomposers to Substrate Carbon, Nitrogen, and Phosphorus Stoichiometry. Chin. J. Plant Ecol. 2016, 40, 620–630. [Google Scholar] [CrossRef]
- Tian, X.-F.; Hu, H.-W.; Ding, Q.; Song, M.-H.; Xu, X.-L.; Zheng, Y.; Guo, L.-D. Influence of Nitrogen Fertilization on Soil Ammonia Oxidizer and Denitrifier Abundance, Microbial Biomass, and Enzyme Activities in an Alpine Meadow. Biol. Fertil. Soils 2014, 50, 703–713. [Google Scholar] [CrossRef]
- Wu, X.; Wang, F.; Li, T.; Fu, B.; Lv, Y.; Liu, G. Nitrogen Additions Increase N2O Emissions but Reduce Soil Respiration and CH4 Uptake during Freeze-Thaw Cycles in an Alpine Meadow. Geoderma 2020, 363, 114157. [Google Scholar] [CrossRef]
- Fu, G.; Shen, Z.-X. Response of Alpine Soils to Nitrogen Addition on the Tibetan Plateau: A Meta-Analysis. Appl. Soil Ecol. 2017, 114, 99–104. [Google Scholar] [CrossRef]
- Elser, J.J.; Dobberfuhl, D.R.; MacKay, N.A.; Schampel, J.H. Organism Size, Life History, and N:P Stoichiometry. BioScience 1996, 46, 674–684. [Google Scholar] [CrossRef]
- Manzoni, S.; Taylor, P.; Richter, A.; Porporato, A.; Ågren, G.I. Environmental and Stoichiometric Controls on Microbial Carbon-use Efficiency in Soils. New Phytol. 2012, 196, 79–91. [Google Scholar] [CrossRef]
- Chen, X.; Hao, B.; Jing, X.; He, J.-S.; Ma, W.; Zhu, B. Minor Responses of Soil Microbial Biomass, Community Structure and Enzyme Activities to Nitrogen and Phosphorus Addition in Three Grassland Ecosystems. Plant Soil 2019, 444, 21–37. [Google Scholar] [CrossRef]
- Xiong, Q.; Pan, K.; Zhang, L.; Wang, Y.; Li, W.; He, X.; Luo, H. Warming and Nitrogen Deposition Are Interactive in Shaping Surface Soil Microbial Communities near the Alpine Timberline Zone on the Eastern Qinghai–Tibet Plateau, Southwestern China. Appl. Soil Ecol. 2016, 101, 72–83. [Google Scholar] [CrossRef]
- Luo, R.; Luo, J.; Fan, J.; Liu, D.; He, J.-S.; Perveen, N.; Ding, W. Responses of Soil Microbial Communities and Functions Associated with Organic Carbon Mineralization to Nitrogen Addition in a Tibetan Grassland. Pedosphere 2020, 30, 214–225. [Google Scholar] [CrossRef]
- Wei, C.; Yu, Q.; Bai, E.; Lü, X.; Li, Q.; Xia, J.; Kardol, P.; Liang, W.; Wang, Z.; Han, X. Nitrogen Deposition Weakens Plant-Microbe Interactions in Grassland Ecosystems. Glob. Change Biol. 2013, 19, 3688–3697. [Google Scholar] [CrossRef] [PubMed]
- Strickland, M.S.; Rousk, J. Considering Fungal:Bacterial Dominance in Soils—Methods, Controls, and Ecosystem Implications. Soil Biol. Biochem. 2010, 42, 1385–1395. [Google Scholar] [CrossRef]
- Ball, B.A.; Virginia, R.A. Microbial Biomass and Respiration Responses to Nitrogen Fertilization in a Polar Desert. Polar Biol. 2014, 37, 573–585. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil Bacterial and Fungal Communities across a PH Gradient in an Arable Soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Chen, D.; Lan, Z.; Hu, S.; Bai, Y. Effects of Nitrogen Enrichment on Belowground Communities in Grassland: Relative Role of Soil Nitrogen Availability vs. Soil Acidification. Soil Biol. Biochem. 2015, 89, 99–108. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; Hobbie, S.E.; Knops, J.M.H.; Reich, P.B. Nitrogen Deposition and Plant Species Interact to Influence Soil Carbon Stabilization. Ecol. Lett. 2004, 7, 1192–1198. [Google Scholar] [CrossRef]
