Understanding Recovery Is as Important as Understanding Decline: The Case of the Crested Ibis in China
Abstract
:1. Introduction
2. Methods
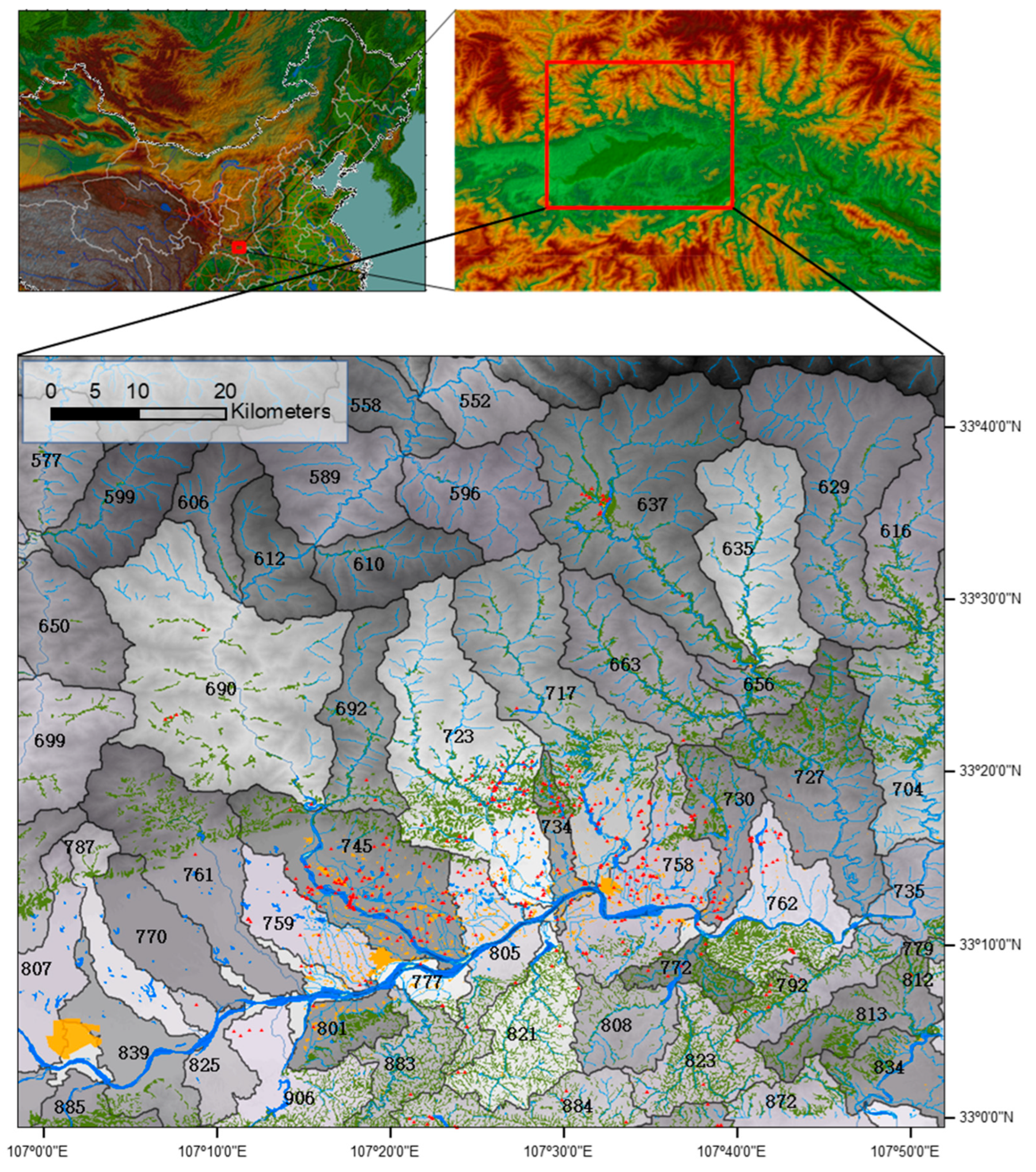
2.1. Study Area and Nest Data Collection
2.2. Using Watersheds as the Spatial Scale for Habitat Analysis
2.3. Envirenmental Variables
2.4. Species Distribution Modelling
2.5. Nest Density and Carrying Capacity Estimation
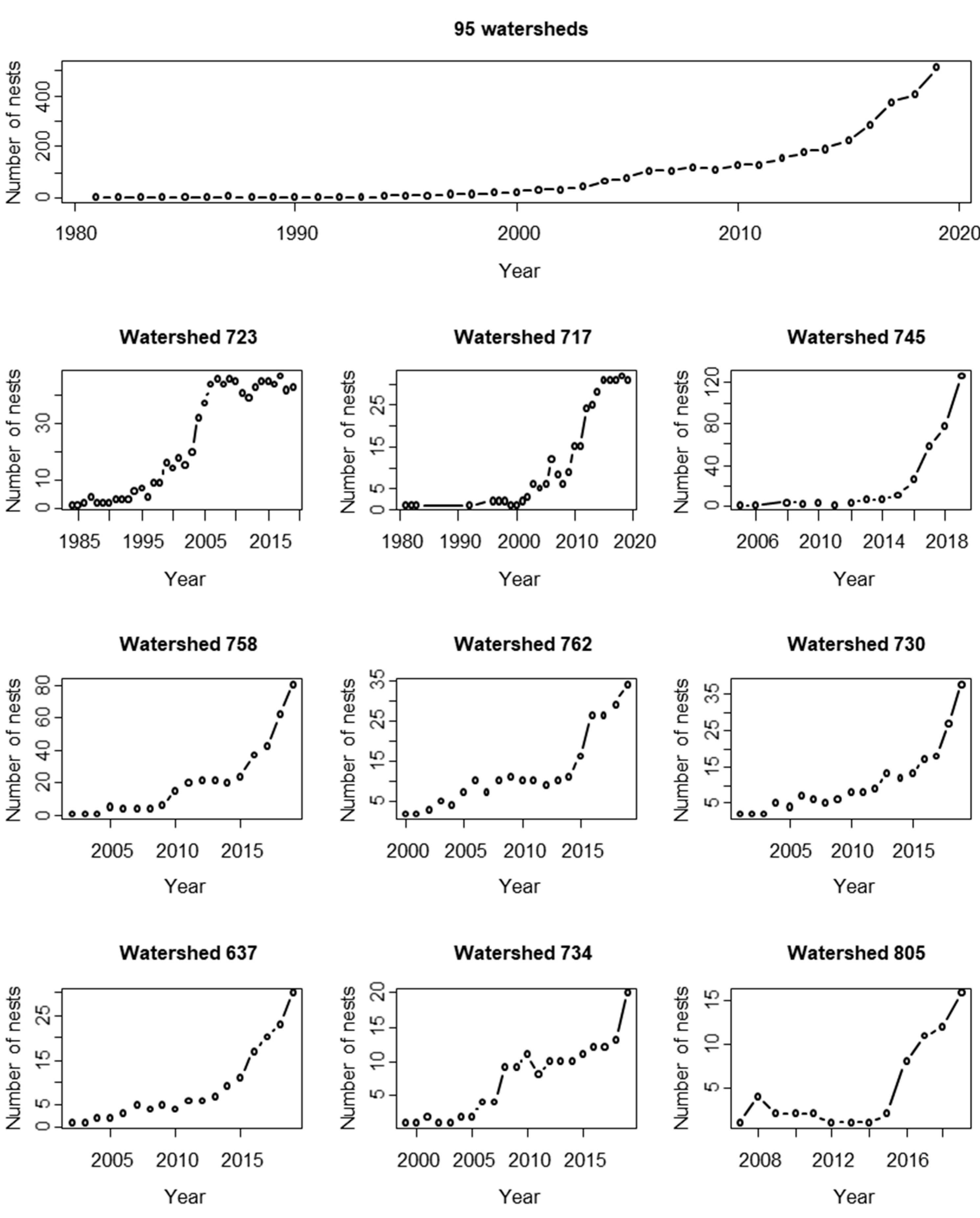
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Channell, R.; Lomolino, M.V. Dynamic biogeography and conservation of endangered species. Nature 2000, 403, 84–86. [Google Scholar] [CrossRef] [PubMed]
- Novacek, M.J.; Cleland, E.E. The current biodiversity extinction event: Scenarios for mitigation and recovery. Proc. Natl. Acad. Sci. USA 2001, 98, 5466–5470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rjf, A.; Scott, J.M. Examining differences between recovered and declining endangered species. Conserv. Biol. 2001, 15, 1274–1284. [Google Scholar] [CrossRef]
- Tear, T.H.; Scott, J.M.; Hayward, P.H.; Griffith, B. Recovery plans and the endangered-species-act—Are criticisms supported by data. Conserv. Biol. 1995, 9, 182–195. [Google Scholar] [CrossRef]
- Vredenburg, V.T. Reversing introduced species effects: Experimental removal of introduced fish leads to rapid recovery of a declining frog. Proc. Natl. Acad. Sci. USA 2004, 101, 7646–7650. [Google Scholar] [CrossRef] [Green Version]
- Fleming, W.J.; Grue, C.E. Recovery of cholinesterase activity in 5 avian species exposed to dicrotophos, an organo-phosphorus pesticide. Pestic. Biochem. Physiol. 1981, 16, 129–135. [Google Scholar] [CrossRef]
- Kauffman, M.J.; Frick, W.F.; Linthicum, J. Estimation of habitat-specific demography and population growth for peregrine falcons in California. Ecol. Appl. 2003, 13, 1802–1816. [Google Scholar] [CrossRef]
