Spatial Pattern and Key Environmental Determinants of Vegetation in Sand Mining and Non-Mining Sites along the Panjkora River Basin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Vegetation Sampling and Diversity Indices
2.3. Environmental Variables and Soil Analysis
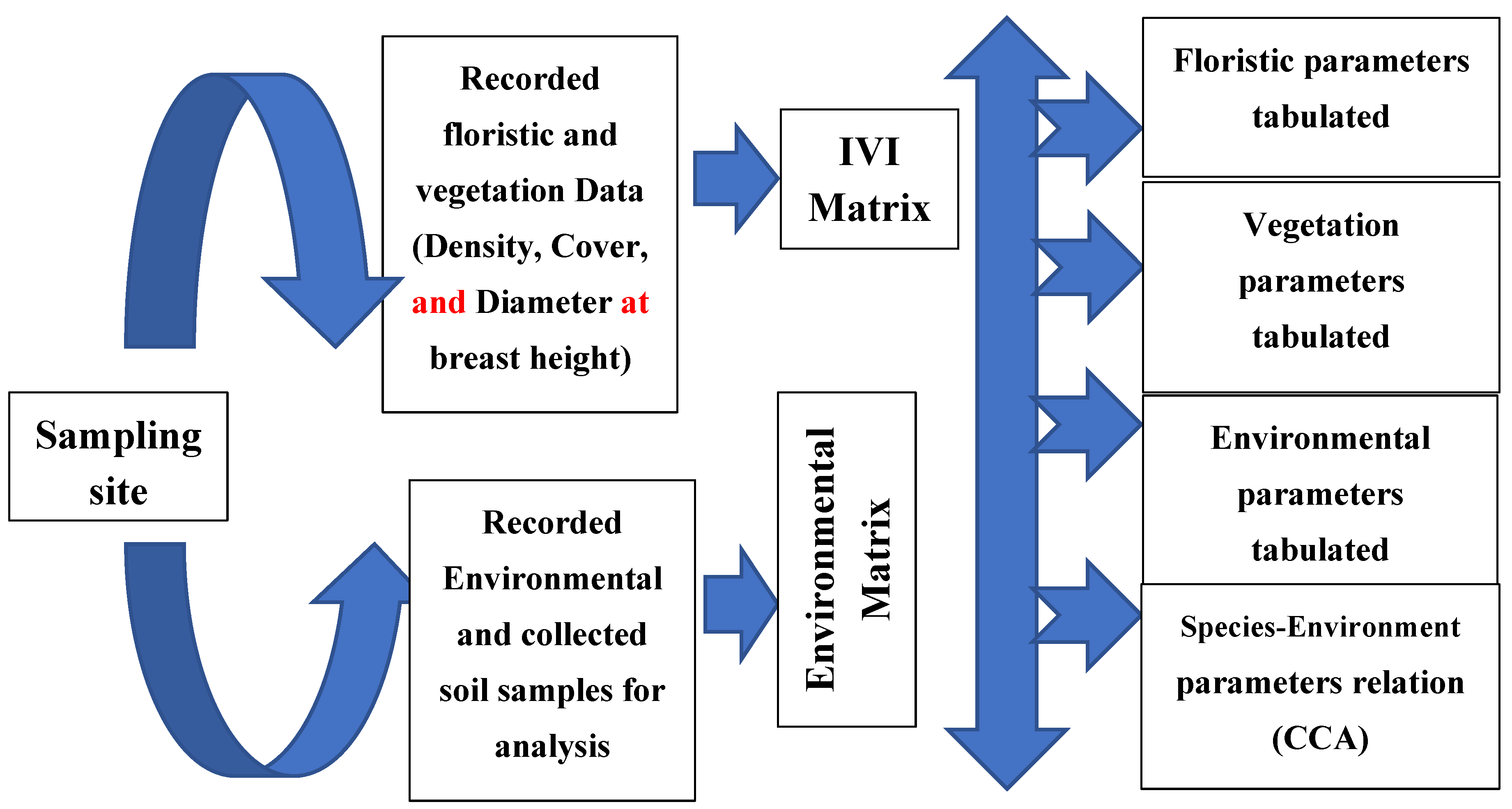
2.4. Data Analysis
3. Results
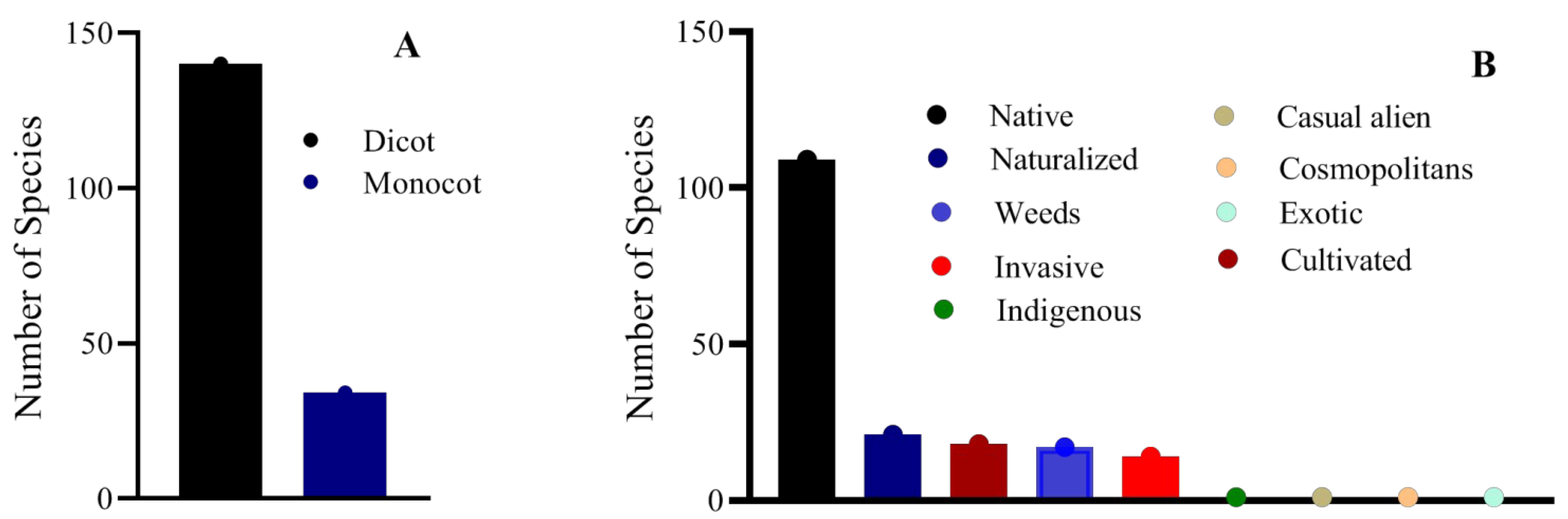
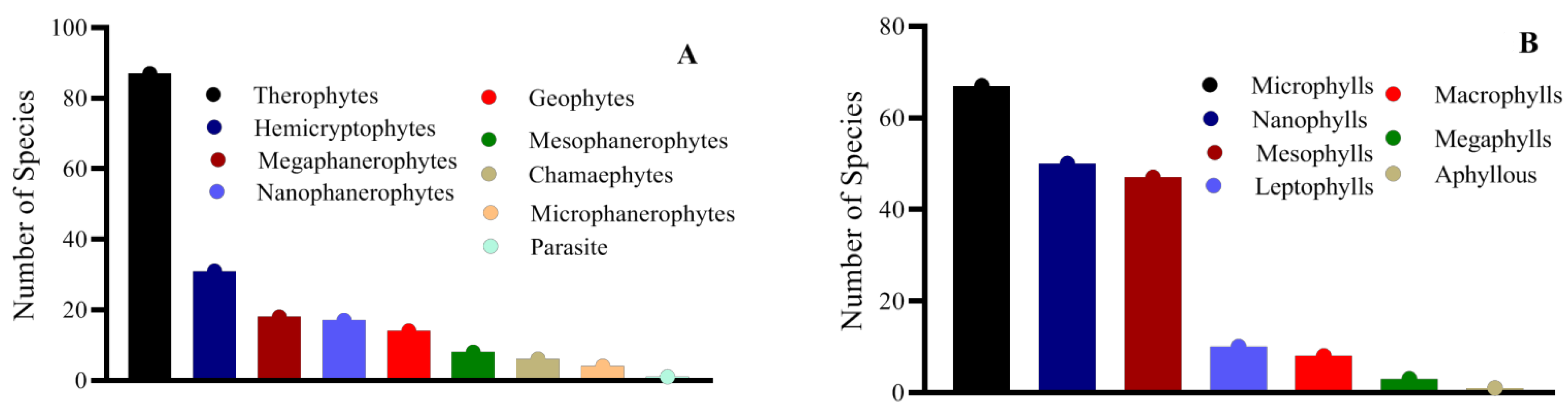
3.1. Floristic and Phytosociological Diversity
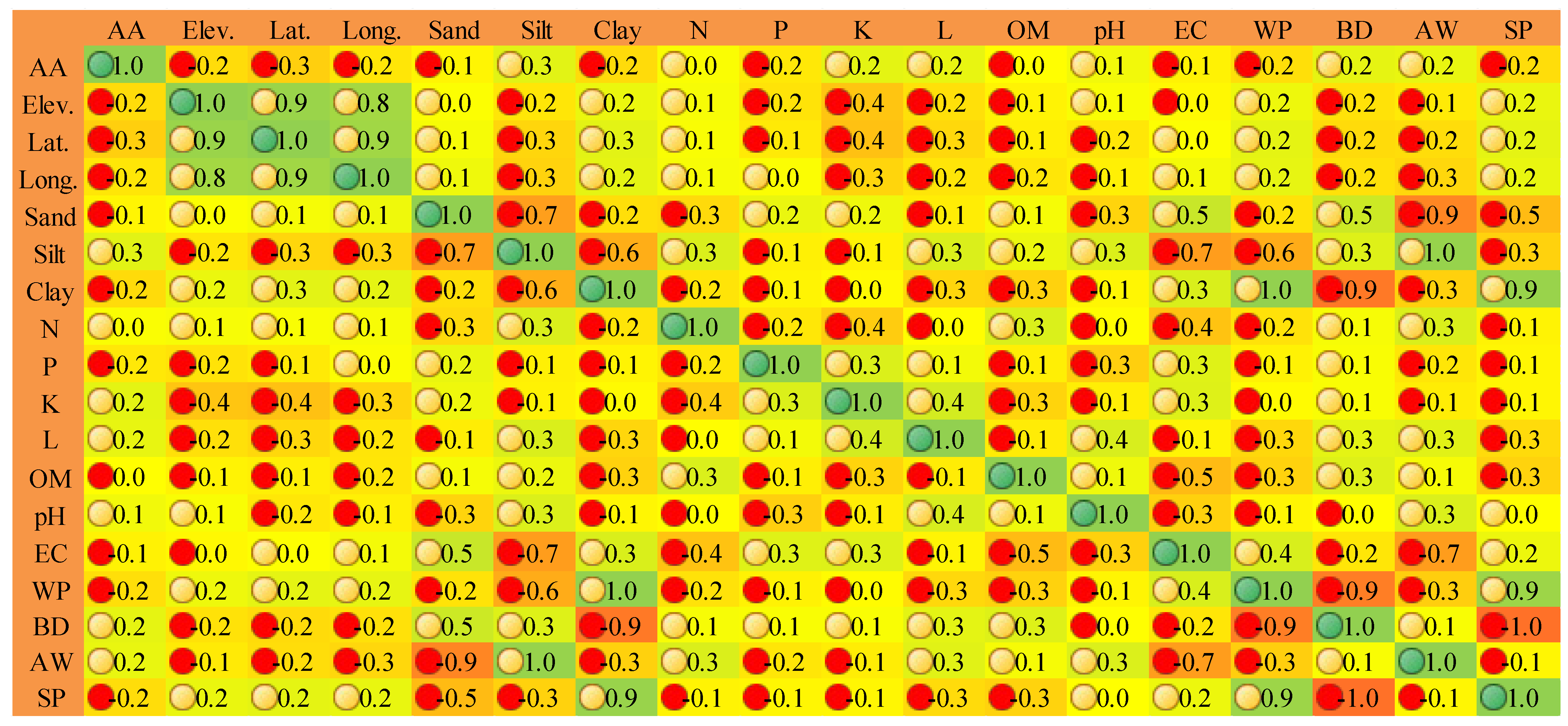
3.2. Environmental Variables and Its Influence
3.3. Species-Environment Correlation
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, S.A.; Khan, S.M.; Ullah, Z.; Ahmad, Z.; Alam, N.; Shah, S.N.; Khan, R.; Zada, M. Phytogeographic classification using multivariate approach; a case study from the Jambil Valley Swat, Pakistan. Pak. J. Bot. 2020, 52, 279–290. [Google Scholar] [CrossRef]
- Teixeira, A.M.C.; Pinto, J.R.R.; Amaral, A.G.; Munhoz, C.B.R. Angiosperm species of “Cerrado” sensu stricto in Terra Ronca State Park, Brazil: Floristics, phytogeography and conservation. Braz. J. Bot. 2017, 40, 225–234. [Google Scholar] [CrossRef]
- Mota, G.S.; Luz, G.R.; Mota, N.M.; Coutinho, E.S.; Veloso, M.d.D.M.; Fernandes, G.W.; Nunes, Y.R.F. Changes in species composition, vegetation structure, and life forms along an altitudinal gradient of rupestrian grasslands in south-eastern Brazil. Flora 2018, 238, 32–42. [Google Scholar] [CrossRef]
- Zeb, S.A.; Khan, S.M.; Ahmad, Z. Phytogeographic elements and vegetation along the river Panjkora-Classification and ordination studies from the Hindu Kush Mountains range. Bot. Rev. 2021, 87, 518–542. [Google Scholar] [CrossRef]
- Gashaw, T.; Tulu, T.; Argaw, M.; Worqlul, A.W. Modeling the hydrological impacts of land use/land cover changes in the Andassa watershed, Blue Nile Basin, Ethiopia. Sci. Total Environ. 2018, 619, 1394–1408. [Google Scholar] [CrossRef]
- Bejarano, M.D.; Jansson, R.; Nilsson, C. The effects of hydropeaking on riverine plants: A review. Biol. Rev. 2018, 93, 658–673. [Google Scholar] [CrossRef]
- Meragiaw, M.; Woldu, Z.; Martinsen, V.; Singh, B.R. Woody species composition and diversity of riparian vegetation along the Walga River, Southwestern Ethiopia. PLoS ONE 2018, 13, e0204733. [Google Scholar] [CrossRef]
- Sunil, C.; Somashekar, R.K.; Nagaraja, B.C. Diversity and composition of riparian vegetation across forest and agro-ecosystem landscapes of river Cauvery, southern India. Trop. Ecol. 2016, 57, 343–354. [Google Scholar]