- Guo, H.; Ye, C.; Zhang, H.; Pan, S.; Ji, Y.; Li, Z.; Liu, M.; Zhou, X.; Du, G.; Hu, F.; et al. Long-Term Nitrogen & Phosphorus Additions Reduce Soil Microbial Respiration but Increase Its Temperature Sensitivity in a Tibetan Alpine Meadow. Soil Biol. Biochem. 2017, 113, 26–34. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Carreiro, M.M.; Repert, D.A. Allocation of Extracellular Enzymatic Activity in Relation to Litter Composition, N Deposition, and Mass Loss. Biogeochemistry 2002, 60, 1–24. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Gallo, M.E.; Lauber, C.; Waldrop, M.P.; Zak, D.R. Extracellular Enzyme Activities and Soil Organic Matter Dynamics for Northern Hardwood Forests Receiving Simulated Nitrogen Deposition. Biogeochemistry 2005, 75, 201–215. [Google Scholar] [CrossRef]
- Yu, Q.; Wilcox, K.; Pierre, K.L.; Knapp, A.K.; Han, X.; Smith, M.D. Stoichiometric Homeostasis Predicts Plant Species Dominance, Temporal Stability, and Responses to Global Change. Ecology 2015, 96, 2328–2335. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, C.; Zheng, M.; Jiang, L.; Luo, Y. Patterns and Mechanisms of Responses by Soil Microbial Communities to Nitrogen Addition. Soil Biol. Biochem. 2017, 115, 433–441. [Google Scholar] [CrossRef]
- Gutknecht, J.L.M.; Field, C.B.; Balser, T.C. Microbial Communities and Their Responses to Simulated Global Change Fluctuate Greatly over Multiple Years. Glob. Change Biol. 2012, 18, 2256–2269. [Google Scholar] [CrossRef]
- Sundqvist, M.K.; Liu, Z.; Giesler, R.; Wardle, D.A. Plant and Microbial Responses to Nitrogen and Phosphorus Addition across an Elevational Gradient in Subarctic Tundra. Ecology 2014, 95, 1819–1835. [Google Scholar] [CrossRef]
- Pietikäinen, A.; Kytöviita, M.-M.; Vuoti, U. Mycorrhiza and Seedling Establishment in a Subarctic Meadow: Effects of Fertilization and Defoliation. J. Veg. Sci. 2005, 16, 175–182. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Li, Y. Contrasting Effects of Nitrogen and Phosphorus Additions on Soil Nitrous Oxide Fluxes and Enzyme Activities in an Alpine Wetland of the Tibetan Plateau. PLoS ONE 2019, 14, e0216244. [Google Scholar] [CrossRef]
- Hrynkiewicz, K.; Baum, C.; Leinweber, P. Mycorrhizal Community Structure, Microbial Biomass P and Phosphatase Activities under Salix Polaris as Influenced by Nutrient Availability. Eur. J. Soil Biol. 2009, 45, 168–175. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.-O.; Roberts, D.; Skea, J.; Shukla, P.R.; Pirani, A.; Moufouma-Okia, W.; Péan, C.; Pidcock, R.; et al. Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Chen, J.; Luo, Y.; Xia, J.; Jiang, L.; Zhou, X.; Lu, M.; Liang, J.; Shi, Z.; Shelton, S.; Cao, J. Stronger Warming Effects on Microbial Abundances in Colder Regions. Sci. Rep. 2016, 5, 18032. [Google Scholar] [CrossRef]