- Bowerman, W.W.; Giesy, J.P.; Best, D.A.; Kramer, V.J. A review of factors affecting productivity of bald eagles in the Great Lakes region: Implications for recovery. Environ. Health Perspect. 1995, 103, 51–59. [Google Scholar] [CrossRef]
- Ma, L.; Li, X.; Zhai, T.; Zhang, Y.; Song, K.; Holyoak, M.; Sun, Y. Changes in the Habitat Preference of Crested Ibis (Nipponia nippon) during a Period of Rapid Population Increase. Animals 2021, 11, 2626. [Google Scholar] [CrossRef]
- Shi, D.; Yu, X.; Fan, C.; Cao, Y. The description of the decline of the crested ibis. J. Northwest Univ. 1991, 21, 25–30. [Google Scholar]
- Zhang, Y.; Wang, F.; Cui, Z.; Li, M.; Li, X.; Ye, X.; Yu, X. Can we reestablish a self-sustaining population? A case study on reintroduced Crested Ibis with population viability analysis. Avian Res. 2021, 12, 14. [Google Scholar] [CrossRef]
- Li, X.; Tian, H.; Li, D. Why the crested ibis declined in the middle twentieth century. Biodivers. Conserv. 2009, 18, 2165–2172. [Google Scholar] [CrossRef]
- Liu, Y. Rediscovery of the crested ibis in Qinling Mountains. Acta Zool. Sin. 1981, 27, 273. [Google Scholar]
- BirdLife International. Nipponia nippon. In 1994 IUCN Red List of Threatened Species; IUCN: Gland, Switzerland, 1994. [Google Scholar]
- BirdLife International. Species Factsheet: Nipponia nippon. 2018. Available online: http://www.birdlife.org (accessed on 17 April 2018).
- Wang, M.; Ye, X.-P.; Li, Y.-F.; Huo, Z.-P.; Li, X.; Yu, X.-P. On the sustainability of a reintroduced crested ibis population in Qinling Mountains, Shaanxi, Central China. Restor. Ecol. 2017, 25, 261–268. [Google Scholar] [CrossRef]
- Yoshio, M.; Kato, N.; Miyashita, T. Landscape and local scale effects on the orthopteran assemblages in the paddy agroecosystems on the Sado Island, Japan with implications for the habitat management for the crested ibis. Ecol. Civ. Eng. 2009, 12, 99–107. [Google Scholar] [CrossRef]