- Njue, N.; Koech, E.; Hitimana, J.; Sirmah, P. Influence of land use activities on riparian vegetation, soil and water quality: An indicator of biodiversity loss, South West Mau Forest, Kenya. Open J. For. 2016, 6, 373–385. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, A.L.; Nilsson, C.; Jansson, R. Restoration effects on germination and survival of plants in the riparian zone: A phytometer study. Plant Ecol. 2015, 216, 465–477. [Google Scholar] [CrossRef]
- Corenblit, D.; Davies, N.S.; Steiger, J.; Gibling, M.R.; Bornette, G. Considering river structure and stability in the light of evolution: Feedbacks between riparian vegetation and hydrogeomorphology. Earth Surf. Process. Landf. 2015, 40, 189–207. [Google Scholar] [CrossRef]
- Caiafa, A.N.; Martins, F.R. Taxonomic identification, sampling methods, and minimum size of the tree sampled: Implications and perspectives for studies in the Brazilian Atlantic Rainforest. Funct. Ecosyst. Communities 2007, 1, 95–104. [Google Scholar]
- Arruda, D.M.; Ferreira-Junior, W.G.; Duque-Brasil, R.; Schaefer, C.E. Phytogeographical patterns of dry forests sensu stricto in northern Minas Gerais State, Brazil. An. Acad. Bras. Ciências 2013, 85, 623–634. [Google Scholar] [CrossRef] [Green Version]
- De Azevedo, I.F.P.; Nunes, Y.R.F.; de Avila, M.A.; da Silva, D.L.; Fernandes, G.W.; Veloso, R.B. Phenology of riparian tree species in a transitional region in southeastern Brazil. Braz. J. Bot. 2014, 37, 47–59. [Google Scholar] [CrossRef]
- Rodrigues, P.M.S.; Azevedo, I.; Veloso, M.; Santos, R.; Menino, G.; Nunes, Y.; Fernandes, G. Riqueza Florística da Vegetação ciliar do rio Pandeiros, norte de Minas Gerais. MG. Biota 2009, 2, 18–35. [Google Scholar]
- Bennett, A.F.; Nimmo, D.G.; Radford, J.Q. Riparian vegetation has disproportionate benefits for landscape-scale conservation of woodland birds in highly modified environments. J. Appl. Ecol. 2014, 51, 514–523. [Google Scholar] [CrossRef]
- Purnomo, M.; Utomo, M.R.; Pertiwi, V.i.A.; Laili, F.; Pariasa, I.I.; Riyanto, S.; Andriatmoko, N.D.; Handono, S.Y. Resistance to mining and adaptation of Indonesia farmer’s household to economic vulnerability of small scale sand mining activities. Local Environ. 2021, 26, 1498–1511. [Google Scholar] [CrossRef]
- Peduzzi, P. Sand, rarer than one thinks. Environ. Dev. 2014, 11, 208–218. [Google Scholar]
- Ikhsan, J.; Rezanaldy, A.; Rozainy, M.Z.M.R. Analysis of Sand Mining Impacts on Riverbed in the Downstream of the Progo River, Indonesia. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1144, 012065. [Google Scholar] [CrossRef]
- Saviour, M.N. Environmental impact of soil and sand mining: A review. Int. J. Sci. Environ. Technol. 2012, 1, 125–134. [Google Scholar]
- Ahmad, M.S.A.; Ashraf, M.; Ali, Q. Soil salinity as a selection pressure is a key determinant for the evolution of salt tolerance in Blue Panicgrass (Panicum antidotale Retz.). Flora-Morphol. Distrib. Funct. Ecol. Plants 2010, 205, 37–45. [Google Scholar] [CrossRef]