- Zhang, X.-Z.; Shen, Z.-X.; Fu, G. A Meta-Analysis of the Effects of Experimental Warming on Soil Carbon and Nitrogen Dynamics on the Tibetan Plateau. Appl. Soil Ecol. 2015, 87, 32–38. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, S.Y.; Zhang, J.F.; He, X.Y.; Liu, W.J.; Zhao, Q.; Zhao, L.; Tian, C.J. Depth-Related Responses of Soil Microbial Communities Toexperimental Warming in an Alpine Meadow on the Qinghai-Tibet Plateau. Eur. J. Soil Sci. 2015, 66, 496–504. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, B.; Chen, C.; Zhang, Z.; Wang, Q.-B.; He, J.-S. Precipitation Overrides Warming in Mediating Soil Nitrogen Pools in an Alpine Grassland Ecosystem on the Tibetan Plateau. Sci. Rep. 2016, 6, 31438. [Google Scholar] [CrossRef] [PubMed]
- Ganjurjav, H.; Gao, Q.; Gornish, E.S.; Schwartz, M.W.; Liang, Y.; Cao, X.; Zhang, W.; Zhang, Y.; Li, W.; Wan, Y.; et al. Differential Response of Alpine Steppe and Alpine Meadow to Climate Warming in the Central Qinghai–Tibetan Plateau. Agric. For. Meteorol. 2016, 223, 233–240. [Google Scholar] [CrossRef]
- Bai, E.; Li, S.; Xu, W.; Li, W.; Dai, W.; Jiang, P. A Meta-analysis of Experimental Warming Effects on Terrestrial Nitrogen Pools and Dynamics. New Phytol. 2013, 199, 441–451. [Google Scholar] [CrossRef]
- Wang, X.; Dong, S.; Gao, Q.; Zhou, H.; Liu, S.; Su, X.; Li, Y. Effects of Short-Term and Long-Term Warming on Soil Nutrients, Microbial Biomass and Enzyme Activities in an Alpine Meadow on the Qinghai-Tibet Plateau of China. Soil Biol. Biochem. 2014, 76, 140–142. [Google Scholar] [CrossRef]
- Yin, H.; Chen, Z.; Liu, Q. Effects of Experimental Warming on Soil N Transformations of Two Coniferous Species, Eastern Tibetan Plateau, China. Soil Biol. Biochem. 2012, 50, 77–84. [Google Scholar] [CrossRef]
- Rui, J.; Li, J.; Wang, S.; An, J.; Liu, W.; Lin, Q.; Yang, Y.; He, Z.; Li, X. Responses of Bacterial Communities to Simulated Climate Changes in Alpine Meadow Soil of the Qinghai-Tibet Plateau. Appl. Environ. Microbiol. 2015, 81, 6070–6077. [Google Scholar] [CrossRef]
- Rinnan, R.; Michelsen, A.; Bååth, E.; Jonasson, S. Fifteen Years of Climate Change Manipulations Alter Soil Microbial Communities in a Subarctic Heath Ecosystem. Glob. Change Biol. 2007, 13, 28–39. [Google Scholar] [CrossRef]
- Van Diepen, L.T.A.; Lilleskov, E.A.; Pregitzer, K.S.; Miller, R.M. Decline of Arbuscular Mycorrhizal Fungi in Northern Hardwood Forests Exposed to Chronic Nitrogen Additions. New Phytol. 2007, 176, 175–183. [Google Scholar] [CrossRef]
- Xiong, J.; Sun, H.; Peng, F.; Zhang, H.; Xue, X.; Gibbons, S.M.; Gilbert, J.A.; Chu, H. Characterizing Changes in Soil Bacterial Community Structure in Response to Short-Term Warming. FEMS Microbiol. Ecol. 2014, 89, 281–292. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Wang, M. Linkages of C: N: P Stoichiometry between Soil and Leaf and Their Response to Climatic Factors along Altitudinal Gradients. J. Soils Sediments 2019, 19, 1820–1829. [Google Scholar] [CrossRef]