- Kim, K.-a.; Car, J.; Gimtae, L.; Bak, H. Sex Identification of the First Incubated Chicks of the Crested Ibis Nipponia nippon in Korea. J. Life Sci. 2011, 21, 626–630. (In Korean) [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Wang, T.; Skidmore, A.K.; Palmer, S.C.F.; Ye, X.; Ding, C.; Wang, Q. Predicting and understanding spatio-temporal dynamics of species recovery: Implications for Asian crested ibis Nipponia nippon conservation in China. Divers. Distrib. 2016, 22, 893–904. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, D. Current state and the future of the crested ibis (Nipponia nippon): A case study by population viability analysis. Ecol. Res. 1998, 13, 323–333. [Google Scholar] [CrossRef]
- Nicoll, M.A.C.; Jones, C.G.; Norris, K. Declining survival rates in a reintroduced population of the Mauritius kestrel: Evidence for non-linear density dependence and environmental stochasticity. J. Anim. Ecol. 2003, 72, 917–926. [Google Scholar] [CrossRef]
- Ye, Y.; Jiang, Y.; Hu, C.; Liu, Y.; Qing, B.; Wang, C.; Fernandez-Juricic, E.; Ding, C. What makes a tactile forager join mixed-species flocks? A case study with the endangered Crested Ibis (Nipponia nippon). Auk 2017, 134, 421–431. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, D.; Ma, Z.; Zhai, T.; Drummond, H. Ritualized aggression and unstable dominance in broods of crested ibis (Nipponia nippon). Wilson Bull. 2004, 116, 172–176. [Google Scholar] [CrossRef]
- Li, X.; Li, D.; Ma, Z.; Schneider, D.C. Nest site use by crested ibis: Dependence of a multifactor model on spatial scale. Landsc. Ecol. 2006, 21, 1207–1216. [Google Scholar] [CrossRef]
- Teng, S.N.; Xu, C.; Sandel, B.; Svenning, J.-C. Effects of intrinsic sources of spatial autocorrelation on spatial regression modelling. Methods Ecol. Evol. 2018, 9, 363–372. [Google Scholar] [CrossRef] [Green Version]
- Segurado, P.; Araujo, M.B.; Kunin, W.E. Consequences of spatial autocorrelation for niche-based models. J. Appl. Ecol. 2006, 43, 433–444. [Google Scholar] [CrossRef]
- Lehner, C.; Verdin, K.; Jarvis, A. HydroSHEDS Technical Documentation; World Wildlife Fund US: Washington, DC, USA, 2006. [Google Scholar]