- Mtero, F. Rural livelihoods, large-scale mining and agrarian change in Mapela, Limpopo, South Africa. Resour. Policy 2017, 53, 190–200. [Google Scholar] [CrossRef]
- Bhatawdekar, R.M.; Singh, T.N.; Tonnizam Mohamad, E.; Armaghani, D.J.; Binti Abang Hasbollah, D.Z. River sand mining vis a vis manufactured sand for sustainability. In Proceedings of the International Conference on Innovations for Sustainable and Responsible Mining, Hanoi, Vietnam, 15–17 October 2020; pp. 143–169. [Google Scholar]
- Madeira, B.G.; Espírito-Santo, M.M.; Neto, S.D.Â.; Nunes, Y.R.; Arturo Sánchez Azofeifa, G.; Wilson Fernandes, G.; Quesada, M. Changes in tree and liana communities along a successional gradient in a tropical dry forest in south-eastern Brazil. In Forest Ecology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 291–304. [Google Scholar]
- Ullah, K.; Zhang, J. GIS-based flood hazard mapping using relative frequency ratio method: A case study of Panjkora River Basin, eastern Hindu Kush, Pakistan. PLoS ONE 2020, 15, e0229153. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, D. Khyber Pakhtunkhwa State of the Environment; Environmental Protection Agency, Government of Khyber Pakhtunkhwa: Peshawar, Pakistan, 2012. [Google Scholar]
- Ali, A.; Khan, T.A.; Ahmad, S. Analysis of climate data of Khyber Pakhtunkhwa, Pakistan. Int. Res. J. Eng. Technol. 2018, 5, 4266–4282. [Google Scholar]
- Curtis, J.T.; Mcintosh, R.P. The interrelations of certain analytic and synthetic phytosociological characters. Ecology 1950, 31, 434–455. [Google Scholar] [CrossRef]
- Martínez-Falcón, A.P.; Zurita, G.A.; Ortega-Martínez, I.J.; Moreno, C.E. Populations and assemblages living on the edge: Dung beetles responses to forests-pasture ecotones. PeerJ 2018, 6, e6148. [Google Scholar] [CrossRef]
- Ali, S.I.; Qaiser, M. A phytogeographical analysis of the phanerogams of Pakistan and Kashmir. Proc. R. Soc. Edinb. Sect. B Biol. Sci. 1986, 89, 89–101. [Google Scholar] [CrossRef]
- Aeschimann, D.; Rasolofo, N.; Theurillat, J.-P. Analyse de la flore des Alpes. 1: Historique et biodiversité. Candollea 2011, 66, 27–55. [Google Scholar] [CrossRef]
- Maan, I.; Kaur, A.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Exotic avenue plantations turning foe: Invasive potential, distribution and impact of Broussonetia papyrifera in Chandigarh, India. Urban For. Urban Green. 2021, 59, 127010. [Google Scholar] [CrossRef]
- Castillón, E.E.; Arévalo, J.R.; Quintanilla, J.Á.V.; Rodríguez, M.M.S.; Encina-Domínguez, J.A.; Rodríguez, H.G.; Ayala, C.M.C. Classification and ordination of main plant communities along an altitudinal gradient in the arid and temperate climates of northeastern Mexico. Sci. Nat. 2015, 102, 59. [Google Scholar] [CrossRef]