- Dawes, M.A.; Schleppi, P.; Hättenschwiler, S.; Rixen, C.; Hagedorn, F. Soil Warming Opens the Nitrogen Cycle at the Alpine Treeline. Glob. Change Biol. 2017, 23, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Xue, X.; You, Q.; Xu, M.; Chen, X.; Guo, J.; Wang, T. Intensified Plant N and C Pool with More Available Nitrogen under Experimental Warming in an Alpine Meadow Ecosystem. Ecol. Evol. 2016, 6, 8546–8555. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, I.K.; Jonasson, S.; Shaver, G.R.; Michelsen, A.; Nordin, A. Mineralization and Distribution of Nutrients in Plants and Microbes in Four Arctic Ecosystems: Responses to Warming. Plant Soil 2002, 242, 93–106. [Google Scholar] [CrossRef]
- Zhang, W.; Parker, K.M.; Luo, Y.; Wan, S.; Wallace, L.L.; Hu, S. Soil Microbial Responses to Experimental Warming and Clipping in a Tallgrass Prairie. Glob. Change Biol. 2005, 11, 266–277. [Google Scholar] [CrossRef]
- Sistla, S.A.; Schimel, J.P. Seasonal Patterns of Microbial Extracellular Enzyme Activities in an Arctic Tundra Soil: Identifying Direct and Indirect Effects of Long-Term Summer Warming. Soil Biol. Biochem. 2013, 66, 119–129. [Google Scholar] [CrossRef]
- Salazar, A.; Rousk, K.; Jónsdóttir, I.S.; Bellenger, J.; Andrésson, Ó.S. Faster Nitrogen Cycling and More Fungal and Root Biomass in Cold Ecosystems under Experimental Warming: A Meta-analysis. Ecology 2020, 101, e02938. [Google Scholar] [CrossRef]
- Hawkes, C.V.; Hartley, I.P.; Ineson, P.; Fitter, A.H. Soil Temperature Affects Carbon Allocation within Arbuscular Mycorrhizal Networks and Carbon Transport from Plant to Fungus. Glob. Change Biol. 2008, 14, 1181–1190. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, H.; Chen, W.; Wu, Y.; Qiao, L.; Yan, Z.; Liu, G.; Xue, S. Long-Term Warming Does Not Affect Soil Ecoenzyme Activity and Original Microbial Nutrient Limitation on the Qinghai-Tibet Plateau. Soil Ecol. Lett. 2021, 4, 383–398. [Google Scholar] [CrossRef]
- Barcenas-Moreno, G.; Gomez-Brandon, M.; Rousk, J.; Baath, E. Adaptation of Soil Microbial Communities to Temperature: Comparison of Fungi and Bacteria in a Laboratory Experiment. Glob. Change Biol. 2009, 15, 2950–2957. [Google Scholar] [CrossRef]
- Romero-Olivares, A.L.; Allison, S.D.; Treseder, K.K. Soil Microbes and Their Response to Experimental Warming over Time: A Meta-Analysis of Field Studies. Soil Biol. Biochem. 2017, 107, 32–40. [Google Scholar] [CrossRef]
- Yu, C.-Q.; Shen, Z.-X.; Zhang, X.-Z.; Sun, W.; Fu, G. Response of Soil C and N, Dissolved Organic C and N, and Inorganic N to Short-Term Experimental Warming in an Alpine Meadow on the Tibetan Plateau. Sci. World J. 2014, 2014, 152576. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Wang, Y.; Chung, H.; Mi, Z.; Wang, S.; Zeng, H.; He, J.-S. No Temperature Acclimation of Soil Extracellular Enzymes to Experimental Warming in an Alpine Grassland Ecosystem on the Tibetan Plateau. Biogeochemistry 2014, 117, 39–54. [Google Scholar] [CrossRef]
- Bradford, M.A. Thermal Adaptation of Decomposer Communities in Warming Soils. Front. Microbiol. 2013, 4, 333. [Google Scholar] [CrossRef]
- Peng, F.; Zhang, W.; Lai, C.; Li, C.; You, Q.; Xue, X.; Ma, S.; Tsunekawa, A. Legacy Effect of Warming on the Heterotrophic Respiration of Alpine Grassland on the Qinghai-Tibet Plateau. Appl. Soil Ecol. 2021, 166, 104093. [Google Scholar] [CrossRef]