- Guth, P.L. Drainage basin morphometry: A global snapshot from the shuttle radar topography mission. Hydrol. Earth Syst. Sci. 2011, 15, 2091–2099. [Google Scholar] [CrossRef] [Green Version]
- Rabus, B.; Eineder, M.; Roth, A.; Bamler, R. The shuttle radar topography mission—A new class of digital elevation models acquired by spaceborne radar. ISPRS J. Photogramm. Remote Sens. 2003, 57, 241–262. [Google Scholar] [CrossRef]
- Chinese Academy of Surveying and Mapping. China Basic Geographical Data: 1:1 Million Landcover; Chinese Academy of Surveying and Mapping: Beijing, China, 2004. [Google Scholar]
- Data Center for Resources and Environmental Sciences. China’s Gross Domestic Product Data (1 km Resolution); Institute of Geographic Sciences and Natural Resources Research, CAS: Beijing, China, 2006. [Google Scholar]
- Data Center for Resources and Environmental Sciences. China’s Population Density Data (1 km Resolution); Institute of Geographic Sciences and Natural Resources Research, CAS: Beijing, China, 2006. [Google Scholar]
- Sanderson, E.W.; Jaiteh, M.; Levy, M.A.; Redford, K.H.; Wannebo, A.V.; Woolmer, G. The Human Footprint and the Last of the Wild. Bioscience 2002, 52, 891–904. [Google Scholar] [CrossRef] [Green Version]
- China Meteorological Administration. The Meteorological Data for China during the Period 1971–2000; China Meteorological Administration: Beijing, China, 2012.
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Chinese Academy of Surveying and Mapping. China Basic Geological Data: 1:1 Million Raster Data of Vegetation and Land Cover; Chinese Academy of Surveying and Mapping: Beijing, China, 2004. [Google Scholar]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, N.; Li, B.; Sun, Y.; Gao, E. abundanceR: A novel method for estimating wildlife abundance based on distance sampling and species distribution models. Land 2022, 11, 660. [Google Scholar] [CrossRef]