- Kamrani, A.; Jalili, A.; Naqinezhad, A.; Attar, F.; Maassoumi, A.A.; Shaw, S.C. Relationships between environmental variables and vegetation across mountain wetland sites, N. Iran. Biologia 2011, 66, 76–87. [Google Scholar] [CrossRef]
- Reis, A.T.; Coelho, J.P.; Rucandio, I.; Davidson, C.M.; Duarte, A.C.; Pereira, E. Thermo-desorption: A valid tool for mercury speciation in soils and sediments? Geoderma 2015, 237, 98–104. [Google Scholar] [CrossRef] [Green Version]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. Methods Soil Anal. Part 3 Chem. Methods 1996, 5, 961–1010. [Google Scholar]
- Yadav, R.; Tomar, S.; Sharma, U. Output: Input ratios and apparent balances of N, P and K inputs in a rice-wheat system in North-West India. Exp. Agric. 2002, 38, 457–468. [Google Scholar] [CrossRef]
- Robin, V.; Cuisinier, O.; Masrouri, F.; Javadi, A. Chemo-mechanical modelling of lime treated soils. Appl. Clay Sci. 2014, 95, 211–219. [Google Scholar] [CrossRef] [Green Version]
- Saxton, K.; Rawls, W.J.; Romberger, J.S.; Papendick, R. Estimating generalized soil-water characteristics from texture. Soil Sci. Soc. Am. J. 1986, 50, 1031–1036. [Google Scholar] [CrossRef]
- Malik, Z.; Hussain, F.; Malik, N. Life form and leaf size spectra of plant communities Harbouring Ganga Chotti and Bedori Hills during 1999–2000. Int. J. Agric. Biol. 2007, 9, 833–838. [Google Scholar]
- Khan, M.; Hussain, F.; Musharaf, S. Biological characteristics of plant species in Tehsil Takht-e-Nasrati Pakistan. J. Biodivers. Environ. Sci. 2012, 2, 42–47. [Google Scholar]
- Biondi, E.; Allegrezza, M.; Casavecchia, S.; Galdenzi, D.; Gasparri, R.; Pesaresi, S.; Soriano, P.; Tesei, G.; Blasi, C. New insight on Mediterranean and sub-Mediterranean syntaxa included in the Vegetation Prodrome of Italy. Flora Mediterr. 2015, 25, 77–102. [Google Scholar]
- Barua, N.; Aziz, M.A.I.; Tareq, A.M.; Sayeed, M.A.; Alam, N.; ul Alam, N.; Uddin, M.A.; Lyzu, C.; Emran, T.B. In vivo and in vitro evaluation of pharmacological activities of Adenia trilobata (Roxb.). Biochem. Biophys. Rep. 2020, 23, 100772. [Google Scholar] [CrossRef]
- Devi, K.; Singh, H.; Gupta, A. Life form in hill forest of Manipur, North-East, India. J. Agroecol. Nat. Resour. Manag. 2014, 1, 7–13. [Google Scholar]
- Díaz, S.; Kattge, J.; Cornelissen, J.H.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Colin Prentice, I. The global spectrum of plant form and function. Nature 2016, 529, 167–171. [Google Scholar] [CrossRef]
- Khwaja, M.A.; Saeed, S.; Urooj, M. Preliminary Environmental Impact Assessment (EIA) Study of China-Pakistan Economic Corridor (CPEC) Northern Route Road Construction Activities in Khyber Pakhtunkhwa (KPK), Pakistan; Sustainable Development Policy Institute: Islamabad, Pakistan, 2018. [Google Scholar]