- Bradford, M.A.; Davies, C.A.; Frey, S.D.; Maddox, T.R.; Melillo, J.M.; Mohan, J.E.; Reynolds, J.F.; Treseder, K.K.; Wallenstein, M.D. Thermal Adaptation of Soil Microbial Respiration to Elevated Temperature. Ecol. Lett. 2008, 11, 1316–1327. [Google Scholar] [CrossRef]
- Craine, J.M.; Fierer, N.; McLauchlan, K.K. Widespread Coupling between the Rate and Temperature Sensitivity of Organic Matter Decay. Nat. Geosci. 2010, 3, 854–857. [Google Scholar] [CrossRef]
- Romero-Olivares, A.L.; Melendrez-Carballo, G.; Lago-Leston, A.; Treseder, K.K. Soil Metatranscriptomes Under Long-Term Experimental Warming and Drying: Fungi Allocate Resources to Cell Metabolic Maintenance Rather Than Decay. Front. Microbiol. 2019, 10, 1914. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, J.; Yuan, X.; Zhu, B. Effects of Warming on Carbon and Nitrogen Cycling in Alpine Grassland Ecosystems on the Tibetan Plateau: A Meta-Analysis. Geoderma 2020, 370, 114363. [Google Scholar] [CrossRef]
- Stark, S.; Ylänne, H.; Tolvanen, A. Long-Term Warming Alters Soil and Enzymatic N:P Stoichiometry in Subarctic Tundra. Soil Biol. Biochem. 2018, 124, 184–188. [Google Scholar] [CrossRef]
- McLaren, J.R.; Buckeridge, K.M. Enhanced Plant Leaf P and Unchanged Soil P Stocks after a Quarter Century of Warming in the Arctic Tundra. Ecosphere 2021, 12, e03838. [Google Scholar] [CrossRef]
- Schimel, J. The Implications of Exoenzyme Activity on Microbial Carbon and Nitrogen Limitation in Soil: A Theoretical Model. Soil Biol. Biochem. 2003, 35, 549–563. [Google Scholar] [CrossRef]
- Huntington, T.G. Evidence for Intensification of the Global Water Cycle: Review and Synthesis. J. Hydrol. 2006, 319, 83–95. [Google Scholar] [CrossRef]
- Xu, S.; Geng, W.; Sayer, E.J.; Zhou, G.; Zhou, P.; Liu, C. Soil Microbial Biomass and Community Responses to Experimental Precipitation Change: A Meta-Analysis. Soil Ecol. Lett. 2020, 2, 93–103. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Sun, G.; Luo, P.; Mou, C.-X.; Wang, J. A Study of Soil-Dynamics Based on a Simulated Drought in an Alpine Meadow on the Tibetan Plateau. J. Mt. Sci. 2013, 10, 833–844. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Chen, H.Y.H.; Luo, X.; Qiu, N.; Ruan, H. Asymmetric Responses of Terrestrial C:N:P Stoichiometry to Precipitation Change. Glob. Ecol. Biogeogr. 2021, 30, 1724–1735. [Google Scholar] [CrossRef]
- Zeglin, L.H.; Bottomley, P.J.; Jumpponen, A.; Rice, C.W.; Arango, M.; Lindsley, A.; McGowan, A.; Mfombep, P.; Myrold, D.D. Altered Precipitation Regime Affects the Function and Composition of Soil Microbial Communities on Multiple Time Scales. Ecology 2013, 94, 2334–2345. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, S.; Schimel, J.P.; Porporato, A. Responses of Soil Microbial Communities to Water Stress: Results from a Meta-analysis. Ecology 2012, 93, 930–938. [Google Scholar] [CrossRef]
- Evans, S.E.; Wallenstein, M.D. Soil Microbial Community Response to Drying and Rewetting Stress: Does Historical Precipitation Regime Matter? Biogeochemistry 2012, 109, 101–116. [Google Scholar] [CrossRef]