- Boyce, M.S.; Johnson, C.J.; Merrill, E.H.; Nielsen, S.E.; Solberg, E.J.; van Moorter, B. Can habitat selection predict abundance? J. Anim. Ecol. 2016, 85, 11–20. [Google Scholar] [CrossRef]
- Wang, F.; Li, M.; Zhang, Y.-S.; Zhao, W.-A.; Liu, D.-N.; Zhang, Y.-Z.; Zhang, H.; Ye, X.-P.; Yu, X.-P. Post-release dispersal and breeding site suitability of reintroduced populations of the Crested Ibis in Shaanxi Province, China. Restor. Ecol. 2021, 29, e13383. [Google Scholar] [CrossRef]
- Hu, C.; Song, X.; Ding, C.; Ye, Y.; Qing, B.; Wang, C. The size of winter-flooded paddy fields no longer limits the foraging habitat use of the endangered crested ibis (Nipponia nippon) in winter. Zool. Sci. 2016, 33, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, T.; Skidmore, A.K.; Wang, Q.; Ding, C. Decline of traditional rice farming constrains the recovery of the endangered Asian crested ibis (Nipponia nippon). Ambio 2015, 44, 803–814. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Skidmore, A.K.; Wang, T.; van Gils, H.A.M.J.; Wang, Q.; Qing, B.; Ding, C. Reduced dependence of crested ibis on winter-flooded rice fields: Implications for their conservation. PLoS ONE 2014, 9, e98690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Li, B.; Wang, G.; Zhan, X.; Holyoak, M. Deeply digging the interaction effect in multiple linear regressions using a fractional-power interaction term. MethodsX 2020, 7, 101067. [Google Scholar] [CrossRef] [PubMed]
- Albert, C.H.; Yoccoz, N.G.; Edwards, T.C., Jr.; Graham, C.H.; Zimmermann, N.E.; Thuiller, W. Sampling in ecology and evolution—Bridging the gap between theory and practice. Ecography 2010, 33, 1028–1037. [Google Scholar] [CrossRef] [Green Version]
- Jones, C.G.; Heck, W.; Lewis, R.E.; Mungroo, Y.; Slade, G.; Cade, T. The restoration of the Mauritius kestrel falco-punctatus population. Ibis 1995, 137, S173–S180. [Google Scholar] [CrossRef]