- Khera, N.; Kumar, A.; Ram, J.; Tewari, A. Plant biodiversity assessment in relation to disturbances in mid-elevational forest of Central Himalaya, India. Trop. Ecol. 2001, 42, 83–95. [Google Scholar]
- Devi, L.S.; Yadava, P. Floristic diversity assessment and vegetation analysis of tropical semievergreen forest of Manipur, north east India. Trop. Ecol. 2006, 47, 89–98. [Google Scholar]
- Chowdhury, M.; Huda, M.; Islam, A. Phytodiversity of Dipterocarpus turbinatus Gaertn. F. (garjan) undergrowths at Dulahazara Garjan Forest, Cox’s Bazar, Bangladesh. Indian For. 2000, 126, 674–684. [Google Scholar]
- Kadavul, K.; Parthasarathy, N. Plant biodiversity and conservation of tropical semi-evergreen forest in the Shervarayan hills of Eastern Ghats, India. Biodivers. Conserv. 1999, 8, 419–437. [Google Scholar] [CrossRef]
- Pande, P. Comparative vegetation analysis and sal (Shorea robusta) regeneration in relation to their disturbance magnitude in some sal forests. Trop. Ecol. 1999, 40, 51–61. [Google Scholar]
- Fox, B.J.; Taylor, J.E.; Fox, M.D.; Williams, C. Vegetation changes across edges of rainforest remnants. Biol. Conserv. 1997, 82, 1–13. [Google Scholar] [CrossRef]
- Ohlson, M.; Söderström, L.; Hörnberg, G.; Zackrisson, O.; Hermansson, J. Habitat qualities versus long-term continuity as determinants of biodiversity in boreal old-growth swamp forests. Biol. Conserv. 1997, 81, 221–231. [Google Scholar] [CrossRef]
- Nautiyal, M.; Nautiyal, B.; Prakash, V. Phenology and growth form distribution in an alpine pasture at Tungnath, Garhwal, Himalaya. Mt. Res. Dev. 2001, 21, 168–174. [Google Scholar] [CrossRef] [Green Version]
- Qian, H.; Guo, Q. Linking biotic homogenization to habitat type, invasiveness and growth form of naturalized alien plants in North America. Divers. Distrib. 2010, 16, 119–125. [Google Scholar] [CrossRef]
- Tappeiner, J.; Zasada, J.; Ryan, P.; Newton, M. Salmonberry clonal and population structure: The basis for a persistent cover. Ecology 1991, 72, 609–618. [Google Scholar] [CrossRef]
- Milchunas, D.G.; Sala, O.E.; Lauenroth, W.K. A generalized model of the effects of grazing by large herbivores on grassland community structure. Am. Nat. 1988, 132, 87–106. [Google Scholar] [CrossRef]
- Timsina, B.; Shrestha, B.B.; Rokaya, M.B.; Münzbergová, Z. Impact of Parthenium hysterophorus L. invasion on plant species composition and soil properties of grassland communities in Nepal. Flora-Morphol. Distrib. Funct. Ecol. Plants 2011, 206, 233–240. [Google Scholar] [CrossRef]