- Schimel, J.; Balser, T.C.; Wallenstein, M. Microbial Stress-Response Physiology and Its Implications for Ecosystem Function. Ecology 2007, 88, 1386–1394. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Ding, C.; Liu, Y.; Wu, K.; Jiang, F.; Su, D. Water Addition Promotes Vegetation Recovery of Degraded Alpine Meadows by Regulating Soil Enzyme Activity and Nutrients in the Qinghai-Tibetan Plateau. Ecol. Eng. 2020, 158, 106047. [Google Scholar] [CrossRef]
- Li, H.; Xu, Z.; Yang, S.; Li, X.; Top, E.M.; Wang, R.; Zhang, Y.; Cai, J.; Yao, F.; Han, X.; et al. Responses of Soil Bacterial Communities to Nitrogen Deposition and Precipitation Increment Are Closely Linked with Aboveground Community Variation. Microb. Ecol. 2016, 71, 974–989. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, J.; Li, G.; Yan, L.; Wei, X. Effects of Rainfall Frequency on Soil Labile Carbon Fractions in a Wet Meadow on the Qinghai-Tibet Plateau. J. Soils Sediments 2022, 22, 1489–1499. [Google Scholar] [CrossRef]
- Wood, J.M.; Bremer, E.; Csonka, L.N.; Kraemer, R.; Poolman, B.; van der Heide, T.; Smith, L.T. Osmosensing and Osmoregulatory Compatible Solute Accumulation by Bacteria. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001, 130, 437–460. [Google Scholar] [CrossRef]
- Placella, S.A.; Brodie, E.L.; Firestone, M.K. Rainfall-Induced Carbon Dioxide Pulses Result from Sequential Resuscitation of Phylogenetically Clustered Microbial Groups. Proc. Natl. Acad. Sci. USA 2012, 109, 10931–10936. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, S.; Gao, Q.; Liu, S.; Ganjurjav, H.; Wang, X.; Su, X.; Wu, X. Soil Bacterial and Fungal Diversity Differently Correlated with Soil Biochemistry in Alpine Grassland Ecosystems in Response to Environmental Changes. Sci. Rep. 2017, 7, 43077. [Google Scholar] [CrossRef]
- Li, P.; Yang, Y.; Han, W.; Fang, J. Global Patterns of Soil Microbial Nitrogen and Phosphorus Stoichiometry in Forest Ecosystems: Stoichiometric Patterns in Microbial System. Glob. Ecol. Biogeogr. 2014, 23, 979–987. [Google Scholar] [CrossRef]
- Warren, C.R. Soil Microbial Populations Substitute Phospholipids with Betaine Lipids in Response to Low P Availability. Soil Biol. Biochem. 2020, 140, 107655. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.I.; et al. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021. [Google Scholar]
- Drigo, B.; Kowalchuk, G.A.; van Veen, J.A. Climate Change Goes Underground: Effects of Elevated Atmospheric CO2 on Microbial Community Structure and Activities in the Rhizosphere. Biol. Fertil. Soils 2008, 44, 667–679. [Google Scholar] [CrossRef]
- Pausch, J.; Kuzyakov, Y. Carbon Input by Roots into the Soil: Quantification of Rhizodeposition from Root to Ecosystem Scale. Glob. Change Biol. 2018, 24, 1–12. [Google Scholar] [CrossRef]