- Finkelstein, M.E.; Doak, D.F.; George, D.; Burnett, J.; Brandt, J.; Church, M.; Grantham, J.; Smith, D.R. Lead poisoning and the deceptive recovery of the critically endangered California condor. Proc. Natl. Acad. Sci. USA 2012, 109, 11449–11454. [Google Scholar] [CrossRef]
| Variables Measured within Each Watershed | Parameters | Unit | Source | |||
|---|---|---|---|---|---|---|
| Mean | Minimum | Maximum | SD | |||
| Area | 154.11 | 32.65 | 474.66 | 99.59 | square km | (Rabus et al. 2003) [29] |
| Mean elevation | 1018.32 | 508.43 | 1891.37 | 394.91 | m | (Rabus et al. 2003) [29] |
| SD of elevation | 243.48 | 28.84 | 601.06 | 121.95 | m | (Rabus et al. 2003) [29] |
| Area of rice paddies | 1.67 | 0.00 | 12.95 | 2.31 | square km | (Chinese Academy of Surveying and Mapping 2004) [36] |
| Area of water bodies | 0.12 | 0.00 | 1.03 | 0.19 | square km | (Chinese Academy of Surveying and Mapping 2004) [36] |
| Population density in 2000 | 190.36 | 3.93 | 1387.31 | 234.86 | person per square km | (Data Center for Resources and Environmental Sciences 2006b) [32] |
| GDP in 2000 | 45.34 | 0.69 | 453.23 | 78.75 | 1000 RMB per capita | (Data Center for Resources and Environmental Sciences 2006a) [31] |
| Human footprint index | 25.04 | 14.62 | 47.62 | 6.67 | / | (Sanderson et al. 2002) [33] |
| Annual mean temperature from 1971 to 2000 | 12.37 | 7.61 | 14.78 | 1.98 | degree Celsius | (China Meteorological Administration 2012) [34] |
| Annual total precipitation from 1971 to 2000 | 878.44 | 803.36 | 1049.88 | 54.57 | mm | (China Meteorological Administration 2012) [34] |
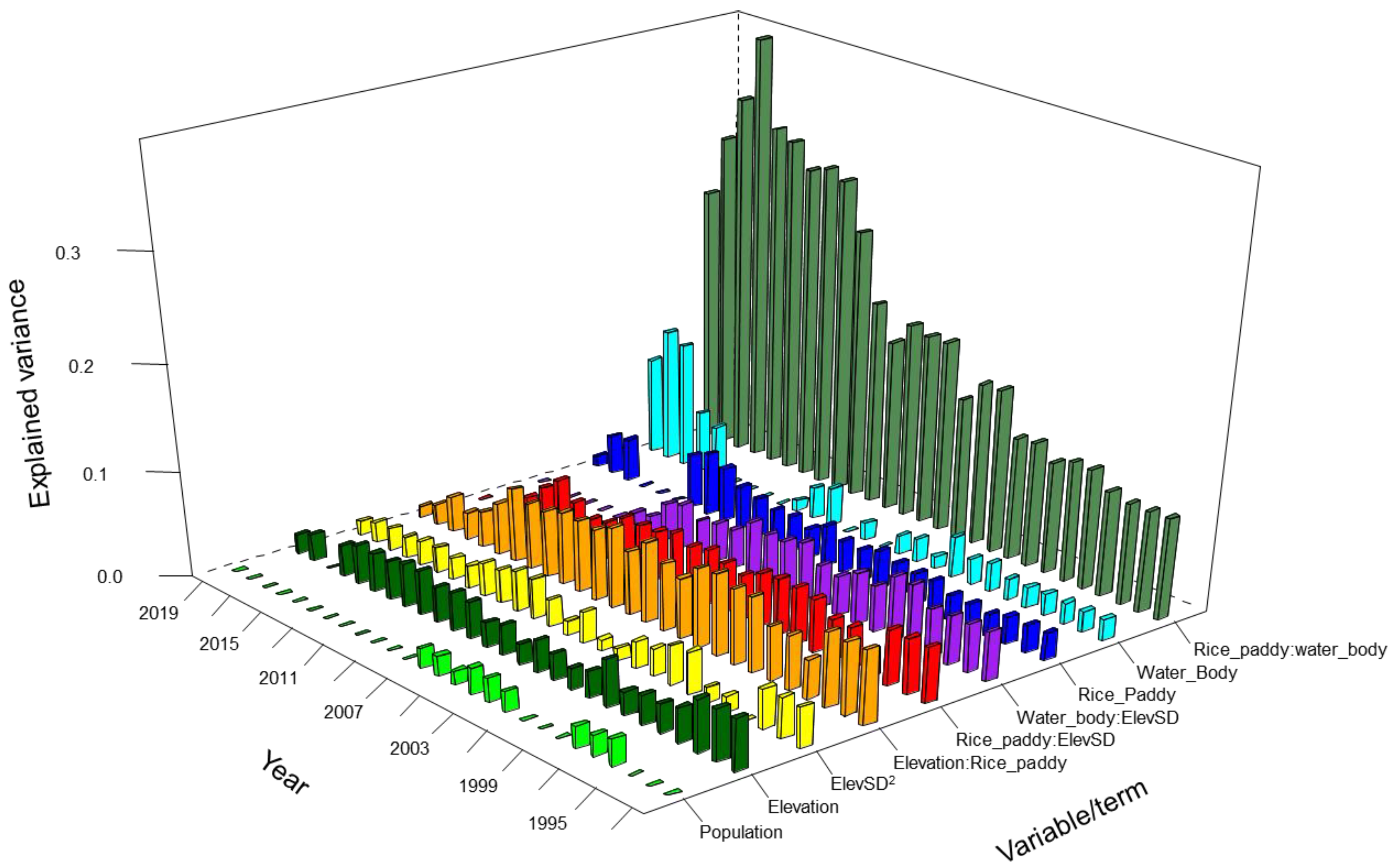
| Negative Binomial Regressions | Multiple Regression | ||||
|---|---|---|---|---|---|
| Variable/Term | Proportion of Deviance (Mean Value) * | Significance in 27 Years ** | Variable/Term | R Square (Mean Value) | Significance in 27 Years ** |
| Elevation | 0.064 | 19 | Elevation | 0.033 | 26 |
| Elevation:Population | 0.538 | 5 | Elevation:Rice_paddy | 0.058 | 27 |
| ElevSD | 0.122 | 24 | ElevSD2 | 0.026 | 26 |
| Elevation2 | 0.144 | 18 | Population | 0.021 | 9 |