- Amjad, M.S.; Arshad, M.; Sadaf, H.M.; Akrim, F.; Arshad, A. Floristic composition, biological spectrum and conservation status of the vegetation in Nikyal valley, Azad Jammu and Kashmir. Asian Pac. J. Trop. Dis. 2016, 6, 63–69. [Google Scholar] [CrossRef]
- Nazir, A.; Malik, Z. Life-form and index of similarity of plant communities recorded at Sarsawa Hills, District Kotli. J. Res. Sci 2006, 17, 27–33. [Google Scholar]
- Shah, M.; Hussain, F. Phytosociological Study of the Vegetation of Hayat Abadpeshawar, Pakistan. Pak. J. Plant Sci. 2009, 15. [Google Scholar]
- Traiser, C.; Klotz, S.; Uhl, D.; Mosbrugger, V. Environmental signals from leaves–a physiognomic analysis of European vegetation. New Phytol. 2005, 166, 465–484. [Google Scholar] [CrossRef]
- Nasir, Z.A.; Sultan, S. Floristic, biological and leaf size spectra of weeds in gram, lentil, mustard and wheat fields of district Chakwal, Pakistan. Pak. J. Biol. Sci. (Pak.) 2002, 5, 758–762. [Google Scholar]
- Hussain, T. A floristic description of flora and ethnobotany of Samahni Valley (AK), Pakistan. Ethnobot. Leafl. 2009, 2009, 6. [Google Scholar]
- Samad, M.; Badshah, L.; Khan, S.M. Biological spectra of Lala Kalay area distrist Peshawar Khyber Pakhtunkhwa province Pakistan. Pak. J. Weed Sci. Res. 2018, 24, 353–362. [Google Scholar] [CrossRef]
- Samreen, U.; Ibrar, M.; Badshah, L. Diversity and Ecological Characteristics of Weed Flora at Darazinda, Frontier Region Dera Ismail Khan Pakistan. Pak. J. Weed Sci. Res. 2018, 24, 223–229. [Google Scholar] [CrossRef]
- McIntosh, R.P. An index of diversity and the relation of certain concepts to diversity. Ecology 1967, 48, 392–404. [Google Scholar] [CrossRef]
- Singh, K.; Kumar, A.; Lal, B.; Todaria, N. Species diversity and population status of threatened plants in different landscape elements of the Rohtang Pass, Western Himalaya. J. Mt. Sci. 2008, 5, 73–83. [Google Scholar] [CrossRef]
- Ayyappan, N.; Parthasarathy, N. Biodiversity inventory of trees in a large-scale permanent plot of tropical evergreen forest at Varagalaiar, Anamalais, Western Ghats, India. Biodivers. Conserv. 1999, 8, 1533–1554. [Google Scholar] [CrossRef]
- Veloso, M.D.D.M.; Nunes, Y.R.F.; Azevedo, I.F.P.; Rodrigues, P.M.S.; Fernandes, L.A.; Santos, R.M.D.; Fernandes, G.W.; Pereira, J.A.A. Floristic and structural variations of the arboreal community in relation to soil properties in the Pandeiros river riparian forest, Minas Gerais, Brazil. Interciencia 2014, 39, 628–636. [Google Scholar]
- De Lima Dantas, V.; Batalha, M.A. Vegetation structure: Fine scale relationships with soil in a cerrado site. Flora-Morphol. Distrib. Funct. Ecol. Plants 2011, 206, 341–346. [Google Scholar] [CrossRef]