- Albert, K.R.; Ro-Poulsen, H.; Mikkelsen, T.N.; Michelsen, A.; van der Linden, L.; Beier, C. Interactive Effects of Elevated CO2, Warming, and Drought on Photosynthesis of Deschampsia flexuosa in a Temperate Heath Ecosystem. J. Exp. Bot. 2011, 62, 4253–4266. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.C.; Angelard, C.; Sanders, I.R.; Kiers, E.T. Predicting Community and Ecosystem Outcomes of Mycorrhizal Responses to Global Change. Ecol. Lett. 2013, 16, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Hagedorn, F.; Hiltbrunner, D.; Streit, K.; Ekblad, A.; Lindahl, B.; Miltner, A.; Frey, B.; Handa, I.T.; Haettenschwiler, S. Nine Years of CO2 Enrichment at the Alpine Treeline Stimulates Soil Respiration but Does Not Alter Soil Microbial Communities. Soil Biol. Biochem. 2013, 57, 390–400. [Google Scholar] [CrossRef]
- Niklaus, P.A.; Korner, C. Responses of Soil Microbiota of a Late Successional Alpine Grassland to Long Term CO2 Enrichment. Plant Soil 1996, 184, 219–229. [Google Scholar] [CrossRef]
- Gifford, R.M.; Barrett, D.J.; Lutze, J.L. The Effects of Elevated [CO2] on the C:N and C:P Mass Ratios of Plant Tissues. Plant Soil 2000, 224, 1–14. [Google Scholar] [CrossRef]
- Bu, X.; Ruan, H.; Wang, L.; Ma, W.; Ding, J.; Yu, X. Soil Organic Matter in Density Fractions as Related to Vegetation Changes along an Altitude Gradient in the Wuyi Mountains, Southeastern China. Appl. Soil Ecol. 2012, 52, 42–47. [Google Scholar] [CrossRef]
- Morgan, J.A.; LeCain, D.R.; Pendall, E.; Blumenthal, D.M.; Kimball, B.A.; Carrillo, Y.; Williams, D.G.; Heisler-White, J.; Dijkstra, F.A.; West, M. C4 Grasses Prosper as Carbon Dioxide Eliminates Desiccation in Warmed Semi-Arid Grassland. Nature 2011, 476, 202–205. [Google Scholar] [CrossRef]
- Austin, A.T.; Yahdjian, L.; Stark, J.M.; Belnap, J.; Porporato, A.; Norton, U.; Ravetta, D.A.; Schaeffer, S.M. Water Pulses and Biogeochemical Cycles in Arid and Semiarid Ecosystems. Oecologia 2004, 141, 221–235. [Google Scholar] [CrossRef]


| Region | Ecosystem Type | MBC:MBN | MBC:MBP | MBN:MBP | MBC:MBN:MBP | Reference |
|---|---|---|---|---|---|---|
| Tibet Plateau | alpine meadow | 14.53 | 117.02 | 8.13 | 118:8:1 | [40] |
| 10.23 | 48.0 | 4.68 | 47.9:4.68:1 | [41] | ||
| 12.78 | [42] | |||||
| alpine wetland | 50.56 | 184.89 | 5.42 | 275:5:1 | [40] | |
| alpine steppe | 13.49 | 80.0 | 6.03 | 81.3:6.03:1 | [41] | |
| Polar | Polygonal tundra | 9.7 | [43] | |||
| low arctic tundra | 11.8 | 22.93 | 1.97 | [44] | ||
| Subarctic | tundra | 14.4 | [45] | |||
| The Rocky Mountains | tundra | 8.3 | [46] | |||
| Global average | 7.6 | 42.4 | 5.6 | 42:6:1 | [47] | |
| 8.6 | 59.5 | 6.9 | 60:7:1 | [22] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Gao, Y. Global Climate Change Effects on Soil Microbial Biomass Stoichiometry in Alpine Ecosystems. Land 2022, 11, 1661. https://doi.org/10.3390/land11101661
Chen L, Gao Y. Global Climate Change Effects on Soil Microbial Biomass Stoichiometry in Alpine Ecosystems. Land. 2022; 11(10):1661. https://doi.org/10.3390/land11101661
Chicago/Turabian StyleChen, Luyun, and Yongheng Gao. 2022. "Global Climate Change Effects on Soil Microbial Biomass Stoichiometry in Alpine Ecosystems" Land 11, no. 10: 1661. https://doi.org/10.3390/land11101661
APA StyleChen, L., & Gao, Y. (2022). Global Climate Change Effects on Soil Microbial Biomass Stoichiometry in Alpine Ecosystems. Land, 11(10), 1661. https://doi.org/10.3390/land11101661