| Population2 | 0.050 | 19 | Rice_paddy | 0.033 | 24 |
| Rice_paddy | 0.061 | 24 | Rice_paddy:ElevSD | 0.044 | 25 |
| Rice_paddy:Wter_body | 0.340 | 10 | Rice_paddy:Waer_body | 0.198 | 27 |
| Water_body | 0.169 | 24 | Water_body | 0.038 | 21 |
| Water_body:ElevSD | 0.042 | 21 | |||
| Year | No. of Nests | No. of Distance <10,000 m | No. of Distance <1000 m | No. of Distance <100 m | No. of Distance <10 m | No. of Distance <1 m |
|---|---|---|---|---|---|---|
| 1993 | 3 | 2 | 0 | 0 | 0 | 0 |
| 1994 | 6 | 14 | 1 | 1 | 0 | 0 |
| 1995 | 7 | 21 | 2 | 1 | 0 | 0 |
| 1996 | 6 | 10 | 0 | 0 | 0 | 0 |
| 1997 | 11 | 44 | 2 | 0 | 0 | 0 |
| 1998 | 11 | 54 | 2 | 0 | 0 | 0 |
| 1999 | 18 | 147 | 14 | 1 | 0 | 0 |
| 2000 | 20 | 117 | 13 | 3 | 0 | 0 |
| 2001 | 30 | 220 | 19 | 3 | 0 | 0 |
| 2002 | 31 | 153 | 8 | 2 | 0 | 0 |
| 2003 | 42 | 312 | 22 | 3 | 0 | 0 |
| 2004 | 62 | 721 | 60 | 6 | 0 | 0 |
| 2005 | 78 | 956 | 77 | 7 | 1 | 0 |
| 2006 | 105 | 1655 | 114 | 2 | 1 | 0 |
| 2007 | 106 | 1510 | 86 | 13 | 9 | 0 |
| 2008 | 118 | 1736 | 80 | 5 | 0 | 0 |
| 2009 | 112 | 1765 | 78 | 3 | 0 | 0 |
| 2010 | 128 | 2373 | 79 | 0 | 0 | 0 |
| 2011 | 129 | 2178 | 61 | 1 | 1 | 0 |
| 2012 | 153 | 2796 | 61 | 1 | 1 | 0 |
| 2013 | 177 | 3588 | 78 | 3 | 2 | 0 |
| 2014 | 189 | 3902 | 100 | 5 | 2 | 0 |
| 2015 | 220 | 4759 | 137 | 11 | 4 | 0 |
| 2016 | 286 | 6992 | 191 | 35 | 14 | 1 |
| 2017 | 368 | 9695 | 271 | 53 | 26 | 1 |
| 2018 | 405 | 14,115 | 543 | 75 | 40 | 2 |
| 2019 | 511 | 26,194 | 1019 | 162 | 58 | 2 |
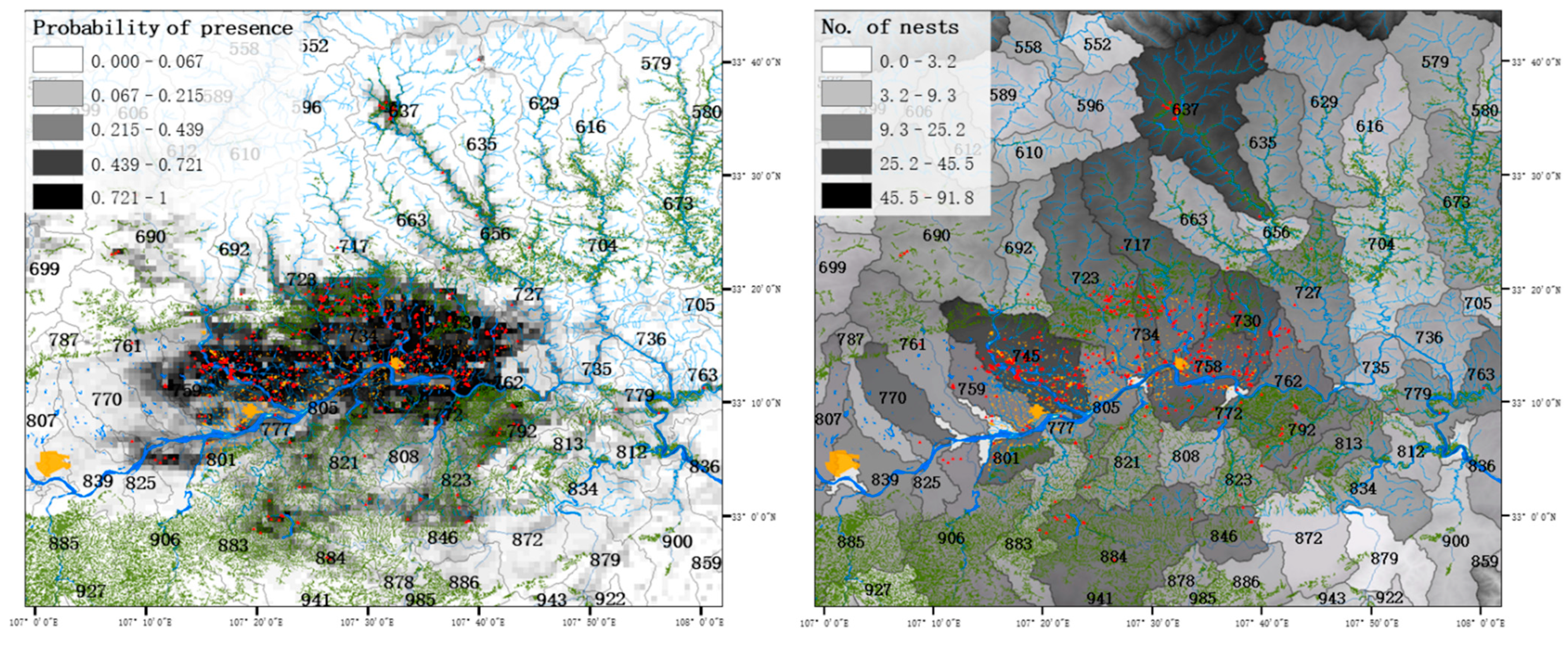
| Watershed | No. Nests in 2019 | Predicted_Point * | Predicted_Polygon ** | Adjusted_Point * | Adjusted_Polygon ** |
|---|---|---|---|---|---|
| 745 | 126 | 196.5 | 76.0 | 53.0 | 91.8 |
| 758 | 81 | 188.1 | 67.0 | 50.7 | 81.0 |
| 723 | 43 | 154.4 | 37.6 | 41.6 | 45.5 |
| 730 | 38 | 70.1 | 20.9 | 18.9 | 25.2 |
| 762 | 34 | 97.9 | 27.9 | 26.4 | 33.7 |
| 717 | 31 | 119.0 | 24.2 | 32.1 | 29.2 |
| 637 | 30 | 37.7 | 27.8 | 10.2 | 33.6 |
| 734 | 20 | 44.1 | 14.7 | 11.9 | 17.8 |