- Fagundes, N.C.A.; de Ávila, M.A.; de Souza, S.R.; de Azevedo, I.F.P.; Nunes, Y.R.F.; Fernandes, G.W.; Fernandes, L.A.; dos Santos, R.M.; Veloso, M.d.D.M. Riparian vegetation structure and soil variables in Pandeiros river, Brazil. Rodriguésia 2019, 70, e01822017. [Google Scholar] [CrossRef]



| Group I (Mining Sites) | Group II (Non-mining Sites) | ||
|---|---|---|---|
| Plant Binomial | Mean ± SE | Plant Binomial | Mean ± SE |
| Cynodon dactylon (L.) | 4.52 ± 0.70 | Cannabis sativa L. | 4.65 ± 1.28 |
| Cannabis sativa L. | 2.7 ± 0.87 | Cirsium arvense (L.) Scop. | 1.88 ± 0.67 |
| Cirsium arvense (L.) Scop. | 2.4 ± 0.73 | Corynephorus canescens (L.) P Beauv. | 2.13 ± 1.18 |
| Corynephorus canescens (L.) P. Beauv. | 2.52 ± 1.22 | Cynodon dactylon (L.) | 2.8 ± 0.94 |
| Datura metal L. | 2.07 ± 0.86 | Dodonaea viscosa (L.) Jacq. | 2.1 ± 1.43 |
| Helianthus annus L. | 2.22 ± 1.01 | Duchesnea indica (Andr.) Focke | 1.79 ± 1.01 |
| Mentha longifolia (L.) Huds | 3.12 ± 0.91 | Ficus carica L. | 1.62 ± 0.71 |
| Persicaria hydropiper (L.) Delarbre. | 2.06 ± 0.74 | Monotheca buxifolia (Falc.) A. DC. | 1.86 ± 1.15 |
| Populus nigra L. | 3.41 ± 0.98 | Olea ferruginea Royle. | 2.21 ± 1.12 |
| Ricinus communis L. | 2.24 ± 0.94 | Pinus roxburghii Sargent. | 2.86 ± 1.23 |
| Tagetes minuta L. | 2.61 ± 0.72 | Rumex dentatus L. | 1.68 ± 0.49 |
| Xanthium strumarium L. | 4.23 ± 1.12 | Ficus carica L. | 1.62 ± 0.71 |
| Factor | Code | Mining Sites | Non-Mining Sites | t-Value | p-Value |
|---|---|---|---|---|---|
| Aspect Angle (°) | AA | 257.5 ± 24.13 | 237.21 ± 21.21 | 0.68 | 0.25 |
| Elevation (m) | ELE | 1189.4 ± 179.68 | 1081.5 ± 111.02 | 1.13 | 0.13 |
| Latitude (°) | Lat. | 35.04 ± 0.08 | 35.00 ± 0.05 | 1.1 | 0.14 |
| Longitude (°) | Long | 71.95 ± 0.04 | 71.94 ± 0.02 | 0.17 | 0.43 |
| Sand (%age) | Sand | 47.41 ± 2.10 | 43.17 ± 2.27 | 1.2 | 0.12 |
| Silt (%age) | Silt | 29.82 ± 2.58 | 32.51 ± 2.87 | 0.79 | 0.22 |
| Clay (%age) | Clay | 22.75 ± 1.90 | 23.93 ± 2.19 | 0.66 | 0.33 |
| Nitrogen (%age) | N | 0.15 ± 0.05 | 0.34 ± 0.07 | 2.66 | 0.009 |
| Phosphorus (mg/Kg) | P | 5.12 ± 0.51 | 6.34 ± 0.54 | 1.76 | 0.05 |
| Potassium (mg/Kg) | K | 137.35 ± 18.06 | 110.92 ± 7.17 | 1.63 | 0.06 |
| Lime (%age) | L | 13.09 ± 1.02 | 11.17 ± 0.70 | 1.9 | 0.033 |
| Organic matter (%age) | OM | 0.86 ± 0.11 | 0.84 ± 0.09 | 0.14 | 0.44 |
| pH (1:5) | pH | 6.84 ± 0.14 | 6.47 ± 0.21 | 1.39 | 0.09 |
| Electrical conductivity | EC | 36.42 ± 2.61 | 37.5 ± 2.5 | 0.55 | 0.29 |
| Wilting point | WP | 0.13 ± 0.00 | 0.14 ± 0.01 | 0.65 | 0.22 |
| Bulk density (g/cm) | BD | 1.41 ± 0.01 | 1.42 ± 0.01 | 1.09 | 0.14 |
| Available water | AW | 0.12 ± 0.00 | 0.13 ± 0.00 | −1.04 | 0.16 |
| Saturation point | SP | 0.46 ± 0.00 | 0.47 ± 0.00 | −1.07 | 0.15 |
| Axes | Axis 1 | Axis 2 | Axis 3 | ||||
|---|---|---|---|---|---|---|---|
| Eigenvalue | 0.701 | 0.504 | 0.438 | ||||
| Species data variance | |||||||
| Variance explained (%) | 8.9 | 6.4 | 5.6 | ||||
| Cumulative variance (%) | 8.9 | 15.3 | 20.9 | ||||
| Correlation (Pearson). Spp-Envt | 0.995 | 0.969 | 0.989 | ||||
| Correlation (Kendall). Spp-Envt | 0.825 | 0.847 | 0.899 | ||||
| Variable | Correlation | Biplot scores | |||||
| Axis 1 | Axis 2 | Axis 3 | Axis 1 | Axis 2 | Axis 3 | ||
| 1 | AA | −0.188 | 0.02 | −0.331 | −0.157 | 0.014 | −0.224 |
| 2 | ELE | 0.955 | −0.192 | −0.034 | 0.795 | −0.133 | −0.023 |
| 3 | Lat. | 0.803 | −0.436 | 0.066 | 0.669 | −0.302 | 0.045 |
| 4 | Long | 0.752 | −0.417 | 0.199 | 0.626 | −0.289 | 0.134 |
| 5 | Sand | −0.003 | 0.03 | 0.07 | −0.003 | 0.021 | 0.047 |
| 6 | Silt | −0.111 | −0.024 | −0.302 | −0.092 | −0.017 | −0.204 |
| 7 | Clay | 0.145 | −0.016 | 0.291 | 0.121 | −0.011 | 0.196 |
| 8 | N | 0.146 | 0.125 | 0.268 | 0.122 | 0.087 | 0.181 |
| 9 | P | −0.188 | 0.076 | 0.383 | −0.156 | 0.053 | 0.258 |
| 10 | K | −0.351 | 0.262 | −0.03 | −0.292 | 0.181 | −0.02 |
| 11 | L | −0.188 | 0.032 | −0.255 | −0.156 | 0.022 | −0.172 |
| 12 | OM | −0.003 | −0.063 | −0.11 | −0.002 | −0.043 | −0.074 |
| 13 | pH | 0.147 | 0.207 | −0.538 | 0.122 | 0.143 | −0.363 |
| 14 | EC | −0.04 | −0.036 | 0.352 | −0.034 | −0.025 | 0.238 |
| 15 | WP | 0.115 | −0.025 | 0.265 | 0.095 | −0.017 | 0.179 |
| 16 | BD | −0.184 | −0.001 | −0.316 | −0.153 | 0 | −0.213 |
| 17 | AW | −0.091 | −0.029 | −0.252 | −0.076 | −0.02 | −0.17 |
| 18 | SP | 0.176 | 0 | 0.313 | 0.147 | 0 | 0.211 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, K.; Khan, N.; Ullah, R.; Shah, M.; Khan, M.E.H.; Jones, D.A.; Dewidar, M. Spatial Pattern and Key Environmental Determinants of Vegetation in Sand Mining and Non-Mining Sites along the Panjkora River Basin. Land 2022, 11, 1801. https://doi.org/10.3390/land11101801
Ali K, Khan N, Ullah R, Shah M, Khan MEH, Jones DA, Dewidar M. Spatial Pattern and Key Environmental Determinants of Vegetation in Sand Mining and Non-Mining Sites along the Panjkora River Basin. Land. 2022; 11(10):1801. https://doi.org/10.3390/land11101801
Chicago/Turabian StyleAli, Kishwar, Nasrullah Khan, Rafi Ullah, Muzammil Shah, Muhammad Ezaz Hasan Khan, David Aaron Jones, and Maha Dewidar. 2022. "Spatial Pattern and Key Environmental Determinants of Vegetation in Sand Mining and Non-Mining Sites along the Panjkora River Basin" Land 11, no. 10: 1801. https://doi.org/10.3390/land11101801
APA StyleAli, K., Khan, N., Ullah, R., Shah, M., Khan, M. E. H., Jones, D. A., & Dewidar, M. (2022). Spatial Pattern and Key Environmental Determinants of Vegetation in Sand Mining and Non-Mining Sites along the Panjkora River Basin. Land, 11(10), 1801. https://doi.org/10.3390/land11101801