| 805 | 16 | 83.9 | 11.1 | 22.6 | 13.5 |
| 884 | 15 | 95.9 | 17.7 | 25.8 | 21.4 |
| 759 | 10 | 69.2 | 15.3 | 18.7 | 18.4 |
| 792 | 8 | 41.7 | 6.0 | 11.2 | 7.2 |
| 821 | 6 | 68.9 | 7.1 | 18.6 | 8.6 |
| 690 | 5 | 51.9 | 3.7 | 14.0 | 4.5 |
| 777 | 5 | 22.1 | 14.3 | 6.0 | 17.3 |
| 772 | 4 | 35.3 | 4.7 | 9.5 | 5.7 |
| 846 | 4 | 26.7 | 5.5 | 7.2 | 6.7 |
| 906 | 4 | 30.6 | 7.7 | 8.3 | 9.3 |
| 727 | 3 | 51.3 | 2.9 | 13.8 | 3.5 |
| 801 | 3 | 20.7 | 7.6 | 5.6 | 9.2 |
| 823 | 3 | 49.2 | 4.2 | 13.3 | 5.1 |
| 692 | 2 | 12.0 | 1.1 | 3.3 | 1.3 |
| 779 | 2 | 18.6 | 3.4 | 5.0 | 4.1 |
| 813 | 2 | 19.6 | 3.5 | 5.3 | 4.2 |
| 629 | 1 | 7.7 | 1.2 | 2.1 | 1.4 |
| 663 | 1 | 16.6 | 1.1 | 4.5 | 1.4 |
| 748 | 1 | 6.9 | 1.4 | 1.9 | 1.7 |
| 761 | 1 | 47.5 | 2.3 | 12.8 | 2.8 |
| 763 | 1 | 11.6 | 4.0 | 3.1 | 4.8 |
| 808 | 1 | 31.7 | 2.0 | 8.5 | 2.4 |
| 825 | 1 | 14.7 | 4.2 | 4.0 | 5.0 |
| 883 | 1 | 44.2 | 2.4 | 11.9 | 2.9 |
| 770 | 0 | 17.7 | 5.8 | 4.8 | 7.0 |
| 807 | 0 | 7.3 | 5.4 | 2.0 | 6.5 |
| 635 | 0 | 7.8 | 4.6 | 2.1 | 5.6 |
| 774 | 0 | 0.1 | 3.9 | 0.0 | 4.8 |
| 839 | 0 | 9.2 | 3.7 | 2.5 | 4.5 |
| 876 | 0 | 11.8 | 3.4 | 3.2 | 4.1 |
| Total | 503 | 2144 | 499 | 578 | 602 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Fu, J.; Zhai, T.; Zhang, Y.; Bruford, M.W.; Sun, Y.; Zhan, X. Understanding Recovery Is as Important as Understanding Decline: The Case of the Crested Ibis in China. Land 2022, 11, 1817. https://doi.org/10.3390/land11101817
Li X, Fu J, Zhai T, Zhang Y, Bruford MW, Sun Y, Zhan X. Understanding Recovery Is as Important as Understanding Decline: The Case of the Crested Ibis in China. Land. 2022; 11(10):1817. https://doi.org/10.3390/land11101817
Chicago/Turabian StyleLi, Xinhai, Jiayu Fu, Tianqing Zhai, Yazu Zhang, Michael W. Bruford, Yuehua Sun, and Xiangjiang Zhan. 2022. "Understanding Recovery Is as Important as Understanding Decline: The Case of the Crested Ibis in China" Land 11, no. 10: 1817. https://doi.org/10.3390/land11101817






